| Research Article | ||
Open Vet. J.. 2024; 14(5): 1206-1215 Open Veterinary Journal, (2024), Vol. 14(5): 1206–1215 Research Article Canine transmissible venereal tumor in Morocco: Clinical and pathological findings in 64 dogs—insights from a descriptive epidemiological study (2020-2023)Nadia Laissaoui1,2*, Yolanda Millán3, Daniela Simon Betz4, Meryem El Mrini5, Ghita Bouayad1, Najat Lamalmi6, Noursaid Tligui2 and Rahma Azrib11Department of Medicine, Surgery and Reproduction, Agronomy and Veterinary Institute Hassan II, Rabat, Morocco 2Department of Veterinary Pathology and Public Health, Agronomy and Veterinary Institute Hassan II, Rabat, Morocco 3Department of Comparative Pathology, Faculty of Veterinary Medicine, University of Córdoba, Córdoba, Spain 4University of Veterinary Medicine Hannover, Independent Scientific Writing, Translation & Consultancy Clinical Oncology, Hannover, Germany 5Department of Animal Health, National Office of Sanitary Safety of Food Products, Rabat, Morocco 6Department of Pathology, Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco *Corresponding Author: Nadia Laissaoui. Department of Medicine, Surgery and Reproduction, Agronomy and Veterinary Institute Hassan II, Rabat, Morocco. Email: nadialsi.iav [at] gmail.com Submitted: 21/02/2024 Accepted: 21/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
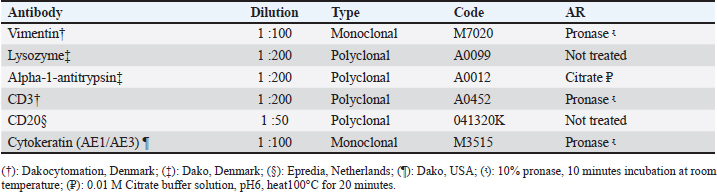
AbstractBackground: Canine transmissible venereal tumor (CTVT) is a widely spread, contagious neoplasm commonly found in dogs. Mostly affects the external genitalia, however, it may also exhibit unusual clinical presentations. Aim: To describe the epidemiology, clinical appearance, cytologic and histopathologic features of dogs with TVT in Morocco. Methods: Within the realm of a nation-wide study on canine and feline tumors in Morocco between September 2020 and March 2023, dogs with histologically diagnosed TVT were identified and data on epidemiologic, clinical as well as cytologic, and histologic features were compiled and analyzed. Results: A total of 64 cases of canine TVT were diagnosed. 52 dogs were cross-breed (81.2%) while 4 Siberian Huskies (6.2%) and 3 German shepherds (4.7%) were the most affected pure-breed dogs. The median age of dogs at diagnosis was 3 years (range, 1–10years) and male gender was more common (male:female ratio; 1.3:1). Tumor was located exclusively in the genital area in 58 cases (90.6%), whereas 6 dogs (9.4%) had an atypical occurrence of TVT with locations including skin and nasal cavity. Cytology allowed for an early diagnosis in 2 cases. Histology revealed no differences between the genital and extragenital forms. Immunohistochemistry was necessary in 4 cases and revealed positive staining for vimentin and Alpha-1-antitrypsin, negative marking for CD3, CD20, and AE1/AE3, and low cytoplasmic labeling for lysozyme. Conclusion: CTVT is a widely distributed neoplasm in Morocco, mostly showing presence in young, cross-breed, and oftentimes stray dogs. An adequate understanding of this tumor’s epidemiological features is necessary for its management and eradication. Keywords: Diagnosis, Dogs, Epidemiology, Transmissible venereal tumor. IntroductionCanine transmissible venereal tumor (CTVT) is a particular, naturally, occurring cancer affecting dogs (Ostrander et al., 2016). It is characterized by its unique capacity of transmission from one individual to another by implantation of living neoplastic cells in unaffected tissues, mainly during coitus or social behavior such as sniffing, licking, or biting (Ganguly et al., 2016; Schectman et al., 2022). CTVT is reported to be the oldest canine neoplasia discovered in nature. Its origin dates back to several 1000 years and contrary to the usual, cancer in the case of CTVT did not disappear with the death of the host, but rather spread widely between individuals (Strakova and Murchison, 2014). This neoplasia constitutes a significant worldwide health issue among dogs and it is found to be more prevalent in countries with large populations of stray dogs with uncontrolled sexual activity (Ganguly et al., 2016; Pimentel et al., 2021). Transmission is reported, most commonly, among young dogs of breeding age, with no breed or sex predilection (Chikweto et al., 2013; Strakova and Murchison, 2014). The external genitalia of both sexes is the most frequently affected site (Araujo et al., 2016; Abedin, 2020). Less commonly, atypical cases of CTVT are present, including cases with extragenital lesions reported at various sites such as the skin, eyes, oral, and nasal cavity (Komnenou et al., 2015; Thatcher et al., 2020; Costa et al., 2023), and metastasis involving, regional lymph nodes, brain, muscles, skin, liver, spleen, and kidney (Park et al., 2006; Pinczowski et al., 2015; Alkan et al., 2017). Table 1. Primary antibodies and AR methods used in immunohistochemical characterization of CTVT.
Table 2. Distribution of canine TVT in different regions of Morocco.
Table 3. Breed distribution of dogs diagnosed with TVT, expressed in absolute value and percentage.
Tumors in genital location exhibit distinct clinical features. These are usually localized, hemorrhagic, possess a cauliflower-like growth pattern, and a friable texture. Tumors may be single or multiple, ranging from a small nodule to a large mass (Muste et al., 2012; Abedin, 2020; Murchison, 2021; Costa et al., 2023). Genital lesions are mostly associated with preputial and vaginal hemorrhagic discharge, as well as increased licking of the affected region. Dysuria and ulceration have also been observed (Muste et al., 2012; Araujo et al., 2016; Ganguly et al., 2016; Abedin, 2020). Given the almost pathognomonic clinical signs, cases of genital CTVT are less complicated to diagnose. However, when the tumor is situated beyond the external genital tract, signs vary according to the localization of the tumor, and may encompass epistaxis, sneezing, epiphora, conjunctival congestion, halitosis, anorexia, weight loss, and dysphagia (Komnenou et al., 2015; Alkan et al., 2017; Ojeda et al., 2018). This may mislead the attending clinician in the search for a diagnosis. Unusual sites of this neoplasia may be confused with several other pathologies and, therefore, render the diagnosis more challenging (Abedin, 2020). Similarly to other neoplasms, CTVT requires meticulous anamnesis and clinical examination as the first steps in the diagnostic procedure. Additionally, this tumor exhibits characteristic cytological features; hence, cytology is often used to provide a prompt diagnosis of CTVT. Histological examination and immunohistochemistry (IHC) may also be performed to confirm the diagnosis, especially in atypical cases (Ganguly et al., 2016; Pimentel et al., 2021). To our knowledge, this study is the first report presenting an evaluation of the occurrence of CTVT in Morocco. Through this work, we aim to describe the epidemiology, clinical manifestations, cytologic, and histopathologic findings of dogs with CTVT in Morocco. Materials and MethodsAnimals and data preparationDuring a descriptive epidemiological study conducted between September 2020 and March 2023, samples of CTVT from 12 private veterinary clinics, distributed across 5 regions in Morocco, and from the veterinary teaching hospital of the Agronomy and Veterinary Institute Hassan II in Rabat, were submitted to the department of veterinary pathology of the institute for free histopathologic examination. Table 4. Distribution of CTVT according to lesion site and gender.
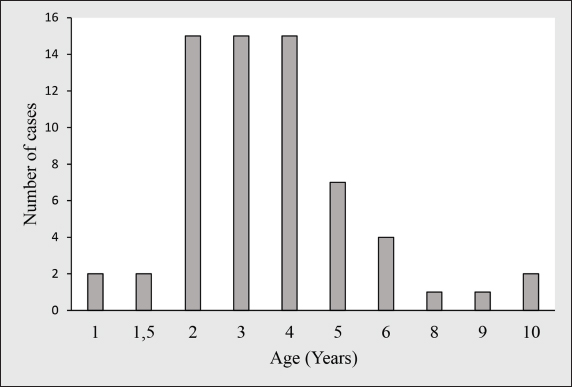
Fig. 1. Age distribution of dogs affected with CTVT in this study. Samples were obtained from owned and sheltered dogs only, following therapeutic resection or diagnostic biopsy of tumors. Informed consent was acquired from all dog owners and shelter managers before submitting tumor tissue for histopathological analysis and inclusion of dogs in the study. For each case, forms with information on epidemiological and clinical data were sent by email to the corresponding referring veterinarian for completion. The form’s items involved anamnesis, species, age, breed, sex, castration status and ownership, and roaming possibilities of the animal, geographic region, anatomical site of both genital and non-genital lesions, clinical description, and date of excision of the tumor. Collected data were then compiled in Microsoft Excel® spreadsheet database and analyzed as simple descriptive statistics using pivot tables. Pathological examinationFine needle aspirates were carried out for cytologic examination in two cases with atypical locations of CTVT at two veterinary practices. Slides were stained by the referring veterinarians with a Diff-Quick kit before submission for cytologic evaluation. Biopsy samples were fixed in 10% buffered formalin, embedded in paraffin, cut into 4 µm thick sections, and stained with hematoxylin and eosin (HE) for histopathological analysis.
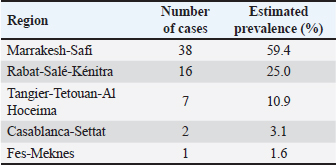
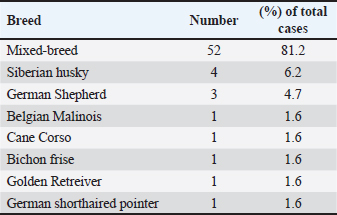
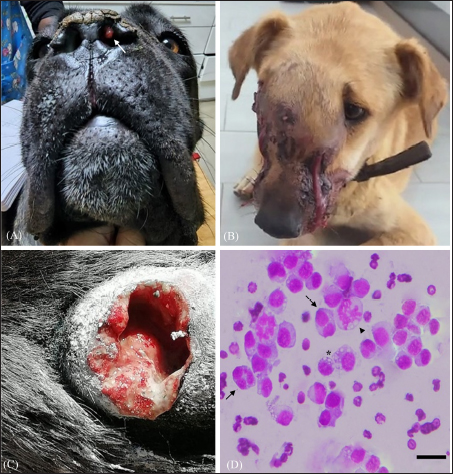
Fig. 2. Atypical cases of canine transmisible venereal tumour (A) Red, solitary nodule at the left nasal cavity (arrow). (B) Large fistulated mass at the nasal region, causing facial deformation and discharging sanguinopurulent secretion. (C) Ulcerated cutaneous mass at the thoracic region draining a seropurulent secretion. (D) Cytology of extragenital transmissible venereal tumor, nasal mucosa. Presence of a population of round to oval cells, with eccentric nucleus and abundant light basophilic cytoplasm containing the typical intracytoplasmic clear vacuoles of TVT cells (*). Multinucleation (Arrow) and mitotic figures(arrowhead) are frequent. Diff-Quick. Bar=20 μm. IHC was later performed at the Department of Comparative Pathology of the Faculty of Veterinary Medicine—University of Córdoba, Spain, to confirm the diagnosis in four atypical cases (Skin and nasal without genital involvement, skin metastasis, and nasal with genital lesions). Immunohistochemical evaluation was performed on 3 µm thick tissue sections of extragenital lesions, using the avidin-biotin-peroxidase complex (ABC) method. Antibodies used in this work were validated for canine tissues in a previous study (Mozos et al., 1996). Characteristics of the primary antibodies and the corresponding antigen retrieval (AR) methods are shown in table 1. Additionally, the detailed protocol of the immunohistochemical technique followed during this study is provided in Supplementary File 1. Ethical approvalNo experimental animals were used in this work. The studied cases were part of routine clinical practice, and surgical resection of tumors was done by treating veterinarians under the request of owners/ shelter managers, and not for reasons related to the study. Only samples were manipulated during this work, after the owner/shelter manager’s approval of sample donation to our laboratory. ResultsEpidemiology and clinical findingsDuring the study period, a total of 64 cases of CTVT were diagnosed. Most were encountered in the Marrakech-Safi region with 38 cases (59.4%), followed by Rabat-Salé-Kénitra with 16 cases (25%) and the region of Tangier-Tetouan-Al Hoceima with 7 cases (10.9 %), 2 cases were reported in Casablanca-Settat and 1 case in Fes-Meknes region with an estimated prevalence of (3.1%) and (1.6%), respectively (Table 2). Cross-breed dogs were mostly affected with 52 cases (81.2%), followed by 4 cases in Siberian Husky (6.2%), and 3 cases in German Shepherd (4.7%); other breeds presenting 1 case each (1.6%) were also noted and the total distribution of the observed breeds can be found in Table 3.
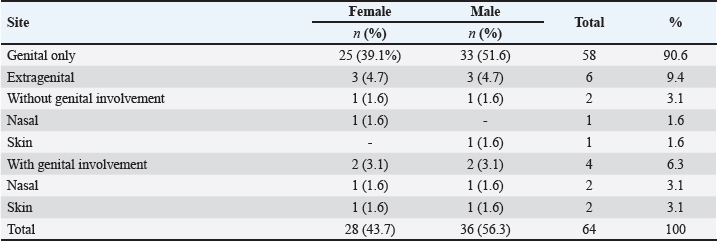
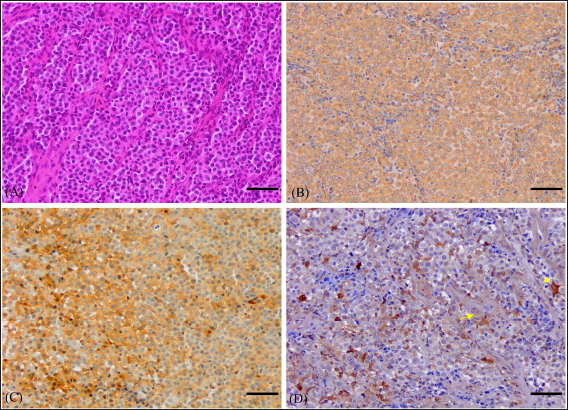
Fig. 3. Histological and immunohistochemical characterization of CTVT (A) Histological section shows round neoplastic cells with eosinophilic cytoplasm, arranged in solid sheets and supported by a thin fibrovascular stroma. Mitotic figures are frequent (38 in 2.37mm2). Hematoxylin and Eosin. Bar=50 μm. (B) Tumor cells are intensely stained with vimentin antibody. ABC method, Mayer’s hematoxylin counterstain. Bar=50 μm. (C) Majority of neoplastic cells had positive labeling for AAT antibody. ABC method, Mayer’s hematoxylin counterstain. Bar=50 μm. (D) Poor and variable immunoreactivity with lysozyme antibody is observed. Rare neoplastic cells have positive cytoplasmic labeling (Arrow). Notice intense staining of numerous infiltrative macrophages (Arrowhead). ABC method, Mayer’s hematoxylin counterstain. Bar=50 μm. The age range was 1 to 10 years with a median age of 3 years. The most afflicted dogs were between 2 and 5 years old, accounting for 81.2% (52/64) of total cases, while 6.2% (4/64) of dogs were aged less than 2 years and 12.5% (8/64) were older than 5 years (Fig. 1). Regarding the dogs’ gender, 36 (56.3%) of the animals were male, and 28 (43.7%) were female, and all dogs were intact. Stray dogs taken up by shelters accounted for 41 cases (64.1%), of the privately owned dogs 13 (20.3%) had poor breeding management and contacted dogs without prior examinations, 6 (9.4%) were allowed to roam free and 4 (6.3%) were declared to have had a runaway incident prior to clinical manifestation of TVT. The location of tumors was exclusively in the genital area in 58 cases (90.6%). 6 dogs (9.4%) had an extragenital location (three in the skin and three in the nasal cavity) including one case with multiple skin metastasis located in the thoracic region. 2 cases (3.1%) with extragenital form showed no genital involvement, whereas 4 cases (6.3%) had consecutive genital and non-genital lesions (Table 4). Bleeding in the external genital area was the most prominent clinical sign in both male and female dogs with a genital form of TVT, accounting for a total of 49 cases (80.3%). Macroscopically, genital tumors ranged from 0.7 cm in diameter to 13 × 10 × 5cm. 48 cases (78.7%) had a cauliflower-like proliferation, 10 (17.2%) were single or multiple nodules, 6 (9.4%) presented multilobulated masses, 37 (60.7%) were friable, 24 (39.3%) presented ulcerate, and 13 (21.3%) were hyperemic. Dogs with the extragenital location of TVT in the nasal area had foul-smelling, hemorrhagic, and purulent nasal discharge and respiratory difficulty. Lesions varied from small nodules in two cases (Fig. 2A) to a large fistulated mass causing facial deformation in one case (Fig. 2B). Dermal tumors were presented as a solitary, hyperemic, and friable nodule in one case (head), a unique ulcerative mass in another dog (neck), and multiple, firm, ulcerated masses draining a seropurulent secretion in one case with skin metastasis (thoracic region) (Fig. 2C). Pathological featuresThe cytologic evaluation revealed the presence of large cells, round to oval in shape with a high nucleus to cytoplasmic ratio. Multinucleation was noted in various cells, and mitotic figures were numerous. Cells had an eccentric large nucleus, with prominent centrally located nucleoli and lightly basophilic to clear cytoplasm containing frequent intracytoplasmic clear vacuoles (Fig. 2D). In general, all cases presented similar histopathological characteristics. Tumors were composed of relatively uniform, large, round to polyhedral neoplastic cells, arranged in solid sheets or rows and supported by septa of connective tissue. Tumoral infiltration by lymphocytes, macrophages, plasma cells, and eosinophils was noted in variable amounts. Tumor cells had indistinguishable cell borders and an eosinophilic, finely granular, and variably vacuolated cytoplasm. The nucleus was large and round with marginal chromatin and centrally placed nucleoli. Mitotic figures were frequent (38 in 2.37mm2) (Fig. 3A). No differences were noted between the genital and extragenital forms in the histologic evaluation. IHC conducted on the 4 atypical cases of CTVT, revealed intense immunostaining for vimentin and Alpha-1-antitrypsin (AAT) (Fig. 3B and C) and negative marking for CD3, CD20, and AE1/AE3. For lysozyme, neoplastic cells showed very low marking in 3 cases, and no immunoreaction was noted in 1 case; however, neutrophils and macrophages were highly marked in the 4 cases, serving as an internal positive control (Fig. 3D). DiscussionCTVT exhibits a worldwide distribution. In fact, it has been reported throughout over 90 countries (Strakova and Murchison, 2014). Interestingly, related epidemiological data are still lacking from several countries, including Morocco. A situation that warrants further investigations, to contribute to the understanding of this pathology. In the present study, we show that CTVT is a common neoplastic condition in Morocco. Similar findings were reported from other countries as Colombia, Brazil, and Grenada (Arcila-Villa et al., 2018; Pimentel et al., 2021; Schectman et al., 2022), whereas, in developed countries where populations of stray dogs have been controlled, enzootic CTVT has been eradicated, and occasional cases were reported only due to dogs importation from abroad (Murchison, 2021). A total of 64 cases were diagnosed in our study. This is not a large sample when compared to 3622 cases reported by Pimentel et al (2021) and 1135 reported by Arcila-Villa et al (2018). The higher number of dogs affected by CTVT in these studies can be attributed to more important veterinarian collaboration, longer study period, and variability of inclusion criteria. CTVT was more prevalent in Marrakech-Safi region (59.4%), due to an important veterinarian collaboration with shelters rescuing stray dogs. The region of Rabat-Salé-Kénitra presented the second highest prevalence (25%) due to the large number of veterinarians contributing to the study and the presence of the veterinary teaching hospital in Rabat that serves as a referral center especially concerning atypical cases of CTVT. Tangier-Tetouan-Al Hoceima (10.9 %), followed by Casablanca-Settat (3.1%) and Fes-Meknes (1.6%) regions showed the lowest number of CTVT mainly in consequence of few veterinarians participating in the study and owners’ frequent tendency to neuter their animals and control their breeding in these regions, therefore, the study does not give a representative geographical distribution on CTVT throughout the country and that more widespread epidemiologic studies are needed for this in the future. Affected dogs were mostly cross-breed, which is mainly due to the large population of stray dogs associated with a higher risk of transmission. This correlates with findings from several other studies (Araujo et al., 2016; Schectman et al., 2022; Costa et al., 2023). Additionally, pure-breed dogs were also reported in our study. The German shepherd and husky were the most affected, however, this may likely be caused by the breed preference in the country and suggests no breed predisposition as it has been reported in previous studies (Ganguly et al., 2016; Fathi et al., 2018). A higher prevalence of TVT was found in dogs classified in the age range of 2 to 5 years old (81.3%). This was in accordance with the findings of similar studies and is thought to be due to greater sexual activity in these age groups (Chikweto et al., 2013; Costa et al., 2023). In any case, this is in contrast to most other neoplastic entities that represent more geriatric diseases, and in this may aid in diagnosis, especially of atypical tumors. Data concerning the sexual predisposition of CTVT are still conflicting and vary considerably. Some studies showed no significant differences regarding the sex of affected animals (Chikweto et al., 2013; Schectman et al., 2022; Costa et al., 2023), meanwhile, other reports revealed higher prevalence in female dogs (Araujo et al., 2016). However, in our study, CTVT was more prevalent in males, similar to what has been reported by Arcila-Villa et al (2018). These findings can be explained by the easier detection of genital lesions in males in comparison to females, in which lesions are usually located deeply within the vestibule-vagina junction or vagina (Ganguly et al., 2016). In fact, external genitalia were the most affected site in our study. This is most likely due to the predominant transmission of CTVT during mating (Fathi et al., 2018). In addition, clinical signs predominantly consisted of genital bleeding as well as friable, ulcerative, hyperemic, multinodular, cauliflower-like masses, all also identified in previous research (Ganguly et al., 2016; Fathi et al., 2018; Costa et al., 2023). In the present study, extragenital lesions were found in 9.4% of dogs diagnosed with TVT and were located either in the skin or nasal cavity, both with or without genital involvement. In fact, the nasal cavity and skin are reported to be the most affected extragenital sites (Chikweto et al., 2013; Araujo et al., 2016; Thatcher et al., 2020; Costa et al., 2023), and atypical manifestations of CTVT may occur independently from genital lesions (Pimentel et al., 2021) Transmission in these cases may be either primary due to direct inoculation of living tumor cells following social behavior such as sniffing, licking, and biting or metastatic from a primary genital lesion (Chikweto et al., 2013; Komnenou et al., 2015). The associated clinical signs of the nasal location of CTVT, including nasal discharge, facial deformation, and dyspnea, were similar to those described in previous reports (Singh and Sood, 2016; Ojeda et al., 2018; Costa et al., 2023). Similarly to other studies (Araujo et al., 2016; Costa et al., 2023), skin tumors in this report were reminiscent of the characteristic hyperemic, friable, and ulcerative aspects of CTVT. Although it is considered a rare condition, cases of skin metastasis were also mentioned in previous studies, with clinical presentations varying from localized solitary nodules to multiple disseminated masses, usually located on the back, flank, and forehead region and presenting at times ulceration, as it has been seen in this study (Park et al., 2006; Behera et al., 2012; Chikweto et al., 2013; Fathi et al., 2018; Costa et al., 2023). Cytology is considered to be more advantageous in establishing a diagnosis of CTVT than histology (Ganguly et al., 2016). The characteristic cytological features of CTVT, allow differentiating this from other round cell tumors including histiocytomas, mast cell tumors, lymphomas, plasma cell tumors, and amelanotic melanomas (Hendrick, 2017). Cytology was the most employed method for diagnosing CTVT in previous reports (Arcila-Villa et al., 2018; Pimentel et al., 2021). However, in our study, this technique was only used in 2 atypical cases to establish an early diagnosis. Actually, cytology was not frequently used by local veterinarians, possibly as many still base their diagnosis on clinical observation of the clinical presentation of CTVT. Cytologic findings were consistent with descriptions in the literature (Behera et al., 2012; Komnenou et al., 2015; Singh and Sood, 2016; Ojeda et al., 2018). Round to polyhedral-shaped cells, with an eccentrically located nucleus, multiple mitosis, occasional multinucleation, and the characteristic intracytoplasmic vacuolations were observed. Histological evaluation showed findings comparable to previous studies, including a homogenous, highly cellular population of round cells with high mitotic rate, arranged in solid sheets, separated by scant fibrous stroma and noticeable lymphocytic infiltration (Behera et al., 2012; Hendrick, 2017; Mascarenhas et al., 2017; Fathi et al., 2018; Costa et al., 2023). The cytoplasm of neoplastic cells varied between moderately to non-vacuolated. In fact, the presence of cytoplasmic vacuolization is not a regular feature in histology (Hendrick, 2017), which renders the distinction between CTVT and other pleomorphic round-cell tumors on a histological basis challenging. Therefore, complementary immunohistochemical analysis is often required for an accurate diagnosis. In this study, IHC was especially used in 4 atypical cases. An intense staining for vimentin and AAT, and negative marking for CD3, CD20, and AE1/AE3 were observed. These results were also described in previous studies (Mozos et al., 1996; Park et al., 2006; Pinczowski et al., 2015; Mascarenhas et al., 2017; Thatcher et al., 2020). However, staining for lysozyme has been shown to be variable. In our report, only occasional neoplastic cells possessed positive cytoplasmic labeling for lysozyme, whereas it showed significant staining in other reports (Mozos et al., 1996; Park et al., 2006; Pinczowski et al., 2015; Flórez et al., 2016), and was considered consistently positive in a research conducted by Marchal et al (1997). Variability of lysozyme expression has been related to several factors, including the degree of cytoplasmic differentiation of neoplastic cells, with poorly differentiated cells often lacking lysozyme (Mozos et al., 1996), variability in protein expression in tumor cells from different geographic regions (Mascarenhas et al., 2017), and modification of antigenic sites due to inadequate tissue fixation. However, the latter hypothesis was ruled out in our report, since neutrophils and macrophages serving as an internal positive control, were highly marked. Overall, the positive expression of vimentin and AAT combined with negative labeling for CD3, CD20, and AE1/AE3 was helpful in excluding lymphomas, amelanotic melanomas, poorly differentiated carcinomas, and mast cell tumors. However, since the immunohistochemical panel did not exclude histiocytomas, differential diagnosis of CTVT should be based on history, clinical signs, and histopathologic criteria (Hendrick, 2017; Thatcher et al., 2020). In conclusion, CTVT is a common neoplasm in Morocco and is mostly detected in young dogs with uncontrolled sexual activity. Knowledge of the diagnostic options available and their corresponding characteristic findings is essential to establish an accurate diagnosis of CTVT, especially considering its atypical manifestations which remain underdiagnosed. Rigorous population control of stray dogs and pre-breeding examination of owned dogs may aid in minimizing the risk of transmission and eradicate this potentially detrimental disease. AcknowledgmentsThe authors express their gratitude to the veterinary clinicians in Morocco who submitted samples, which made this and further studies possible. The authors are also grateful to all staff members of the Department of Pathology of the Faculty of Veterinary Medicine of Córdoba, Spain, the Faculty of Medicine and Pharmacy of Rabat, and the Agronomy and Veterinary Institute Hassan II, Rabat, Morocco. The authors thank Dr. Meryem Mahoudi and Dr. ELhassania Lakhel for supplying them with photographs of clinical cases used in Figure 2. Conflict of interestThe authors declare that they have no conflict of interest. Author contributionNL: Conceptualization, investigation, data curation, and analysis, visualization, writing original draft; YM: Supervision, methodology, validation, review, and editing; DSB: Conceptualization, supervision, validation, review, and editing; MEM: Methodology, investigation; GB: Investigation; NL: Resources; NT: Conceptualization, methodology, supervision, review, and editing; RA: conceptualization, validation, supervision, review, and editing. FundingThis work was supported by the National Center for Scientific and Technical Research (CNRST) [Grant number 4IAV2021, 2021]. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided by the corresponding author upon reasonable request. ReferencesAbedin, S.N. 2020. Canine transmissible venereal tumor: a review. J. Entomol. Zool. Stud. 8(2), 596–599. Alkan, H., Satilmis, F., Alcigir, M.E., Kivrak, M.B. and Aydin, I. 2017. Clinicopathological evaluation of disseminated metastases of transmissible venereal tumor in a spayed bitch. Acta. Sci. Vet. 45, 6–6. Araujo, D.C.C., Antonioli, T., Costa, T.S., de Carvalho, J.R.G., Laguna, A.G.V., Ramadinha, R.H.R. and Fernandes, J.I. 2016. Occurrence and location of transmissible venereal tumors in dogs seen at the Universidade Federal Rural do Rio de Janeiro Veterinary Hospital: oncology Sector between 2010 and 2014. Rev. Bras. Med. Vet. 38(3), 277–280. Arcila-Villa, A., Dussán-Lubert, C. and Pedraza-Ordoñez, F. 2018. Distribution and prevalence of transmissible venereal tumor in the Colombian canine population. Rev. Colomb. Cienc. Pecu. 31(3), 180–187. Behera, S.K., Kurade, N.P., Monsang, S.W., Das, D.P., Mishra, K.K. and Mohanta, R.K. 2012. Clinico-pathological findings in a case of canine cutaneous metastatic transmissible venereal tumor. Vet. Arh. 82(4), 401–410. Chikweto, A., Kumthekar, S., Larkin, H., Deallie, C., Tiwari, K.P., Sharma, R.N. and Bhaiyat, M.I. 2013. Genital and extragenital canine transmissible venereal tumor in dogs in Grenada, West Indies. Open. J. Vet. Med. 03, 111–4. Costa, T.S., Paiva, F.N., Manier, B.S., Araújo, D.C., Ribeiro, G.B. and Fernandes, J.I. 2023. Epidemiological, clinical, and therapeutic aspects of canine transmissible venereal tumor in Rio de Janeiro, Brazil (2015-2020). Pesqui. Veterinária. Bras. 43, e07189. Fathi, M., Ashry, M., Ali, K.M., Hassan, A. and Elkarmoty, A.F. 2018. Clinico-pathological evaluation and treatment outcomes of canine transmissible venereal tumor using three different protocols. Pak. Vet. J. 38(2), 204–208. Flórez, L.M., Ballestero, H.F., Duzanski, A.P., Bersano, P.R., Lima, J.F., Cruz, F.L., Mota, L.S. and Rocha, N.S. 2016. Immunocytochemical characterization of primary cell culture in canine transmissible venereal tumor. Pesqui. Vet. Bras. 36, 844–850. Ganguly, B., Das, U. and Das, A.K. 2016. Canine transmissible venereal tumour: a review. Vet. Comp. Oncol. 14(1), 1–12. Hendrick, M.J. 2017. Mesenchymal tumors of the skin. In tumors in domestic animals, Fifth edition: Eds., Meuten, D.J. Ames, IA: John Wiley & Sons, pp: 173–174. Komnenou, A.T., Thomas, A.L.N., Kyriazis, A.P., Poutahidis, T. and Papazoglou, L.G. 2015. Ocular manifestations of canine transmissible venereal tumour: a retrospective study of 25 cases in Greece. Vet. Rec. 176(20), 523–523. Mascarenhas, M.B., Peixoto, P.V., Ramadinha, R.R., Armien, A.G., Costa, S.Z., Miranda, I.C., Nogueira, V.A. and França, T.N. 2017. Immunohistochemical, lectin histochemical and ultrastructural studies of canine transmissible venereal tumor in Brazil. Pesqui. Vet. Bras. 37, 613–620. Marchal, T., Chabanne, L., Kaplanski, C., Rigal, D. and Magnol, J.T.P. 1997. Immunophenotype of the canine transmissible venereal tumour. Vet. Immunol. Immunopathol. 57(1-2), 1–11. Mozos, E., Mendez, A., Gómez-Villamandos, J.C., Martín De Las Mulas, J and Pérez, J. 1996. Immunohistochemical characterization of canine transmissible venereal tumor. Vet. Pathol. 33(3), 257–263. Murchison, E.P. 2021. Rising incidence of canine transmissible venereal tumours in the UK. Vet. Rec. 189(12), 472–474. Muste, A., Beteg, F., Muste, M., Stroe, T. and Lăcătuş, R. 2012. Research and observation on clinical and therapeutic aspects regarding transmissible venereal tumor in dogs. Vet. Med. 58(4), 272–277. Ojeda, J., Mieres, M., Soto, F., Arnes, V., Paredes, E. and Navarrete, M. 2018. Computer tomographic imaging in 4 dogs with primary nasal canine transmissible venereal tumor and differing cellular phenotype. J. Vet. Intern. Med. 32(3), 1172–1177. Ostrander, E.A., Davis, B.W. and Ostrander, G.K. 2016. Transmissible tumors: breaking the cancer paradigm. Trends. Genet. 32(1), 1–15. Park, M.S., Kim, Y., Kang, M.S., Oh, S.Y., Cho, D.Y., Shin, N.S. and Kim, D.Y. 2006. Disseminated transmissible venereal tumor in a dog. J. Vet. Diagn. Invest. 18(1), 130–133. Pimentel, P.A., Oliveira, C.S. and Horta, R.S. 2021. Epidemiological study of canine transmissible venereal tumor (CTVT) in Brazil, 2000–2020. Prev. Vet. Med. 197, 105526. Pinczowski, P., Gimeno, M., Aceña, C., Villegas, A., de Martino, A. and Luján, L. 2015. Brain metastasis in a case of canine transmissible venereal tumor after a supposed successful treatment with vincristine sulfate. Acta. Vet. (Beogr). 65(1), 137–142. Schectman, S.J., Khanam, A., Walters, M.N., Kirwan, E., Sylvester, W.R. and Khan, F.A. 2022. A retrospective study of canine transmissible venereal tumour in Grenada, West Indies. Vet. Med. Sci. 8(3), 1008–1012. Singh, R.S. and Sood, N.K. 2016. Management of primary transmissible venereal tumor in nasal cavity of a dog. Intas. Polivet. 17(2), 546–548. Strakova, A. and Murchison, E.P. 2014. The changing global distribution and prevalence of canine transmissible venereal tumour. BMC. Vet. Res. 10(1), 1–11. Thatcher, G., Robat, C. and Bell, C. 2020. Diagnosis and management of an oronasal fistula secondary to nasal transmissible venereal tumor in a dog. J. Vet. Dent. 37(4), 220–226. Supplementary file 1. Detailed protocol of the immunohistochemical technique conducted during this studyImmunohistochemical evaluation was performed on 3 µm thick tissue sections and processed as follows in 2-day steps: Day 1Step 1: Dewaxing and Hydration
Step 2: AR methods– Slides destined for Vimentin, CD3, and Cytokeratin (AE1/AE3) antibodies, were incubated for 10 minutes at room temperature in 10% pronase (Sigma life science, P5459-5G). – Slides destined for AAT were incubated in 0.01 M Citrate buffer solution, pH6, and heated to 100°C for 20 minutes. – Slides destined for Lysozyme and CD20 were not treated and were kept in distillated water. Step 3: Rinsing1x bath of 5minutes in distillated water 3x baths of 5minutes in PBS (Phosphate-Buffered Saline) (PBS=1000ml distillated water + 1,48g Na2HPO4 + 0,43g KH2PO4 + 7,2g NaCl). Step 4: Blocking non-specific antibody bindingAfter removing the slides from PBS solution, 10% normal goat serum (NGS) (vidraFOC, Barcelona, Spain) was deposited on each preparation and incubated for 30 minutes at room temperature. (1 preparation=10µl NGS + 90 µl PBS). Step 5: Primary AntibodiesThe slides were then incubated for 18 hours at 4°C with the following primary antibodies:
Dilution was carried out with an Antibody Diluent (Ref S0809, Dako, USA). PS: Each preparation=100 µl (It includes antibody + diluent added depending on the consecutive dilution). Day 2Step 1: Rising3x baths of 10minutes in PBS Step 2: Secondary antibodiesThe slides were then incubated for 30 minutes at 4°C with the following secondary antibodies:
Step 3: Rising3x baths of 10 minutes in PBS Step 4: Application of The Elite ABC (Avidin-Biotin Complex)The Vectastain® Elite ABCPeroxidase kit (Vector Laboratories, Burlingame, CA, USA) wasapplied at a dilution of 1:50. Slides were later incubated in the dark for 60 minutes. Dilution was carried out with PBS solution. (1 preparation=2µl solution A + 2µl solution B + 96 µl PBS). Step 5: Revealing peroxidase activityThe immunoreaction was observed in the light of microscopy after the application of the Peroxidase DAB Substrate Chromogen System (Dako, North America, CA, USA). Once the immunoreaction was revealed slides were placed in distillated water. Step 6: Preparation of slides for interpretation3x baths of 5 minutes in distillated water 1 bath of 1 minutes in Mayer’s Hematoxylin 3 baths of water Dehydration
Mounting the slides | ||
| How to Cite this Article |
| Pubmed Style Laissaoui N, Millán Y, Betz DS, Mrini ME, Bouayad G, Lamalmi N, Tligui N, Azrib R. Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Vet. J.. 2024; 14(5): 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 Web Style Laissaoui N, Millán Y, Betz DS, Mrini ME, Bouayad G, Lamalmi N, Tligui N, Azrib R. Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). https://www.openveterinaryjournal.com/?mno=191799 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i5.16 AMA (American Medical Association) Style Laissaoui N, Millán Y, Betz DS, Mrini ME, Bouayad G, Lamalmi N, Tligui N, Azrib R. Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Vet. J.. 2024; 14(5): 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 Vancouver/ICMJE Style Laissaoui N, Millán Y, Betz DS, Mrini ME, Bouayad G, Lamalmi N, Tligui N, Azrib R. Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Vet. J.. (2024), [cited January 24, 2026]; 14(5): 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 Harvard Style Laissaoui, N., Millán, . Y., Betz, . D. S., Mrini, . M. E., Bouayad, . G., Lamalmi, . N., Tligui, . N. & Azrib, . R. (2024) Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Vet. J., 14 (5), 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 Turabian Style Laissaoui, Nadia, Yolanda Millán, Daniela Simon Betz, Meryem El Mrini, Ghita Bouayad, Najat Lamalmi, Noursaid Tligui, and Rahma Azrib. 2024. Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Veterinary Journal, 14 (5), 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 Chicago Style Laissaoui, Nadia, Yolanda Millán, Daniela Simon Betz, Meryem El Mrini, Ghita Bouayad, Najat Lamalmi, Noursaid Tligui, and Rahma Azrib. "Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023)." Open Veterinary Journal 14 (2024), 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 MLA (The Modern Language Association) Style Laissaoui, Nadia, Yolanda Millán, Daniela Simon Betz, Meryem El Mrini, Ghita Bouayad, Najat Lamalmi, Noursaid Tligui, and Rahma Azrib. "Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023)." Open Veterinary Journal 14.5 (2024), 1206-1215. Print. doi:10.5455/OVJ.2024.v14.i5.16 APA (American Psychological Association) Style Laissaoui, N., Millán, . Y., Betz, . D. S., Mrini, . M. E., Bouayad, . G., Lamalmi, . N., Tligui, . N. & Azrib, . R. (2024) Canine transmissible venereal tumour in Morocco: Clinical and pathological findings in 64 dogs – Insights from a descriptive epidemiological study (2020-2023). Open Veterinary Journal, 14 (5), 1206-1215. doi:10.5455/OVJ.2024.v14.i5.16 |