| Research Article | ||
Open Vet. J.. 2024; 14(8): 1819-1835 Open Veterinary Journal, (2024), Vol. 14(8): 1819–1835 Research Article Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in EgyptDina A. Mobarak1, Elzahara K. Elbaz1, Samar M. Atwa1,2, Mohamed I. Eisa3, Ahmed M. El-Sebaey4 and Ahmed M. Selim1*1Department of Internal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 2Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan 3Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Clinical Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt *Corresponding Author: Ahmed Magdy Selim. Internal Medicine and Infectious Diseases Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt. Email: ahmedmagdy201017 [at] mans.edu.eg; ahmedmagdygamalselim [at] gmail.com Submitted: 08/04/2024 Accepted: 21/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Canine monocytic ehrlichiosis (CME) is considered a multisystemic, life-threatening, rickettsial, and tick-borne disease that affects canine species and is caused by Ehrlichia canis (E. canis). Clinical signs of CME vary from asymptomatic to severe illness with three clinical phases. E. canis has the potential to infect humans. Aim: This study aimed to provide recent information as there is limited data about the disease in Egypt. Therefore, this work was conducted to study the molecular prevalence of E. canis and evaluate the corresponding risk factors, hematology, biochemistry, and molecular characterization of the genus Ehrlichia and E. canis species among Egyptian dogs. Methods: One hundred eighty dogs of both sexes from 3 months to 8 years from different breeds: stray and foreign breeds were examined for clinical signs in all seasons in two delta governorates: El-Dakahlia and El-Gharbia. Blood samples were collected from dogs for microscopic and haemato-biochemical analysis, and then molecular characterization of the genus Ehrlichia and species-specific E. canis was performed, followed by sequencing and phylogenetic analysis. Results: Out of 180 samples examined by polymerase chain reaction (PCR) assay, 42 (23.33%) were positive for the genus of Ehrlichia and the species-specific E. canis. Only twenty-four dogs (13.33%) were positive for PCR, infested with ticks, and showed fever, anemia, loss of body weight, pale mucous membrane of gum and conjunctiva, blindness, paralysis, hemoglobinuria, and Melena. The univariate logistic regression revealed that all variables, including age, season, tick infestation, hemorrhage from natural orifices, and ectoparasitic treatments per year, showed statistical significance (p ≤ 0.05), except breed and sex, which also did not exhibit any relation between CME infection in multivariate logistic regression. The presence of morulae inside leukocytes in 66 dogs out of the total examined 180 (36.67%), only 39 (59.1%) were positive for morulae and PCR-positive for E. canis. Dogs positive for E. canis suffered from anemia, severe thrombocytopenia, the absolute value of WBCs and their fractions, alanine aminotransferas (ALT), AST, ALKP, γ-GT, total. P, T.BIL, urea, globulin, and creatinine were significantly increased in dogs infected with E. canis when compared to those with negative PCR results, while the levels of albumin and A: G ratios were significantly decreased. Conclusion: The current study proves the existence of E. canis in El-Dakahlia and El-Gharbia governorates, and this is the first large-scale study concerning the epidemiological, clinicopathological examination, molecular characterization, sequencing, and phylogenetic analysis of reported from the center of the Delta of the Nile in Egypt. Keywords: Dogs, Egypt, Ehrlichia canis, Molecular, Risk factors. IntroductionCanine monocytic ehrlichiosis (CME) is considered a multisystemic, life-threatening, rickettsial, and tick-borne disease that affects canine species all over the world caused by Ehrlichia canis (E. canis), which is an obligate intracellular Gram-negative, (Harrus and Waner, 2011; Sainz et al., 2015), and transmitted by the brown tick of dogs (Rhipicephalus sanguineus) from the genus Rhipicephalus which related to family Ixodidae (Groves et al., 1975; Aguirre et al., 2004). Ehrlichia canis has the potential to infect humans and cause clinical signs similar to CME (Maeda et al., 1987; Perez et al., 2006). Clinical signs of CME vary from asymptomatic to severe life-threatening illness, so there are three clinical phases of CME; Acute, chronic, and subclinical. The acute phase, which lasts 2–4 weeks and is characterized clinically by anorexia, depression, lethargy, pyrexia, loss of body weight, generalized lymphadenopathy, pale mucous membranes, and hemorrhagic tendencies with/without ocular signs like anterior uveitis (Skotarczak, 2003; Mylonakis et al., 2019; Piratae et al., 2019). Some dogs may spontaneously clinically improve without treatment but remain persistent asymptomatic carriers for months or even years, which is called the subclinical phase that is characterized by absence or mild clinical signs (Sainz et al., 2015). Sometimes, the acute and chronic phases may be difficult to distinguish clinically. Otherwise, the chronic stage is usually more severe (Mylonakis et al., 2004; Harrus and Waner, 2011). Ehrlichia canis can be diagnosed initially by microscopic examination of blood smears for the demonstration of the morula inside leucocytes (especially monocytes), but it does not appear in all positive cases due to the low levels of parasitemia (Salib and Farghali, 2015). The presence of multiple rounded and relatively large morulae within macrophages or engulfing nuclear material and the formation of typical vacuoles are clear indications of CME (El-Dakhly et al., 2021). Hematological findings are diagnostic as thrombocyte count significantly decreased. During the acute stage, there are distinctive hematological findings which are true thrombocytopenia (from 20,000 to 52,000/µl), mild anemia, and mild leukopenia (Harrus and Waner, 2011), while the chronic stage is characterized by marked pancytopenia because of the hypoplasia of the bone marrow (Aziz et al., 2023). Many biochemical alterations aid in the diagnosis of CME including; hyperproteinemia, hypergammaglobulinemia, hyperglobulinemia, hypoalbuminemia, a slight increase in hepatic enzymes (alanine aminotransferase (ALT), ALP), and also an increase of both creatinine and blood urea nitrogen (BUN) values (Aziz et al., 2023). Polymerase chain reaction (PCR) is a highly reliable and sensitive molecular method that can detect DNA of E. canis (4–10 days after infection) before any other traditional methods. It could be hard to detect the disease during the acute stage by microscopical examination because of the limited number of morulae (Salem et al., 2014; Chua et al., 2020). Recently, CME has been reported in Egyptian dogs, so this study aimed to provide recent information as there is limited data about the disease in Egypt. Therefore, this work was conducted to study the molecular prevalence of E. canis and evaluate the corresponding risk factors, hematology, biochemistry, and molecular characterization of the genus Ehrlichia and E. canis species among Egyptian dogs. Materials and MethodsStudy areaOur study was conducted on two delta governorates: El-Dakahlia (31°2′25″N, 31°22′58″E) and El-Gharbia (30°58′07″N, 31°09′49″E), which are located in the center of the Delta of the Nile in Egypt (Fig. 1). These Governorates are characterized by warm temperatures most of the year (between 25:40°C), which is considered a suitable condition for the multiplication and growth of ticks which are the principal vector of CME. AnimalsThis study was conducted from July 2022 to December 2023 in all seasons on 180 dogs (clinically affected dogs exhibited signs such as fever, anorexia, epistaxis, and paralysis and apparently healthy dogs) of both sexes from 3 months to 8 years from different breeds: stray and foreign breeds (Table 1). Dog’s localities were dog farms, streets, the Veterinary Teaching Hospital (Mansoura University), and various veterinary clinics with the owners’ consent in El-Dakahlia and El-Gharbia governorates. Sample collectionBlood samples were collected from the cephalic or saphenous veins of 180 dogs from different breeds reared in Egypt. Data of each examined animal was recorded, such as age, sex, breed, presence of ticks, season, presence of clinical signs, pregnancy, lactation, and the number of ectoparasitic treatments per year. Each animal’s blood was drawn aseptically twice: in a 2 ml VACUETTE® plain tube (Greiner Bio-One, GmbH, Austria) for serum separation by using Luxiangyi TD4 centrifuge (Shanghai, China) for further biochemical tests and in a 2 ml VACUETTE® tube containing Tri potassium-EDTA for blood smear examination, complete blood count, and molecular characterization. After collection, blood samples were stored in an icebox and finally brought to the Infectious Diseases Laboratory, Faculty of Veterinary Medicine, Mansoura University, for immediate hematology and biochemical studies, and then whole blood was kept at −20°C until molecular examination at ِAnimal Health Research Institute of Dokki, Giza, Egypt. Microscopic and clinicopathological examinationsBlood smears on positively charged glass slides were stained with Diff-Quik for the microscopical examination for demonstration of intracytoplasmic morulae of E. canis inside monocytes. Total erythrocytes, total leukocytes, and thrombocytes were counted by an automated hematology analyzer. Hb, PCV, MCV, MCH, and MCHC were estimated. Serum samples were analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (Ɣ-GT), alkaline phosphatase (ALKP), total protein (Total. P), albumin, total bilirubin (T.BIL), BUN, and creatinine using AGAPPE Diagnostics LTD ® kits (Ernakulam, Kerala, India) with the aid of chem-7 analyzer (Erba Mannheim, Germany) as outlined by the supplier company.
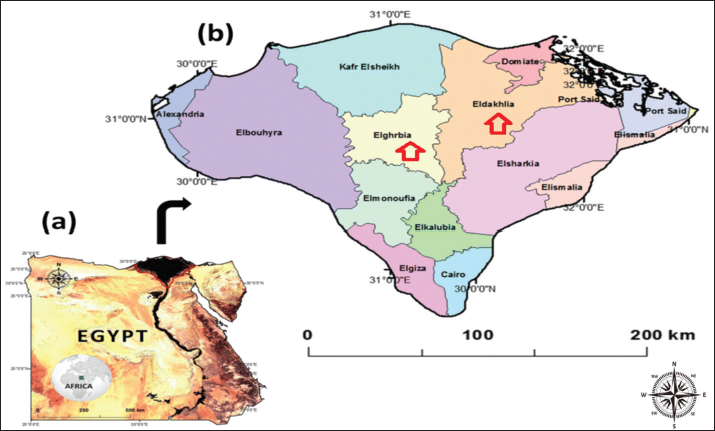
Fig. 1. Map of Egypt (a) showing El-Dakahlia and El-Gharbia governorates in the center of the Delta of the Nile in Egypt (b) where animals included in the study were sampled. Table 1. Shows the two groups of dogs and their number.
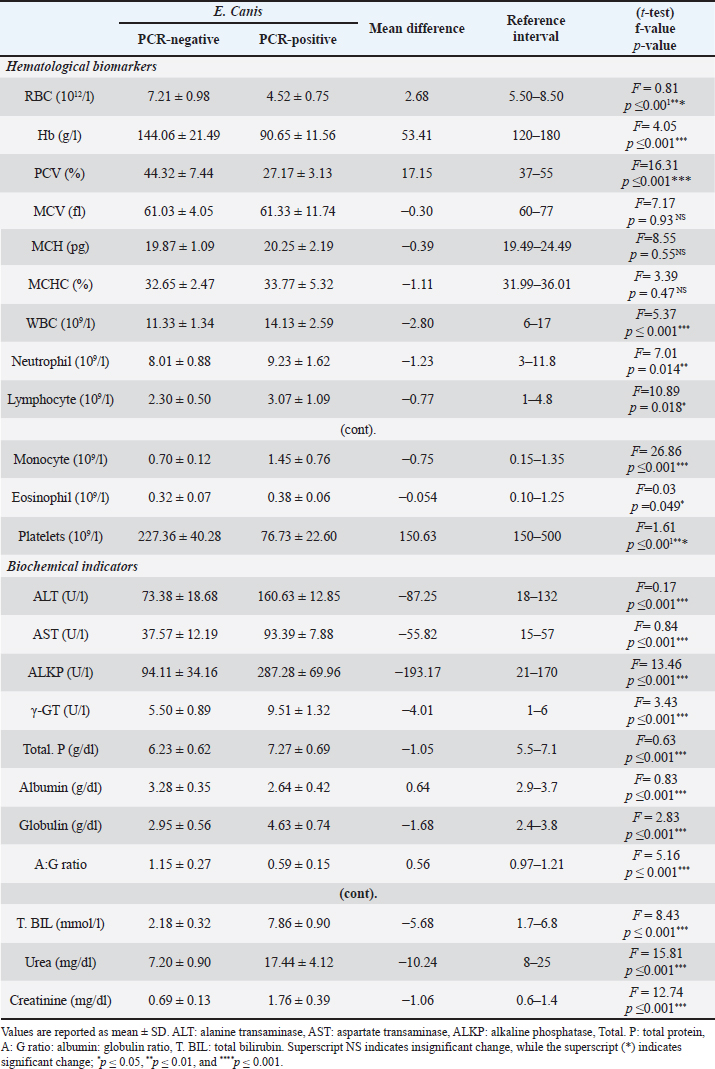
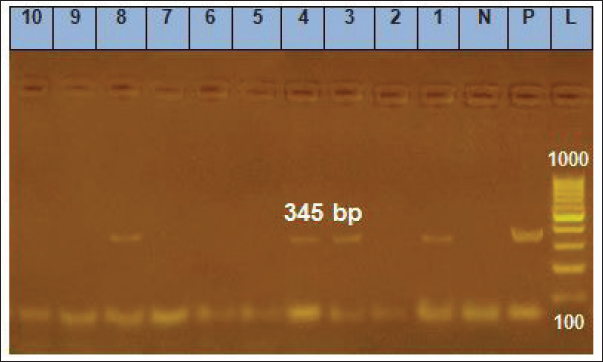
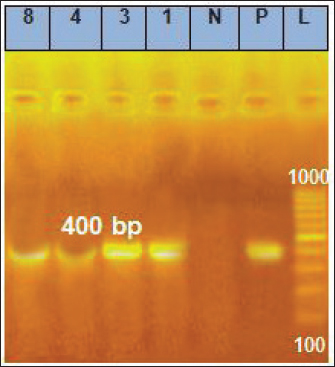
PCR and SequencingDNA extraction and PCR amplification Genomic DNA was extracted from 200 μl of anti-coagulated blood from each dog using a commercially available DNA mini kit (QIAamp DNA mini kit) following the manufacturer’s instructions. The purity and integrity of genomic DNA were examined using nanodrop and by running on 1% agarose gel. After quality checking, the extracted DNA was stored at −20°C until being used in the PCR assay. Conventional PCR was carried out using two pairs of primers: EHR16SD (5’-GGTACCYACAGAAGAAGTCC-3’) combined with the reverse primer EHR16SR (5’-TAGCACTCATCGTTTACAGC-3’) to amplify Ehrlichia genus 16SrRNA and ECA (5’-AACACATGCAAGTCGAACGGA-3’) combined with the reverse primer HE3 (5’-TATAGGTACCGTCATTATCTTCCCTAT-3’) to amplify species-specific E. canis 16SrRNA, the lengths of amplified products were 345 bp and 400 bp, respectively (Gal et al., 2008). Preparation of PCR Master Mix for each of the tested genes according to Emerald Amp GT PCR mastermix (Takara) Code No. RR310A kit. The PCR amplification was done in a 25 μl reaction volume, containing 12.5 μl of Emerald Amp GT PCR mastermix (2x premix), 5.5 μl of PCR grade water, 1.0 μl of 20 pmol of each primer (forward and reverse), and 5 μl of template DNA. The DNA of an Ehrlichia genus-free dog was used as a negative control. The PCR amplifications were performed under the following cycling conditions: initial denaturation of one cycle of 94°C for 3 minutes, followed by two cycles of 94°C for 30 seconds, 62°C for 30 seconds, and 72°C for 30 seconds. Then, two more cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, followed by two cycles of 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds, two more cycles of 94°C for 30 seconds, 56°C for 30 seconds 72°C for 30 seconds, two cycles of 94°C for 30 seconds, 54°C for 30 s, and 72°C for 30 seconds. Finally, thirty-nine cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 30 seconds, followed by the final extension step at 72°C for 10 minutes. Twenty μl of each uniplex PCR product were loaded to electrophoresis on a 1% agarose gel containing ethidium bromide dye in Tris borate EDTA (TBE) buffer. The power supply was 1-5 volts/cm of the tank length for about 30 min, then the gel was transferred to a UV cabinet, photographed by a gel documentation system, and the data were analyzed through computer software. The highly bright thick band from positive samples of E. canis was selected for DNA sequencing. QIAquick PCR Product extraction kit. (Qiagen Inc. Valencia CA): was used for PCR product purification and Bigdye Terminator V3.1 cycle sequencing kit. (Perkin-Elmer, Foster City, CA) cat-number 4336817: was used for performing gene sequencing using an Applied Biosystems 3,130 genetic analyzer (HITACHI, Japan). The sequences were aligned using the programs BioEdit and MEGA6. The partial E. canis gene sequence was compared and analyzed to other published gene sequences in the GenBank. Furthermore, the analysis of DNA sequencing data was by nucleotide basic local alignment search tool (nBLAST) provided in the National Center for Biotechnology Information (NCBI) database to ascertain the homology of E. canis genes. A comparative analysis of sequences was performed using the CLUSTAL W multiple sequence alignment program, version 12.1 of MegAlign module of Lasergene DNAStar software Pairwise (Madison, WI). Phylogenetic analyses were done using maximum likelihood, neighbor-joining, and maximum parsimony in MEGA6 (Tamura et al., 2013). The sequence discovered during this work was imported into the GenBank database. Statistical analysisThe data were analyzed with IBM SPSS Statistics® version 20 (Armonk, NY: IBM Corporation). The normal (Gaussian) distribution of obtained haemato-biochemical data were determined by performing the Shapiro-Wilk test. Data were clustered toward the mean, and p values exceeded 0.05, which confirms the assumption of data homogeneity. Therefore, the result outlined as mean ± standard deviation (SD) and independent samples t-test was applied to get the significance of the difference between groups. Descriptive statistics of risk factor distribution were conducted to determine the prevalence of Ehrlichiosis in dogs using the chi-square test. Logistic regression was used to assess the association between the occurrence of CME and risk factors. First, univariate logistic regression was performed where the dependent dichotomous variable was the category of the dogs, either infected positive-PCR or non-infected negative-PCR, while the suggested risk factors were the independent variables. The multivariate logistic regression was performed for the independent factors that showed a significant correlation (p<0.1) in univariate analysis. All statistical analysis findings were regarded as significant when p < 0.05 was reached. The Eta (η) coefficient test was performed to find the strength of the association between nominal independent variables (risk factors) and interval dependent variables (haemato-biochemical parameters) where ≤0.19 is no association, 0.2 to 0.39 is a weak association, 0.4 to 0.69 is a medium association, and ≥0.7 is a strong association (Field et al., 2009). Determined Eta values, the pattern of association between risk factors, and measured parameters were collectively represented as a Chord Diagram in OriginPro 2022 software for Windows. Ethical approvalSamples collection in the current study was performed with the owner’s permission. All procedures of the current study were carried out under the ethical standards of the Faculty of Veterinary Medicine, Mansoura University, since the samples were taken under physical restrictions and according to the ethical rules with Application No. M/159. ResultsClinical signsOut of 180 dogs clinically examined, 24 showed clinical signs with a prevalence of (13.33%). The affected dogs were infested with ticks and displayed various clinical signs such as fever of more than 40°C, lethargy, anemia, loss of body weight, incoordination, tachycardia, pale mucous membrane of gum and conjunctiva, shivering, blindness, paralysis, hemoglobinuria, black and tar-like feces with a jelly-like consistency (Melena), and some dogs died (Fig. 2). Microscopic examinationExamination of Diff-Quik blood smears under an oil immersion lens showed the presence of intracytoplasmic inclusion bodies (morulae) of E. canis inside leukocytes (Fig. 3). The total number of blood smears examined was 180. Out of which, 66 (36.67%) dogs were positive for morulae, either infected or non-infected cases, and only 39 (59.1%) were positive for morulae and infected with E. canis, which was confirmed by PCR. Clinicopathological examinationsAs illustrated in Table 2, dogs positive for E. canis were suffered from anemia of normocytic normochromic (non-regenerative) type which outlined by the significant decrease of RBCs (p ≤ 0.001), Hb (p ≤ 0.001) and PCV (p ≤ 0.001) together with the insignificant variation of RBCs indices such as MCV (normocytic, p= 0.93) and MCHC (normochromic, p= 0.47) which were within the reference intervals. Regarding the leukogram, the absolute value of WBCs and their fractions (neutrophils, lymphocytes, monocytes, and eosinophils) were significantly increased in dogs infected with E. canis when compared to those with negative PCR results. Furthermore, the mean platelets count was significantly decreased (p ≤ 0.001) in the E. canis infected group and reached 76.73 ± 22.60, which was below the minimum normal value of the reference interval, indicating severe thrombocytopenia. In terms of serum biochemical profile for E. canis infected dogs (Table 2), the values of ALT, AST, ALKP, γ-GT, total. P, T.BIL, urea, globulin, and creatinine exceeded the reference values and were significantly increased (p ≤ 0.001) in comparison with the non-infected dogs. However, the levels of albumin and A: G ratios were significantly decreased (p ≤ 0.001).
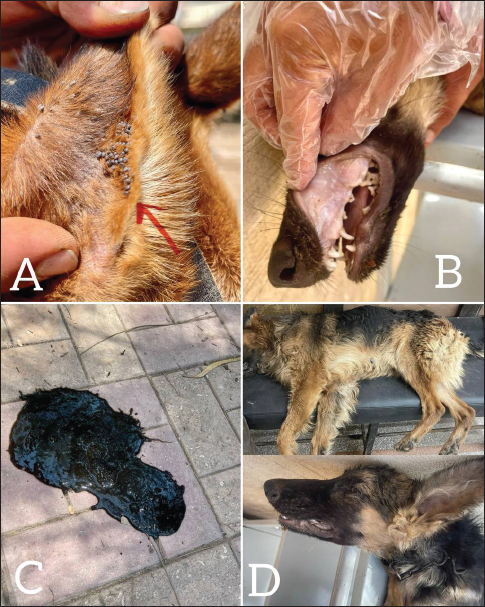
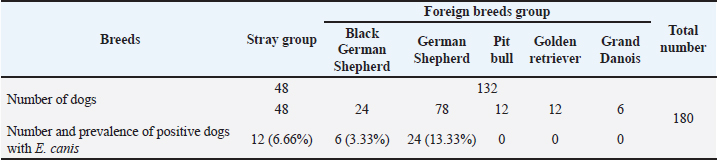
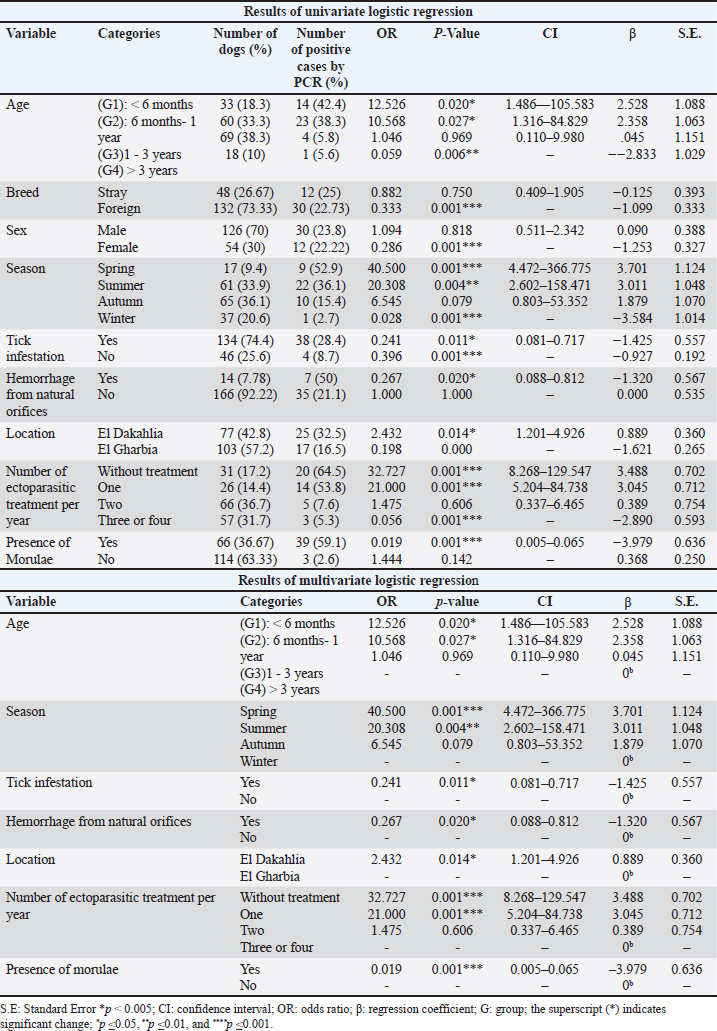
Fig. 2. Shows various symptoms of infected dogs with E.canis. (A) Severe infestation of ticks on dog’s ear. (B) Pale gum. (C) Melena of an infected dog. (D) Lethargy EpidemiologyThe number and prevalence of positive cases of E. canis in different breeds are demonstrated in Table 3, while Table 4 displays the epidemiological data of dogs that were gathered and presented in the Table. The use of univariate logistic regression revealed that all variables showed statistical significance (p ≤ 0.05), except breed and sex, which also did not exhibit any relation between CME infection in multivariate logistic regression, and the prevalence was higher in stray breeds (25%, p > 0.05, OR=0.882, 95% CI: 0.409–1.905) than in foreign breeds and higher in males (23.8%, p > 0.05, OR=1.094, 95% CI: 0.511–2.342) than females. Meanwhile, there is a significant association between the age and occurrence of CME (p < 0.05), and the highest incidence of CME was observed in the group of dogs less than six months (42.4%, p < 0.05, OR=12.526, 95% CI: 1.486–105.583), followed by the group of dogs between 6 months–1 year (38.3%, p <0.05, OR=10.568, 95% CI: 1.316–84.829), and the lowest incidence was in the group of dogs between > 3 years (5.6%). A significant association between the season and occurrence of CME was found, and the higher incidence was in the spring season (52.9%, p=0.001, OR=40.500, 95% CI: 4.472–366.775) followed by the summer (36.1%, p < 0.01, OR=20.308, 95% CI: 2.602–158.471), and the lowest incidence was in the winter (2.7%). Another significant association between the tick infestation and the occurrence of CME was noticed, and the higher incidence was in dogs suffering from tick infestations (28.4%, p < 0.05, OR=0.241, 95% CI: 0.081–0.717).
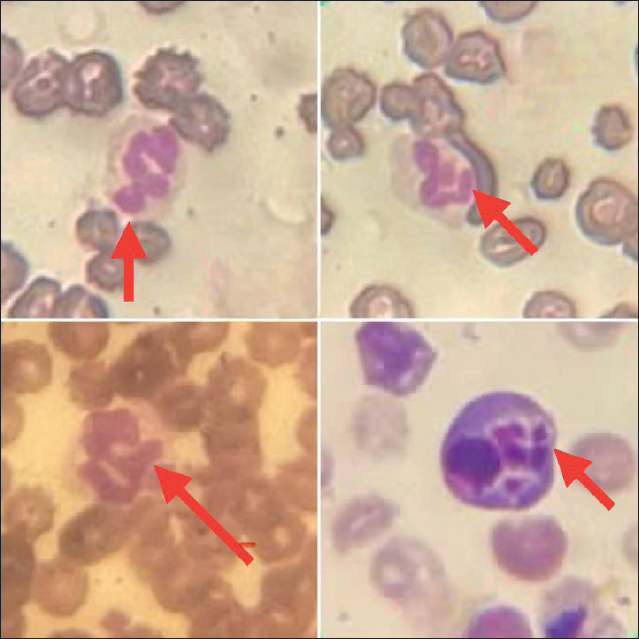
Fig. 3. Diff-Quik blood smears under an oil immersion lens showing morulae within leukocytes of dogs infected with E. canis. Table 2. The haemato-biochemical profile in dogs with PCR-negative or PCR-positive E. canis.
Table 3. shows the number and prevalence of positive cases with E. canis in different breeds.
Table 4. shows the results of univariate and multivariate logistic regression.
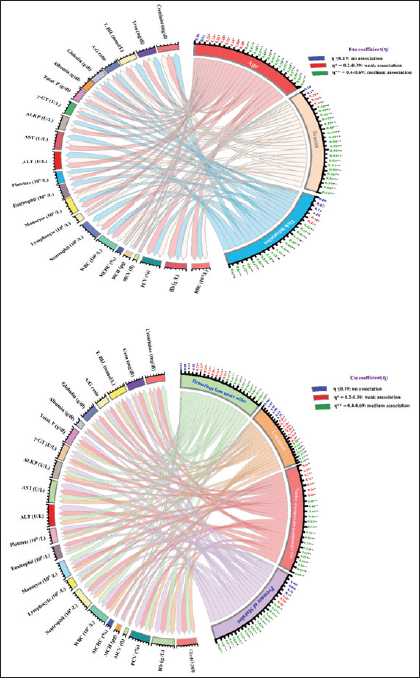
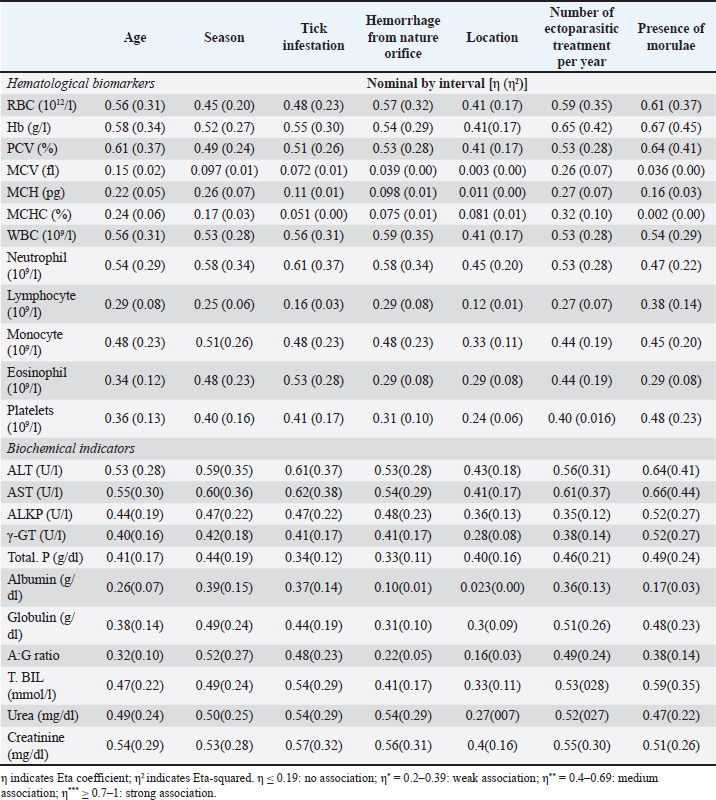
Also, hemorrhage from natural orifices was found to be significantly associated between the presence of the disease as the highest incidence occurred in dogs with bleeding (50%, p < 0.05, OR=0.267, 95% CI: 0.088–0.812), the location had a role in the disease occurrence, and the prevalence was higher in El Dakahlia (32.5%, p < 0.05, OR=2.432, 95% CI: 1.201–4.926) than El Gharbia (16.5), and also the number of ectoparasitic treatments had a significant association with the occurrence of CME, and the highest ratio was seen in the group of dogs that did not receive any ectoparasitic treatment per year (64.5%, p=0.001, OR=32.727, 95% CI: 8.268–129.547) followed by the group that received ectoparasitic treatment once per year (53.8%, p=0.001, OR=21.000, 95% CI: 5.204–84.738) while the lowest ratio was seen in the group of dogs that received ectoparasitic treatment three or four times per year (5.3%). It was found that there is a significant relation between the presence of morulae and the emergence of CME, and it was observed in the majority of infected dogs (59.1%, p=0.001, OR=0.019, 95% CI: 0.005–0.065). Correlation of risk factors with haemato-biochemical findingsAs visualized in Figure 4 and Table S1, the value of the Eta coefficient (η=0.4–0.69) suggested a moderate correlation between the levels of RBCs, Hb, PCV, WBC, neutrophil, ALT, AST, creatinine, and all investigated risk factors. Conversely, RBCs indices (MCV, MCH, and MCHC) declare a weak or no association with these factors. Furthermore, η values for the presence of Morula in blood film were like that of season factor, tick infestation, and number of ectoparasitic treatments per year. As such, these risk factors declared a moderate correlation with the count of monocytes, eosinophils, and platelets as well as the levels of ALKP, γ-GT, total. P, globulin, A:G ratio T. BIL and urea. Whereas a weak or no correlation was detected between lymphocyte count, albumin levels, and these risk factors. Likewise, age and hemorrhage from natural orifice showed a moderate association with the values of monocyte, ALKP, γ-GT, total. P, T. BIL, and urea. However, a weak correlation was detected with the count of lymphocytes, eosinophils, and platelets, together with the levels of albumin, globulin, and A:G ratio. Finally, the location of the sample collection showed either a weak or ineffective correlation with most variables.
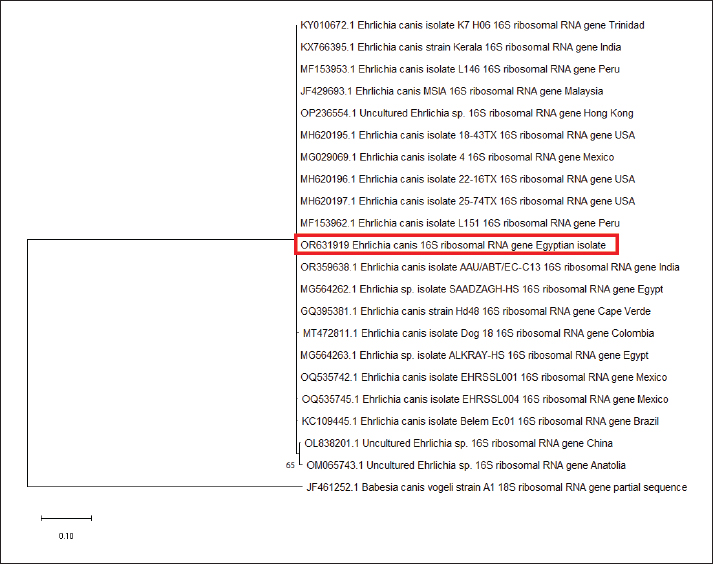
Fig. 4. Chord diagrams visually outline the intensity of the correlation between risk factors and the levels of haemato-biochemical variables in overall investigated dogs (N=180). Links flow with colored nodes show the association between risk factors and blood variables. Risk factors are at the center of each node. Each link represents a haemato-biochemical parameter and parameters with no weak or strong association are visualized based on the width of the link. The numbers outside nodes reflect the calculated Eta coefficient (η) value for each parameter. PCROut of 180 samples examined by PCR assay, 42 (23.33%) were positive for the genus of Ehrlichia (Fig. 5), and the same samples (42) were positive for the species-specific E. canis (Fig. 6). The prevalence of CME was (32.5%) in El Dakahlia and (16.5%) in El Gharbia. Sequencing and phylogenetic analysisBy searching the National Center for Biotechnology Information (NCBI) database using a tool called BLAST to analyze the DNA sequence of the E. canis 16SrRNA gene and identify genes with similar sequences. The partial 16SrRNA gene (400 bp) of E. canis that was obtained in our study (GenBank accession no. OR631919) had a nucleotide identity of 100% with other sequences that had been published before from Hong Kong (OP236554), Mexico (MG029069), USA (MH620197), India (OR359638), and Colombia (MT472811), while Egypt (MG564262), Egypt (MG564263), and Brazil (KC109445) shared 99.75% identity of nucleotide sequence, in addition to China that showed 99.02% nucleotide identity, as shown in the phylogenetic tree (Fig. 7). DiscussionCanine vector-borne diseases (CVBDs) are of global importance (Nguyen et al., 2020). Among CVBPs, tick-borne Ehrlichia species are of great importance for dogs and pose a threat to public health as they can infect humans (Gokmen et al., 2019). There are limited data available regarding the occurrence of CME in Egyptian dogs. In our study, the prevalence of E. canis was 23.33% in the two governorates, but the prevalence was more significant in El Dakahlia (32.5%) than in El Gharbia (16.5%), suggesting that the climate role in the multiplication of ticks while in 2015, the prevalence was 5.15% but based on detection of E. canis antibodies (Salib and Farghali, 2015). In 2019 and 2020, the prevalence was reported at 15.8% and 9.7%, respectively, based on PCR identification in other governorates in Egypt (Giza, Cairo, and Qalyubia) due to the veterinary care in these governorates (Abdelfattah et al., 2019; Selim et al., 2020). The higher prevalence rate based upon PCR was closely related to our results in Alexandria, Northern Egypt, which was 20% (El-Dakhly et al., 2021), in St. Kitts, West Indies was 27% (Kelly et al., 2013), in Pakistan was 28% (Malik et al., 2018) and in Senegal was 18.8% (Dahmani et al., 2019). The lower infection rates of E. canis were recorded at 1.7%–8% in Central Italy, 2.9% in Northern Italy, and 9.7% in Southern Italy (Ebani et al., 2015), and a higher prevalence rate was recorded in Colombia at 40.6% because R. sanguineus and E. canis are mostly found in Colombia (Vargas-Hernández et al., 2012). In our study, infected dogs with E. canis showed multisystemic signs such as fever, lethargy, anemia, tachycardia, paleness of gum and conjunctiva, shivering, blindness, paralysis, hemoglobinuria, Melena, and some dogs died, and the prevalence of dogs which showed symptoms and PCR-positive was 13.33% (24/180), and out of 180, 18 dogs were asymptomatic and PCR-positive (10%), attributing to the subclinical phase CME as the infected dogs apparent healthy although the pathogen present in its blood and different tissues (Rodríguez-Alarcón et al., 2020). The results during the examination of Diff-Quik blood smears in this study revealed the presence of morulae inside leukocytes in 66 dogs out of the total examined 180 (36.67%), and only 39 (59.1%) were positive for morulae and PCR-positive for E. canis. The presence of morulae and PCR-negative for E. canis in 27 samples (40.9%) may suggest infection with other pathogens related to the Anaplasmatacea family such as Anaplasma phagocytophilum, Anaplasma platys, and Neorickettsia risticii (Harrus and Waner, 2011; Sainz et al., 2015) or misdiagnosed with nuclear remnants of megakaryocytes and products raised from Platelet activation (Lara et al., 2020) or due to the artifacts during the staining process so the definite diagnosis must be by using PCR (El-Dakhly et al., 2021). On the other hand, the PCR-positive samples with the absence of morulae suggest a low level of E. canis in the bloodstream, even during the acute stage (Lara et al., 2020; Hegab et al., 2022). The prevalence of CME was the highest in dogs less than six months 42.4% and the lowest percentage in the elderly dogs like that was reported (Malik et al., 2018; El-Dakhly et al., 2021) as puppies infested with ticks in their early stage of life or maybe the potential for E. canis to be passed from a pregnant bitch to its puppy through the placenta (Astigarraga, 2023; Sukara et al., 2023). This result conflicts with another study, which reported a higher incidence among elderly dogs aged more than five years (Selim et al., 2021). There was no significant association between the breed and sex and the occurrence of CME, and this was agreed with (Selim et al., 2020). In contrast, previous studies recorded that the prevalence was higher in mixed breeds than in foreign purebreds due to the environmental condition that makes these dogs more susceptible to infestation of ticks and the less use of ectoparasitic treatment (Barrantes-González et al., 2016). Some published studies have found greater prevalence in males than females, which can be explained by the behavioral characteristics that make a chance of tick infestations (Costa Jr et al., 2007), while some other studies have found that the disease was most prevalent in female dogs (Selim et al., 2021). Concerning the season, it has been found that the infection rate of CME reached its peak in the spring season (52.9%), followed by the summer season (36.1%), and our finding was closely related to (Selim et al., 2021) because the ticks’ activation and multiplication take place from spring to the beginning of autumn, and the highest infestations occur during this time (Sainz et al., 2015). On the contrary, another study reported that the high prevalence of the Anaplasmataceae family was in summer, followed by spring (Hegab et al., 2022). In our study, tick infestation was associated with E. canis infection, and this result agreed with previous studies that considered it as a risk factor owing to its great role as a main vector for CME transmission (Martínez-Vega et al., 2016; Selim et al., 2020). Moreover, some studies did not consider tick infestation a risk factor (Pérez-Macchi et al., 2019; Mitpasa et al., 2022). Among the different clinical findings, the presence of hemorrhages from natural orifices like hemoglobinuria or melena was the most characteristic sign and observed in 50% of total infected dogs with E. canis based on PCR confirmation, so it was significantly associated with the disease since thrombocytopenia and immune complex deposition cause vascular wall damage (Salib and Farghali, 2015; Parashar et al., 2016). On the other hand, the results of the previous study indicated that these bleeding disorders were not associated with the occurrence of CME (Malik et al., 2018).
Fig. 5. Gel electrophoresis results for PCR of the genus of Ehrlichia gene, Lanes 1, 3, 4, 8=positive samples; Lanes 2, 5, 6, 7, 9, 10=negative samples; N=Negative control; P=Positive control; L=Ladder marker.
Fig. 6. Gel electrophoresis results for PCR of the same previous samples but by using the species-specific gene of E. canis, Lanes 1, 3, 4, 8=Positive samples; N=Negative control; P=Positive control; L=Ladder marker. According to our findings, the highest frequency of CME 64.5% occurred in the dogs’ group that did not receive any ectoparasitic treatment, either spray, shampoo, spot-on, or collar, which decreased the infestation of the different stages of ticks. The absence of veterinary care, sanitary measures, and the awareness of owners make them more susceptible to CME and other CVBDs as mentioned before (Selim et al., 2020; Selim et al., 2021) while the lowest frequency was 5.3% occurred in the dogs’ group that received three or more ectoparasitic treatment per year suggested that the perfect veterinary care will eradicate these vectors (El-Dakhly et al., 2021). Reported normocytic normochromic anemia and profound thrombocytopenia, which are considered the hallmark of ehrlichiosis in dogs confirmed to be naturally infected with E.canis, were in accordance with earlier findings (Harrus and Waner, 2011; Salem et al., 2014). In contrast to our results, (Bai et al., 2017) reported that hypochromic normocytic anemia was predominant among dogs positive for E.canis. In our study, hemorrhage from the natural orifice was moderately associated with RBCs and platelets count (η > 0.39). Henceforth, the authors agree with the recent findings (Espino-Solís et al., 2023), who attributed the non-regenerative anemia and thrombocytopenia either to the bone marrow depletion by E.canis associated hemorrhage or to the immune-mediated destruction of RBCs and platelets. Apart from this, (Harrus, 2015) stated that infection with E. canis usually enhances the systemic release of inflammatory mediators, which have an inhibitory action on bone marrow erythropoiesis and upregulate the migration and consumption of circulatory platelets at sites of tissue injury during inflammation.
Fig. 7. A phylogenetic tree was created using nucleotide sequences of the E. canis 16SrRNA gene. The red rectangle indicates the sequence obtained in our study. The evolutionary relationships were inferred using a maximum likelihood method based on the MEGA 11 software. The analysis was then repeated 1,000 times to generate bootstrap values. The findings of our study declared that the leukogram pattern was within the normal reference intervals but showed a significant elevation of absolute WBC values and their fractions (neutrophil, lymphocyte, monocytes, eosinophil) in dogs with positive PCR results for E. canis when compared to those with negative PCR result. Similar findings have been reported recently by Gianopoulos et al., (2016), Malik et al. (2018), and Thongsahuan et al. (2020), who declared that these leukocyte changes are indicative of the acute stage of CME and infected dogs try to evade infection by enhancing the synthesis and release of leukocyte fractions at the central pool of blood in response to multiple inflammatory pathways. In contrast, many retrospective studies (Moreira et al., 2003; Assarasakorn et al., 2008; Chochlios et al., 2019) of naturally infected dogs demonstrated that significant decrease of leukocyte and neutrophil counts with a mild shift to the left of neutrophil. The finding of leukopenia in natural cases might reveal the development of mild transient inflammatory response concurrent with impaired neutrophil production, accelerated neutrophil utilization, or immune-mediated destruction of neutrophils (Waner et al., 2000; Harrus et al., 2001). Considering hepatorenal indicators, hypoalbuminemia and enhanced activity of hepato-biliary enzymes (ALT, AST, ALP, and γ-GT) within the PCR-positive group are probably related to immune-mediated RBCs destruction, which causes ischemic necrosis of hepatic tissue leading to enzyme leakage and decreased intravascular albumin mass. In earlier studies, this hypothesis was supported by finding different degrees of hepatocellular damage in dogs naturally infected with E. canis (Nicholson et al., 2010; Bai et al., 2017). In fact, albumin is a negative acute-phase protein and can be slightly decreased in response to acute tissue injury. However, in our study, the decrease in total. P is not observed due to the compensatory synthesis and release of globulins to act as positive acute phase reactants and trigger the inflammatory process. Furthermore, noted hypoalbuminemia in conjunction with high creatinine and urea levels suggests a transient renal impairment. A few recent studies (Ziliani et al., 2019; Chawla et al., 2020) supported our findings and reported the incidence of kidney failure in dogs positive for E. canis. The phylogenetic analysis revealed that there is a clear relationship between the E. canis 16SrRNA gene of Egyptian isolate (OR631919) and other isolates submitted in GenBank, such as Hong Kong (OP236554), Mexico (MG029069), USA (MH620197), India (OR359638), and Colombia (MT472811) that shared 100% of nucleotide identity, in addition to Egypt (MG564262), Egypt (MG564263), and Brazil (KC109445) that showed 99.75% identity of nucleotide sequence and China with nucleotide identity of 99.02%. Our study confirmed the previous research that utilized the same E. canis 16SrRNA gene as a precise identification method (Wen et al., 1997; Gal et al., 2008). The current study proves the existence of E. canis in El-Dakahlia and El-Gharbia governorates, and this is the first large-scale study concerning the epidemiological, clinicopathological examination, molecular characterization, sequencing, and phylogenetic analysis of reported from the center of the Delta of the Nile in Egypt. AcknowledgmentThe authors would like to thank the Deanship of the Faculty of Veterinary Medicine at Mansoura University for supporting this work. Conflict of interestThe authors of this paper have declared that no competing interests exist. Authors’ contributionMohamed Eisa and Samar atwa conceived and planned the study; Ahmed Selim and Dina Mobark collaborated in the writing and revision of the manuscript; Dina mobark and Ahmed sebaay conducted laboratory testing; Ahmed Selim and Elzahraa elbaz collaborated in sequencing of genes and phylogenetic analysis. Mohamed Eisa and Samar atwa revised the manuscript. All authors’ read and endorsed the final manuscript. FundingThis study was conducted without external funding. Data availabilityAll data are provided in the manuscript. ReferencesAbdelfattah, S., Ahmed, S. and Elsayed, G. 2019. Epidemiological and molecular diagnosis of Ehrlichia canis infection among dogs. BVMJ. 37, 169–171. Aguirre, E., Sainz, A., Dunner, S., Amusategui, I., López, L., Rodríguez-Franco, F., Luaces, I., Cortés, O. and Tesouro, M.A. 2004. First isolation and molecular characterization of Ehrlichia canis in Spain. Vet. Parasitol. 125, 365–372. Assarasakorn, S., Kaewthamasorn, M. and Manachai, N. 2008. A retrospective study of clinical use of recombinant human erythropoietin for treatment in anemic dogs with canine monocytic ehrlichiosis from an animal hospital in Bangkok, Thailand. Comp. Clin. Path. 17, 237–243. Astigarraga, M.J.T. 2023. Vertical transmission of canine ehrlichiosis. Res. J. Agric. Sci. 3, 1–5. Aziz, M.U., Hussain, S., Song, B., Ghauri, H.N., Zeb, J. and Sparagano, O.A. 2023. Ehrlichiosis in dogs: a comprehensive review about the pathogen and its vectors with emphasis on South and East Asian Countries. Vet. Sci. 10, 21. Bai, L., Goel, P., Jhambh, R., Kumar, P. and Joshi, V.G. 2017. Molecular prevalence and haemato-biochemical profile of canine monocytic ehrlichiosis in dogs in and around Hisar, Haryana, India. J. Parasit. Dis. 41, 647–654. Barrantes-González, A.V., Jiménez-Rocha, A.E., Romero-Zuñiga, J.J. and Dolz, G. 2016. Serology, molecular detection and risk factors of Ehrlichia canis infection in dogs in Costa Rica. Ticks Tick-Borne Dis. 7, 1245–1251. Chawla, H., Katoch, A., Sharma, D., Wadhwa, D.R. and Sharma, A., 2020. Diagnosis and therapeutic management of Ehrlichiosis induced chronic kidney disease in dogs. Vet. Pract. 21, 71–75. Chochlios, T.A., Angelidou, E., Kritsepi-Konstantinou, M., Koutinas, C.K. and Mylonakis, M.E. 2019. Seroprevalence and risk factors associated with Ehrlichia canis in a hospital canine population. Vet. Clin. Pathol. 48, 305–309. Chua, A.P.B., Galay, R.L., Tanaka, T. and Yamazaki, W. 2020. Development of a loop-mediated isothermal amplification (LAMP) assay targeting the citrate synthase gene for detection of Ehrlichia canis in Dogs. Vet. Sci. 7, 156. Costa Jr, L.M., Rembeck, K., Ribeiro, M.F.B., Beelitz, P., Pfister, K. and Passos, L.M.F. 2007. Sero-prevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Vet. J. 174, 673–676. Dahmani, M., Davoust, B., Sambou, M., Bassene, H., Scandola, P., Ameur, T., Raoult, D., Fenollar, F. and Mediannikov, O. 2019. Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal. Parasit. Vectors. 12, 1–15. Ebani, V.V., Nardoni, S., Fognani, G., Mugnaini, L., Bertelloni, F., Rocchigiani, G., Papini, R.A., Stefani, F. and Mancianti, F. 2015. Molecular detection of vector-borne bacteria and protozoa in healthy hunting dogs from Central Italy. Asian Pac. J. Trop. Biomed. 5, 108–112. El-Dakhly, K.M., Tawfik, M.M., Aboshinaf, A.S., Mahrous, L.N. and Arafa, W.M., 2021. Detection of anaplasmosis and ehrlichiosis in blood of owned dogs in Alexandria, Northern Egypt. Adv. Anim. Vet. Sci. 9, 1383–1389. Espino-Solís, G.P., Flores-Lira, E.A., Barreras-Serrano, A., García-Reynoso, I.C., De la Mora Covarrubias, A., Vega, F.J. and Escárcega-Ávila, A., 2023. Clinical and pathological factors associated with Ehrlichia canis in companion dogs. JIDC. 17, 1598–1605. Field, A., Miles, J. and Field, Z. 2009. Discovering statistics using SPSS, 3rd edition. New York, NY: SAGE Publications, pp: 81. Gal, A., Loeb, E., Yisaschar-Mekuzas, Y. and Baneth, G. 2008. Detection of Ehrlichia canis by PCR in different tissues obtained during necropsy from dogs surveyed for naturally occurring canine monocytic ehrlichiosis. Vet. J. 175, 212–217. Gianopoulos, A., Mylonakis, M.E., Theodorou, K. and Christopher, M.M. 2016. Quantitative and qualitative leukocyte abnormalities in dogs with experimental and naturally occurring acute canine monocytic ehrlichiosis. Vet. Clin. Pathol. 45, 281–290. Gokmen, T.G., Gunaydin, E., Turut, N., Bünyamin, A., Özgür, K. and Ütük, A.E. 2019. A serosurvey on some canine vector-borne zoonoses (Anaplasma spp., Ehrlichia spp., Borrelia burgdorferi, Dirofilaria immitis and Leishmania spp.) in Osmaniye. Ataturk Univ. Vet. Bilim. Derg. 14, 151–158. Groves, M., Dennis, G., Amyx, H. and Huxsoll, D. 1975. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res. 36, 937–940. Harrus, S. 2015. Perspectives on the pathogenesis and treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet. J. 3, 239–240. Harrus, S., Day, M.J., Waner, T. and Bark, H. 2001. Presence of immune-complexes, and absence of antinuclear antibodies, in sera of dogs naturally and experimentally infected with Ehrlichia canis. Vet. Microbiol. 83, 343–349. Harrus, S. and Waner, T., 2011. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet. J. 187, 292–296. Hegab, A.A., Omar, H.M., Abuowarda, M., Ghattas, S.G., Mahmoud, N.E. and Fahmy, M.M. 2022. Screening and phylogenetic characterization of tick-borne pathogens in a population of dogs and associated ticks in Egypt. Parasit. Vectors. 15, 222. Kelly, P.J., Xu, C., Lucas, H., Loftis, A., Abete, J., Zeoli, F., Stevens, A., Jaegersen, K., Ackerson, K. and Gessner, A., 2013. Ehrlichiosis, babesiosis, anaplasmosis and hepatozoonosis in dogs from St. Kitts, West Indies. PLoS One 8, e53450. Lara, B., Conan, A., Thrall, M.A., Ketzis, J.K., Branford, G.C. and Rajeev, S. 2020. Serologic and molecular diagnosis of Anaplasma platys and Ehrlichia canis infection in dogs in an endemic region. Pathogens. 9, 488. Maeda, K., Markowitz, N., Hawley, R.C., Ristic, M., Cox, D. and McDade, J.E. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316, 853–856. Malik, M.I., Qamar, M., Ain, Q., Hussain, M.F., Dahmani, M., Ayaz, M., Mahmood, A.K., Davoust, B., Shaikh, R.S. and Iqbal, F. 2018. Molecular detection of Ehrlichia canis in dogs from three districts in Punjab (Pakistan). Vet. Med. Sci. 4, 126–132. Martínez-Vega, P.P., Bolio-Gonzalez, M.E., Rodríguez-Vivas, R.I., Gutierrez-Blanco, E., Pérez-Osorio, C., Villegas-Perez, S.L. and Sauri-Arceo, C.H. 2016. Associated factors to seroprevalence of Ehrlichia spp. in dogs of Quintana Roo, Mexico. J. Trop. Med. 2016, 4109467. Mitpasa, T., Sarker, B.R., Macotpet, A., Bupata, P.-A., Sangmaneedet, S. and Taweenan, W. 2022. First report on molecular characteristics and risk factor analysis of Ehrlichia canis in dogs in Khon Kaen, Thailand. Vet. World. 15, 232. Moreira, S., Bastos, C., Araújo, R., Santos, M., and Passos, L. 2003. Retrospective study (1998–2001) on canine ehrlichiosis in Belo Horizonte, MG, Brazil. Arq. Bras. Med. Vet. Zootec. 55, 141–147. Mylonakis, M.E., Harrus, S. and Breitschwerdt, E.B. 2019. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet. J. 246, 45–53. Mylonakis, M.E., Koutinas, A.F., Breitschwerdt, E.B., Hegarty, B.C., Billinis, C.D., Leontides, L.S. and Kontos, V.S. 2004. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J. Am. Anim. Hosp. Assoc. 40, 174–184. Nguyen, V.-L., Colella, V., Greco, G., Fang, F., Nurcahyo, W., Hadi, U.K., Venturina, V., Tong, K.B.Y., Tsai, Y.-L. and Taweethavonsawat, P. 2020. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit. Vectors. 13, 1–11. Nicholson, W.L., Allen, K.E., McQuiston, J.H., Breitschwerdt, E.B. and Little, S.E., 2010. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 26, 205–212. Parashar, R., Sudan, V., Jaiswal, A.K., Srivastava, A. and Shanker, D. 2016. Evaluation of clinical, biochemical and haematological markers in natural infection of canine monocytic ehrlichiosis. J. Parasit. Dis. 40, 1351–1354. Pérez-Macchi, S., Pedrozo, R., Bittencourt, P. and Müller, A. 2019. Prevalence, molecular characterization and risk factor analysis of Ehrlichia canis and Anaplasma platys in domestic dogs from Paraguay. Comp. Immunol. Microbiol. Infect. Dis. 62, 31–39. Perez, M., Bodor, M., Zhang, C., Xiong, Q., and Rikihisa, Y. 2006. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 1078, 110–117. Piratae, S., Senawong, P., Chalermchat, P., Harnarsa, W. and Sae-Chue, B. 2019. Molecular evidence of Ehrlichia canis and Anaplasma platys and the association of infections with hematological responses in naturally infected dogs in Kalasin, Thailand. Vet. World. 12, 131. Rodríguez-Alarcon, C.A., Beristain-Ruiz, D.M., Olivares-Munoz, A., Quezada-Casasola, A., Perez-Casio, F., Álvarez-Martinez, J.A., Tapia-Alanis, J., Lira-Amaya, J.J., Rivera-Barreno, R. and Cera-Hurtado, O.S., 2020. Demonstrating the presence of Ehrlichia canis DNA from different tissues of dogs with suspected subclinical ehrlichiosis. Parasit. Vectors. 13, 1–7. Sainz, A., Roura, X., Miro, G., Estrada-Peña, A., Kohn, B., Harrus, S. and Solano-Gallego, L. 2015. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors. 8, 1–20. Salem, N.Y., Rakha, G.H. and Baraka, T.A. 2014. Naturally occurring ehrlichiosis in Egyptian dogs. Iran. J. Vet. Res. 15, 54–57. Salib, F.A. and Farghali, H.A. 2015. Epidemiological, surgical and therapeutic studies on canine ehrilichiosis in Giza governorate, Egypt. Int. J. Livestock. Res. 5, 82–91. Selim, A., Abdelhady, A. and Alahadeb, J. 2020. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak. Vet. J. 41, 117–121. Selim, A., Alanazi, A.D., Sazmand, A. and Otranto, D. 2021. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasit. Vectors. 14, 1–11. Skotarczak, B. 2003. Canine ehrlichiosis. Ann. Agric. Environ. Med. 10, 137–141. Sukara, R., Andrić, N., Andrić, J.F., Mihaljica, D., Veinović, G., Ranković, V. and Tomanović, S., 2023. Autochthonous infection with Ehrlichia canis and Hepatozoon canis in dogs from Serbia. Vet. Med. Sci. 9, 111–118. Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Evol. 30, 2725–2729. Thongsahuan, S., Chethanond, U., Wasiksiri, S., Saechan, V., Thongtako, W. and Musikacharoen, T. 2020. Hematological profile of blood parasitic infected dogs in Southern Thailand. Vet. World. 13(11), 2388–2394. Vargas-Hernández, G., André, M., Faria, J., Munhoz, T., Hernandez-Rodriguez, M., Machado, R., and Tinucci-Costa, M. 2012. Molecular and serological detection of Ehrlichia canis and Babesia vogeli in dogs in Colombia. Vet. Parasitol. 186, 254–260. Waner, T., Leykin, I., Shinitsky, M., Sharabani, E., Buch, H., Keysary, A., Bark, H., and Harrus, S. 2000. Detection of platelet-bound antibodies in beagle dogs after artificial infection with Ehrlichia canis. Vet. Immunol. Immunopathol. 77, 145–150. Wen, B., Rikihisa, Y., Mott, J.M., Greene, R., Kim, H.-Y., Zhi, N., Couto, G.C., Unver, A. and Bartsch, R. 1997. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 35, 1852–1855. Ziliani, T.F., Castilho, A.R., Poletto, D., Mendonca, A.J., Sousa, V.R.F., Dutra, V. and de Almeida, A.d.B.P.F. 2019. Kidney disease in natural infection by Ehrlichia canis in dogs. Cien. Agrar. 40, 981–986. Supplementary MaterialsTable S1 outlines the obtained Eta values that inform the association degree between each risk factor and haematobiochemical indicators. Calculated Eta-squared (η2) that describes the amount of variance in each parameter that can be explained by one or more risk factors. Table S1. Eta values for the correlation between risk factors and levels of haemato-biochemical variables in overall investigated dogs (N=180).
| ||
| How to Cite this Article |
| Pubmed Style Mobark DA, Elbaz EK, Atwa SM, Eisa MI, El-sebaey AM, Selim AM. Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Vet. J.. 2024; 14(8): 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 Web Style Mobark DA, Elbaz EK, Atwa SM, Eisa MI, El-sebaey AM, Selim AM. Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. https://www.openveterinaryjournal.com/?mno=195517 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.10 AMA (American Medical Association) Style Mobark DA, Elbaz EK, Atwa SM, Eisa MI, El-sebaey AM, Selim AM. Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Vet. J.. 2024; 14(8): 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 Vancouver/ICMJE Style Mobark DA, Elbaz EK, Atwa SM, Eisa MI, El-sebaey AM, Selim AM. Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 Harvard Style Mobark, D. A., Elbaz, . E. K., Atwa, . S. M., Eisa, . M. I., El-sebaey, . A. M. & Selim, . A. M. (2024) Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Vet. J., 14 (8), 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 Turabian Style Mobark, Dina A., Elzahara K. Elbaz, Samar M. Atwa, Mohamed I. Eisa, Ahmed M. El-sebaey, and Ahmed M. Selim. 2024. Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Veterinary Journal, 14 (8), 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 Chicago Style Mobark, Dina A., Elzahara K. Elbaz, Samar M. Atwa, Mohamed I. Eisa, Ahmed M. El-sebaey, and Ahmed M. Selim. "Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt." Open Veterinary Journal 14 (2024), 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 MLA (The Modern Language Association) Style Mobark, Dina A., Elzahara K. Elbaz, Samar M. Atwa, Mohamed I. Eisa, Ahmed M. El-sebaey, and Ahmed M. Selim. "Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt." Open Veterinary Journal 14.8 (2024), 1819-1835. Print. doi:10.5455/OVJ.2024.v14.i8.10 APA (American Psychological Association) Style Mobark, D. A., Elbaz, . E. K., Atwa, . S. M., Eisa, . M. I., El-sebaey, . A. M. & Selim, . A. M. (2024) Molecular, epidemiological, and hematological evaluation in Ehrlichia canis infected dogs from an endemic region in Egypt. Open Veterinary Journal, 14 (8), 1819-1835. doi:10.5455/OVJ.2024.v14.i8.10 |