| Review Article | ||
Open Vet. J.. 2024; 14(7): 1526-1537 Open Veterinary Journal, (2024), Vol. 14(7): 1526–1537 Review Article Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunityHanar Azad Abdulrahman1, Hiewa Othman Dyary2, Rebar N. Mohammed3,4*, Darya Shorsh Hamad4, Faraidoon Abdul-Star5 and Nahla Mohammad Saeed31Department of Pharmacy, Kurdistan Technical Institute, Sulaymaniyah, Iraq 2Department of Basic Sciences, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq 3Department of Microbiology, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq 4Medical Laboratory Analysis Department, College of Health Sciences, Cihan University of Sulaymaniyah, Sulaymaniyah, Iraq. 5Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq *Corresponding Author: Rebar Nawzad Mohammed. Department of Microbiology, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq. Email: rebar.mohammed [at] univsul.edu.iq Submitted: 24/03/2024 Accepted: 18/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
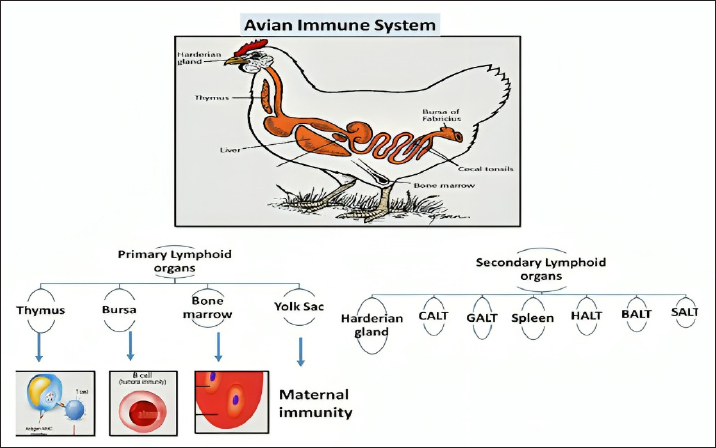
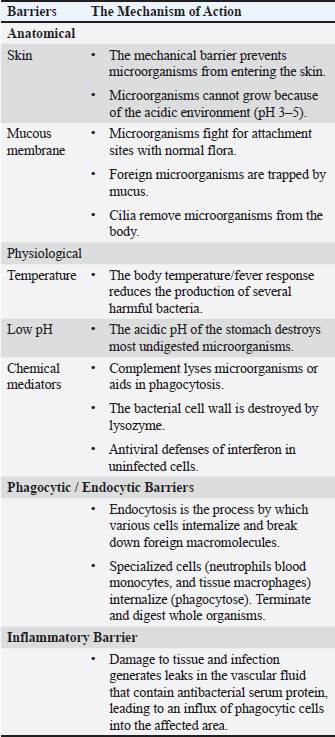
AbstractFree radicals (FRs), also known as reactive oxygen species (ROS), are usually established in the body when adequate oxygen depletion occurs. Oxidative stress and the establishment of FRs in the body are mainly caused by high metabolic activity, the need for rapid growth, inadequate flock management, exposure to viral and bacterial microorganisms, and adverse environmental conditions. Furthermore, FRs can also be produced during the activity of phagocytes when they depend on the action of ROS to kill the engulfed pathogen. FRs have very adverse effects on all cells, particularly the cells of the immune system. They are extremely erratic and reactive molecules that directly harm DNA, cellular proteins, lipids, and carbohydrates within cells. Antioxidants are substances that can eliminate and neutralize FRs within the body and free the body from the oxidative stress that occurs due to the accumulation of FRs. Many vitamins and minerals support the activity and effect of the immune system in fighting against microbes and cancer, which mostly depend on their antioxidant elements to diminish the negative impact of FRs in the body. Examples are vitamin C, vitamin E, superoxide dismutase, selenium, glycine, cofactors of glutathione peroxidase, manganese, essential oils, and phenolic compounds. Keywords: Free radicals, Antioxidants, Oxidative stress, Nutrition, Immune system. IntroductionThe poultry industry remains one of the most important sources of income for many countries, especially developing ones. Chickens can supply a high-quality and reasonably priced source of animal proteins for human consumption, fulfilling daily protein requirements in compliance with dietary guidelines. Many factors, particularly environmental elements like cold, heat, and overcrowding, can negatively impact the poultry industry. Moreover, other factors, including physiological (internal), technological, and dietary stressors, can influence the production rate, performance, and protein content quality of poultry meat (Akinyemi and Adewole, 2021). Environmental stress continues to be a key danger to the immune system’s ability to respond to different diseases, even though several factors influence the immune system’s strength and performance in the body. Among the mechanisms impaired by environmental stress are the cytokine-receptor interaction pathway, toll-like receptor signaling pathways, and the production of excessive reactive oxygen species (ROS) in response to multiple impaired immune pathways (Hofmann et al., 2020). Oxidative stress, one of the main causes of immune dysfunction and a common stressor in poultry, arises from an imbalance between the rate of ROS production and accumulation in cells and interstitial tissues and its detoxification and clearance by the body’s available antioxidants. Fortunately, scientific advancements, particularly in studying free radicals (FRs), ROS, and antioxidants, have improved the immune system and defense mechanisms. These enhancements have been achieved by manipulating the nutritional content of poultry diets to strike a balance (Lobo et al., 2010). This review focuses on the mechanisms of oxidative stress in poultry and highlights the importance of including antioxidants in poultry feed to reduce the detrimental effects of ROS on the different body systems, especially the immune system. Avian immune systemThe immune system comprises a complicated network of chemicals, cells, and organs. Together, they provide and mount an immune response against many pathogens they encounter. Apart from defense against microbes, it is the responsibility of the immune system to eliminate any developing abnormal cells categorized under various tumor-forming cells. Moreover, homeostasis and scavenging dead cells and debris are among the functions of the immune system (Bonilla and Oettgen, 2010). The avian physiology and immune system resemble those of other mammals, which all developed from the same reptilian ancestors and have many commonalities (Surai, 2002). The immune system of avian species is primarily composed of primary and secondary lymphoid organs together with lymphatic tissues (Fig. 1). The pivotal role of the immune system in avian species and all other mammals is to maintain homeostasis, in which harmful substances can be distinguished from non-harmful substances and elicit an appropriate response in the event of the entry of a toxic substance. The innate and adaptive immune systems work together to eradicate disease-causing agents, such as pathogenic bacteria and viruses (Bhuiyan et al., 2021). Innate immunityIn the avian world, the innate immune system is similar to other mammals, providing the first line of defense. These range from external defenses, such as physical barriers like feathers, skin, and other parts of the bird’s body, to chemical barriers, such as nasal discharges, antimicrobial peptides in sweat, and acids in the GIT. Other components of the innate immune system that contribute to the bird’s overall protection against invaders include fluctuations in body temperature and acidity levels, particularly at low pH (Luo et al., 2010). The internal defenses of the innate immune system play the most important role in initiating immune responses. While the innate immune system does not respond as specifically as the adaptive immune system, which cannot differentiate between the specificities of pathogens, it does possess a range of receptors designed to recognize common pathogenic structures (Table 1) (Gombart et al., 2020). Another important role of the innate immune system is stimulating and shaping the adaptive immune system. The adaptive immune system cannot target the pathogen without the support of the innate immune system. The functions of the innate immune system include secreting various cytokines and chemokines and presenting antigens to antigen-specific lymphocytes of the adaptive immune system (Ohteki and Koyasu, 2001).
Fig. 1. Primary and Secondary lymphoid organs. Enmeshed in the immune complex, mature B and T cells are transferred from primary or lymphoid tissue for the development stage, termed immune movement or immune peripheralization. Table 1. The nonspecific host defense mechanisms.
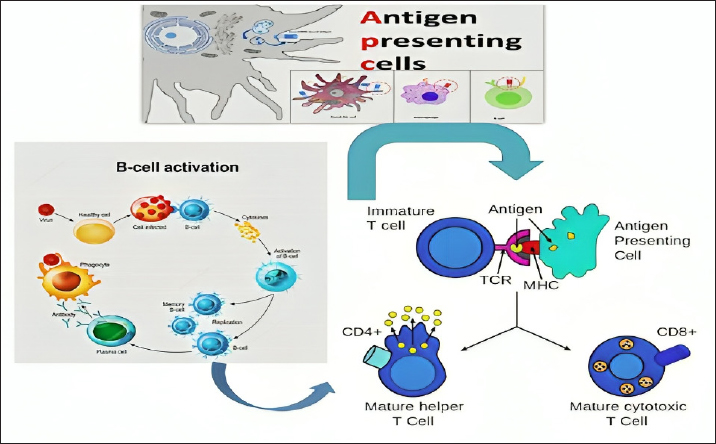
Adaptive immunityThe adaptive immune system is another part of the bird’s immune system, which mainly responds to pathogen invasions and the development of tumor cells via a range of cells called antigen-specific lymphocytes. The response is initiated by the cells of the innate immune system, and lymphocytes respond through adaptation and clonal selection. The immune system’s evolution is important in that it can compensate for any missing parts and cells if they exist. When cells of the innate immune system are defective or missing due to any genetic or acquired defect, the cells of the adaptive immune system can compensate for the mechanisms that are supposed to be provided by the defective cell (Marshall et al., 2018; Martin, 2001). The adaptive immune system relies on a specific sequence of an antigen’s amino acids, available either on the pathogen’s surface or internally. T lymphocytes are born in the bone marrow and mature in the thymus in most mammals, including birds. Upon their maturation and subsequent release into circulation, these cells are responsible for performing immune surveillance, a process in which the immune cells look for the entry of any pathogens. Once there is an invasion by a pathogen, there will be clonal selection and subsequent expansion of the antigen-specific T lymphocytes, which usually occurs in a nearby secondary lymphoid organ. This antigen-specific effector T cell wave is responsible for dealing specifically with the pathogens and their elimination from the body. These so-called effector cells use several mechanisms to kill the pathogens, such as the secretion of cytotoxic cytokines that perforate infected cells and later lyse the microbes. One of the most important advantages of the adaptive immune response over the innate immune system is the development of immunological memory. The memory cells, distributed throughout the bird’s body and specifically found in the secondary lymphoid tissues, are responsible for re-initiating the immune response in case of an invasion from the same pathogen for the second time (Bonilla and Oettgen, 2010). The development of immunological memory by adaptive immune system cells is the basis for vaccination and immunization. Vaccination remains one of the greatest achievements in medicine for saving both humans and animals, and it is the safest method for their protection and overall survival on the planet. The main goal of vaccination is to generate a large pool of antigen-specific antibodies that can neutralize a specific pathogen in the future. In addition, the vaccination process will also lead to the creation of a huge repertoire of antigen-specific memory T and B cells. B cells are the generators of antibodies. They are born in the bone marrow and mature in the bursa of Fabricius, which is only available in avian species. Upon maturation, B cells circulate via blood and lymphatics, and once a vaccine is introduced or the body is invaded with a pathogen, mature B cells, also called naïve B cells, will internalize the antigen and differentiate into plasma cells. Plasma cell maturation also takes time, going through plasmablasts and eventually differentiating into mature plasma cells. Hundreds to thousands of antibodies can be secreted by a fully mature plasma cell per second to generate a huge repertoire (Marshall et al., 2018) (Fig. 2). Poultry production stressorsA wide range of external factors, such as environmental factors, can cause stress for the avian species and others. In addition, internal factors can also cause stress. The stress level can vary based on the type of stressor the bird encounters. Homeostasis, maintaining a steady balance of internal mechanisms and cell numbers, can be restored in cases of moderate stress through the function of the hypothalamic-pituitary-adrenal gland. Neurological involvement and the immune system can also contribute to the restoration of the effects caused by moderate stress. However, intense stress is difficult to restore and can result in a complex cascade of metabolic modifications, such as metabolic shifts and increased energy mobilization. The long-term effects of intense stress can lead to other complications and primarily result in reduced poultry and livestock production, the business’s primary goal of production management (Bureau et al., 2009).
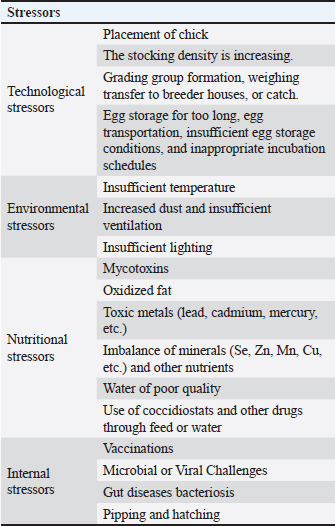
Fig. 2. Adaptive immune system response, showing the flow from antigen to APC, MHC II, CD4+ T cells, B cells, antibodies, macrophages, and cytotoxic CD8+ T cells. Although nutritional stress that leads to various metabolic diseases such as muscular dystrophy, encephalomalacia, and exudative diathesis is rare in commercial poultry production, mainly due to providing well-controlled and balanced nutrition, livestock management remains an important factor to be investigated and monitored (Surai, 2002). Due to the genetic selection of specific strains of broilers, layers, and turkeys that can provide a rapid growth rate, improved feed conversion, domestication, and a high egg production rate, these birds can be exposed to oxidative stress, which eventually leads to a marked reduction in production and reproduction capacity. A sharp or moderate reduction curve in production ability in these livestock management practices is an important factor contributing to substantial economic losses (Soleimani et al., 2011). Environmental, technological, internal, and nutritional stresses are considered among the four most important types of stresses that can affect poultry production. These various stress types can impact the bird at a molecular/cellular level and then have effects at a physiological level, ultimately influencing the production capability and performance of commercial livestock, especially in terms of reproductive capacity (Surai et al., 2018). According to several research studies (P. F. Surai and Fisinin, 2016; Peter F. Surai et al., 2018), oxidative stress is the main type of commercial stress influencing poultry production during livestock management (Table 2). Free radicalsFRs are defined as electrons that are not paired. Many of these radicals are highly reactive and unstable; they can serve as reducing or oxidizing agents by taking an electron from another molecule or giving it to them (Cheeseman and Slater, 1993; Lobo et al., 2010). ROS are radicals centered around oxygen, forming a subset of reactive nitrogen species (RNS). They all emerge due to regular cellular metabolism (Phaniendra et al., 2015). ROS and RNS have been categorized as advantageous and detrimental due to their positive and adverse impacts on biological systems (Di Meo et al., 2016). Positive outcomes are observed when ROS/RNS levels are within low to moderate concentrations, yet detrimental effects arise at higher levels, surpassing the overall antioxidant capacity required to regulate them (Agarwal et al., 2005). Table 2. Main stressors in poultry production.
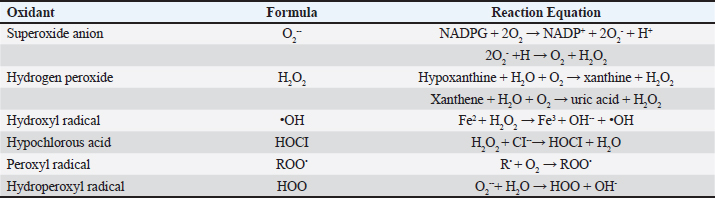
FRs are generated through three distinct mechanisms: (a) via the hemolytic rupture of a normal molecule’s covalent bond, (b) by extracting an electron from an ordinary molecule, and (c) by introducing an electron into a regular molecule (Chen et al., 2021). FRs can target various molecules within the body. The detrimental impacts of ROS/RNS leading to biological harm are termed oxidative stress and nitrosative stress, respectively (Lobo et al., 2010; Phaniendra et al., 2015). FRs can cause cellular damage and disrupt homeostasis by attacking essential macromolecules. Excessive ROS can impair the proper functioning of cellular lipids, proteins, or DNA. Redox homeostasis is maintained through “redox regulation,” establishing a balanced equilibrium between FRs’ beneficial and detrimental effects within a living organism (Birben et al., 2012). Oxidizing agents in poultryAlthough genetics play an important role in poultry production, the environment and nutrition should be optimal for maximum growth and health statuses (Kachungwa Lugata et al., 2022). Stressors are agents capable of producing stress at any time (Rostagno, 2020). Stress initiates the defense system and increases energy and nutritional demands, especially amino acids, trace elements, and vitamins. Oxidative stress results from ROS overproduction or insufficient antioxidant defense systems (Halliwell, B. and Gutteridge, J.M. (2015). Various environmental, nutritional, and biological stressors can impact poultry production, reducing reproductive and growth performances and compromising the general health of the birds (Surai et al., 2019). Many metals, such as calcium, magnesium, potassium, sodium, and iron, are essential animal micronutrients. However, they may be toxic at high concentrations by increasing ROS formation (Koivula and Eeva, 2010). Nonoptimal temperature, lighting (Huth and Archer, 2015), and ventilation impact the overall growth rate and are considered environmental stressors for poultry. Moreover, nutritional stressors of poultry include mycotoxins, oxidized fats, low water quality, and toxic metals such as lead, cadmium, and mercury (Guo et al., 2019). Also, microbial and viral infections and disturbances in gut microbiomes are some of the internal stressors that negatively affect the growth performance of poultry. Endogenous sources of ROSROS are generated through the ordinary metabolic processes of cells, originating from molecular oxygen. ROS can be categorized into two types: FRs and nonradicals. FRs are molecules possessing one or more unpaired electrons that confer reactivity upon the molecule. Nonradical forms emerge when two FRs exchange their unpaired electrons. The superoxide anion (O2•−), hydroxyl radical (•HO), and hydrogen peroxide (H2O2) are the three most physiologically significant ROS (Table 3) (Phaniendra et al., 2015). The superoxide anion is formed by adding a single electron to molecular oxygen (Miller et al., 1990). The fundamental components include nicotinamide adenine dinucleotide phosphate [NAD(P)H) oxidase, xanthine oxidase, and the mitochondrial electron transport system. The mitochondria, responsible for ATP synthesis, act as the primary source of the superoxide anion. Typically, electrons move through the mitochondrial electron transport chain to convert oxygen into water. However, a small fraction, roughly 1%–3%, escapes, generating superoxide. NAD(P)H oxidase is found in polymorphonuclear leukocytes, monocytes, and macrophages. These cells create a surge of superoxide during phagocytosis, contributing to bactericidal action. Superoxide dismutases (SODs) are pivotal in converting superoxide to hydrogen peroxide. The plasma membrane facilitates the easy diffusion of hydrogen peroxide. Table 3. Major endogenous sources of ROS.
Moreover, xanthine oxidase, amino acid oxidase, and NAD(P)H oxidase contribute to hydrogen peroxide production (Dupuy et al., 1991). Peroxisomes utilize molecular oxygen for metabolic processes. In the presence of transition metals such as Fe2+ or Cu2+, H2O2 can participate in a sequence of reactions known as the Haber-Weiss and Fenton reactions, ultimately leading to the creation of OH- (Fenton, 1894).
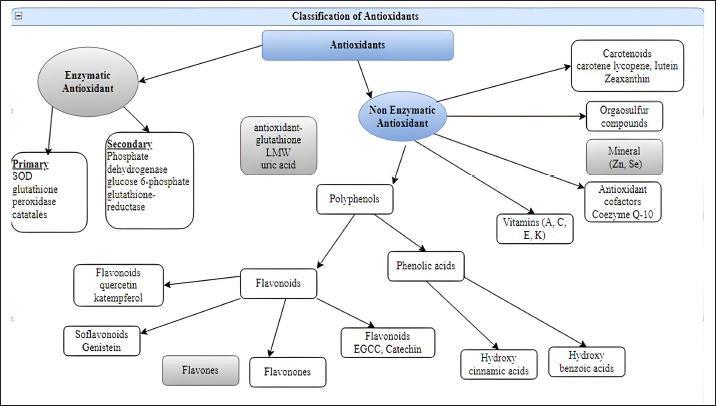
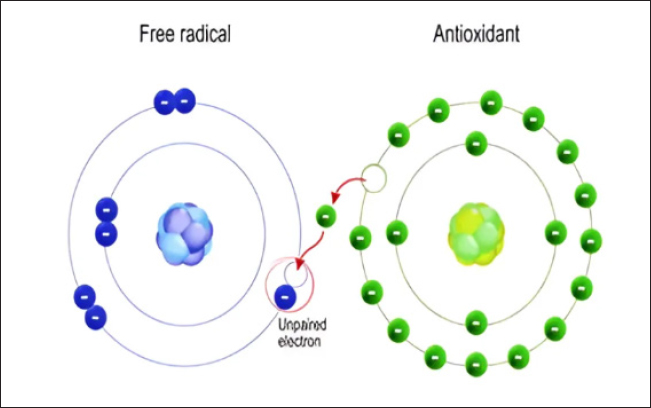
O2- can also produce OH- when it interacts with H2O2 (Haber and Weiss, 1934). The hydroxyl radical, the most reactive type of ROS, can damage proteins, carbohydrates, lipids, and DNA. Furthermore, by extracting an electron from polyunsaturated fatty acids, it can initiate lipid peroxidation (Phaniendra et al., 2015). Granulocytic enzymes, specifically eosinophil peroxidase and myeloperoxidase (MPO), further heighten the reactivity of H2O2. When activated neutrophils are present, MPO absorbs H2O2. In the presence of chloride ions, H2O2 transforms into hypochlorous acid (HOCl). HOCl possesses potent oxidative properties and plays a pivotal role in eradicating pathogens in the airways (Klebanoff, 2005). However, HOCl can also interact with DNA, leading to DNA-protein interactions, pyrimidine oxidation products, and adding chloride to DNA bases (Kulcharyk and Heinecke, 2001). During processes such as halogenation, nitration, and the formation of protein cross-links through tyrosyl radicals, eosinophil peroxidase, and MPO contribute to oxidative stress. Furthermore, these enzymes induce oxidative stress by modifying proteins through halogenation, nitration, and forming protein cross-links via tyrosyl radicals (Davies, 2011). Peroxyl radicals (ROO•) constitute another class of oxygen-derived FRs. The hydroperoxyl radical (HOO•) is particularly significant, as it participates in fatty acid peroxidation. By extracting a hydrogen atom from a side-chain methylene carbon, FRs initiate a chain reaction in the process of lipid peroxidation. The peroxyl radical arises when the lipid radical encounters oxygen, converting polyunsaturated fatty acids into lipid hydroperoxides and initiating a chain reaction. These lipid hydroperoxides are highly unstable and rapidly degrade into aldehydes (e.g., 4-hydroxy-2,3-nonenal) and malondialdehydes (MDA). Another category of products stemming from lipid peroxidation are isoprostanes, formed when arachidonic acid undergoes peroxidation. Research has shown that elevated levels of isoprostanes in both asthmatic plasma and breath condensate the integrity of cell membranes is disrupted by lipid peroxidation, which results in membrane reorganization (Wood et al., 2000). Standardized tests can easily measure hydrogen peroxide, superoxide radical, oxidized glutathione (GSSG), MDAs, isoprostanes, carbonyls, and nitrotyrosine, acting as biomarkers in plasma, blood, or bronchoalveolar lavage samples (Birben et al., 2012). Antioxidants can directly counteract ROS, indirectly enhance the body’s defenses against oxidation, or inhibit ROS production. These compounds include monohydroxy/polyhydroxy phenols (Mann et al., 2018). Guttering and Halliwell have classified antioxidants into three distinct groups: primary antioxidants, crucial for preventing the production of oxidants; secondary antioxidants, recognized for their ability to scavenge ROS; and tertiary antioxidants, responsible for mending damaged molecules through dietary or sequential antioxidants (Singh et al., 2003). Moreover, they can be further categorized into various groups based on their properties, origins, and mechanisms of action (Fig. 3). Antioxidants have two types classified as enzymatic or nonenzymatic antioxidants. RH, ROH, ROOH, and a relatively unreactive phenoxyl radical are formed when antioxidants (AH) react with R, RO, and ROO (RH). Antioxidants provide electrons for FRs, allowing them to be neutralized (Table 4). When cells are exposed to oxidative stress, a defense system is activated, promoting the production and regulation of numerous antioxidant enzymes to shield cells from damage caused by FRs (Pham-Huy Lien Ai et al., 2008).
Fig. 3. Enzymatic and nonenzymatic classification of antioxidants. Table 4. Antioxidant defensive agents.
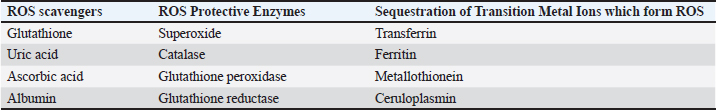
AntioxidantsThe body’s antioxidant systems can tolerate mild oxidative stress in poultry. However, oxidative stress can be reduced by reducing exposure to oxidizing agents, increasing endogenous and exogenous antioxidant levels, and stabilizing mitochondrial energy production efficiency. Enzymatic antioxidantsThe direct enzymatic antioxidant system and the indirect enzymatic antioxidant system serve as crucial defenses against ROS. Enzymatic antioxidants include catalase, SOD, glutathione peroxidase, glutathione reductase, and other relevant enzymes (Ighodaro and Akinloye, 2018). There are two categories of enzyme antioxidants: primary and secondary defenses. Glutathione peroxidase plays a role in reducing peroxides by contributing two electrons, forming selenols, and also neutralizing peroxides. On the other hand, catalase converts hydrogen peroxide into water and molecular oxygen, utilizing them as substrates. Additionally, SOD converts superoxide anions into hydrogen peroxide, which subsequently acts as a substrate for catalase (Rahman, 2007). Nonenzymatic antioxidantsDifferent classes of nonenzymatic antioxidants are present, encompassing vitamins (A, C, E, and K), enzyme cofactors (Q10), minerals (Zn and Se), organosulfur compounds (like allium and allium sulfur), nitrogen compounds (such as uric acid), peptides (like glutathione), and polyphenols (which include flavonoids and phenolic acid)(Mann et al., 2018; Tan et al., 2018). Endogenous antioxidantsEndogenous antioxidants can be categorized into primary antioxidants, which deactivate ROS by converting them into intermediate forms. Primary antioxidant enzymes include SOD, catalase, and glutathione peroxidase. These antioxidants are found in water-soluble forms like ascorbate, glutathione, and uric acid, as well as lipid-soluble forms such as tocopherols, ubiquinols, and carotenoids (Shu, 1998). Secondary antioxidant enzymes directly eliminate ROS. They modulate their functions by reducing peroxide levels and providing a constant supply of NADPH (nicotinamide adenine dinucleotide phosphate) and glutathione to primary antioxidant enzymes. Notable secondary antioxidants comprise glutathione reductase, glucose-6-phosphate dehydrogenase, glutathione-s-transferase, and ubiquinone. Moreover, the activity of antioxidant enzymes is enhanced by the presence of iron, copper, zinc, manganese, and selenium (Gale, 2001; Vertuani et al., 2004). Exogenous antioxidantsExogenous antioxidants are present in various herbs, spices, foods, vitamins, plants, and other sources. These agents with antioxidant properties have gained significant importance in both the clinical and research realms, as they can be utilized to treat various pathological conditions (Lourenço et al., 2019). A multitude of polyphenolic compounds, including flavonoids, isoflavones, flavones, isocatechins, anthocyanins, coumarins, lignans, catechins, and epicatechins, as well as phenolic acids like hydrocinnamic acid, hydrobenzoic acid, gallic acid, ellagic acid, and others, have emerged as crucial phytochemical antioxidants. Clinical and preclinical trials are currently in progress to explore the bioactive characteristics of these compounds (Farag et al., 2020). Table 5. Antioxidant enzymes that scavenge ROS.
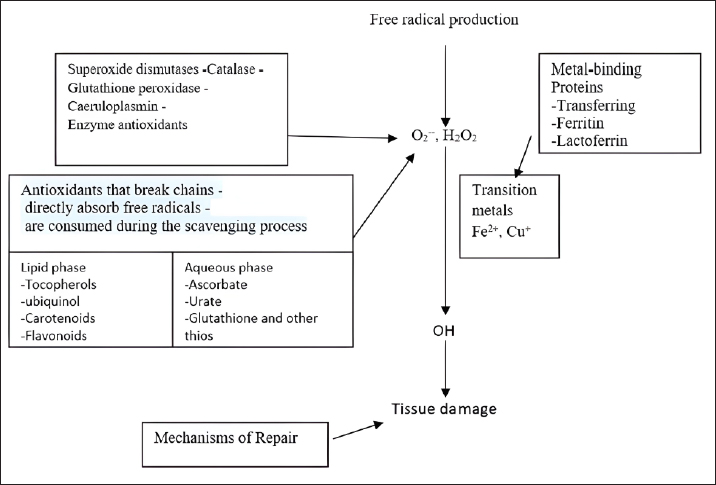
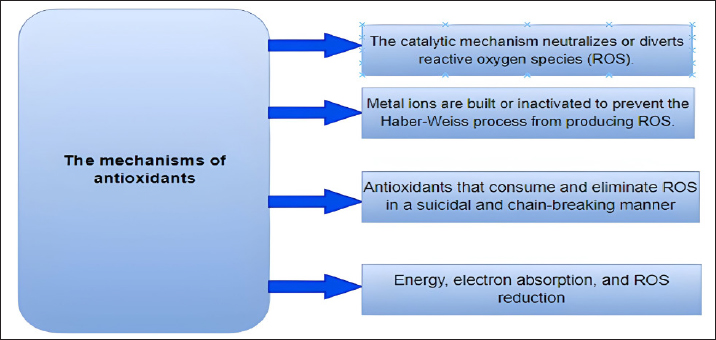
Fig. 4. A schematic diagram of antioxidant defense mechanisms. Plant-derived remedies hold medicinal value and are pivotal in research due to their rich content of phytochemicals such as terpenoids, glycosides, alkaloids, polyphenolics, and steroids (Fuhrman et al., 2004; Wu et al., 1999). Antioxidant enzymes are synthesized from dietary minerals, proteins, and amino acids and play an important part in the body’s defense mechanism. GSH, creatine, and uric acid are notable direct scavengers of reactive metabolites (Rassaf et al., 2002). Natural antioxidants like polyphenols, flavonoids, and tannins assist in alleviating lipid peroxidation by donating electrons to intermediate radicals generated during periods of oxidative stress or tissue damage (Sharifi-Rad et al., 2020). Antioxidant defense mechanismThe endogenous antioxidant defense systems effectively eliminate FRs generated through various activities. These include scavenging FRs, breaking down peroxides, and interacting with pro-oxidant metal ions. (Table 5; Fig. 4). Antioxidants are classified into three categories: Primary antioxidants obstruct the generation of oxidants by averting the formation of FRs. These include glutathione peroxidase, catalase, selenoprotein, transferrin, ferritin, lactoferrin, and carotenoids (Fig. 3). Secondary antioxidants serve as scavengers for ROS. They disrupt chain reactions and reduce chain initiation, functioning as radical scavengers (Fig. 3). Tertiary antioxidants participate in the restoration of oxidized molecules. This process is facilitated by specific proteolytic enzymes, DNA enzymes, and similar agents, with the support of dietary or subsequent antioxidants (Nimse and Pal, 2015). Mechanism of action of antioxidantsAntioxidants utilize a range of mechanisms to counteract FRs. These encompass catalytic systems that either neutralize or divert ROS (Fig. 5). Another aspect of antioxidant function involves AH reacting with R, RO, and ROO, leading to the creation of RH, ROH, ROOH, and a relatively unreactive phenoxyl radical (RH, ROH, ROOH) (RH). Through the donation of electrons, antioxidants possess the ability to neutralize FRs (Kurutas, 2016) (Fig. 6). Mode of action of antioxidantsAntioxidants that are produced chemically through synthesis are not found in nature. Synthetic antioxidants are categorized into many groups based on how they function. Primary synthetic antioxidants operate by hindering the formation of FRs during the oxidation process. Conversely, secondary antioxidants function by breaking down hydroperoxides, which result from lipid oxidation, into forms that are steadier and more intricate (Kurutas, 2016). It is important to mention that while synthetic antioxidants can have positive effects, they also have the potential to exert detrimental biological effects on molecules. PhytochemicalsFlavones, flavonoids, isoflavones, gallic acid, anthocyanins, coumarins, catechins, lignans, isocatechins, and esculin are some of the phytochemicals found in medicinal plants (Panche et al., 2016). These plants have antioxidant properties, as phytochemicals are abundant in various sources, including grapes, berries, tea, herbs, and nutmeg. Many medicinal plants also contain valuable compounds like gallic acids. Furthermore, plants such as Terminalia chebula, Terminalia bellerica, Terminalia muelleri, Phyllanthus emblica, Hemidesmus indicus, Cichorium indicum, Withania somnifera, Ocimum sanctum, Mangifera indica, and Punica granatum have been identified to possess antioxidant capabilities (Das et al., 2020).
Fig. 5. Various mechanisms of antioxidants against FRs.
Fig. 6. Antioxidant mechanism of action. R (alkyl radical), RO (alkoxyl radical), ROO (alkylperoxyl radical), AH (antioxidants), A (nonradical products), ROOH (alkyl hydroperoxidase). ConclusionVarious stressors impact poultry’s health and poultry enterprises’ productivity, from hatching to slaughter. Oxidative stress is considered one of the most important stressors in poultry production. Excess ROS production can negatively affect the immune system and overall health status of birds. The most important ROS include superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2), while the principal antioxidant enzyme systems are SOD, glutathione peroxidase, and catalase. The common antioxidant agents in poultry’s diet include vitamins C and E, selenium, glycine, manganese, essential oils, and phenolic compounds. Ethical approvalThe College of Veterinary Medicine Research Committee, University of Sulaimani, Kurdistan Regional Government, Kurdistan, Iraq, approved the study (no. 142: on 20/January/2024). Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsAll authors were involved in the planning and hypothesizing of this review article. All the authors were involved in the study’s data collection and literature review. All were involved in drafting and writing the manuscript. RNM was responsible for the final revision and proofing. ReferencesAgarwal, A., Gupta, S. and Sharma, R. K. 2005. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3(28), 1–21. Akinyemi, F. and Adewole, D. 2021. Environmental Stress in Chickens and the Potential Effectiveness of Dietary Vitamin Supplementation. Front. Anim. Sci. 2, 1–21. Bhuiyan, M. S. A., Amin, Z., Rodrigues, K. F., Saallah, S., Shaarani, S. M., Sarker, S. and Siddiquee, S. 2021. Infectious bronchitis virus (Gammacoronavirus) in poultry farming: Vaccination, immune response and measures for mitigation. Vet. Sci. 8(273), 1–22. Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. and Kalayci, O. 2012. Oxidative stress and antioxidant defense. World Allergy Organ. J. 5(1), 9–19. Bonilla, F. A. and Oettgen, H. C. 2010. Adaptive immunity. J. Allergy Clin. Immunol. 125(2), S33–S40. Bureau, C., Hennequet-Antier, C., Couty, M. and Guémené, D. 2009. Gene array analysis of adrenal glands in broiler chickens following ACTH treatment. BMC Genom. 10(430), 1471–2164. Cheeseman, K. H. and Slater, T. F. 1993. Introduction to free radical biochemistry. Br. Med. Bull. 49(3), 481–493. Chen, P., Fatayer, S., Schuler, B., Metz, J. N., Gross, L., Yao, N. and Zhang, Y. 2021. The Role of Methyl Groups in the Early Stage of Thermal Polymerization of Polycyclic Aromatic Hydrocarbons Revealed by Molecular Imaging. Energ. Fuels. 35(3), 2224–2233. Das, G., Kim, D. Y., Fan, C., Gutiérrez-Grijalva, E. P., Heredia, J. B., Nissapatorn, V., Mitsuwan, W., Pereira, M. L., Nawaz, M., Siyadatpanah, A., Norouzi, R., Sawicka, B., Shin, H. S. and Patra, J. K. 2020. Plants of the Genus Terminalia: An Insight on Its Biological Potentials, Pre-Clinical and Clinical Studies. Front. Pharmacol. 11, 1–30. Davies, M. J. 2011. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutri. 48(1), 8–19. Di Meo, S., Reed, T. T., Venditti, P. and Victor, V. M. 2016. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev, 2016, 1–44. Dupuy, C., Virion, A., Ohayon, R., Kaniewski, J., Dème, D. and Pommier, J. 1991. Mechanism of hydrogen peroxide formation catalyzed by NADPH oxidase in thyroid plasma membrane. J. Biol. Chem. 266(6), 3739–3743. Farag, R. S., Abdel-Latif, M. S., Abd El Baky, H. H. and Tawfeek, L. S. 2020. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 28, e00536. Fenton, H. J. H. 1894. Oxidation of Tartaric Acid in the Presence of Iron. J. Chem. Soc. 65, 899–910. Fuhrman, M. P., Charney, P. and Mueller, C. M. 2004. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 104(8), 1258–1264. Gale, C. R. 2001. Dietary antioxidants and dementia. Int. Psychogeriatr. 13(3), 259–262. Gombart, A. F., Pierre, A. and Maggini, S. 2020. A review of micronutrients and the immune system—Working in harmony to reduce the risk of infection. Nutrients. 12(236), 1–41. Guo, Q., Majeed, S., Xu, R., Zhang, K., Kakade, A., Khan, A., Hafeez, F. Y., Mao, C., Liu, P. and Li, X. 2019. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Envir. Manag. 240, 266–272. Haber, F. and Weiss, J. 1934. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Math. Phys. Sci. 147(861), 332–351. Hofmann, T., Schmucker, S. S., Bessei, W., Grashorn, M. and Stefanski, V. 2020. Impact of housing environment on the immune system in chickens: A review. Animals 10(7), 1–26. Huth, J. C. and Archer, G. S. 2015. Comparison of Two LED Light Bulbs to a Dimmable CFL and their Effects on Broiler Chicken Growth, Stress, and Fear. Poul. Sci. 94(9), 2027–2036. Ighodaro, O. M. and Akinloye, O. A. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 54(4), 287–293. Kachungwa Lugata, J., Ortega, A. D. S. V. and Szabó, C. 2022. The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agricult. 12(10), 689-694. Klebanoff, S. J. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77(301), 598–625. Koivula, M. J. and Eeva, T. 2010. Metal-related oxidative stress in birds. Environ. Poll. 158(7), 2359–2370. Kulcharyk, P. A. and Heinecke, J. W. 2001. Hypochlorous acid produced by the myeloperoxidase system of human phagocytes induces covalent cross-links between DNA and protein. Biochemistry 40(12), 3648–3656. Kurutas, E. B. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutri. J, 15(71), 1–22. Lobo, V., Patil, A., Phatak, A. and Chandra, N. 2010. Free radicals, antioxidants and functional foods: Impact on human health. Phcog Rev. 4(8), 118–126. Lourenço, S. C., Moldão-Martins, M. and Alves, V. D. 2019. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 24(22), 14–16. Luo, J., Xu, T., Wang, X., Ba, X., Feng, X., Deepak, V. and Zeng, X. 2010. Cellular Biochemistry. J. Cell. Biochem. 110, 910–919. Mann, M., Mehta, A., de Boer, C. G., Kowalczyk, M. S., Lee, K., Haldeman, P., Rogel, N., Knecht, A. R., Farouq, D., Regev, A. and Baltimore, D. 2018. Heterogeneous Responses of Hematopoietic Stem Cells to Inflammatory Stimuli Are Altered with Age. Cell Rep. 25(11), 2992-3005.e5. Marshall, J. S., Warrington, R., Watson, W. and Kim, H. L. 2018. An introduction to immunology and immunopathology. Allergy, Asthma Clin. Immunol. 14(49), 5–14. Martin, M. A. 2001. The Future of the World Food System. Outlook Agri, 30(1), 11–19. Miller, D. M., Buettner, G. R. and Aust, S. D. (1990). Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 8(1), 95–108. Nimse, S. B. and Pal, D. 2015. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5(35), 27986–28006. Ohteki, T. and Koyasu, S. 2001. Role of antigen-presenting cells in innate immune system. Arch. Immunol. Ther. Exp. 49(1), S47–S52. Panche, A. N., Diwan, A. D. and Chandra, S. R. 2016. Flavonoids: An overview. Journal of Nutri. Sci. 5(e47), 1–15. Pham-Huy Lien Ai, He, H. and Pham-Huay, C. 2008. Free Radicals, Antioxidants in Disease and Health Lien. Int. J. Biomed. Sci, 4(2), 89–96. Phaniendra, A., Jestadi, D. B. and Periyasamy, L. 2015. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 30(1), 11–26. Rahman, K. 2007. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2(2), 219–236. Rassaf, T., Preik, M., Kleinbongard, P., Lauer, T., Heiß, C., Strauer, B.-E., Feelisch, M. and Kelm, M. 2002. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J. Clin. Invest. 109(9), 1241–1248. Rostagno, M. H. 2020. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 98(4), 98–104. Sharifi-Rad, M., Anil Kumar, N. V., Zucca, P., Varoni, E. M., Dini, L., Panzarini, E., Rajkovic, J., Tsouh Fokou, P. V., Azzini, E., Peluso, I., Prakash Mishra, A., Nigam, M., El Rayess, Y., Beyrouthy, M. El, Polito, L., Iriti, M., Martins, N., Martorell, M., Docea, A. O. and Sharifi-Rad, J. 2020. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 11(July), 1–21. Shu, Y. 1998. Recent Natural Products Based Drug Development: A Pharmaceutical Industry Perspective. J. Nat. Products. 61, 1053–1071. Singh, R. P., Khanna, R., Kaw, J. L., Khanna, S. K. and Das, M. 2003. Comparative effect of benzanthrone and 3-bromobenzanthrone on hepatic xenobiotic metabolism and anti-oxidative defense system in guinea pigs. Arch.Toxicology 77(2), 94–99. Soleimani, A. F., Zulkifli, I., Omar, A. R. and Raha, A. R. 2011. Physiological responses of 3 chicken breeds to acute heat stress. Poul. Sci. 90(7), 1435–1440. Surai, P. F., Kochish, I. I., Fisinin, V. I. and Kidd, M. T. 2019. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 8(7), 235. Surai, P. F., Kochish, I. I., Fisinin, V. I. and Velichko, O. A. 2018. Selenium in poultry nutrition: From sodium selenite to organic selenium sources. J. Poul. Sci. 55(2), 79–93. Tan, B. L., Norhaizan, M. E., Liew, W. P. P. and Rahman, H. S. 2018. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 9, 1–28. Vertuani, S., Angusti, A. and Manfredini, S. 2004. The Antioxidants and Pro-Antioxidants Network: An Overview. Curr. Pharm. Des. 10(14), 1677–1694. Wood, L. G., Fitzgerald, D. A., Gibson, P. C., Cooper, D. M. and Garg, M. L. 2000. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids, 35(9), 967–974. Wu, G., Flynn, N. E., Flynn, S. P., Jolly, C. A. and Davis, P. K. 1999. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J. Nutri, 129(7), 1347–1354. | ||
| How to Cite this Article |
| Pubmed Style Abdulrahman HA, Dyary HO, Mohammed RN, Hamad DS, Abdul-star F, Saeed NM. Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Vet. J.. 2024; 14(7): 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 Web Style Abdulrahman HA, Dyary HO, Mohammed RN, Hamad DS, Abdul-star F, Saeed NM. Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. https://www.openveterinaryjournal.com/?mno=194650 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i7.2 AMA (American Medical Association) Style Abdulrahman HA, Dyary HO, Mohammed RN, Hamad DS, Abdul-star F, Saeed NM. Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Vet. J.. 2024; 14(7): 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 Vancouver/ICMJE Style Abdulrahman HA, Dyary HO, Mohammed RN, Hamad DS, Abdul-star F, Saeed NM. Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Vet. J.. (2024), [cited January 24, 2026]; 14(7): 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 Harvard Style Abdulrahman, H. A., Dyary, . H. O., Mohammed, . R. N., Hamad, . D. S., Abdul-star, . F. & Saeed, . N. M. (2024) Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Vet. J., 14 (7), 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 Turabian Style Abdulrahman, Hanar Azad, Hiewa Othman Dyary, Rebar N. Mohammed, Darya Shorsh Hamad, Faraidoon Abdul-star, and Nahla Mohammad Saeed. 2024. Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Veterinary Journal, 14 (7), 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 Chicago Style Abdulrahman, Hanar Azad, Hiewa Othman Dyary, Rebar N. Mohammed, Darya Shorsh Hamad, Faraidoon Abdul-star, and Nahla Mohammad Saeed. "Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity." Open Veterinary Journal 14 (2024), 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 MLA (The Modern Language Association) Style Abdulrahman, Hanar Azad, Hiewa Othman Dyary, Rebar N. Mohammed, Darya Shorsh Hamad, Faraidoon Abdul-star, and Nahla Mohammad Saeed. "Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity." Open Veterinary Journal 14.7 (2024), 1526-1537. Print. doi:10.5455/OVJ.2024.v14.i7.2 APA (American Psychological Association) Style Abdulrahman, H. A., Dyary, . H. O., Mohammed, . R. N., Hamad, . D. S., Abdul-star, . F. & Saeed, . N. M. (2024) Preventing free radical damage: The significance of including antioxidants in diet to strengthen immunity. Open Veterinary Journal, 14 (7), 1526-1537. doi:10.5455/OVJ.2024.v14.i7.2 |