| Research Article | ||
Open Vet. J.. 2024; 14(5): 1191-1198 Open Veterinary Journal, (2024), Vol. 14(5): 1191–1198 Research Article Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activityGabriel Alvarez1,2, Sofia Villanueva3, Elizabeth Breininger1,4, Marisa Geller5, Claudio Ruhlmann2, Gabriel Dalvit6, Pablo Cetica1,4*† and Masashige Kuwayama7†1CONICET-Universidad de Buenos Aires, Instituto de Investigaciones en Producción Animal (INPA), Buenos Aires, Argentina 2Fertilidad San Isidro, Buenos Aires, Argentina 3Freelance Embryologist, Buenos Aires, Argentina 4Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Instituto de Investigación y Tecnología en Reproducción Animal (INITRA), Buenos Aires, Argentina 5In vitro Buenos Aires, Buenos Aires, Argentina 6Advanced Fertility Center Cancun, Cancun, Mexico 7Repro-Support Medical Research Center, Tokyo, Japan *Corresponding Author: Pablo Cetica. Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Cátedra de Química Biológica, Buenos Aires, Argentina. Email: pcetica [at] fvet.uba.ar †Equal senior contribution Submitted: 10/02/2024 Accepted: 25/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
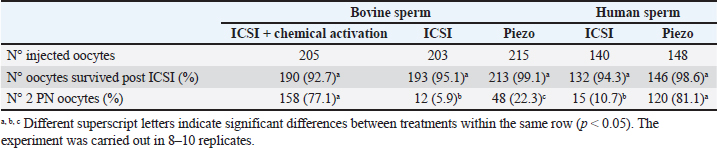
AbstractBackground: The intracytoplasmic sperm injection (ICSI) technique has low efficiency in cattle. This has mainly been attributed to the oocyte activation failure due to oocyte and/or sperm factors. Aim: Our aim was to evaluate the effect of conventional ICSI and Piezo-ICSI with bull or human sperm on bovine oocyte activation and embryo development and to assess its relationship with the phospholipase C zeta (PLCɀ) activity of both species. Methods: In vitro matured bovine oocytes were randomly divided into five groups and were fertilized as follows: conventional ICSI using bovine sperm with chemical activation (control), conventional ICSI using bovine sperm, Piezo-ICSI using bovine sperm, conventional ICSI using human sperm, and Piezo-ICSI using human sperm. PLCɀ activity was determined in bull and human sperm samples. Results: Within the groups using bull sperm, the oocytes fertilized by conventional ICSI had the lowest values of 2 pronuclei (PN) formation and cleavage, Piezo-ICSI increased both percentages and ICSI + chemical activation presented the highest 2 PN, cleavage, and blastocyst rates (p < 0.05). Within the groups using human sperm, the oocytes fertilized by Piezo-ICSI presented higher 2 PN and cleavage rates than those activated by conventional ICSI (p < 0.05). Piezo-ICSI with human sperm increased bovine oocyte activation as much as conventional ICSI + chemical activation with bovine sperm (p < 0.05). Higher values of PLCɀ activity were found in human sperm compared with bovine sperm (p < 0.05). Conclusion: Our results suggest that the higher stability of the bovine sperm in combination with its relatively low content of PLCɀ impairs bovine oocyte activation after ICSI. Keywords: Bovine, ICSI, Piezo-ICSI, PLCɀ, Oocyte activation. IntroductionIntracytoplasmic sperm injection (ICSI) is an in vitro technique that consists of the perforation of the zona pellucida and the oolemma using sharp micropipettes to inject a single sperm with a crushed tail into the cytoplasm of a matured oocyte. ICSI is the selected fertilization technique in cases of low sperm quality in humans (Kang et al., 2018). It can also represent an interesting alternative for its use with sex-sorted semen or in species in which in vitro fertilization (IVF) does not work properly. However, this technique has low efficiency in cattle and it needs to be improved (Unnikrishnan et al., 2021). In several species such as human, mice, and hamster, the sperm injection procedure itself is enough to induce Ca2+ oscillations in the oocyte required for oocyte activation and subsequent embryo development. Even though in Goto et al. (1990) reported the first calf obtained by ICSI, very few groups have been able to obtain similar results (García-Roselló et al., 2009). The low efficiency of ICSI in bovine has mainly been attributed to the characteristics of the oocyte of this species, which has problems related to the decondensation of the sperm head, pronuclei (PN) formation, and delays in the beginning of Ca2+ oscillations (Malcuit et al., 2006; Zambrano et al., 2016). Due to these problems, bovine ICSI is usually accompanied by the chemical activation of the oocyte to stimulate embryo development (Unnikrishnan et al., 2021). Nevertheless, it has been reported that activation protocols significantly affect developmental rates and bovine embryo quality (Felmer and Arias, 2015). A common protocol for the chemical activation of bovine oocytes is the use of ionomycin in combination with 6-dimethylaminopurine (6-DMAP), resulting in PN formation and blastocyst development (Bevacqua et al., 2010; Ohlweiler et al., 2013). Unfortunately, this protocol also causes DNA synthesis alterations, chromosomal abnormalities, accelerated PN formation in comparison with embryos produced by IVF, and high rates of parthenogenetic activation (Liu and Yang, 1999; Keskintepe et al., 2002; Canel et al., 2010). Another strategy to stimulate oocyte activation is the use of an electric piezo drill coupled to a blunt injection pipette (Piezo-ICSI). This device causes micro vibrations and electrical pulses that facilitate not only the zona pellucida and the oolemma penetration, but also the rupture of the sperm plasma membrane with the consequent release of sperm content into the ooplasm (Devito et al., 2010; Salgado et al., 2018). It has been suggested that the low efficiency of ICSI in bovine may also be related to the features of the bull sperm (Morozumi et al., 2006). A principal event of the oocyte activation process consists of an increase of intracellular Ca2+ from the endoplasmic reticulum in response to inositol 1, 4, 5-triphosphate (IP3) produced by phospholipase C zeta (PLCɀ). This enzyme has been identified in the sperm of several mammalian species. After oocyte-sperm fusion, PLCɀ is delivered by the fertilizing sperm to the ooplasm, resulting in the release of IP3 and the subsequent increase in the intracellular cytosolic calcium concentrations that activate the oocyte (Nomikos et al., 2013). Heterologous fertilization, using human sperm with bovine oocytes, has been used to study human sperm function (Nakamura et al., 2001). This approach can also be used to study bovine oocyte activation after fertilization with the sperm of a species in which chemical activation is not used, like human sperm. We hypothesize that the bovine oocyte activation failure after ICSI is in part due to the low PLCɀ release and/or activity in bull sperm. The aim of the present study was to evaluate the effect of conventional ICSI and Piezo-ICSI with bull or human sperm on bovine oocyte activation and embryo development, and to assess its relationship with PLCɀ activity of both species. Materials and MethodsMaterialsUnless specified, all chemicals and reagents were purchased from Sigma (Sigma Chemical Co., St. Louis, MO). Experimental designFirst, we wanted to verify in vitro bovine oocyte maturation, IVF, and embryo development in our laboratory system. For this purpose, in vitro matured bovine oocytes were divided into two groups to evaluate oocyte nuclear and cytoplasmic maturation and bovine sperm quality using a standard protocol for in vitro embryo production. The experiment was carried out in three replicates. To evaluate 2 PN formation or cleavage and blastocyst rates, ICSI trials were carried out using in vitro matured bovine oocytes which were randomly divided into five groups: conventional ICSI using bovine sperm with chemical activation (control), conventional ICSI using bovine sperm, Piezo-ICSI using bovine sperm, conventional ICSI using human sperm, and Piezo-ICSI using human sperm. The experiment was replicated 8 to 10 times. Sperm samples from bovine and human were also used to measure PLCɀ activity. The experiment was carried out in three replicates. Recovery of bovine cumulus-oocyte complexes (COCs)Bovine ovaries were collected from an abattoir within 30 minutes after slaughter and kept warm (30°C) during the 2 hours journey to the laboratory. Ovaries were washed with physiological saline containing 100,000 IU/l penicillin and 100 mg/l streptomycin. Cumulus-oocyte complexes (COCs) were recovered by aspiration of antral follicles (3–5 mm in diameter) and collected directly in the maturation medium. COCs were washed three times before being placed in the definitive maturation medium. Only oocytes surrounded by a compact and multilayered cumulus oophorus were used. In vitro maturation of bovine COCsCOCs were cultured in medium 199 (Earle’s salts, L-glutamine, 2.2 mg/l sodium bicarbonate; GIBCO, Grand Island, NY) supplemented with 5%(v/v) fetal bovine serum (FBS; GIBCO), 0.2 mg/l porcine FSH (Folltropin-V; Bioniche, Belleville, Ontario, Canada), 2mg/l porcine LH (Lutropin-V; Bioniche), and 50 mg/l gentamicin sulfate under mineral oil at 39°C for 22 hours in a humidified atmosphere containing 5% CO2 in air. Before ICSI or evaluation of maturation rate, matured oocytes were denuded by repeated pipetting in modified synthetic oviductal fluid with HEPES (mSOF-HEPES) with 1 g/l hyaluronidase. For the evaluation of maturation rate, denuded oocytes were fixed after 22 hours of culture on a glass slide with 2%(v/v) glutaraldehyde and stained with Hoechst 33342 (1 mg/ml in distilled water). The oocytes were then observed under an ultraviolet (UV) fluorescence microscope (excitation and emission wavelengths of 330–380 and 410 nm, respectively) at a magnification of x400. Bovine sperm preparationWe used straws from three Holstein bulls of proven fertility that were thawed at 37°C in a water bath, then resuspended in mSOF-HEPES (107.7 mM sodium chloride, 7.16 mM potassium chloride, 1.19 mM potassium monobasic phosphate, 1.71 mM calcium chloride, 0.49 mM magnesium chloride, 25.07 mM sodium bicarbonate, 3.30 mM sodium lactate, 0.30 mM sodium pyruvate, and 25 mM HEPES). Sperm cells were immediately used after selection by centrifugation in mini-Percoll (Machado et al., 2009) and then washed in mSOF-HEPES. Human sperm preparationSperm samples from three donors with normal parameters according to the World Health Organization (WHO, 2021) and offspring were used. Donors consented to donate sperm cells for scientific research. Sperm samples were frozen with Freezing Medium-TYB with Glycerol and Gentamicin (Irvine, IR-90128). Semen was thawed for 1 minute at room temperature and then placed for 5 minutes at 37°C in a water bath, then resuspended in Quinn’s™ Sperm Washing Medium (Sage, ART-1006). Sperm cells were immediately used after selection by density gradient Isolate (Irvine, IR-99264) and washed in a sperm washing medium. Bovine IVFAfter in vitro maturation, COCs were co-cultured with a final concentration of 1 × 106 motile bovine spermatozoa. Fertilization was performed in 500 µl IVF-mSOF (mSOF supplemented with 3 g/l bovine serum albumin (BSA) and 10,000 U/l heparin) under mineral oil at 39°C, in 5% CO2 in air for 20 hours. Putative zygotes were denuded by repeated pipetting and placed in 500 µl in IVC-mSOF, consisting of mSOF supplemented with 30 ml/l amino acid MEM (GIBCO), 10 ml/l non-essential amino acid MEM (GIBCO), 2 mmol/L-glutamine, 6 g/L BSA, and 5%(v/v) FBS (GIBCO). Conventional ICSIWe used commercially available ICSI micropipettes with a beveled and spiked tip (Fig. 1a) (MIC-9-30, ORIGIO, USA). We aspirated 7% polyvinylpyrrolidone (7% PVP Solution, Irvine Scientific, USA) in the micropipette applying negative pressure using the pneumatic injector. A bovine or human motile sperm was immobilized by crushing the tail with the tip of the micropipette and aspirating the tail first into the micropipette with 7% PVP. With the polar body at 12 or 6 o’clock, the micropipette was inserted through the zona pellucida far into the oocyte (~90% of the oocyte diameter) to stretch the membrane. Aspiration into the micropipette was applied to create negative pressure and suction onto the membrane. At first, the membrane was slowly aspirated into the micropipette until a sudden flow of cytoplasm into the micropipette occurred (membrane breakage). After membrane breakage, we introduced positive pressure to transfer the sperm into the oocyte. Oocyte survival was evaluated after 18 hours of conventional ICSI procedure by observing cell lysis under an inverted microscope at 400 magnification. In lysed cells, release of cytoplasmic content into the perivitelline space, reduction in oocyte diameter, and/or loss of plasma membrane integrity were seen. After ICSI, oocytes fertilized with bovine sperm were randomly allocated into two groups: direct in vitro culture or chemical activation. In the chemical activation group, oocytes were placed back in the incubator for 1 hour in maturation medium, then they were chemically activated for 5 minutes in 5 µmol/l ionomycin followed by 2.5 hours in 2 mmol/l 6-DMAP, at 39°C in a humidified atmosphere containing 5% CO2 in air. After activation, oocytes were subjected to the same in vitro culture as the first group (Ohlweiler et al., 2013). After ICSI, oocytes fertilized with human sperm were cultured in vitro without further treatment. A proportion corresponding to 20% of the oocytes was subjected to a sham test (the same conventional ICSI procedure but without a sperm present in the micropipette). Piezo-ICSIWe used commercially available Piezo-ICSI micropipettes with a flat tip (Fig. 1b) (PIN07-20FT, PRIME TECH Ltd., Japan). Fluorinert (FC- 770, 3 M), 6.25 μl was placed in the middle of the micropipette. After Fluorinert was pushed to the tip of the micropipette, 7% PVP was aspirated into the micropipette. Bovine or human sperm was then immobilized by applying one Piezo pulse (speed 1, intensity 2) in the tail and aspirating the tail first into the micropipette. Then the micropipette was gently placed on the zona pellucida while Piezo pulses were applied (speed 6, intensity 2), to allow the pipette to break through the zona pellucida and not the oocyte membrane. The sperm was advanced until the sperm head was near the tip of the micropipette, and the micropipette was advanced forward (to ~90% of the oocyte diameter) to stretch the membrane. The breakage of the membrane was performed by applying one Piezo pulse (speed 1, intensity 2) without aspirating the cytoplasm into the micropipette, and the sperm was injected into the oocyte. Oocyte survival was evaluated after 18 hours of the Piezo-ICSI procedure by observing cell lysis under an inverted microscope at 400 magnification as described above. After Piezo-ICSI, oocytes were cultured in vitro without further treatment. A proportion corresponding to 20% of the oocytes was subjected to a sham test (the same Piezo-ICSI procedure but without a sperm present in the micropipette). In vitro culture of embryos and fertilization evaluationPutative zygotes were placed in 500 ml in IVC-mSOF and cultured under mineral oil at 39°C in a humidified atmosphere with 90% N2:5% CO2:5% O2. In the experiment performed to evaluate pronuclear formation, the oocytes were fixed after 18 hours of culture on a glass slide with 2%(v/v) glutaraldehyde and stained with Hoechst 33342 (1 mg/ml in distilled water). The oocytes were then observed under a UV fluorescence microscope (excitation and emission wavelengths of 330–380 and 410 nm, respectively) at a magnification of x400. In the experiment performed to evaluate embryo development, the proportion of cleaved embryos after 48 h of culture was evaluated by the number of embryos that presented two or more blastomeres, and the proportion of blastocysts produced was determined on days 7 and 8 following insemination. Determination of PLCɀ activity in bovine and human sperm cellsSemen was thawed and washed as described above and sperm cells were suspended in distilled water. The suspension was frozen at −20°C, thawed, homogenized, and sonicated at 100 W in a 50% cycle at 4°C using a VibraCell sonicator model 600 W (Sonics and Materials Inc., Newton, CT) for 4 minutes. After centrifugation of the homogenate (10,000×g, 20 minutes, 4°C), the supernatants were collected and maintained at 4°C until enzyme assay. PLCɀ activity was measured using a commercial kit based on the cleavage of a substrate (glycero-phosphoethanolamine with a dye-labeled sn-2 acyl chain) releasing the dye-labeled diacylglycerol, which produces a positive fluorescence signal (EnzChek® Direct Phospholipase C Assay Kit, E10215, Molecular Probes). Fluorescence was measured at 492 nm with an EZ Read 400 Microplate Reader, Biochrom. The enzymatic activity was expressed as mIU/106 spermatozoa. Statistical analysisTwo PN, cleavage, and blastocyst rates were compared using a Chi-square analysis for nonparametric data. PLCɀ activity values are expressed as mean ± S.E.M and compared using the Student´s t-test. p < 0.05 was considered significant. Ethical approvalNo animals were used during this study. Bovine ovaries were obtained from an officially registered and approved local slaughterhouse and frozen bull sperm straws were donated by a local bovine semen collection center. This study was performed in line with the principles of the Declaration of Helsinki for human subjects. Approval was granted by the Ethics Committee of Fertilidad San Isidro (Date 2022-06-10/No. CESI V1 031022). ResultsBovine oocyte maturation, fertilization, and embryo development in vitroPrevious to ICSI trials, we wanted to verify oocyte nuclear and cytoplasmic maturation and sperm fertilizing capacity in our laboratory system, so a standard protocol for in vitro production of bovine embryos was carried out. Oocyte maturation rate was 88.2% (97 oocytes with polar body out of 110 cultured COCs), 2 PN formation rate 82.9% (87 2 PN zygotes out of 105 inseminated COCs), cleavage rate 79.2% (95 cleaved embryos out of 120 inseminated COCs), and blastocyst rate 37.5% (45 blastocysts out of 120 inseminated COCs) (Fig. 2). Our system presented an adequate gamete quality, interaction and embryo culture conditions, similar to those reported in the literature (Lonergan et al., 2003; Choudhary et al., 2016). Bovine oocyte activation with conventional ICSI and Piezo-ICSI with bovine or human spermWithin the groups of oocytes fertilized with bovine sperm, Piezo-ICSI increased 2 PN formation with respect to conventional ICSI (p < 0.05). However, conventional ICSI with chemical activation presented the highest rates (p < 0.05). Although no differences in 2 PN formation using conventional ICSI with bovine or human sperm were observed, the 2 PN formation rate was higher using Piezo-ICSI with human sperm than with bovine sperm (p < 0.05; Table 1). Sham-injected oocytes disclosed 20%, 0%, and 0% of 2 PN formation for conventional ICSI with chemical activation, conventional ICSI, and Piezo-ICSI, respectively (n=180 oocytes).
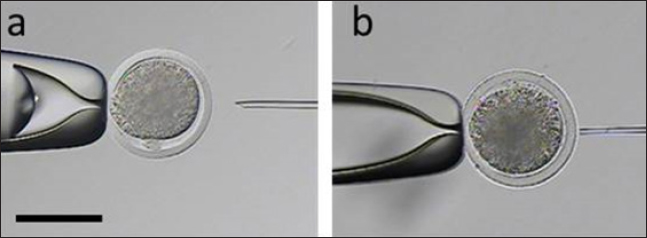
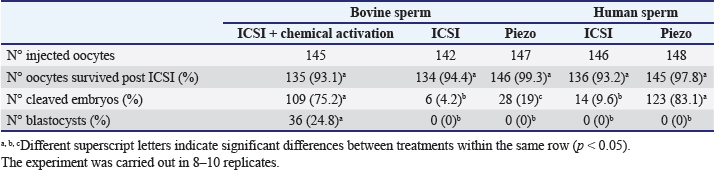
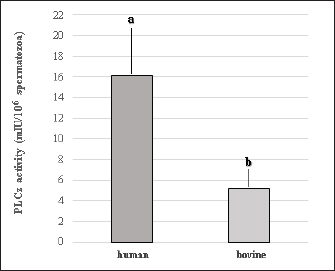
Fig. 1. Representative photographs of the beveled spiked micropipette used for conventional ICSI (a) and the flat-tipped micropipette used for Piezo-ICSI (b). Scale bar represents 100 μm. In oocytes fertilized with bovine sperm, the use of Piezo-ICSI increased the cleavage rate with respect to conventional ICSI (p < 0.05), but no blastocysts were obtained. However, conventional ICSI with chemical activation presented the highest cleavage rate, and blastocysts were obtained in this group. Although no differences in cleavage rates using conventional ICSI with bovine or human sperm were detected, the cleavage rate was higher using Piezo-ICSI with human sperm than with bovine sperm (p < 0.05; Table 2). Sham-injected oocytes disclosed 42%, 0 %, and 0% of cleavage and 12%, 0%, and 0% of blastocysts for conventional ICSI with chemical activation, conventional ICSI, and Piezo-ICSI, respectively (n=150 oocytes). Interestingly, Piezo-ICSI with human sperm increased the number of activated bovine oocytes as much as with conventional ICSI + chemical activation with bovine sperm, obtaining similar 2 PN formation and cleavage rates (Tables 1 and 2), but also considering that some of the latter oocytes are parthenogenetically activated ones (see sham-injected oocytes data). PLCɀ activity in bovine and human sperm cellsAs regards PLCɀ activity, significantly higher values were detected in human sperm compared with bull sperm (16.1 ± 4.4 and 5.2 ± 1.8 mIU/106 spermatozoa, respectively, p < 0.05, n=9 for each treatment in three replicates) (Fig. 3). DiscussionWe proposed that the bovine oocyte activation failure after ICSI may be in part due to the low PLCɀ release and/or activity in bull sperm. For this purpose, we used conventional ICSI and Piezo-ICSI with bull or human sperm to induce bovine oocyte activation, considering that fertilization with human sperm does not use chemical activation. Additionally, PLCɀ activity was determined in both bovine and human spermatozoa. Our results demonstrated that the use of Piezo-ICSI with bovine sperm can significantly increase the rates of 2 PN formation and cleavage in comparison with conventional ICSI using bovine sperm without any additional activation process for the oocyte. Similar results have been obtained in other small ruminants where the mechanical force exerted by the Piezo system on the sperm plasma membrane and acrosome substantially improved embryonic development (Anzalone et al., 2016). Unfortunately, these rates continue to be significantly lower than those obtained when performing the chemical activation of the bovine oocyte. In a coincidence, Devito et al. (2010by micromanipulation, of a single sperm into the cytoplasm of a mature egg. This technique is particularly advantageous when only a few sperm are available for fertilization, representing an important tool in preserving genetic material, especially from poorly fertile males. The results from ICSI in cattle are very often unsatisfactory and difficult to reproduce. Thus, the goal of this study was to evaluate the effect of the use of a Piezo drill (PD) reported that the independent use of Piezo or chemical activation was not enough to produce an adequate morulae formation, but when both techniques were combined embryos could develop to the blastocyst stage. However, as previously mentioned, bovine oocyte chemical activation has limitations. According to our sham test results, about half of the blastocysts obtained were parthenogenetically activated ones, and therefore unsuitable to produce calves, as other authors also reported (Keskintepe et al., 2002).
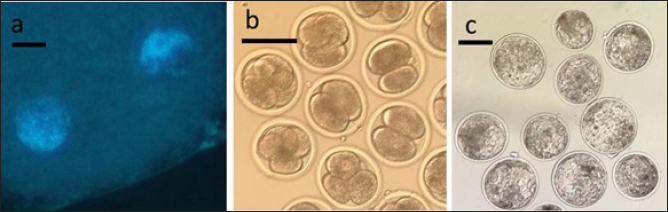
Fig. 2. Representative photographs of 2 PN zygotes (a, scale bar represents 10 μm), cleaved embryos after 48 h-culture (b, scale bar represents 100 μm), and blastocyst after 7 day-culture (c, scale bar represents 100 μm). Table 1. Oocyte survival and 2 PN formation rates in oocytes activated using bovine or human sperm. Numbers in parentheses represent percentages.
Table 2. Cleavage and blastocyst rates in oocytes activated using bovine or human sperm. Numbers in parentheses represent percentages.
Fig. 3. PLCɀ activity in human and bovine sperm cells. a, b Different superscript over bars indicate significant differences (p < 0.05, mean ± S.E.M., n=9 for each treatment in three replicates). Conventional ICSI using bovine or human sperm resulted in similar 2 PN formation and cleavage rates. But surprisingly, Piezo-ICSI using human sperm resulted in significantly higher two PN formation and initial embryo development in comparison with bovine sperm, indicating that human sperm cells possess some physical or chemical characteristics that favor oocyte activation and therefore ICSI outcomes. Interestingly, the release of some sperm factors seems to be higher when using Piezo pulse during ICSI in both sperm species, improving the results obtained with conventional ICSI procedures. To successfully activate the oocyte, sperm oocyte activation factors (SOAFs) must be released from the post-acrosomal perinuclear theca (PT). This event triggers calcium release from the oocyte’s endoplasmic reticulum, initiating a signaling cascade leading to meiotic resumption, activation of anti-polyspermy mechanisms, PN formation, and finally embryo cleavage and embryo development (Sutovsky, 2018). Although ICSI can apparently produce normal offspring in several species, this process circumvents some biological processes necessary for normal fertilization, such as the loss of the acrosomal vesicle and sperm plasma membrane, and the oocyte-sperm interaction and fusion. The retention of these structures may be harmful to the oocyte or delay its activation (Morozumi et al., 2006). The use of the Piezo system may promote a faster dissolution of the sperm membrane facilitating the efflux of SOAFs after the injection (Salgado et al., 2018). Sperm-specific PLCɀ is widely considered the main SOAF responsible for generating calcium oscillations that induce oocyte activation and embryonic development during mammalian fertilization (Saleh et al., 2020). We compared PLCɀ activity between bovine and human sperm cells, finding that human sperm possess thrice as high PLCɀ activity as bovine sperm. This higher PLCɀ activity may explain the higher fertilization rates obtained when using Piezo-ICSI and human sperm to activate bovine oocytes. Therefore, we can suggest that the low results obtained with ICSI are not just a consequence of bovine oocyte activation problems (Malcuit et al., 2006; Aguila et al., 2017), but also a lower PLCɀ activity in the bovine sperm than the needed for oocyte activation after ICSI. On the other hand, the oocyte is fully competent when activated by a different signal mechanism during IVF, where sperm and oocyte membranes interact and fuse with each other. Although the molecular events underlying these processes are not fully understood, previous sperm capacitation and acrosome reaction would be involved (Breitbart, 2003). We observed a considerable increase in bovine oocyte activation when human sperm was used for Piezo-ICSI, but not with bovine sperm. This suggests that Piezo-ICSI could facilitate PLCɀ release from human sperm and, therefore, allow PLCɀ dependent cell signaling pathway activation. It has been reported that the complete solubilization of post-acrosomal PT, where PLCɀ is stored, is necessary for oocyte activation. Therefore, the greater rigidity of the PT and the higher nuclear stability of the bovine sperm could difficult the solubilization of the PT contents (Sutovsky et al., 2003), impairing the replacement of sperm protamines by oocyte-derived histones, an essential requirement for male PN formation (Unnikrishnan et al., 2021). The tight condensation of the sperm nucleus is the result of the binding of DNA to protamines. In mammalian spermatozoa, two different types of protamines exist, protamine 1 and protamine 2, the former presenting a greater affinity for DNA due to its higher cysteine content than the latter (Brewer et al., 2003; Hutchison et al., 2017). Human and mouse sperm present protamine 1 and 2, while bovine sperm have only protamine 1, making the nuclear chromatin very stable (Unnikrishnan et al., 2021). In this way, sperm with a higher proportion of protamine 2, as human sperm, decondensed faster in comparison with bovine sperm, being this a possible explanation for the higher two PN formation and cleavage rates observed when bovine oocytes were Piezo injected with human sperm. This increased stability of the bovine sperm may compromise or delay the release of PLCɀ within the oocyte and therefore may not trigger oocyte activation in the proper way. Considering this, previous treatment of the spermatozoa to remove the acrosome and the plasma membrane may facilitate sperm decondensation, PN formation, and a faster release of PLCɀ (Malcuit et al., 2006; Zambrano et al., 2016). In conclusion, our results indicate that the higher stability of the bovine sperm in combination with its relatively low content of PLCɀ would not allow efficient oocyte activation after ICSI. The Piezo system increases bovine ICSI efficiency, augmenting two PN formation and cleavage rates, but outcomes are still lower in comparison with the results obtained after the chemical activation of the oocyte. Further studies are necessary to increase the efficiency of ICSI in the bovine, considering that the failure after ICSI seems to be in part due to the incomplete activation of PLCɀ dependent cell signaling pathway by bovine sperm. An alternative could be the evaluation of the combined use of the Piezo system with PLCɀ injection or supplementation during ICSI. Therefore, an earlier beginning of calcium oscillations, oocyte activation, and consequently a more coordinated formation of female and male PN could be obtained. AcknowledgmentsThe authors acknowledge Centro de Reproducción Bovina SRL for donating frozen bovine sperm straws. Conflict of interestThe authors have declared that there is no conflict of interest concerning this study. FundingThis research was supported by funds of UBACyT 20020170100379BA-2018 and PIP CONICET 11220170100813CO-2017. Authors’ contributionsAll authors contributed to the study’s conception and design. Bovine oocyte in vitro maturation, bovine semen preparation, bovine oocyte conventional ICSI and Piezo-ICSI with bovine or human sperm, and embryo in vitro culture were performed by Gabriel Alvarez and Sofia Villanueva, human sperm preparation was performed by Marisa Geller and Claudio Ruhlmann and determination of PLCɀ activity in bovine and human sperm cells was performed by Elizabeth Breininger. Gabriel Dalvit, Masashige Kuwayama, and Pablo Cetica contributed with resources, supervision, and project administration. Pablo Cetica also contributed to funding acquisition. The first draft of the manuscript was written by Gabriel Alvarez and all authors commented on previous versions of the manuscript. Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request. ReferencesAguila, L., Felmer, R., Arias, M.E., Navarrete, F., Martin-Hidalgo, D., Lee, H.C., Visconti, P. and Fissore R. 2017. Defective sperm head decondensation undermines the success of ICSI in the bovine. Reproduction 154, 307–318. Anzalone, D.A., Iuso D., Czernik M., Ptak G. and Loi P. 2016. Plasma membrane and acrosome loss before ICSI is required for sheep embryonic development. J. Assist. Reprod. Genet. 33, 757–763. Bevacqua, R.J., Pereyra-Bonnet, F., Fernandez-Martin, R. and Salamone, D.F. 2010. High rates of bovine blastocyst development after ICSI-mediated gene transfer assisted by chemical activation. Theriogenology 74, 922–931. Breitbart, H. 2003. Signaling pathways in sperm capacitation and acrosome reaction. Cell. Mol. Biol. 49, 321–327. Brewer, L., Corzett, M., Lau, E.Y. and Balhorn, R. 2003. Dynamics of protamine 1 binding to single DNA molecules. J. Biol. Chem. 27843, 42403–42408. Canel, N., Bevacqua, R., Fernández-Martín, R. and Salamone, D.F. 2010. Activation with ionomycin followed by dehydroleucodine and cytochalasin b for the production of parthenogenetic and cloned bovine embryos. Cell. Reprogram. 12, 491–499. Choudhary, K.K., Kavya, K.M., Jerome, A. and Sharma, R.K. 2016. Advances in reproductive biotechnologies. Vet. World 9, 388–395. Devito, L.G., Fernandes, C.B., Blanco, I.D.P., Tsuribe, P.M. and Landim-Alvarenga, F.C. 2010. Use of a piezo drill for intracytoplasmic sperm injection into cattle oocytes activated with ionomycin associated with roscovitine. Reprod. Domest. Anim. 45, 654–658. Felmer, R. and Arias, M.E. .2015. Activation treatment of recipient oocytes affects the subsequent development and ploidy of bovine parthenogenetic and somatic cell nuclear transfer (SCNT) embryos. Mol. Reprod. Dev. 82, 441–449. García-Roselló, E., García-Mengual, E., Coy, P., Alfonso, J. and Silvestre, M.A. 2009. Intracytoplasmic sperm injection in livestock species: an update. Reprod. Domest. Anim. 44, 143–151. Goto K., Kinoshita A., Takuma Y. and Ogawa K. 1990. Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet. Rec. 127, 517–520. Hutchison, J.M., Rau, D.C. and DeRouchey, J.E. 2017. Role of disulfide bonds on DNA packaging forces in bull sperm chromatin. Biophys. J. 113, 1925–1933. Kang, Y.N., Hsiao, Y.W., Chen, C.Y. and Wu C.C. 2018. Testicular sperm is superior to ejaculated sperm for ICSI in cryptozoospermia: an update systematic review and meta-analysis. Sci. Rep. 8, 7874. Keskintepe, L., Pacholczyk, G., Machnicka, A., Norris, K., Curuk, M.A., Khan, I. and Brackett, B. G. 2002. Bovine blastocyst development form oocytes injected with freeze-dried spermatozoa. Biol. Reprod. 67, 409–415. Liu L. and Yang X. 1999. Interplay of maturation-promoting factor and mitogen-activated protein kinase inactivation during metaphase-to-interphase transition of activated bovine oocytes. Biol. Reprod. 61, 1–7. Lonergan, P., Rizos, D., Gutierrez-Adan, A., Fair, T. and Boland, M.P. 2003. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod. Domest. Anim. 38, 259–267. Machado, G.M., Carvalho, J.O., Filho, E.S., Caixeta, E.S., Franco, M.M., Rumpf, R. and Dode, M.A.N. 2009. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology 71, 1289–1297. Malcuit, C., Maserati, M., Takahashi, Y., Page, R. and Fissore, R.A. 2006. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca 2+] responses and oocyte activation. Reprod. Fertil. Dev. 18, 39–51. Morozumi, K., Shikano, T., Miyazaki, S. and Yanagimachi, R. 2006. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci USA 103, 17661–17666. Nakamura, S.I., Terada, Y., Horiuchi, T., Emuta, C., Murakami, T., Yaegashi, N. and Okamura K. 2001. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a piezo-driven pipette: a novel assay for human sperm centrosomal function. Biol. Reprod. 65, 1359–1363. Nomikos, M., Kashir, J, Swann, K. and Lai, F.A. 2013. Sperm PLCζ: From structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett. 587, 3609–3616. Ohlweiler, L.U., Brum, D.S., Leivas, F.G., Moyses, A.B., Ramos, R.S., Klein, N., Mezzalira, J.C. and Mezzalira A. 2013. Intracytoplasmic sperm injection improves in vitro embryo production from poor quality bovine oocytes. Theriogenology 79, 778–783. Saleh, A., Kashir, J., Thanassoulas, A., Safieh-Garabedian, B., Lai, F.A. and Nomikos, M. 2020. Essential role of sperm-specific PLC-Zeta in egg activation and male factor infertility: an update. Front. Cell Dev. Biol. 8, 28. Salgado, R.M., Brom-De-luna, J.G., Resende, H.L., Canesin H.S. and Hinrichs K. 2018. Lower blastocyst quality after conventional vs. Piezo-ICSI in the horse reflects delayed sperm component remodeling and oocyte activation. J. Assist. Reprod. Genet. 35, 825–840. Sutovsky P. 2018. Review: sprem-oocyte interactions and their implications for bull fertility, with emphasis on the ubiquitin-proteasome system. Animal 12, s121–s132. Sutovsky, P, Manandhar, G, Wu, A. and Oko R. 2003. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc. Res. Tech. 61, 362–378. Unnikrishnan V., Kastelic J. and Thundathil J. 2021. Intracytoplasmic sperm injection in cattle. Genes (Basel) 12, 198. WHO. 2021. World Health Organization manual for the examination and processing of human semen. 2021. Sixth ed., pp: 276. Zambrano, F., Aguila, L., Arias, M.E., Sánchez, R. and Felmer, R. 2016. Improved preimplantation development of bovine ICSI embryos generated with spermatozoa pretreated with membrane-destabilizing agents lysolecithin and Triton X-100. Theriogenology 86, 1489–1497. | ||
| How to Cite this Article |
| Pubmed Style Alvarez GM, Villanueva S, Breininger E, Geller M, Ruhlmann C, Dalvit GC, Cetica PD, Kuwayama M. Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Vet. J.. 2024; 14(5): 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 Web Style Alvarez GM, Villanueva S, Breininger E, Geller M, Ruhlmann C, Dalvit GC, Cetica PD, Kuwayama M. Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. https://www.openveterinaryjournal.com/?mno=190174 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.14 AMA (American Medical Association) Style Alvarez GM, Villanueva S, Breininger E, Geller M, Ruhlmann C, Dalvit GC, Cetica PD, Kuwayama M. Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Vet. J.. 2024; 14(5): 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 Vancouver/ICMJE Style Alvarez GM, Villanueva S, Breininger E, Geller M, Ruhlmann C, Dalvit GC, Cetica PD, Kuwayama M. Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 Harvard Style Alvarez, G. M., Villanueva, . S., Breininger, . E., Geller, . M., Ruhlmann, . C., Dalvit, . G. C., Cetica, . P. D. & Kuwayama, . M. (2024) Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Vet. J., 14 (5), 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 Turabian Style Alvarez, Gabriel Martín, Sofía Villanueva, Elizabeth Breininger, Marisa Geller, Claudio Ruhlmann, Gabriel Carlos Dalvit, Pablo Daniel Cetica, and Masashige Kuwayama. 2024. Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Veterinary Journal, 14 (5), 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 Chicago Style Alvarez, Gabriel Martín, Sofía Villanueva, Elizabeth Breininger, Marisa Geller, Claudio Ruhlmann, Gabriel Carlos Dalvit, Pablo Daniel Cetica, and Masashige Kuwayama. "Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity." Open Veterinary Journal 14 (2024), 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 MLA (The Modern Language Association) Style Alvarez, Gabriel Martín, Sofía Villanueva, Elizabeth Breininger, Marisa Geller, Claudio Ruhlmann, Gabriel Carlos Dalvit, Pablo Daniel Cetica, and Masashige Kuwayama. "Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity." Open Veterinary Journal 14.5 (2024), 1191-1198. Print. doi:10.5455/OVJ.2024.v14.i5.14 APA (American Psychological Association) Style Alvarez, G. M., Villanueva, . S., Breininger, . E., Geller, . M., Ruhlmann, . C., Dalvit, . G. C., Cetica, . P. D. & Kuwayama, . M. (2024) Bovine oocyte activation with bull or human sperm by conventional ICSI and Piezo-ICSI: Its relationship with PLCɀ activity. Open Veterinary Journal, 14 (5), 1191-1198. doi:10.5455/OVJ.2024.v14.i5.14 |