| Research Article | ||
Open Vet. J.. 2024; 14(8): 1858-1865 Open Veterinary Journal, (2024), Vol. 14(8): 1858–1865 Research Article Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndromeYudit Oktanella*, Hana Ismiawati, Hafizh Zakaria and Handayu UntariFaculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia *Corresponding Author: Yudit Oktanella. Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia. Email: yudito [at] ub.ac.id Submitted: 22/04/2024 Accepted: 25/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
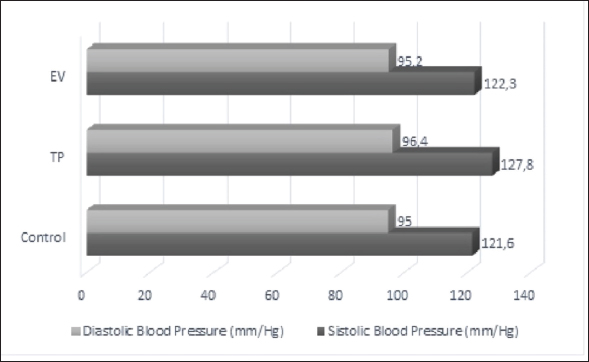
ABSTRACTBackground: Polycystic ovary syndrome (PCOS) is a hormonal disorder characterized by elevated androgen levels, heightened insulin secretion, and dysregulation of luteinizing hormone and follicle-stimulating hormone. This disorder results in metabolic disruptions, while the irregular estrous cycles associated with PCOS impact cellular functions like growth, movement, and alterations in cell adhesion within the tissue matrix. Aim: This study aims to identify the blood tension, serum malondialdehyde (MDA) levels, and serum Metalloproteinase-1 (MMP-1) in rat models of PCOS. The study was conducted using female Wistar rats aged 6 months weighing between 130 and 180 g. Methods: The rats were divided into three treatment groups: negative control, induction of testosterone propionate (TP) at a dose of 100 mg/kg BW IP for 12 days, and induction of estradiol valerate (EV) at a dose of 2 mg/kg BW IP for 2 days. Data were analyzed quantitatively using a one-way analysis of variance followed by a Posthoc Test using the least significant difference with a confidence level of 95%. Results: The research results indicate that the average blood pressure of TP Group and EV Group did not differ significantly from the negative control (p > 0.05). Serum MDA levels were significantly different in the TP Group compared to the negative control (p < 0.05). On the other hand, MMP-1 levels showed no significant difference (p > 0.05) among all the treatment groups. Conclusion: The findings of this study suggest that TP induction in a rat model of PCOS can potentially contribute to oxidative stress and lipid peroxidation, but does not significantly affect blood pressure or serum MMP-1 levels. Keywords: PCOS, Blood-pressure, Animal models, MMP-1, Malondialdehyde. IntroductionPoly cystic ovary syndrome (PCOS) is a globally widespread infertility case, affecting 6%–10% of women in their reproductive years (Chaudhary et al., 2021). The prevalence of PCOS among women of reproductive age is particularly high in various countries. For example, it reaches up to 33% in the UK, 24.6% in Greece, and as high as 45.7% in Indonesia (Panghiyangani et al., 2019; Hestiantoro and Pamungkas, 2020; Bulsara et al., 2021). As noted by Wulandari et al., (2018), insulin resistance (50%–70%) and endometrial hyperplasia (35%) are identified as common causes of PCOS. Clinical manifestations indicate that among a group of 300 women with PCOS, approximately 52% experienced amenorrhea, while around 28% had oligomenorrhea; hirsutism was evident in about 64%, and roughly 35 % were obese. In the context of PCOS, dysfunction in ovarian tissue remodeling and follicular growth has been identified as contributing factors (Smith et al., 2002). In order to understand the pathogenesis and progression of PCOS, it is crucial to investigate the role of matrix metalloproteinases (MMP) in this condition (Zhou et al., 2017). PCOS is a reproductive disorder affecting the endocrine system in women of reproductive age. It is marked by anovulation and high androgen levels. Polycystic ovary syndrome (PCOS) is associated with increased androgen levels and oxidative stress. The elevated androgens can negatively affect the cardiovascular system, leading to conditions such as hypertension by activating the vasoconstrictor Endothelin-1. This process starts with androgens binding to the α1-androgen receptor, stimulating endothelial cells in blood vessels to produce Endothelin-1 as a vasoconstrictor. The presence of Endothelin-1 causes constriction of blood vessels, which hinders blood flow and consequently elevates blood pressure. Constriction of blood vessels can also lead to oxidative stress (Lilyasari, 2007). PCOS can lead to long-term reproductive health issues and cardiovascular problems like increased blood pressure (Chaudhary et al., 2021). Polycystic ovarian syndrome is a common endocrine disorder characterized by chronic hyperandrogenic anovulation, resulting in symptoms such as hirsutism, acne, irregular menstrual cycles, and infertility (Panico et al., 2017). One of the potential mechanisms involved in the pathogenesis of PCOS is an imbalance in MMP activity. MMPs are enzyme molecules involved in the breakdown of extracellular matrix (ECM) components (Rodgers et al., 1994). These enzymes have been found to be dysregulated in women with PCOS, leading to abnormalities in ovarian tissue remodeling and follicular growth (Goldman and Shalev, 2003). Metalloproteinase-1 (MMP-1’s) activity on the ECM affects fundamental cellular processes such as proliferation, differentiation, migration, and changes in cell-matrix adhesion (Gaffney et al., 2015). Therefore, understanding the regulation and activity of MMP-1 in PCOS can provide valuable insights into the pathogenesis of the disease and potential therapeutic targets. Furthermore, hyperandrogenism in PCOS condition causes cardiovascular system disturbances such as hypertension through the activation of the androgen vasoconstrictor, Endothelin-1 (Chistiakov et al., 2018). The active mechanism of Endothelin-1 begins with androgens binding to the androgen receptor, specifically the α1-androgen receptor. Androgen binding to the α1-androgen receptor stimulates endothelial cells to activate Endothelin-1 as an androgen vasoconstrictor. The presence of Endothelin-1 vasoconstrictors leads to blood vessel constriction, which hampers blood flow and results in increased blood pressure (Pecci et al., 1993). Blood vessel constriction can cause oxidative stress conditions to occur (Sena et al., 2018). Oxidative stress arises from an imbalance between reactive oxygen species (ROS) production and antioxidants in the body. ROS are free radicals and have toxic properties to cells. Free radicals cause lipid peroxidation processes in the body. Lipid peroxidation happens in cell membranes, especially in unsaturated fatty acids, and MDA can be determined as the end product of lipid peroxidation. Therefore, measuring MDA levels can be used as an indicator of oxidative stress. Animal models play a crucial role in studying various aspects of reproductive diseases, including their etiology, pathogenesis, and potential treatments. These models offer valuable perspectives on the mechanisms that drive reproductive diseases, enable the evaluation of potential treatments, and aid researchers in gaining a deeper comprehension of disease advancement and its effect on fertility and reproductive results. Materials and MethodsThis research has been approved by the research ethics commission of Brawijaya University with ethics number 090-KEP-UB-2022. The work was carried out in several laboratories at Brawijaya University: the Animal Experimentation Laboratory for maintaining and inducing animal models, the Veterinary Anatomy Laboratory for conducting necropsies and serum sampling on experimental animals, and the Animal Disease Diagnostic Laboratory for measuring blood pressure, serum MDA, and ELISA MMP-1. Preparing and generating animal model of PCOS18 female Wistar strain rats aged 6–8 months and weighing between 130–180 g were acclimatized for about 7 days prior to the treatment, allowing them to adjust to their new surroundings. Throughout the study, the rats had unrestricted access to food and water, with feeding taking place twice daily in the morning and evening. The housing of the animals was arranged according to their respective treatment groups. Testosterone propionate (TP) was administered at a dosage of 100 mg/kg BW, with a volume of 0.13 ml per tail, for a duration of 12 days. Estradiol valerate (EV) was administered at a dosage of 2 mg/kg BW, with a volume of 0.13 ml per head, over the course of 2 days. TP was administered at 100 mg/kg BW in group P1 for 12 days. EV was administered at 2 mg/kg BW in group P2 for 2 days. This method is also mentioned in Oktanella et al, (2023). EV is employed to induce PCOS in animal models through a modification of the method described by Venegas et al. (2019) where 2 mg of EV diluted with sesame oil is administered for a period of 60 days. Similarly, the induction of PCOS using TP follows a modified protocol based on Siahaan et al. (2022) involving the intraperitoneal administration of 100 mg/kg BW TP for 21 days. The vaginal swab method was used to examine the estrous cycle. A cotton swab moistened with NaCl 0.9% was inserted into the vulva up to the part of the vagina adjacent to the cervix and then moved in a circular motion to collect epithelial cells. The collected cells were applied onto a glass slide, stained with methylene blue dye, and observed under a microscope at 400x–1,000x magnification to identify different stages of the estrous cycle. Blood pressure measurementBlood pressure was measured using the Tail cuff method and the CODA™ Non-invasive blood pressure system, USA. The device was activated 30 minutes prior to use, and a medium-sized cuff was chosen and connected to the occlusion cuff and VPR cuff. Subsequently, the rat was placed in a handling and restraint device equipped for rats weighing between 75 g and 200 g. After measuring the rat’s temperature, the occlusion cuff and VPR cuff were fastened to its tail for blood pressure measurements on the last day of TP and EV induction. Euthanizing experimental animals and obtaining blood samplesThe rats were first given a combination of Ketamine-Xylazine for anesthesia, with a dose of 70 mg/kg BW Ketamine and 15 mg/kg BW Xylazine. This was injected intramuscularly (i.m.) at a volume of 0.2 ml per rat. The anesthetized rats were then euthanized using the cervical dislocation method, which involved placing each rat dorsal side down on a surgical board and making an incision to expose the thoracic and abdominal areas before collecting blood samples intracardially using a syringe, totaling 3 ml. Serum preparationThe blood specimens were gathered and preserved in crimson Vacutainer tubes. Following collection, the blood samples underwent centrifugation at a speed of 6,000 rpm for 15 minutes to partition the liquid component from the solid part of the blood cells, yielding serum samples. Subsequently, the obtained serum specimens were transferred into 1.5 ml microtubes, with each sample receiving 100 µl. These samples were then frozen at −20°C prior to performing the MDA assay and ELISA. The measurement of MDA levels was conducted using the Thiobarbituric Acid (TBA) methodThe serum samples were extracted from the 1.5 ml microtubes using a micropipette to draw out 100 µl of each. Afterward, 1 ml of cold 0.9% NaCl solution was introduced into every serum sample. Subsequently, the samples underwent centrifugation at 8,000 rpm for a duration of 20 minutes. Following this process, 100 µl of the supernatant from each specimen was moved to a fresh microtube and combined with 550 µl of aquades and then mixed with an addition of 100 µl of 10% Trichloroacetic acid through homogenization using a vortex agitator. Subsequently, 250 µl of 1 N HCl and 100 µl of 1% Na-Thio were added to each sample, and the serum samples were homogenized again using a vortex. The samples were centrifuged for 10 minutes at 500 rpm, and the supernatant was transferred to a new microtube using a micropipette. The serum samples were heated in a water bath at 100°C for 20–30 minutes and then cooled to room temperature. The absorbance of the serum samples was measured using a microplate reader (iMark™ Microplate Absorbance Reader, USA) at a wavelength of 532 nm. The measurement of MMP-1 serum using ELISAThe MMP-1 analysis was conducted utilizing the sandwich ELISA technique with the Rat MMP-1 ELISA Kit from Bioassay Technology Laboratory, Shanghai Korain Biotech Co Ltd, China. The necessary number of strips was prepared and placed in the frame. Next, 40 µl of serum sample was added to each well along with 10 µl of anti-MMP-1 antibody. Then, 50 µl of streptavidin-HRP was added to each well, mixed thoroughly, and incubated for 60 minutes at 37°C. After the incubation period, the sealer was removed and the wells were washed five times using a wash buffer. Subsequently, each well was soaked with 300 µl of wash buffer for a duration ranging from 30 seconds to 1 minute per washing cycle. Following this step, both substrate solutions A (50 µl) and B (50 µl) were added to each well and left to incubate for 10 minutes at 37°C in darkness. Finally, 50 µl of stop solution was added to each well, and the OD value was read using a microplate reader at a wavelength of 450 nm after 10 minutes. Data analysisThe study examined the parameters of blood pressure, MDA levels, and serum MMP-1 using a one-way analysis of variance at a 95% confidence level (α=0.05) to identify variations between treatment groups. A Post Hoc Test with the Least Significant Difference was then conducted to ascertain the most effective treatment. The Statistical Program for Social Science Version 2.9 for Windows was employed for quantitative data analysis. ResultsPCOS induction using TP and EVThe rats in the TP group exhibited irregular estrous cycles, characterized by persistent phases of both estrus and diestrus upon vaginal swabbing. The persistent estrus phase lasted for more than 12 hours, while the persistent diestrus phase lasted for more than 57 hours. These findings are consistent with the study conducted by Santoso et al., (2013), which stated that PCOS model rats induced with 100 mg/kg BW TP on day 15 exhibited all rats in the diestrus phase. Stener-Victorin et al. (2000) found that irregular estrous cycles in PCOS model rats can disrupt ovulation, resulting in inhibited ovulation processes. Meanwhile, the rats in the EV group showed continued estrus when vaginal swabs were performed. The findings align with the research of Osuka et al., (2019) which demonstrated that PCOS model rats, when induced with 2 mg/kg BW EV after 2 days, exhibited continuous estrus in all subjects. This supports the conclusions of Mardika et al., (2018) who described how elevated estrogen levels during the estrus phase lead to reduced FSH and increased LH levels. The ELISA levels of testosterone in rats from the group induced with propionate were measured at 423.09 ng/l, whereas rats from the group induced with valerate showed a level of 482.32 ng/l. Typically, normal ELISA testosterone levels in female white rats fall within the range of 200–400 ng/l. The data obtained from vaginal swabs and ELISA testosterone levels indicate that rats subjected to propionate induction display symptoms consistent with PCOS, such as irregular estrous cycles and elevated testosterone levels, while those exposed to valerate exhibit persistent estrus and increased testosterone levels. Overall, the data suggests that the administration of TP and EV in rats can induce symptoms of PCOS, including irregular estrous cycles and elevated testosterone levels. Blood pressureFigure 1 shows that induction with TP and EV does not lead to a significant increase in blood pressure. The systolic and diastolic blood pressure in the TP and EV induction groups remains within the normal range based on data from white rat blood pressure, which is reported to have a systolic value of 129 mmHg and a diastolic value of 90 mmHg.
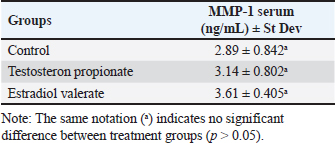
Fig. 1. The average systolic and diastolic blood pressure in several treatment groups. Serum MDA levels using ELISABased on Table 1, it appears that there are significant differences in serum MDA levels between the treatment groups, as indicated by the various notations (a, b) in the Table. These notations suggest that the differences in MDA levels between certain treatment groups are statistically significant (p < 0.05). The TP group has significantly higher MDA levels compared to the control group (p < 0.05), nonetheless, the EV group has significantly lower MDA levels compared to both the Control and TP group. The results of serum MMP-1 level measurements indicate that the TP groups and EV group show no significant difference (p > 0.05) compared to the control group (Table 2). All the treatment groups have an average serum MMP-1 level ranging from 2,89–3,61 ng/ml, which is still within the normal range. According to Masuhara et al., (2002), the normal serum MMP-1 levels range from 2,2 to 22,9 ng/ml. The presence of MMP-1 in control rats is related to the active regulation of MMP function by globulin and Tissue Inhibitors of Metalloproteinase (TIMP). Physiologically, MMP-1 activity is inhibited by TIMP and modulated by various hormones, growth factors, and cytokines. Loss of MMP activity control can lead to abnormalities such as cyst formation, inflammation, and cancer (Visse and Nagase, 2003). DiscussionIn patients with PCOS, increased blood pressure is influenced by factors such as genetics and high levels of androgens. Genetic factors play a crucial role in elevated blood pressure in these individuals (Scicchitano et al., 2012). Patients with inherited hypertension are at a greater risk of elevated blood pressure in comparison to individuals without hereditary hypertension (Duică et al., 2021). Additionally, factors such as high levels of androgens or hyperandrogenism result in increased blood pressure through the mechanism of vasoconstriction of blood vessels. Hyperandrogenism leads to increased blood pressure through the activation of the androgen vasoconstrictor, Endothelin-1 (Dubey et al., 2002). The active mechanism of Endothelin-1 starts with androgens binding to the androgen receptor, α1-Androgen Receptor. The binding of androgens to the α1-Androgen Receptor stimulates endothelial cells of blood vessels to activate Endothelin-1 as an androgen vasoconstrictor. The presence of the vasoconstrictor Endothelin-1 causes the narrowing of blood vessels, resulting in inhibited blood flow and increased blood pressure (Lilyasari, 2007). Table 1. Average serum MDA levels in several treatment groups.
Table 2. Serum MMP-1 levels in treatment groups.
However, our research findings regarding the induction of PCOS animal models with TP and EV show that both compounds do not affect the increase in blood pressure. This is evidenced by the average blood pressure measurements in all groups remaining within normal limits. Additionally, further analysis revealed no significant changes in other cardiovascular parameters such as heart rate and arterial stiffness. The induction group administered with TP at a dosage of 100 mg/kg BW exhibited a significant difference (p < 0.05) compared to the negative control group, leading to an increase in MDA levels attributed to oxidative stress induced by exposure to TP. According to Zhang et al., (2009), TP induction can increase MDA levels by inducing lipid peroxidation and reducing antioxidant activity. Based on Serrano et al., (2018), TP induction at a dose of 100 mg/kg BW causes glucose intolerance, dyslipidemia, and increased oxidative stress levels, which potentially lead to lipid peroxidation, ROS toxicity, and decreased antioxidant function. TP has a half-life of about 33 hours, allowing it to reach the required therapeutic concentration. It can remain in the body for up to 5 days. The longer ester group structure in TP leads to a longer half-life, allowing TP to remain in the bloodstream for an extended period (Turza et al., 2022). The EV induction group at a dose of 2 mg/kg BW showed no significant difference (p > 0.05) compared to the negative control group. EV has a short half-life of about 11–14 hours (Ndefo and Mosely, 2010). Fatty acids containing longer ester groups exhibit higher reactivity to lipid peroxidation compared to those with shorter carbon chains. This is due to the presence of a greater number of double bonds in fatty acids, which can promote the generation of free radicals within cell membranes and enhance their susceptibility to oxidation (Wu et al., 2015). High levels of free radicals in the body can be indicated by low antioxidant enzyme activity and high MDA levels. The higher the MDA level, the more tissue damage and free radical production occur (Mulianto, 2020). Serum MDA concentrations rose in the group that received TP induction at a dosage of 100 mg/kg BW, as a result of its extended half-life, which enables TP to persist in the bloodstream for an extended period. MMPs are a group of zinc-dependent endopeptidases that play a crucial role in proteolytic activity towards ECM components. They are involved in ovarian component division, growth factor release, ECM degradation, and tissue remodeling. MMP-1, specifically, is a collagenase enzyme responsible for degrading the ECM in endothelial cells (Kessenbrock et al., 2010). In ovarian follicles, MMP-1 is localized in granulosa and theca cells, while in the kidneys, it can be found in the glomerulus. MMP-1 influences the pathogenesis of diseases, particularly through inflammatory processes (Wang and Khalil, 2018). PCOS causes irregularities in the reproductive estrous cycle of women, affecting ovarian organs and tissue remodeling during follicle growth, maturation, and ovulation. According to Masuhara et al. (2002), the normal serum MMP-1 level ranges from 2.2 to 22.9 ng/ml. The normal serum MMP-1 levels found in all treatment groups are related to the physiological function of MMP regulated by globulin and TIMP. Physiologically, MMP-1 activity is inhibited by TIMP and modulated by various hormones, growth factors, and cytokines. Loss of MMP activity control can lead to abnormalities such as the formation of cysts, inflammation, and cancer (Visse and Nagase, 2003). Androgen-stimulated collagenase activity (in this case, TP) can release Vascular Endothelial Growth Factor (VEGF) bound to the matrix and angiogenesis factors. According to Eisermann et al. (2013), the angiogenic release process in stimulated tissue production of VEGF is followed by MMP release. MMP mediates to accelerate and provide potential angiogenic responses to testosterone (Kim et al., 2008). Increased MMP-1 levels can be caused by several hormonal profiles including progesterone, glucocorticoids, and androgenic activity. Hormonal imbalance is one of the factors increasing MMP levels in the body (Mardika et al., 2018). The role of ECM enzymes begins after a surge in LH in response to the accumulation of estrogen increases, initiating changes in a number of intraovarian regulatory systems that react, leading to ECM degradation in follicles, increased permeability, and increased blood flow. Increased permeability and blood flow stimulate vascular changes and increase intrafollicular pressure. LH plays a role as the final trigger after the follicle wall degradation process that occurs since the early estrus phase (Shaherawal and Jadav, 2023). PCOS is also characterized by persistent estrus for more than 12 hours and an increase in estrogen hormones. Estradiol plays a crucial role in regulating collagen metabolism. Estrogen increases fibroblast proliferation, expression of Fibroblast associated protein, ECM, and growth factors (Luo et al., 2014). This affects collagen metabolism by stimulating collagen degradation and activating increased Matrix MMP-1 activity. The increase in MMP and the decrease in TIMP by estrogen also result in increased ECM damage (Moalli et al., 2004). Additionally, several factors can cause an increase in MMP-1 levels including hormonal imbalance in the body, oxidative stress, ROS, proinflammatory cytokines, and increased TGF-β (Tency et al., 2012). EV is utilized to induce hormonal alterations from typical conditions in order to generate PCOS animal models. Exposure to EV in rats leads to disruptions in the reproductive cycle, lack of ovulation, and the presence of polycystic ovaries, suggesting a rise in atretic follicles (Mirabolghasemi and Kamyab, 2017). The PCOS condition induced by EV in rats is similar to cases of PCOS in humans. This similarity is also supported by the consistent reproductive cycle with persistent estrus phases and uric acid being one of the metabolic disorder indicators in PCOS (Oktanella et al., 2023). EV has a half-life of about 11–14 hours and also provides a faster onset of PCOS conditions (Ndefo and Mosely, 2010). In conclusion, the findings discussed highlight the intricate interplay between hormonal imbalances, oxidative stress, and the pathogenesis of PCOS. The induction of PCOS animal models using TP and EV demonstrated distinct effects on serum markers such as MMP-1 and malondialdehyde (MDA). TP induction resulted in a significant rise in MDA levels, indicating increased oxidative stress possibly caused by testosterone-induced lipid peroxidation and reduced antioxidant activity. The extended half-life of TP may also contribute to prolonged oxidative stress and tissue damage. EV induction did not significantly affect MDA levels, indicating potential compensatory mechanisms by renal cells to combat ROS formation. Despite the lack of a significant increase in MDA, the EV-induced PCOS model showed irregular reproductive cycles and polycystic ovaries similar to human PCOS manifestations, highlighting its efficacy as an experimental model. Moreover, the dysregulation of MMP-1 levels observed in both induction models underscores the involvement of ECM remodeling in PCOS pathogenesis. Hormonal fluctuations, particularly estrogen and androgen levels, play pivotal roles in modulating MMP-1 activity, which in turn impacts follicular development and tissue integrity. Overall, these findings shed light on the multifaceted mechanisms underlying PCOS development and provide valuable insights into potential therapeutic targets for managing this complex endocrine disorder. Further research elucidating the intricate molecular pathways involved in PCOS pathophysiology is warranted to advance our understanding and improve clinical management strategies for affected individuals. AcknowledgmentThe authors gratefully acknowledge the financial support provided by internal funding from Universitas Brawijaya for the year 2024. This support facilitated the completion of the research variables reported in this article. FundingThe financial support is provided by internal funding from Universitas Brawijaya for the year 2024. Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Authors’ contributionsConceptualization: YO; methodology: YO, HU; software and validation, HU, HZ; data curation: HZ, HU; writing—original draft preparation: YO, HI; writing—review and editing: YO, HU. All authors have read and agreed to the published version of the manuscript. Data availabilityThe data used to support the findings of this study are included within the article and no additional data sources are required. ReferencesBulsara, J., Patel, P., Soni, A. and Acharya, S. 2021. A review: brief insight into polycystic ovarian syndrome. Endocrine Metab. Sci. 3, 100085. Chaudhary, H., Patel, J., Jain, N.K. and Joshi R. 2021. The role of polymorphism in various potential genes on polycystic ovary syndrome susceptibility and pathogenesis. J. Ovarian Res. 14, 1–21. Chistiakov, D.A., Myasoedova, V.A., Melnichenko, A.А., Grechko, A.V. and Orekhov, A.N. 2018. Role of androgens in cardiovascular pathology. Vasc. Health. Risk. Manag. 14, 283–290. Dubey, R.K., Oparil, S., Imthurn, B. and Jackson, E.K. 2002. Sex hormones and hypertension. Cardiovasc. Res. 53, 688–708. Duică, F., Dănilă, C.A., Boboc, A.E., Antoniadis, P., Condrat, C.E., Onciul, S., Suciu, N., Crețoiu, S.M., Varlas, V. and Creţoiu, D. 2021. Impact of increased oxidative stress on cardiovascular diseases in women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne). 12, 614679. Eisermann, K., Broderick, C.J., Bazarov, A., Moazam, M.M. and Fraizer, G.C. 2013. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol Cancer 12, 7. Gaffney, J., Solomonov, I., Zehorai, E., and Sagi, I. 2015. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 44-46, 191–199. Goldman, S., and Shalev, E. 2003. The role of the matrix metalloproteinases in human endometrial and ovarian cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 11, 109–121. Hestiantoro, A. and Pamungkas, D.T. 2020. Assessment of the quality of internet-based health information in the Indonesian language about polycystic ovarian syndrome. Indonesian J. Obstetrics Gynecol. 8, 222–227. Shaherawala, J.G. and Jadav, P.M. 2023. A study of follicle stimulating hormone (FSH) and luteinizing hormone (LH) in women of polycystic ovarian disease (PCOD) at Tertiary Care Hospital of Gujarat, India. Int. J. Clin. Biochem. Res. 5, 112–115. Kessenbrock, K., Plaks, V. and Werb, Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67. Kim, D.H., Lilliehook, C., Roides, B., Chen, Z., Chang, M., Mobashery, S. and Goldman, S. A. 2008. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronal addition to the adult songbird brain. J. Neurosci. 28, 208–216. Lilyasari, O., 2007. Hypertension with obesity: is there a role for endothelin-1? Indonesian J. Cardiol. 2, 460–475. Luo, N., Guan, Q., Zheng, L., Qu, X., Dai, H. and Cheng, Z. 2014. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Transl. Res. 163, 232–241. Mardika, K., Setyawati, I. and Darmadi, A. 2018. Length of estrus cycle and histological structure of white rat ovaries after administration of ethanol extract of red calliandra leaves. J. Vet. 19, 342–350. Masuhara, K., Nakai, T., Yamaguchi, K., Yamasaki, S. and Sasaguri, Y. 2002. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 46, 2625–2631. Mirabolghasemi, G. and Kamyab, Z. 2017. Changes of The uterine tissue in rats with polycystic ovary syndrome induced by estradiol valerate. Int. J. Fertil. Steril. 11, 47–55. Moalli, P.A., Talarico, L.C., Sung, V.W., Klingensmith, W.L., Shand, S.H., Meyn, L.A. and Watkins, S.C. 2004. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am. J. Obstet. Gynecol. 190, 620–627. Mulianto, N. 2020. Malondialdehyde as a marker of oxidative stress in various skin diseases. Cermin Dunia Kedokteran 47, 39–44. Ndefo, U.A., and Mosely, N. 2010. Estradiol valerate and estradiol valerate/dienogest (natazia) tablets: the first four-phasic oral contraceptive. P. T. 35, 614–617. Oktanella, Y., Untari, H., Wuragil, D.K., Ismiawati, H., Hasanah, N.A., Agustina, G.C. and Pratama, D.A.O. 2023. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet. J. 13, 1003–1011. Osuka, S., Nakanishi, N., Murase, T., Nakamura, T., Goto, M., Iwase, A. and Kikkawa, F. 2019. Animal models of polycystic ovary syndrome: a review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod. Med. Biol. 18, 151–160. Panghiyangani, R., Kurniati, M., Soeharso, P., Andrijono, A., Suryandari, D.A. and Wiweko, B. 2019. FSH receptor gene polymorphism in Indonesian women with polycystic ovarian syndrome (PCOS). J. Physics (Conference Series) 1374, 012045. Panico, A., Messina, G., Lupoli, G.A., Lupoli, R., Cacciapuoti, M., Moscatelli, F., Esposito, T., Villano, I., Valenzano, A., Monda, V., Messina, A., Precenzano, F., Monda, M. and Lupoli, G. 2017. Quality of life in overweight (obese) and normal-weight women with polycystic ovary syndrome. Patient Prefer Adherence. 11, 423–429. Pecci, A., Gómez-Sánchez, C.E., Bedners, M.E.D., Lantos, C.P. and Cozza, E.N. 1993. In vivo stimulation of aldosterone biosynthesis by endothelin: Loci of action and effects of doses and infusion rate. J. Steroid Biochem. Mol. Biol. 45, 555–561. Rodgers, W.H., Matrisian, L.M., Giudice, L.C., Dsupin, B.A., Cannon, P.J., Svitek, C., Gorstein, F. and Osteen, K.G. 1994. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J. Clin. Invest. 94, 946–953. Santoso, B., Sulistyono, A., Widjiati. 2013. Effectiveness of rat bone marrow stem cell therapy in rat model of poly cystic ovary syndrome on folliculogenesis and transforming growth factor-β expression. Majalah Obstetri Ginekologi 21, 115–120. Scicchitano, P., Dentamaro, I., Carbonara, R., Bulzis, G., Dachille, A., Caputo, P., Riccardi, R., Locorotondo, M., Mandurino, C. and Ciccone, M M. 2012. Cardiovascular risk in women with PCOS. Int. J. Endocrinol. Metab. 10, 611–618. Sena, C.M., Leandro, A., Azul, L., Sei, R., and Perry, G. 2018. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 9, 1668. Serrano, Lady, Bridi, A., Della Mea, R., Braga Rissi, V., dos Santos Guarda, N., Moresco, R.N., Premaor, M.O., Antoniazzi, A.Q., Gonçalves, P.B.D. and Comim, F.V. 2018. Oxidative stress and metabolic markers in pre- and postnatal polycystic ovary syndrome rat protocols. J. Inflamm. Res. 11, 193–202. Siahaan, S., Santoso, B. and Widjiati. W. 2022. Effectiveness of Moringa oleifera Leaves on TNF-α Expression, Insulin Levels, Glucose Levels and Follicle Count in Rattus norvegicus PCOS Model. Diabetes Metab. Syndr. Obes. Targets Ther. 15(2), 3255–3270. Smith, M.F., Ricke, W.A., Bakke, L.J., Dow, M.P. and Smith, G.W. 2002. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol. Cell Endocrinol. 191, 45–56. Stener-Victorin, E.P., Kirsty, V.A., Walters, R.E., Campbell, A., Benrick, P., Giacobini, D., Dumesic, A. and Abbott, D.H. 2000. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr. Rev. 41(4), 1–39. Tency, I., Verstraelen, H., Kroes, I., Holtappels, G., Verhasselt, B., Vaneechoutte, M. and Temmerman, M. 2012. Imbalances between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) in maternal serum during preterm labor. PLoS One 7(11), e49042. Turza, A., Popescu, V., Mare, L., and Borodi, G. 2022. Structural aspects and intermolecular energy for some short testosterone esters. Materials (Basel), 15, 7245. Venegas, B., De León Gordillo, L.Y., Rosas, G., Espinoza, J.A., Morán, C., Domínguez, R. and Morales-Ledesma, L. 2019. In rats with estradiol valerate-induced polycystic ovary syndrome, the acute blockade of ovarian β-adrenoreceptors improve ovulation. Reprod. Biol. Endocrinol. 17(1), 95. Visse, R. and Nagase, H. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839. Wang, X. and Khalil, R. A. 2018. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 81, 241–330. Wu, X., Guan, Y., Yan, J., Liu, M., Yin, Y., Duan, J., Wei, G., Hu, T., Weng, Y., Xi, M. and Wen, A. 2015. ShenKang injection suppresses kidney fibrosis and oxidative stress via transforming growth factor-β/Smad3 signalling pathway. J. Pharm. Pharmacol. 67(8), 1054–1065. Wulandari, L.P., Santoso, B. and Purwanto, B. 2018. Malondialdehyde levels in polycystic ovary syndrome rat model using moringa leaves (Moringa oleifera). J. Biosains Pascasarjana 19, 224. Zhang, W., Sun, K., Yang, Y., Zhang, H., Hu, F.B. and Hui, R. 2009. Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clin. Chem. 55, 2026–2034. Zhou, F., Shi, L. and Zhang, S. 2017. Ovarian fibrosis. Chin. Med. J. 130, 365–371. | ||
| How to Cite this Article |
| Pubmed Style Oktanella Y, Ismiawati H, Zakaria H, Untari H. Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Vet. J.. 2024; 14(8): 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 Web Style Oktanella Y, Ismiawati H, Zakaria H, Untari H. Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. https://www.openveterinaryjournal.com/?mno=197884 [Access: January 26, 2026]. doi:10.5455/OVJ.2024.v14.i8.14 AMA (American Medical Association) Style Oktanella Y, Ismiawati H, Zakaria H, Untari H. Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Vet. J.. 2024; 14(8): 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 Vancouver/ICMJE Style Oktanella Y, Ismiawati H, Zakaria H, Untari H. Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Vet. J.. (2024), [cited January 26, 2026]; 14(8): 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 Harvard Style Oktanella, Y., Ismiawati, . H., Zakaria, . H. & Untari, . H. (2024) Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Vet. J., 14 (8), 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 Turabian Style Oktanella, Yudit, Hana Ismiawati, Hafizh Zakaria, and Handayu Untari. 2024. Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Veterinary Journal, 14 (8), 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 Chicago Style Oktanella, Yudit, Hana Ismiawati, Hafizh Zakaria, and Handayu Untari. "Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome." Open Veterinary Journal 14 (2024), 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 MLA (The Modern Language Association) Style Oktanella, Yudit, Hana Ismiawati, Hafizh Zakaria, and Handayu Untari. "Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome." Open Veterinary Journal 14.8 (2024), 1858-1865. Print. doi:10.5455/OVJ.2024.v14.i8.14 APA (American Psychological Association) Style Oktanella, Y., Ismiawati, . H., Zakaria, . H. & Untari, . H. (2024) Exploring blood pressure dynamics and oxidative stress markers in an animal model of polycystic ovary syndrome. Open Veterinary Journal, 14 (8), 1858-1865. doi:10.5455/OVJ.2024.v14.i8.14 |