| Research Article | ||
Open Vet. J.. 2024; 14(10): 2551-2563 Open Veterinary Journal, (2024), Vol. 14(10): 2551–2563 Research Article Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, IndonesiaDyah Haryuningtyas Sawitri1*, April Hari Wardhana1,2, Farlin Nefho1, Eko Setyo Purwanto1, Dwi Endrawati1, Yudhi Ratna Nugraheni3, Roza Azizah Primatika4, Ndaru Andri Damayanti5, Rizal Arifin Akbari6, Eni Kusumaningtyas1 and Makoto Matsubayashi71Research Center for Veterinary Science, Research Organization for Health, National Research and Innovation Agency, Cibinong, Indonesia 2Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia 3Department of Parasitology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 5Faculty of Medicine, University of Yarsi, Jakarta, Indonesia 6RVet animal Clinic, Bogor, Indonesia 7Department of Veterinary Science, Graduate School of Veterinary Immunology, Osaka Metropolitan University, Osaka, Japan *Corresponding Author: Dyah Haryuningtyas Sawitri. Research Center for Veterinary Science, Organization for Health, National Research and Innovation Agency, Cibinong, Indonesia. Email: dyah.haryuningtyas [at] gmail.com Submitted: 02/04/2024 Accepted: 17/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
AbstractBackground: Intestinal helminth infections in cats are often neglected major zoonoses spread from pets to humans worldwide. Aim: This study evaluated the prevalence and identified risk factors associated with zoonotic gastrointestinal helminth infections in different cat populations in the most populous megapolitan areas of Indonesia: Jakarta, Bogor, Depok, Tangerang, and Bekasi (Jabodetabek). Methods: Fecal samples from the shelter (stray) and household (owned) cats were analyzed using sugar flotation techniques. Intestinal helminth eggs were detected microscopically based on structural and morphometric characteristics. Risk factors for the occurrence of helminth infection were identified through statistical analysis of cat ownership, breed, migrant status, management practices, caging, feed type, and deworming medications used. Human cases of worm larvae infestation identified during the interviews were reported. Results: Analysis of 354 fecal samples revealed that 37.9% (134/354) of examined cats were infected with Toxocara sp., 22.6% (80/354) with Ancylostoma sp., 25.4% (90/354) with Uncinaria sp., 3.1% (11/354) with Strongyloides sp., 2% (7/354) with Diphyllobothrium sp., and 0.6% (2/354) with Dipylidium sp. Infection with roundworms and hookworms was associated with a variety of factors, including introduction of new animals, management practices, cage cleanliness, feed type, use of deworming medication, routine deworming, and contact with other animals. A human case of cutaneous larva migrans was due to hookworm (Ancylostoma sp./Uncinaria sp.) infection. Conclusion: The prevalence of important zoonotic gastrointestinal nematodes (hookworms and roundworms) is high in cats in Jabodetabek, Indonesia. To reduce the risk of transmission to other animals or humans, adequate measures to control, manage, and prevent zoonotic helminth infections are required. This study presents important baseline information that provides a basis for future epidemiologic studies and the development of strategies to manage zoonotic gastrointestinal helminths in cats in the region. Keywords: Cat, Gastrointestinal helminths, Prevalence, Risk factors, Zoonotic. IntroductionThe established benefits of companion animal ownership on human mental, emotional, and physical health (Paul et al., 2010) are thought to play a significant role in observed increases in pet cat populations worldwide (Ursache et al., 2021). However, pet ownership is also associated with potential health hazards (Overgaauw and van Knapen, 2013). One such hazard is infection with gastrointestinal helminths, which represent a leading cause of morbidity in domestic cats. Some of these helminths are zoonotic and thus threaten public health due to the potential for spread to humans (Yang and Liang 2015; Ursache et al., 2021). Zoonotic helminths include nematodes (Toxocara sp., Ancylostoma sp., Uncinaria sp., and Strongyloides sp.), trematodes (Diphyllobothrium sp.), and cestodes (Dipylidium sp. and Echinococcus sp.) (Overgaauw and van Knapen, 2013; Phosuk et al., 2013). Roundworm species such as Toxocara canis, T. cati, and T. malaysiensis and hookworm species such as Ancylostoma braziliense, A. caninum, A. ceylanicum, and Uncinaria stenocephala are capable of infecting both domestic dogs and cats. Toxocara species can infect humans, who serve as paratenic hosts, via exposure to contaminated soil/water, although the parasites are usually acquired via the fecal-oral route through ingestion of live L2 larvae, embryonated eggs found in the environment, larvae present on unwashed hands or raw vegetables, or through ingestion of larvae found in uncooked organs or muscle tissue from other paratenic hosts (Wolfe and Wright 2003; Wu and Bowman 2020). Toxocara infections can produce a variety of clinical symptoms, including ocular larva migrans, visceral larva migrans, and eosinophilic meningoencephalitis (Despommier 2003; Macpherson 2013) and associated complications, including allergic and neurologic disorders/neurotoxocariasis (Ma et al., 2018). Human ancylostomiasis occurs when zoonotic hookworm larvae penetrate unprotected skin that has contacted contaminated soil or sand (de Mello et al., 2022). These species are regarded as neglected tropical zoonoses because they can induce cutaneous larva migrans (CLMs), a creeping skin eruption affecting humans (Massetti et al., 2020). Four species of Strongyloides have been identified in cats, including S. felis, S. planiceps, S. tumefaciens, and S. stercoralis, among which S. stercoralis is the predominant species affecting humans, dogs, and cats (Wulcan et al., 2019). Infection with Strongyloides sp. occurs predominantly via oral ingestion or percutaneous penetration facilitated by infectious third-stage larvae, although the precise mechanism by which the organism enters the intestines remains unclear (Wulcan et al., 2019). Although the life cycle and diet of host animals play a role in disease transmission, the prevalence of patent infections is often highest in puppies and kittens, lower in juveniles, and lowest in adult dogs and cats (Ridwan et al., 2023). Dogs, cats, and humans can acquire helminth infections by consuming contaminated raw flesh, helminth eggs present in the environment, or an infected paratenic host (including chickens and rodents) (Overgaauw and van Knapen, 2013). Cases of infection with zoonotic helminths in cats are often asymptomatic, and they are usually only identified by fecal screening or spontaneous passage of worms through feces or vomitus (toxocariasis in kittens), as healthy companion cats typically exhibit a low worm burden (Castro and Sapp 2021). Animals with a higher parasite burden, for example, kittens with transmammary toxocariasis infection, may exhibit signs of malnutrition, pot belly appearance, respiratory distress, diarrhea, vomiting, and cachexia (Dantas-Torres et al., 2020). Similarly, worm infections in humans are also generally asymptomatic. Clinically apparent infections may go undetected due to the high cost of diagnostic testing and the potential need for pruritic or unaffordable molecular, imaging, or serologic tests (Macpherson 2013). Routine diagnosis of a worm infection involves demonstrating the presence of characteristic eggs in feces (Overgaauw and van Knapen, 2013). Dryden et al., (2005) proposed a sedimentation and flotation method as an alternative to centrifugation due to the consistently higher egg recovery compared with centrifugation-based methods. Rates of cat and dog ownership are increasing in Indonesia (Ridwan et al., 2023), particularly in urban regions surrounding Jakarta, Bogor, Depok, Tangerang, and Bekasi (Jabodetabek) (direct communication with cat shelter owners in Bogor and Jakarta, 2022). Nevertheless, many pet owners exhibit a significant lack of awareness regarding the risk of diseased animals contaminating their surroundings or serving as carriers for disease transmission to humans. It is, therefore, important to identify the predominant circulating gastrointestinal parasites in order to assess the relevant risk factors for the spread of parasitic diseases to other animals or humans and initiate appropriate mitigation actions. Furthermore, studies in this subject area in Indonesia are extremely limited. The present study evaluated the prevalence of zoonotic gastrointestinal helminths in cats and identified risk factors for infection with these helminths and their zoonotic potential among the cat population in Jabodetabek and surrounding areas. Materials and MethodsSampling sites and sample collectionA cross-sectional study was carried out between November 2022 and April 2023 in the important urban zone located in the Jakarta metropolitan area, known locally as Jabodetabek (an acronym of Jakarta–Bogor–Depok–Tangerang–Bekasi. This is the most populous megapolitan area in Indonesia and includes the national capital (Jakarta Special Capital Region as the core city), five satellite cities, and three complete regencies (Rustiadi et al., 2015). The survey area included cities in Central, South, East, and West Jakarta (DKI Jakarta Province), Bogor and Depok cities (West Java Province), and Tangerang Regency (Banten Province). The study investigated stray cats obtained from cat shelters and door-to-door sampling (owned cats; household companions). Cat shelters in the study area care for stray cats from various locations, especially in the province in which the shelter is located. All cities in the study area are tropical and adjacent to one another. Rainfall in the study area is generally evenly distributed throughout the year. Average annual rainfall at all locations is generally 2,000–4,000 mm, with air temperature in the 24°C–35°C range (Putri and Wibowo, 2023). A total of 354 fecal samples were collected from the following locations: East Jakarta city (10/7 owned/stray cats), South Jakarta city (23/89 owned/stray cats), Central Jakarta city (33 owned cats), Bogor city (55/42 owned/stray cats), Depok city (23 stray cats), Tangerang city/district (20 owned cats), and Bekasi city (52 stray cats). Stool samples were collected from each animal from the litter during initial defecation. Each sample was individually numbered and sealed in a polyethylene container, which was stored at 4°C, with analysis completed within 72 hours. Each cat’s sex, age, breed, housing conditions, deworming history, and food were documented on a data entry sheet on the day of sampling. Human cases of worm larvae infestation noted during the interview were reported to the appropriate authorities. The sampling sites selected in this study are presented in Figure 1. Sample size estimationA random sampling approach was utilized based on a 35% expected prevalence of ancylostomiasis and toxocariasis determined from a previous report that included the study area (Ridwan et al., 2023). The sample size was calculated as follows: n=z2 p (1 − p)/d2, where n=required sample size, p=estimated prevalence, d=95% confidence level, and d=margin of error at 5% (Daniel and Cross 2018). Examination of stool samplesGastrointestinal nematode eggs were isolated from each sample using a sugar flotation technique as previously described, with some modifications (Sawitri et al., 2019). Fecal samples (5 g each) were suspended in distilled water and filtered using a 150 μm sieve. The filtrate was poured into a 50 ml tube and centrifuged at 800 g for 5 minutes, after which as much of the supernatant as possible of each sample was discarded. Sugar solution (specific gravity ~1.2) was added to the sediment of each sample to a scale volume of 45 ml, and 5 ml of distilled water was then added to a scale volume of 50 ml (to form 2 layers). The sample was then centrifuged at 1,200 g for 10 minutes. Worm eggs floating on the surface of the sugar solution (at the border between the saturated sugar and water layers) were collected up to a total of 15 ml using a Pasteur pipette, placed in a new 50 ml centrifuge tube, and washed with distilled water 3 times. Purified eggs were dissolved in 1 ml of phosphate-buffered saline. Finally, samples were stored at 4°C until further analysis. Identification of isolated eggsA total of 10 µl of egg-containing solution was placed on a microscope slide and mixed with 10 µl of saturated sugar solution. A cover glass was placed over the specimen, and the slide was examined under a binocular microscope at magnifications of 40 × and 100 × to identify gastrointestinal helminth eggs based on structural and morphometric features (Thienpont et al., 2003). Data management and statistical analysisData collected during sample collection and the results of fecal examinations were entered into a Microsoft Excel spreadsheet for analysis. Statistical analyses were carried out using SPSS software, version 25.0 (Ghozali 2021). The prevalence and frequency distribution of overall infection with each parasite were determined using the bivariate analysis method. Chi-square tests were used to analyze associations between risk factors. In cases of fewer than 5 total cells, Fisher’s exact test was used. A standardized questionnaire was used to obtain information regarding the management profile of each cat. The following independent variables were considered: age (≤12 months, >12 months), gender, and management (e.g., cat food, outdoor versus indoor housing, contact with other animals, deworming program, and cage cleaning). Dependent variables included eggs of Toxocara sp., Ancylostoma sp., Uncinaria sp., Strongyloides sp., Diphyllobothrium sp., and Dipylidium sp. In statistical tests, p < 0.05 was considered indicative of a statistically significant difference. Human cases of worm larvae infestation identified during interviews were reported to the appropriate authorities.
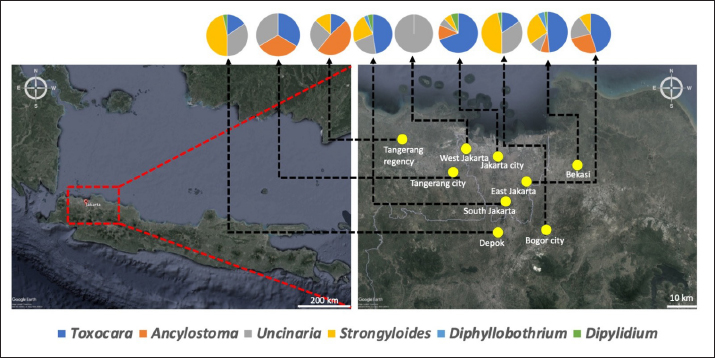
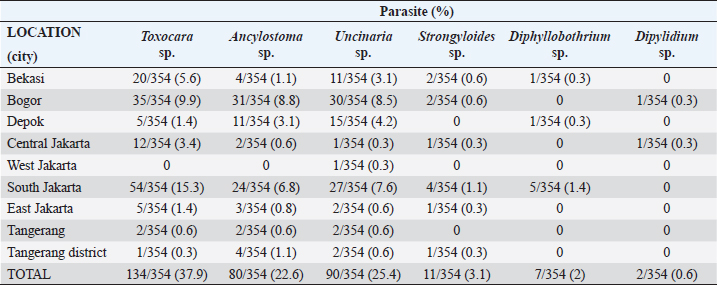
Fig. 1. Map depicting sample collection sites in this study. Ethical approvalThe experimental design was approved by the Ethics Committee on Animal Use and the Sosial Humaniora of the National Research and Innovation Agency (approval numbers 094/KE.02/SK/11/2022 and 524/KE.01/SK/11/2022, respectively). ResultsA total of 354 fecal samples were collected during the survey in the Jabodetabek area. The samples were collected from 213 stray cats and 141 owned cats. The overall prevalence of zoonotic gastrointestinal helminth infection in the study was 53.3%, as 188 of 354 samples tested positive for parasite ova. A total of 6 zoonotic gastrointestinal helminths were identified from the examined fecal samples, including Toxocara sp., Ancylostoma sp., Uncinaria sp., Strongyloides sp., Dipylidium sp., and Diphyllobothrium sp. (Fig. 2). The prevalence of infections in the study varied regionally. The most common helminth infections in cats examined in the study were roundworm (Toxocara sp.) and hookworm (Uncinaria sp. and Ancylostoma sp.), with overall prevalences of 37.9% (134/354), 25.4% (90/354), and 22.6% (80/354), respectively. As infections caused by Strongyloides sp., Dipylidium sp., and Diphyllobothrium sp. are generally uncommon, the prevalence of infection with these organisms was low, at 3.1% (11/354), 1.4% (7/354), and 0.6% (2/354), respectively.
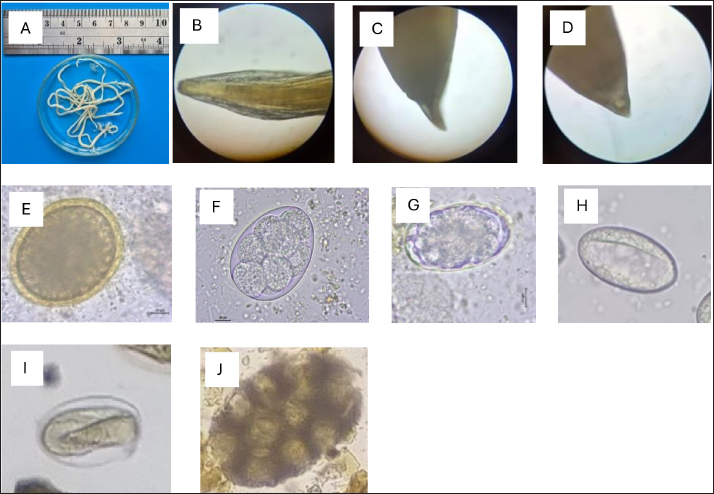
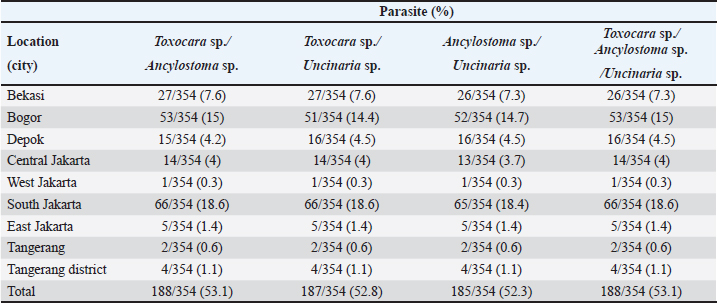
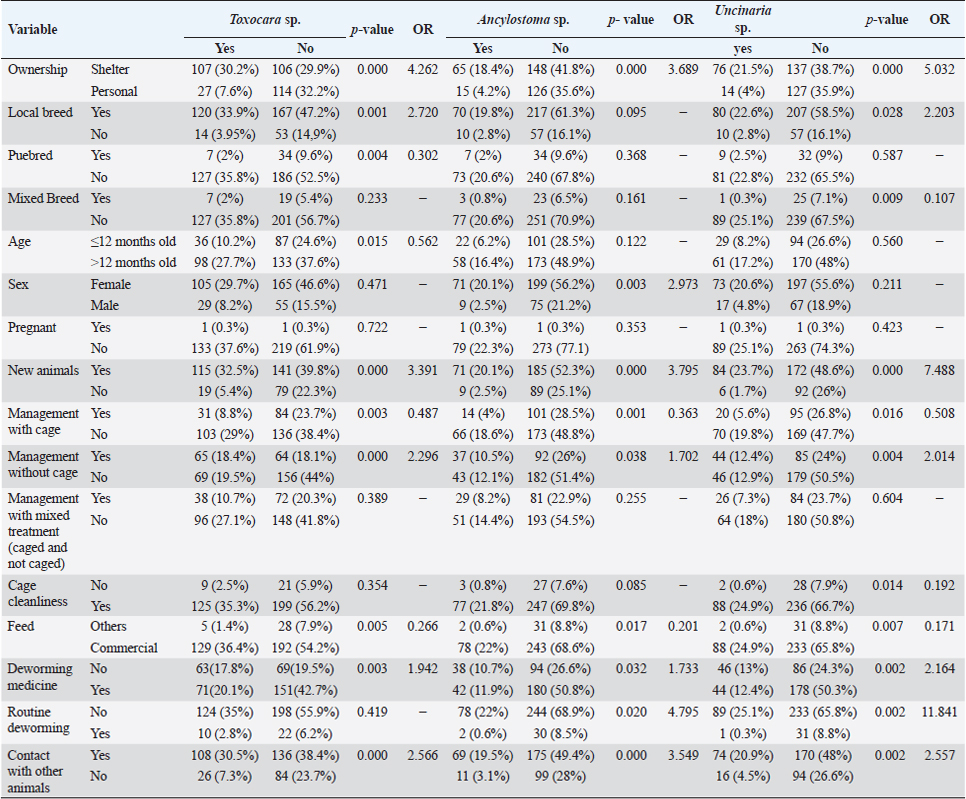
Fig. 2. Helminth worms and eggs found in parasitological examinations of domestic cat feces from the Jabodetabek area, Indonesia. Macroscopic: (A) Toxocara malaysiensis worm; microscopic: (B) cephalic alae of Toxocara malaysiensis; (C) tail-end of Toxocara malaysiensis male (D); tail-end of Toxocara malaysiensis female; (E) egg of Toxocara malaysiensis; (F) egg of Ancylostoma sp.; (G) egg of Uncinaria sp.; (H) egg of Diphyllobothrium sp.; (I) egg of Strongyloides sp.; (J) egg of Dipylidium caninum. Photomicrograph of worm (100 × magnification), egg (400 × magnification). Infection with Toxocara sp. was most prevalent in South Jakarta, at 15.3% (54/354), suggesting that this region bears a greater overall burden of Toxocara infection, followed by Bogor at 9.9% (34/354) and Bekasi at 5.6% (20/354). Bogor city exhibited the highest prevalence of Ancylostoma sp. infection, at 8.8% (31/354), followed by South Jakarta at 6.8% (24/354). The prevalence of Uncinaria sp. infection was 8.5% (30/354) and 7.6% (27/354) in Bogor and South Jakarta, respectively. The importance of implementing targeted public health measures was underscored by the substantial cumulative prevalence observed across all regions (Table 1). The cats examined in the present study were often infected with more than one parasite species. The incidence of mixed infections was higher than that of single infections and ranged from 52.3% (185/354) to 53.1% (188/354) (Table 2). Co-infections involving two worm species (Toxocara sp. and Ancylostoma sp.) or three worm species (Ancylostoma sp., Toxocara sp., and Uncinaria sp.) exhibited similar prevalence, as shown in Table 3. This finding suggests that helminth infections tend to be mixed rather than single. At all sites in the study area, the prevalence rates of infection with cestode and trematode worms were very low. Factors associated with Toxocara sp., Ancylostoma sp., and Uncinaria sp. infectionToxocara sp., Ancylostoma sp., and Uncinaria sp. were the dominant nematode species detected in the study area. The predominant predictors of intestinal helminth infection were pet ownership, breed, pet age, introduction of new animals, housing conditions, feed, use of deworming medicine, and contact with other animals (Table 3). Cats in shelters had a 4.26-fold higher risk of toxocariasis infection than owned cats. The local breed had a 2.72-fold higher risk of toxocariasis than other breeds (purebred and mixed). Cats ≤12 months old had a 0.562-fold higher risk than cats >12 months old. The introduction of new animals increased the risk of transmission of Toxocara sp. by 3.39-fold compared with no introduction of new animals. Feeding cats a non-commercial feed increased the risk of contracting an intestinal parasite infection by 0.266-fold compared with cats fed a commercial feed. Cats that did not receive deworming medicine were at a 1.942-fold higher risk of infection with Toxocara sp. eggs. Contact with other animals increased the risk of contracting Toxocara sp. eggs by 2.566-fold compared with no contact with other animals. No significant associations (p > 0.05) were observed between Toxocara sp. infection and factors such as mixed breed, sex, pregnancy status, presence of new animals, management with mixed treatment (caged versus not caged), cage cleanliness, and routine deworming. However, other factors exhibited statistically significant associations (p < 0.05), as presented in Table 3. Cats not subjected to routine deworming were at a 4.9-fold greater risk of being infected with Ancylostoma sp. than routinely dewormed cats. Other factors, such as the introduction of new animals, cats held in shelters, coming into contact with other animals/wildlife, female cats, free-range cats, and unclean cages were significantly associated with ancylostomiasis, exhibiting 3.79-, 3.69-, 3.5-, 2.9-, and 2.7-fold greater risk of contracting ancylostomiasis, respectively. Significant associations (p < 0.05) were also observed between Ancylostoma infection and factors including ownership, pet sex, presence of new animals, management with cage, management without cage, feed type, deworming medicine, routine deworming, and contact with other animals. By contrast, no significant associations were observed with variables such as breed and age (p > 0.05). Table 1. Prevalence of infections with zoonotic gastrointestinal helminths in this study.
Table 2. Prevalence of dominant mixed infections with zoonotic gastrointestinal helminths in this study.
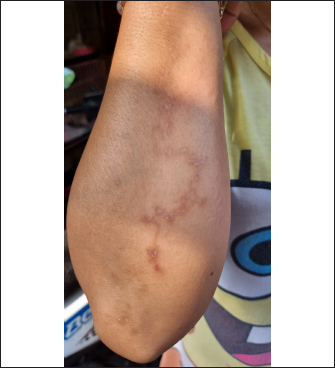
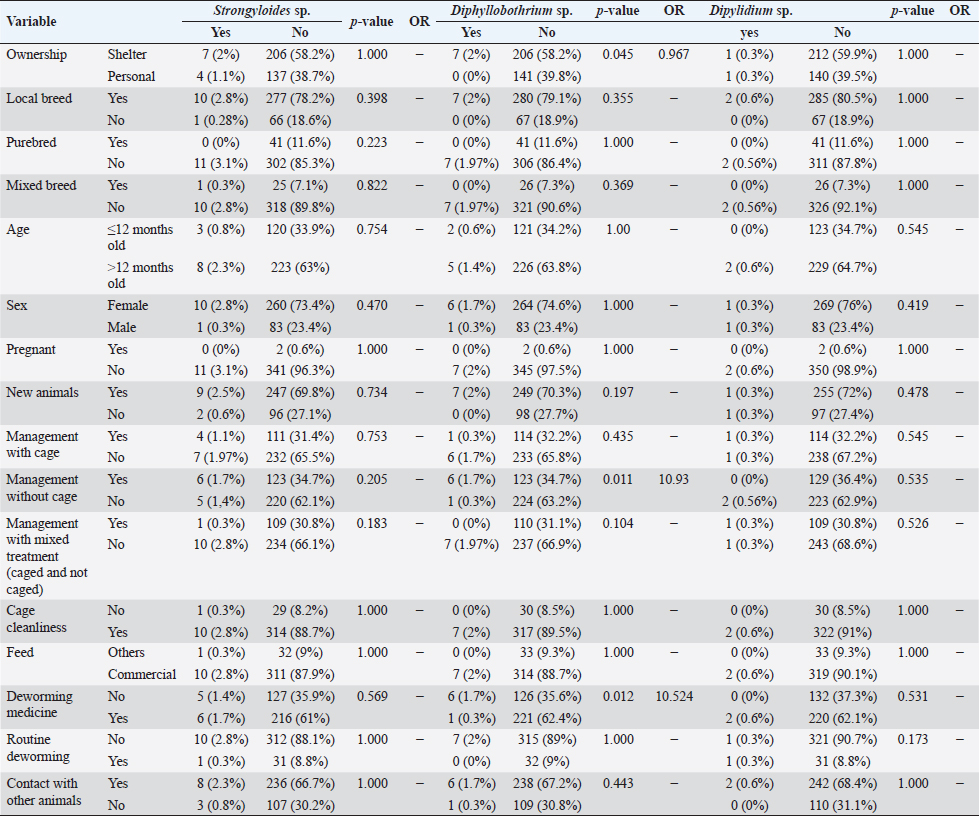
However, routine deworming, the introduction of new animals, ownership type, contact with other animals/wild animals, breed, deworming medicine usage, housing situation, and breed were significantly associated with Uncinaria sp. infection (Table 3). Routine deworming exhibited the strongest association with Uncinaria sp. infection. Cats not routinely dewormed exhibited an 11.8-fold greater risk of Uncinaria sp. infection than cats that were routinely dewormed. Other risk factors associated with Uncinaria sp. infection were the introduction of new animals, ownership type, contact with other animals/wild animals, breed, deworming medicine usage, and housing type, with 7.48-, 5.03-, 2.56-, 2.2-, 2.16, and 2.01-fold greater risk of infection, respectively. Among all variables, significant associations (p < 0.05) were observed between Uncinaria infection and ownership type, local breed, mixed breed, introduction of new animals, management with cage, management without cage, cage cleanliness, feed type, deworming medicine, routine deworming, and contact with other animals. Other factors were not significantly associated with Uncinaria infection (p > 0.05). Factors associated with Strongyloides sp., Diphyllobothrium sp., and Dipylidium sp. infectionStrongyloides sp., Diphyllobothrium sp., and Dipylidium sp. exhibited the lowest prevalence rates in the study area. No significant associations were observed between infection with these organisms and cat breed, age, sex, pregnancy, the introduction of new animals, management practices, cage cleanliness, feed type, and routine deworming status (Table 4). In contrast, animals kept without cages and ownership type were associated with Diphyllobothrium sp. infection, with 10.9- and 1-fold greater risk of infection, respectively. In contrast, these parameters were not associated with Strongyloides sp. and Dipylidium sp. infections. It is possible that the small number of infections in the population under study contributed to the lack of significant associations. CLM in humansDuring interviews conducted at the shelter in Depok city regarding cat feces sampling, a 40-year-old female presented with a 6-week history of severe, migratory, and itching dermatitis accompanied by the formation of blisters on her right hand (Fig. 3). This individual was a cat caretaker at a local shelter. The prevalence rates for hookworm infection among the 23 samples collected at that location were approximately 21% (5/23) for Toxocara sp., 47.8% (11/23) for Ancylostoma sp., and 60.87% (14/23) for Uncinaria sp. DiscussionThis survey found that roundworm (Toxocara sp.) was the most prevalent zoonotic helminth infection in cats in the Jabodetabek area of Indonesia, followed by hookworm (Ancylostoma sp. and Uncinaria sp.). Other worms identified in the study included Strongyloides sp., Diphyllobothrium sp., and Dipylidium sp., but these were uncommonly characterized by low prevalence. The overall prevalence of roundworm Toxocara sp. infection (37.9%), hookworm Ancylostoma sp. infection (22.4%), and Uncinaria sp. infection (25.6%) in cats in Jabodetabek were higher than the previously reported prevalence of roundworm and hookworm infections in cats in China (17.7% and 6.39%, respectively) (Yang and Liang 2015) as well as in Northern Thailand (2.2% and 13.9%, respectively) (Pumidonming et al., 2016). The prevalence of Toxocara sp. in the present study area was comparable to the infection rate in cats in Bogor in a previous study, which was 35%. According to Dantas-Torres (2020) and Phoosangwalthong et al., (2022), the prevalence of toxocariasis in cats in the present study was higher than that previously reported in Brazil and Bangkok (6.7% and 0.6%, respectively). However, the prevalence in the present study was lower than the T. cati prevalence in cats in Vietnam (47.8%) (Anh et al., 2016) and Romania (40.2%) (Ursache et al., 2021) In contrast, the prevalence of hookworm in this study was higher than that reported by Ursache et al., (2021), and Yang and Liang (2015) for central China (6.39%), Romania (3.7%), and Algeria (1.15%), respectively. Hookworm prevalence rates vary regionally due to factors such as climate, living conditions, diagnostic resources, and the quality of veterinarian care (Garcia-Campos et al., 2019). Although the present study found an association between Toxocara sp. and Ancylostoma sp. infection rates and several risk factor parameters, only two parameters were associated with diphyllobothriasis in cats, namely cage management and owned cats. Cats kept outdoors (without cages) or in shelters are at a 10-fold and 1-fold greater risk of infection with Diphyllobothrium sp., respectively, compared with cats kept indoors and owned cats. Table 3. Summary of risk factors associated with Toxocara sp., Ancylostoma sp., and Uncinaria sp. infection in the bivariate analysis.
Fig. 3. Case of CLMs in a human after a 6-week infection. According to Kurnosova et al., (2019), pets can serve as long-term sources of infection due to the subclinical nature of many parasitic diseases, especially helminthiases. The course and duration of the disease depend on a number of factors, including the type and intensity of infestation, the animal’s age and immune status, and the presence of any concurrent underlying diseases (Kurnosova, 2009). Yang and Liang (2015) reported that regional differences in the frequency and availability of veterinary care during different seasons of the year and the type of cat population (household, stray, feral, or shelter cats) can affect the prevalence of intestinal parasites. Table 4. Summary of risk factors associated with Strongyloides sp., Diphyllobothrium sp., and Dipylidium sp. infection in the bivariate analysis.
The case of CLM in a shelter worker in Depok demonstrated that human zoonotic hookworms can be transmitted through contact with cat excrement contaminated with hookworm larvae. The individual was a cat caretaker, and her daily responsibilities included feeding and cleaning cat litter. She did not wear gloves and sometimes went barefoot while cleaning the cages. The cats in this shelter were stray cats removed from streets and markets, and they were not owned and had never been dewormed. Coello et al., (2019) reported that penetration of human skin by infective larvae (stadium III) of Ancylostoma sp. or Uncinaria sp. can lead to CLM. According to Yavuzer et al., (2010), the most common ways people become infected is through contact with contaminated materials during activities such as gardening, cleaning cat litter without gloves, sitting in contaminated sand or dirt, or going barefoot. Typically, these larvae travel through the epidermis and create raised tracks because they are unable to pass through the epidermis basal barrier (Reichert et al., 2018). The incubation period can be >5 months but is most often between 5 and 15 days (Sears et al., 2022). Investigation of the prevalence of Toxocara sp. infection revealed that animals in shelters, local breeds, and recently introduced cats exhibited higher prevalence than owned cats, emphasizing the impact ownership, breeding, and introduction of new animals have on infection rates (Bonilla-Aldana et al., 2024). Additionally, management practices such as enclosed habitat, feed variety, and use of deworming medication are vital. Chi-square analyses of infections with Ancylostoma sp. and Uncinaria sp. demonstrated comparable patterns, underscoring the complex and diverse array of factors that contribute to gastrointestinal infections in animals (Table 3). The present study also showed associations between toxocariasis and ancylostomiasis infection and the introduction of new animals, management practices, cage cleanliness, feed type, deworming medicine, routine deworming, and contact with other animals, similar to the report by Arruda et al. (2022). Prevention of toxocariasis and ancylostomiasis requires targeted management strategies. Dami et al. (2023) reported that if domestic carnivores do not receive routine basic veterinary care and coexist in the same environment as wild animals, there is a greater chance that environmental pollution will spread parasitic infections from domestic carnivores to humans. The absence of significant associations between infection with Strongyloides sp., Dipylidium sp., and Diphyllobothrium sp. and the variables under study may be attributed to the small number of infections in the population under study. According to Vafae Eslahi et al. (2022), cats are the most common carriers of Diphyllobothrium sp. in Asia, whereas dogs are the most common carriers in Africa. Furthermore, no noteworthy correlations were observed between Dipylidium sp. infection and breed, age, gender, introduction of new animals, management practices, cage cleanliness, feed type, deworming medicine, routine deworming, and contact with other animals, which can be attributed to the low prevalence of Dipylidium sp. infection in the sample population. The species of vertebrate and its life cycle can impact the chance of Dipylidium caninum infection. However, animals that are less likely to have access to veterinary treatment, such as animals in shelters and those on the streets, are more likely to become infected (ESCCAP 2021). Overall, the results of the present study provide a more comprehensive understanding of the prevalence of gastrointestinal helminth infections in different regions of Indonesia and shed light on the varied factors that influence these infections. Considering the disparate prevalence rates of various gastrointestinal infections in the studied areas, the results of this study highlight the need for region-specific interventions. In order to effectively prevent and control gastrointestinal infections in animals, targeted interventions that consider ownership, breed, age, management practices, and other relevant factors are crucial, as demonstrated by the present findings. ConclusionIn conclusion, the research findings presented here indicate that the prevalence of infection with Toxocara sp., Ancylostoma sp., and Uncinaria sp. is higher in Jabodetabek, Indonesia. Significant factors contributing to the development of infection with these parasites include ownership, breed, introduction of new animals, management practices, caging, feed type, and deworming medication use. The presence of CLM in humans carrying hookworm larvae in Depok city is due to the high prevalence of hookworm infection in animals in the area. This study emphasized the importance of individualized prevention strategies that can reduce the risk of hookworm and roundworm transmission (e.g., using gloves when handling cat feces, washing hands after handling cats, and wearing footwear when outdoors). However, proper management practices, including providing adequate housing and high-quality feed, and instituting interventions to reduce environmental contamination such as limiting free-roaming cat populations, deworming new arrivals, routine deworming of outdoor cats, preventing owned cats from accessing public places, and preventing contact between owned cats and stray cats are important for controlling and preventing gastrointestinal infections in animals. The results of this study provide important baseline data that can be used by veterinarians and public health officials to establish a framework for epidemiologic studies and the development of strategies for treating and controlling parasites that can potentially cause zoonotic infections of feline origin. AcknowledgmentsThe authors thank owners of the cat shelters, pet owners, and practicing veterinarians in Jakarta, Bogor, Depok, Tangerang, and Bekasi who assisted with the sample collection. Conflict of interestThe authors declare that there are no conflicts of interest. FundingThis research was funded by The National Research and Innovation Agency of the Republic of Indonesia, no. 4/III.9/HK/2023. Authors’ contributionsDHS and AHW conceived of and designed the study. Field sampling, data collection, and laboratory work: DHS, AHW, ESP, FN, DE, YRN, NAD, RAP, and EK. Data entry, analysis, and interpretation: DHS, AHW, DE, RAP, and YRN. The manuscript was drafted by DHS and YRN, and AHW and MM revised the intellectual content. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data used to support the primary findings of this study are included in the article. Complete datasets generated and analyzed during the study are available from the corresponding author upon reasonable request. ReferencesAnh, N.T., Thuy, D.T., Hoan, D.H., Hop, N.T. and Dung, D.T. 2016. Levels of Toxocara infections in dogs and cats from urban vietnam together with associated risk factors for transmission. J. Helminthol. 90(4), 508–510. Arruda, I.F., Mendes, Y.A.C., Bonifácio, T.F., da Silveira Gonçalves, I.M., Millar, P.R., da Silva Barbosa, A., de Souza Abboud, L.C. and Amendoeira, M.R.S. 2022. Socioeconomic Profile, Animal Care, Sanitary Practices, and Knowledge about Parasites among Owners of Domestic Dogs and Cats Treated in Rio de Janeiro City. Revista Brasil. Med. Vet. 44, 1–12. Bonilla-Aldana, J.L., Espinosa-Nuñez, A.C., Bonilla-Aldana, D.K. and Rodriguez-Morales, A.J. 2024. Toxocara cati Infection in Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals 14(7), 1–37. Castro, P.D. and Sapp, S.G. 2021. Role of cats in human toxocarosis. Companion Anim. 26(1), 1–8. Coello, R.D., Pazmiño, B.J., Reyes, E.O., Rodríguez, E.X., Rodas, E.I., Rodas, K.A., Dávila, A.X., Rodas, J.P. and Cedeño, P.P. 2019. A case of cutaneous larva migrans in a child from Vinces, Ecuador. Am. J. Case Rep. 20, 1402–1406. Dami, J.C., Damayanti, L.P.E., Indarjulianto, S., and Priyowidodo, D. 2023. Ancylostomiasis in cats in Yogyakarta, Indonesia, and its causative genetic relations. Biodiversitas 24(5), 2605–2611. Daniel, W.W. and Cross, C.L. 2018. Biostatistics: a foundation for analysis in the health sciences. Hoboken, NJ: Wiley-Blackwell. Dantas-Torres, F. 2020. Toxocara prevalence in dogs and cats in Brazil. Adv. Parasitol. 109, 715–741. Dantas-Torres, F., Ketzis, J., Mihalca, A.D., Baneth, G., Otranto, D., Tort, G.P., Watanabe, M., Linh, B.K., Inpankaew, T., Jimenez Castro, P.D., Borrás, P., Arumugam, S., Penzhorn, B.L., Ybañez, A.P., Irwin, P. and Traub, R.J. 2020. TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Vet. Parasitol. 283, 109167. Despommier, D. 2003. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin. Microbiol. Rev. 16(2), 265–272. Dryden, M.W., Payne, P.A., Ridley, R. and Smith, V. 2005. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 6(1), 15–28. ESCCAP. 2021. Worm control in dogs and cats, 6th ed. Malvern, UK: Malvern Hills Science Park. Garcia-Campos, A., Power, C., O'Shaughnessy, J., Browne, C., Lawlor, A., McCarthy, G., O'Neill, E.J. and de Waal, T. 2019. One-year parasitological screening of stray dogs and cats in County Dublin, Ireland. Parasitology 146(6), 746–752. Ghozali, I. 2021. Aplikasi analisis multivariate dengan program IBM SPSS 26, 10th ed. Semarang, Indonesia: Diponegoro University. Kurnosova, O.P., Arisov, M.V. and Odoyevskaya, I.M. 2019. Intestinal parasites of pets and other house-kept animals in Moscow. Helminthologia 56(2), 108–117. Kurnosova, O.P. 2009. Parasitic diseases in domestic dogs and cats in the megalopolis of Moscow. Med. Parasitol. (Mosk) 4, 31–35. Ma, G., Holland, C.V., Wang, T., Hofmann, A., Fan, C.K., Maizels, R.M., Hotez, P.J. and Gasser, R.B. 2018. Human toxocariasis. Lancet Infect. Dis. 18, e14–24. Macpherson, C.N.L. 2013. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int. J. Parasitol. 43(12–13), 999–1008. Massetti, L., Colella, V., Zendejas, P.A., Ng-Nguyen, D., Harriott, L., Marwedel, L., Wiethoelter, A. and Traub, RJ. 2020. High-throughput multiplex QPCR for the surveillance of zoonotic spesies of canine hookworms. PLoS Negl. Trop. Dis. 15, 1–14. de Mello, C.C.S., Nizoli, L.Q., Ferraz, A., Chagas, B.C., Azario, W.J.D., Motta, S.P.D. and Villela, M.M. 2022. Soil contamination by Ancylostoma Spp. and Toxocara Spp. eggs in elementary school playgrounds in the extreme south of Brazil. Braz. J. Vet. Parasitol. 31(1), 1–5. Overgaauw, P.A.M. and van Knapen, F. 2013. Veterinary and public health aspects of toxocara Spp. Vet. Parasitol. 193(4), 398–403. Paul, M., King, L. and Carlin, E.P. 2010. Zoonoses of people and their pets: a US perspective on significant pet-associated parasitic diseases. Trends Parasitol. 26, 153–154. Phoosangwalthong, P., Luong, N.H., Wongwigkan, J., Kamyingkird, K., Phasuk, J., Pattanatanang, K., Thammasonthijarern, N., Kengradomkij, C., Chimnoi, W., Odermatt, P. and Inpankaew, T. 2022. Toxocara Canis and Toxocara Cati in Stray Dogs and Cats in Bangkok, Thailand: Molecular Prevalence and Risk Factors. Parasitologia 2(2), 88–94. Phosuk, I., Intapan, P.M., Thanchomnang, T., Sanpool, O., Janwan, P., Laummaunwai, P., Aamnart, W., Morakote, N. and Maleewong, W. 2013. Molecular detection of Ancylostoma duodenale, Ancylostoma ceylanicum, and necator americanus in humans in Northeastern and Southern Thailand. Korean J. Parasitol. 51(6), 747–749. Pumidonming, W., Salman, D., Gronsang, D., Abdelbaset, A.E., Sangkaeo, K., Kawazu, S.I. and Igarashi, M. 2016. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower northern Thailand. J. Vet. Med. Sci. 78(12), 1779–1784. Putri, N. and Wibowo, A. 2023. Rainfall maps for the suitability of settlement area in Bogor Raya. Enviro. Scienteae 19(2), 123. Reichert, F., Pilger, D. and Schuster, A. 2018. Epidemiology and morbidity of hookworm related cutaneous larva migrans (HrCLM): results of a cohort study over a period of six months in a resource-poor community in Manaus, Brazil. PLoS Negl. Trop. Dis. 12(7), 1–15. Ridwan, Y., Etih, S.T., Dewi, I.T. and Budiono, N.G. 2023. Gastrointestinal helminth parasites of pets: retrospective study at the Veterinary Teaching Hospital, IPB University, Bogor, Indonesia. Vet. World 16(5), 1043–1051. Rustiadi, E., Pribadi, D.O., Pravitasari, A.E., Indraprahasta, G.S. and Iman, L.S. 2015. Chapter 22 Jabodetabek megacity: from city development toward urban complex management system. In Urban development challenges, risks and resilience in Asian Mega Cities. Ed., Singh, R.B., Berlin, Germany: Springer, pp: 421–445. Sawitri, D.H., Wardhana, A.H., Martindah, E., Ekawasti, F., Dewi, D.A., Utomo, B.N., Shibahara, N., Kusumoto, M., Tokoro, M., Sasai, K. and Matsubayashi, M. 2019. Detections of gastrointestinal parasites, including giardia intestinalis and Cryptosporidium Spp., in Cattle of Banten Province, Indonesia. J. Parasit. Dis. 44(1), 174–179. Sears, W.J., Jorge, C., Joseph, K., Thomas, B.N. and Philip, J.C. 2022. Zoonotic Ancylostoma ceylanicum hookworm infections, Ecuador. Emergi. Infect. Dis. 28(9), 1867–1869. Thienpont, D., Rochette, F. and Vanparijs, O. 2003. Diagnosing helminthiasis through coprological examination, 3rd ed. Beerse, Belgium: Janssen Animal Health. Ursache, A.L., Adriana, G., Viorica, M., Dumitrache, M.O., Andrei, R. and Vasile, C. 2021. Toxocara cati and other parasitic enteropathogens: more commonly found in owned cats with gastrointestinal signs than in clinically healthy ones. Pathogens 10(198), 1–10. Vafae Eslahi, A., Olfatifar, M., Barikbin, F., Zaki, L. and Badri, M. 2022. Systematic review the global prevalence of diphyllobothrium in dogs, and cats: a systematic review and meta-analysis. J. Inflamm. Dis. 26(1), 43–56. Wolfe, A. and Wright, I. 2003. Human toxocariasis and direct contact with dogs. Vet. Rec. 152, 419–422. Wu, T. and Bowman, D.D. 2020. Chapter four - visceral larval migrans of toxocara canis and toxocara cati in non-canid and non-felid hosts. Adv. Parasitol. 109, 63–88. Wulcan, J.M., Michelle, M.D., Jennifer, K.K., Thomas, J.B. and Guilherme, G.V. 2019. Strongyloides Spp. in cats: a review of the literature and the first report of zoonotic strongyloides stercoralis in colonic epithelial nodular hyperplasia in cats. Parasit. Vectors 12(1), 1–12. Yang, Y. and Liang, H. 2015. Prevalence and risk factors of intestinal parasites in cats from China. Bio. Med. Res. Int. 2015, 1–5. Yavuzer, K., Muharrem, A. and Karadag, A.S. 2010. A case report of cutaneous larva migrans. Eurasian J. Med. 42(1), 40–41. | ||
| How to Cite this Article |
| Pubmed Style Sawitri DH, Wardhana AH, Nefho F, Purwanto ES, Endrawati D, Nugraheni YR, Primatika RA, Damayanti NA, Akbari RA, Kusumaningtyas E, Matsubayashi M. Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Vet. J.. 2024; 14(10): 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 Web Style Sawitri DH, Wardhana AH, Nefho F, Purwanto ES, Endrawati D, Nugraheni YR, Primatika RA, Damayanti NA, Akbari RA, Kusumaningtyas E, Matsubayashi M. Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. https://www.openveterinaryjournal.com/?mno=196036 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.5 AMA (American Medical Association) Style Sawitri DH, Wardhana AH, Nefho F, Purwanto ES, Endrawati D, Nugraheni YR, Primatika RA, Damayanti NA, Akbari RA, Kusumaningtyas E, Matsubayashi M. Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Vet. J.. 2024; 14(10): 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 Vancouver/ICMJE Style Sawitri DH, Wardhana AH, Nefho F, Purwanto ES, Endrawati D, Nugraheni YR, Primatika RA, Damayanti NA, Akbari RA, Kusumaningtyas E, Matsubayashi M. Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 Harvard Style Sawitri, D. H., Wardhana, . A. H., Nefho, . F., Purwanto, . E. S., Endrawati, . D., Nugraheni, . Y. R., Primatika, . R. A., Damayanti, . N. A., Akbari, . R. A., Kusumaningtyas, . E. & Matsubayashi, . M. (2024) Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Vet. J., 14 (10), 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 Turabian Style Sawitri, Dyah Haryuningtyas, April Hari Wardhana, Farlin Nefho, Eko Setyo Purwanto, Dwi Endrawati, Yudhi Ratna Nugraheni, Roza Azizah Primatika, Ndaru Andri Damayanti, Rizal Arifin Akbari, Eni Kusumaningtyas, and Makoto Matsubayashi. 2024. Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Veterinary Journal, 14 (10), 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 Chicago Style Sawitri, Dyah Haryuningtyas, April Hari Wardhana, Farlin Nefho, Eko Setyo Purwanto, Dwi Endrawati, Yudhi Ratna Nugraheni, Roza Azizah Primatika, Ndaru Andri Damayanti, Rizal Arifin Akbari, Eni Kusumaningtyas, and Makoto Matsubayashi. "Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia." Open Veterinary Journal 14 (2024), 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 MLA (The Modern Language Association) Style Sawitri, Dyah Haryuningtyas, April Hari Wardhana, Farlin Nefho, Eko Setyo Purwanto, Dwi Endrawati, Yudhi Ratna Nugraheni, Roza Azizah Primatika, Ndaru Andri Damayanti, Rizal Arifin Akbari, Eni Kusumaningtyas, and Makoto Matsubayashi. "Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia." Open Veterinary Journal 14.10 (2024), 2551-2563. Print. doi:10.5455/OVJ.2024.v14.i10.5 APA (American Psychological Association) Style Sawitri, D. H., Wardhana, . A. H., Nefho, . F., Purwanto, . E. S., Endrawati, . D., Nugraheni, . Y. R., Primatika, . R. A., Damayanti, . N. A., Akbari, . R. A., Kusumaningtyas, . E. & Matsubayashi, . M. (2024) Prevalence and risk factors associated with zoonotic gastrointestinal helminths transmitted by cats in Jabodetabek, Indonesia. Open Veterinary Journal, 14 (10), 2551-2563. doi:10.5455/OVJ.2024.v14.i10.5 |