| Research Article | ||
Open Vet. J.. 2024; 14(7): 1614-1624 Open Veterinary Journal, (2024), Vol. 14(7): 1614–1624 Research Article Influence of azathioprine on healing of exposed dogs’ dental pulp capped with three different materialsMokhtar A. Al-Anesi1, Ashraf M. Abu-Seida2,3*, Ehab S. Abd-Elhamid4, Abeer H. Mahran4, Salma H. El Ashry4, Mohamed H. Issa5 and Mohamed M. Nagy3,41Department of Endodontic, Faculty of Dentistry, Thamar University, Dhamar, Yemen 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 3Faculty of Dentistry, Galala University, New Galala City, Suez, Egypt 4Faculty of Dentistry, Ain Shams University, Cairo, Egypt 5Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, University of Tripoli, Tripoli, Libya *Corresponding Author: Ashraf M. Abu-Seida. Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Email: ashrafseida [at] cu.edu.eg Submitted: 16/04/2024 Accepted: 08/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Azathioprine is one of the earliest immunosuppressants prescribed for several autoimmune diseases. Yet there is a lack of research on the impact of azathioprine on pulp healing following the pulp capping procedure. Aim: This study aimed to investigate the effect of azathioprine on the healing ability of mechanically exposed dogs’ dental pulps following direct pulp capping with mineral trioxide aggregate (MTA), bio-aggregates (BA), and Calcium hydroxide (Ca(OH)2). Methods: Four mongrel dogs were randomly assigned to two groups (two dogs/30 teeth in each group): immunosuppressed (group I) and control (group II). Group I received azathioprine for two months before surgical treatments and until the dogs were euthanized. Fifteen class V buccal cavities were performed in each dog. Each group was randomly divided into three subgroups (10 teeth each) based on the pulp capping substance. The pulps in subgroups A, B, and C were immediately capped with MTA, BA, and Ca(OH)2, respectively. Inflammation and dentine bridge development were histopathologically evaluated and scored at one and two months. The data were statistically analyzed. Results: The immunosuppressed group exhibited statistically greater inflammatory cell count and decreased dentine bridge thickness, compared to the control group in all subgroups (p < 0.05). Conclusion: Azathioprine has an adverse effect on the healing of exposed dogs’ dental pulp following direct pulp capping with MTA, BA, and Ca(OH)2. Therefore, patients using azathioprine as an immunosuppressive medication may experience delayed healing of mechanically exposed pulps following capping with MTA, BA, or Ca(OH)2. Keywords: Bio-Aggregates, Calcium hydroxide, Dentine bridge, Immunosuppression, Mineral trioxide aggregate. IntroductionSeveral immunosuppressive medications are administered for several medical conditions, including, but not limited to, autoimmune diseases and organ transplantation. However, the effect of immune suppression is not restricted to a single organ or tissue but rather affects the entire body, including the tooth pulp (Evans, 2004). Up to 40% of immunosuppressed patients experience ulcerative, hemorrhagic, or infectious oral issues as a result of direct or indirect stomatotoxicity (Patel et al., 2006). Furthermore, immunosuppressants have been associated with abnormal secondary dentine growth, pulpal lesions, pulp chamber constriction, increased periapical bone deterioration, and delayed pulpal tissue regeneration (Näsström et al., 1985; Elion, 1993; Oh et al., 2008; Mahmoud et al., 2010; Soliman et al., 2022). Azathioprine is one of the first drugs to be used in immunosuppressive therapy. It is frequently recommended for rheumatoid arthritis, pemphigus, systemic lupus erythematosus, Behçet’s illness, autoimmune hepatitis, atopic dermatitis, myasthenia gravis, neuromyelitis optica (Devic’s disease), restrictive lung disease, post-transplantation, and leukemia (Ayanoglou et al., 1997; Cara et al., 2004; Evans, 2004). Azathioprine has several serious side effects, including bone marrow suppression (leukopenia, thrombocytopenia, and anemia), an increased risk of infection, and excessive bleeding. Long-term azathioprine treatment may potentially cause the formation of malignant lesions (Lopes et al., 1997). Calcium hydroxide (Ca(OH)2) is still widely used for direct and indirect pulp capping. However, it has substantial disadvantages, including its degradation over time, the formation of tunnel flaws in freshly created dentine bridges, and inadequate sealing characteristics (El Ashry et al., 2013). As a result, additional pulp capping materials, such as calcium silicate-based compounds and bio-ceramic aggregates, entered the market and outperformed calcium hydroxide (Al Ashry et al., 2016; Negm et al., 2016; Abo El-Mal et al., 2019, 2021; Kunert and Lukomska-Szymanska, 2020; Al-Sherbiny et al., 2021; Mohamed et al., 2022; Elkhashab et al., 2023, 2024; Mohamed et al., 2023). The current study examined the effect of azathioprine on pulpal tissue healing following direct pulp capping in mechanically exposed dog teeth utilizing mineral trioxide aggregates (MTAs), bio-aggregates (BA), and calcium hydroxide (Ca(OH)2). We expected that azathioprine may delay pulp healing following pulp capping as regards dentin bridge formation and pulp tissue inflammation. Materials and MethodsAnimalsThe sample size was determined based on earlier studies (Mahmoud et al., 2010; Soliman et al., 2022; Seif et al., 2023) using the G*power software 3.1.9.2, where a large effect size of 1.38 was detected. The significance level (α-error) was set at 0.05 and the power (1-β error) was set at 0.8 using a two-sided hypothesis test. The estimated sample size was 30 teeth for each group, summing up a total sample size of 60 teeth at both evaluation periods. The current investigation was conducted on four healthy mongrel male dogs, aging 1.5–2 years and weighed 19–22 kg. Dogs were housed in separate kennels (1.5, 2.5, and 3 m) in a clean environment with a 12-hours light/dark cycle. Dogs were acclimatized to their housing environment and diet for 14 days before conducting the experiment. Dogs were fed twice daily, with soft food and milk. Water was available at all times. In each dog, fifteen maxillary and mandibular teeth, including incisors, canines, and premolars were used. These dogs were randomly divided into two groups (two dogs, 30 teeth each): group I, immunosuppressed animals, and group II, the control. According to the pulp capping material, each group was randomly divided into three subgroups (ten teeth each). MTA (MTA Proroot®-Dentsply, USA), BA (BioAggregate®-Innovative BioCeramix Inc. Vancouver, Canada), and Ca (OH)2 (Dycal®-Dentsply, USA) were employed for direct pulp capping in subgroups A, B, and C, respectively. Each subgroup was further subdivided into two subdivisions (five teeth each) based on the assessment time: subdivision 1 for one month and subdivision 2 for two months (Fig. 1). Azathioprine-immunosuppression protocolDogs in Group I were administered azathioprine (Imuran®—Aspen, Australia) two months before surgery. The initial dose was 4 mg/kg given orally as a single dose at the start of the experiment, followed by a maintenance dose of 2 mg/kg given orally once a day for two months before the pulp capping procedure and throughout the trial (Favrot et al., 2007). Surgical procedureDogs were premedicated with a subcutaneous injection of Atropine sulphate (Atropine®, Sunways Private Ltd., Mumbai, India) at a dose of 0.1 mg/kg and an intravenous injection of Xylazine HCl (Xylamed®, Bimeda Animal Health, Dublin, Ireland) at a dose of 1 mg/kg. The anesthesia was then induced with Ketamine HCl (Ketalar®, JHP Pharmaceuticals, USA) and maintained throughout the surgical operation using Thiopental sodium (Thiopental sodium®, Livealth Biopharma Private Ltd., Mumbai, India).
Fig. 1. Flow diagram showing the classification of samples in different groups, subgroups and subdivisions. MTA: Mineral trioxide aggregate, BA: Bio-aggregates and Ca(OH)2: Calcium hydroxide. All teeth were swapped with 0.5% povidone-iodine solution and isolated with a rubber dam. Deep class V buccal cavities (approximately 2.5 mm width, 3 mm length, and 1.5–2 mm depth) were produced almost 1 mm coronal to the gingival boundary with a # 2 inverted cone carbide bur at high speed while irrigated with normal saline solution (Saleh et al., 2016). For pulp exposure, a sterile sharp probe was used. Rinsing with sterile saline achieved hemostasis, and the capping materials were mixed and applied according to the manufacturer’s instructions. The MTA paste was made by combining three parts of powder and 1 part of water using a sterilized glass slab and a metal spatula. The BA paste was made by thoroughly mixing three parts powder and one part liquid on a sterilized glass slab using a plastic spatula. The MTA carrier administered both MTA and BA pastes to the exposure locations, which were then softly condensed using a moist cotton pellet. The Ca(OH)2 paste was made by combining equal amounts of base and catalyst on a sterile paper pad. A tiny calcium hydroxide applicator was used to apply the soft mix to the exposure site with minimum pressure, after which it was allowed to set. The cavities were eventually sealed with glass ionomer cement (Riva, SDI, Australia). Histopathological examinationFollowing the observation period, the dogs were euthanized by anesthetic overdose using 20 mL of 5% Thiopental sodium solution (Thiopental sodium®, EPICO, Giza, Egypt) administered by fast intravenous injection. A block of each capped tooth and its surrounding bone was preserved in 10% neutrally buffered formalin solution for three days before being decalcified in 20% formic acid and 25% sodium citrate solution for four months (Saleh et al., 2016). The specimens were dried in alcohol, cleaned in xylene, embedded in paraffin wax, and serially sectioned on the buccolingual plane, including the capping site and pulp. Each tooth yielded five cross-sections measuring 5 µm thick. These cross-sections were stained with hematoxylin and eosin to interpret the inflammatory cell response and dentine bridge development in the following way: Qualitative evaluationFour sample fields from each slide were examined at X10 and X40 magnifications for qualitative assessment of pulp tissue features such as inflammatory cell infiltration, pulp architecture, focal necrotic areas, vasodilatations, and dentine bridge formation. The chosen fields should be of good architecture and without any staining artifacts. Quantitative evaluation of the inflammatory cellsThe total number of inflammatory cells in each microscopic area was determined using image analysis software (Image J, 1.41.NIH, USA). The photographs were transformed into 8-bit greyscale images. To avoid counting other cells such as fibroblasts, the threshold for inflammatory cells ranged from 38 to 120 pixels (based on their area). Evaluation of dentinal bridge developmentDentine bridge creation was graded using Mahmoud et al. (2010) criteria. Briefly, values 0, 1, and 2 indicate no, partial, and full dentine bridges, respectively. The dentinal bridge thickness was determined using image analysis software (Image J). A line was drawn across the dentinal bridge’s maximum thickness, measured in pixels, and then translated to micrometers. Statistical analysisThe results were given as means and standard deviations (SDs). The Kruskal-Wallis test was performed to compare control and azathioprine groups with their respective materials (subgroups). The Mann-Whitney test was used to investigate the differences across evaluation times (one and two months). A Tukey HSD test was used for multiple comparisons. Test findings with p < 0.05 were considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics Version 23 (IBM Corporation, Armonk, NY). Ethical approvalThe Institutional Ethics Committee at the Faculty of Dentistry, Ain Shams University-Egypt, accepted the study protocol (Approval No. 17-04-11-Endo). This project followed all international and institutional criteria for animal care and usage, including the Animal Research: Reporting in Vivo Experiments guidelines (ARRIVE). ResultsQualitative findingsGroup I (immunosuppressed group) One month following pulp capping in azathioprine-immunosuppressed dogs, MTA exhibited a damaged odontoblastic layer opposite to the exposure site, areas of superficial necrosis, and loss of normal pulp architecture, without evidence of dentine bridge formation (Fig. 2a). Inflammatory cell infiltrates were seen in the deeper layer of the pulp (Fig. 2b). Subgroup B (BA subgroup) had the same pathological findings found in the MTA subgroup; however, partial dentine bridge formation and areas of newly formed fibrous tissue were seen in the middle part of the pulp (Fig. 2c and d). Subgroup C (Ca(OH)2 subgroup) exhibited the same pathological findings as the MTA subgroup (Fig. 2e) but the pulp tissue was heavily infiltrated with inflammatory cells (Fig. 2f). In subdivision 2 (at two months), subgroup A exhibited an organized odontoblastic layer opposite to the partially formed dentine bridge (Fig. 3a). Newly formed fibrous tissue with inflammatory cells infiltrates was noted in the middle of the pulp (Fig. 3b). Subgroup B exhibited a damaged odontoblastic layer with a partial dentine bridge formation (Fig. 3c). Areas of tissue degeneration and congested blood vessels were also seen (Fig.3d). Subgroup C had a damaged odontoblastic layer with areas of necrosis and no dentine bridge formation (Fig. 3e). Inflammatory cell infiltration and numerous dilated and congested blood vessels were seen in the middle portion of the pulp (Fig. 3f).
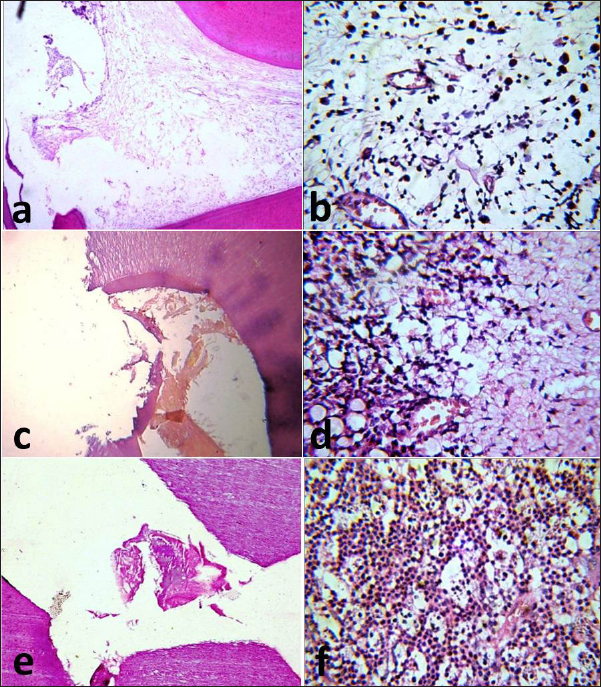
Fig. 2. Photomicrographs of the immunosuppressed group after one month. MTA subgroup showing the exposure site without dentine bridge formation (a) and infiltration of the pulp with inflammatory cells (b). BA subgroup showing the exposure site with partial dentine bridge formation (c) and dilated and congested blood vessels with areas of fibrosis (d). Ca(OH)2 subgroup showing the exposure site without dentine bridge formation (e) and heavy inflammatory cell infiltration (f). The images (a), (c) and (e): H&E, X10 while (b), (d), and (f): H&E, X40. Group II (control group) In subdivision 1 (after one month), subgroup A had a regular architecture in the area opposite the exposure site, a partially degenerated odontoblastic layer, and complete dentine bridge formation. There were numerous congested blood vessels in the superficial and middle portions of the pulp with few infiltrated inflammatory cells. Subgroup B exhibited an irregular odontoblastic layer with complete dentine bridge formation and congested blood vessels in most samples (Fig. 4a). The pulp tissue appeared to regain its integrity with the absence of inflammatory cells in the middle portion of the pulp. Subgroup C had a damaged odontoblastic layer with partial dentine bridge formation. Few inflammatory cell infiltrates and dilated blood vessels were seen in the middle portion of the pulp (Fig. 4b).
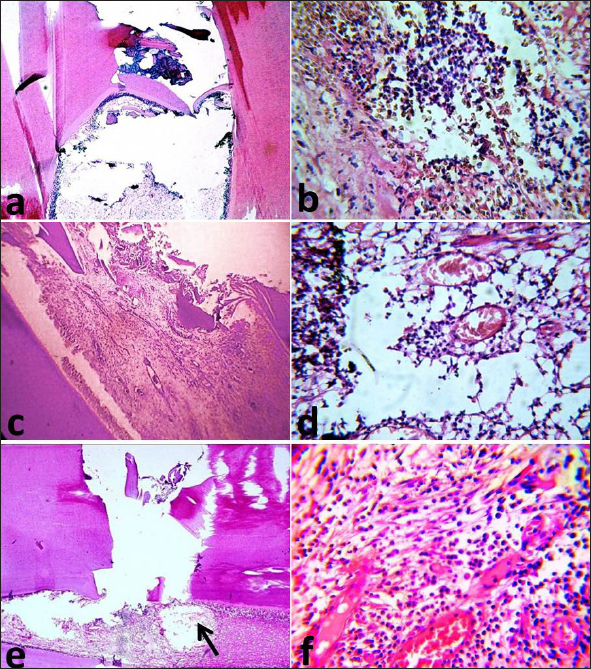
Fig. 3. Photomicrographs of the immunosuppressed group after two months. MTA subgroup showing the exposure site with partial dentine bridge formation (a), newly formed fibrous tissue and inflammatory cell infiltration (b). BA subgroup showing partial dentine bridge formation (c), dilated and congested blood vessels with areas of degeneration (d). Ca(OH)2 subgroup showing absence of dentine bridge formation, an area of necrosis (arrow), a destructed odontoblastic layer (e), dilated and congested blood vessels and inflammatory cell infiltration (f). The images (a), (c) and (e): H&E, X10 while (b), (d), and (f): H&E, X40. In subdivision 2 (after two months), the superficial portion of the pulp regained its integrity and the connective tissue stroma resembled that of normal pulpal architecture in subgroup A. There was a well-organized odontoblastic layer with no inflammatory cell infiltrates and no dilated blood vessels opposite to the completely formed dentine bridge (Fig. 4c). Subgroup B exhibited an irregular odontoblastic layer with areas of degenerations and complete dentine bridge formation. Few inflammatory cell infiltrates in the superficial and middle portions of the pulp were seen (Fig. 4d). In subgroup C, the superficial portion of the pulp exhibited organization and regularity of the odontoblastic layer with areas of degeneration opposite to the completely formed dentine bridge. The connective tissue resembled that of the normal pulp with dilated blood vessels and no inflammatory cells.
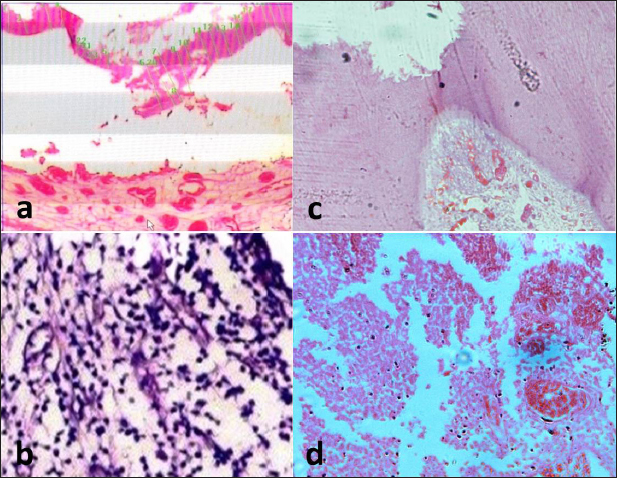
Fig. 4. Representative photomicrograph of the control group after one and two months. (a) BA subgroup after one month showing the exposure site with complete dentine bridge formation. (b) The Ca(OH)2 subgroup showing few inflammatory cell infiltrates in the middle portion of the pulp. (c) MTA subgroup after two months showing well-organized odontoblastic layer with no inflammatory cell infiltrates and no dilated blood vessels opposite to the completely formed dentine bridge. BA subgroup after two months showing dilated blood vessels with few inflammatory cells (d). The images (a) and (c): H&E, X10 while (b) and (d): H&E, X40. Table 1. Mean and SD of inflammatory cell count in all groups, subgroups and subdivisions.
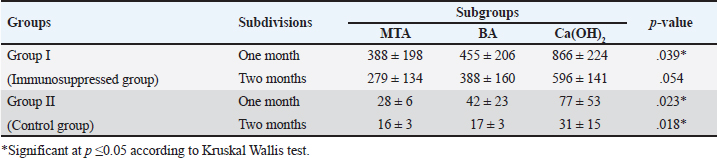
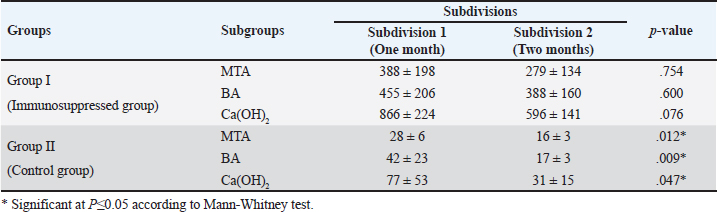
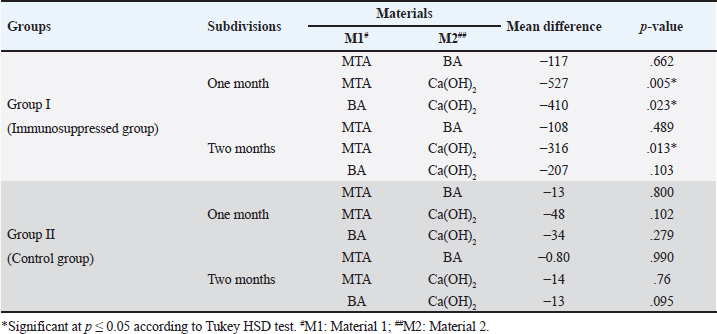
Quantitative findings of the inflammatory cellsThere were significant differences in the mean inflammatory cell count between group I (immunosuppressed group) and group II (control group) in both subdivisions (p < 0.05). Group I (immunosuppressed group)In subdivision 1 (after one month), the mean inflammatory cell count was 388 ± 198, 455 ± 206, and 866 ± 224 in subgroups A, B, and C, respectively (Table 1). There was a significant difference between the three subgroups (p < 0.05). In subdivision 2 (after two months), the mean inflammatory cell count was 279 ± 134, 388 ± 160, and 596 ± 141 in subgroups A, B, and C, respectively (Table 1). There was no significant difference between the subgroups (p > 0.05). In group I, all subgroups showed a decrease in the mean inflammatory cell count over time. The mean cell count decreased dramatically after two months. However, the decrease in the mean inflammatory cell count was statistically not significant in all subgroups (Table 2). Regarding the effect of subgroups on inflammatory response in the two subdivisions, all subgroups showed a difference in the mean inflammatory cell counts in group I after one month. However, there was no significant difference between subgroups A and B (p > 0.05). The difference in the mean inflammatory cell count was statistically significant between subgroups A and C (p < 0.05) and between subgroups B and C in group I after one month (p < 0.05). In group I after two months, all subgroups showed a difference in the mean inflammatory cell counts. There was no significant difference in the mean inflammatory cell counts between subgroups A and B (p > 0.05). However, the difference between subgroups A and C was statistically significant (p < 0.05). There was no significant difference in the mean inflammatory cell counts between subgroups B and C (p > 0.05) as shown in Table 3. Group II (control group)In subdivision 1 (after one month), the mean inflammatory cell count was 28 ± 6, 42 ± 23, and 77 ± 53 in subgroups A, B, and C, respectively (Table 1). There was a significant difference between the three subgroups (p < 0.05(. In subdivision 2 (after two months), the mean count was 16 ± 3, 17 ± 3, and 31 ± 15 in subgroups A, B, and C, respectively (Table 1). However, no significant difference between the subgroups was detected (p > 0.05). The tested subgroups exhibited a significant decrease in the mean inflammatory cell count after two months (p < 0.05). Table 2. Effect of time on inflammatory response (mean ± SD) in all tested groups, subgroups and subdivisions.
Table 3. Comparison of inflammatory response between different subgroups in the two subdivision.
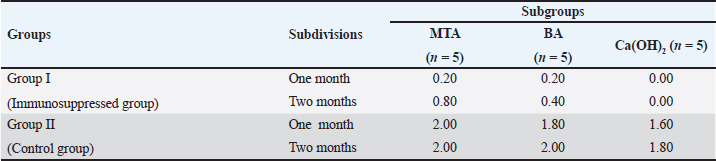
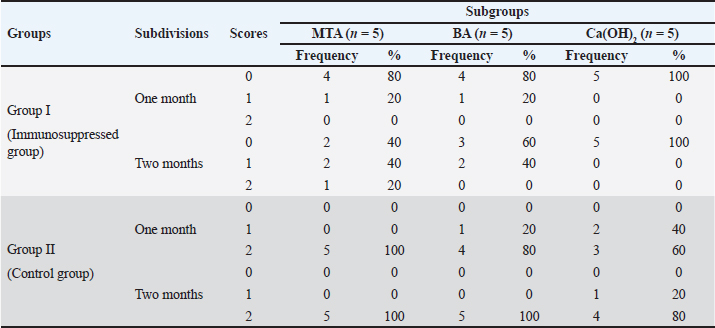
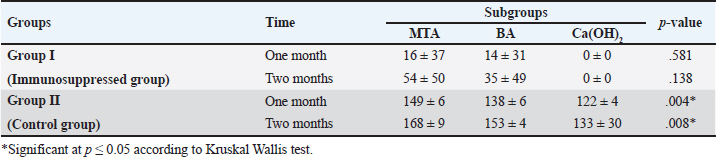
In group II, all subgroups showed a decrease in the mean inflammatory cell count by time. The decrease in the mean inflammatory cell count was statistically significant in both tested groups after two months (p < 0.05) as shown in Table 2. Regarding the effect of different capping materials on inflammatory response in the two tested periods, there was no statistically significant difference between all subgroups in both subdivisions (p > 0.05) as shown in Table 3. Results of dentine bridge formationThere were significant differences in the mean dentine bridge thicknesses between group I (immunosuppressed group) and group II (control group) in both subdivisions (p < 0.05(.The mean scores of dentine bridge formation are presented in Table 4. The frequencies and percentages of dentine bridge formation are shown in Table 5. The mean dentine thickness in micrometers is presented in Table 6. Group I (immunosuppressed group)In subdivision 1 (after one month), the mean dentine bridge thickness (µm) was 16 ± 37, 14 ± 31, and 0.0 in subgroups A, B, and C, respectively. While it was 54 ± 50, 35 ± 49, and 0.0 in subgroups A, B, and C, respectively in subdivision 2 (after two months). No significant differences were observed between the subgroups after one and two months (p > 0.05(. Group II (control group)The mean dentine bridge thickness (µm) was149 ± 6, 138 ± 6, and 122 ± 4 in subgroups A, B, and C, respectively in subdivision 1 and 168 ± 9, 153 ± 4, and 133 ± 30, respectively in subdivision 2. There were significant differences in dentine bridge thickness between the three subgroups after one and two months (p < 0.05). There were significant differences between the two subdivisions in subgroups A and B (p < 0.05). Subdivision 2 had a higher dentine bridge thickness than subdivision 1. DiscussionThe current study investigated the effect of azathioprine as an immunosuppressive drug on dental pulp healing after pulp capping using three commonly used materials. The study’s hypothesis was accepted since our results revealed that azathioprine inhibited the healing of exposed dogs’ dental pulp following direct pulp capping with MTA, BA, and Ca(OH)2. Table 4. Mean scores of dentine bridge formation among the different groups, subgroups and subdivisions.
Table 5. Frequencies and percentages of dentine bridge scores among different groups, subgroups and subdivisions.
Table 6. Mean and SD of dentine bridge thickness (μm) in different groups, subgroups and subdivisions.
Azathioprine was selected for immunosuppression in this study because it was one of the first immunosuppressants utilized in numerous autoimmune inflammatory disorders (Patel et al., 2006). Furthermore, there is a scarcity of information on azathioprine and its effects on pulp healing after the pulp capping process. For more than 50 years, azathioprine has been used to treat hematologic malignancies, rheumatoid arthritis, organ transplantation, inflammatory bowel disease, and other disorders that are associated with increased production of prostaglandin E2 (Patel et al., 2006). It has been reported that prostaglandin E2 production by rabbit retina/choroid was inhibited by azathioprine underlining the drug’s antiinflammatory action (Naveh et al., 1988). Additionally, based on histologic examination, less inflammatory cell infiltration was found in nasal polyps treated with azathioprine compared to controls (Yeo et al., 2019). Furthermore, it has been documented that azathioprine induces gastrointestinal T-cell apoptosis and decreases inflammation by conversion to a purine metabolite that binds to the GTPase Rac1.2. It is effective at maintaining remission in both Crohn’s disease and ulcerative colitis (Ewe et al., 1993). However, the exact mechanism of action of azathioprine is uncertain and might be multifactorial. Maltzman and Koretzky (2003) reported that azathioprine inhibits T and B lymphocyte development and suppresses cytotoxic T cells and plasma cells. Furthermore, azathioprine’s action is delayed for approximately eight weeks (Ewe et al., 1993). Therefore, in the present study, azathioprine was administered for 60 days before the pulp capping procedures. The dog was chosen as the animal model in this study because it has a relatively large number of teeth, making it possible to compare different materials within the same animal. Furthermore, the pulp tissue and the healing process in dogs are identical to those in people, or even faster (Accorinte et al., 2008; Al-anesi et al., 2021). In the current study, a probe was employed to provide evenly sized pulp exposures while avoiding considerable pulp damage caused by bur as was recommended by previous studies (Negm et al., 2017; Emara et al., 2022; Ahmed et al., 2024). The early and late response of pulp tissue was evaluated histologically according to previous experimental studies (Negm et al., 2017; Emara et al., 2022). The immunocompromised dogs responded less favorably to the pulp capping process than the control dogs. Because of azathioprine’s cytotoxic effect on healing, the immunocompromised group had significant inflammation after one month. MTA demonstrated better physiologic responses than BA and Ca(OH)2 due to its higher biocompatibility and hydroxyapatite formation (Roberts et al., 2008; Al-Sherbiny et al., 2020; Mohamed et al., 2023; Elkhashab et al., 2024). Furthermore, the physicochemical and mechanical features of MTA enable good sealing, limit bacterial growth, and facilitate mineralization (Sallay et al., 1984; Tziafas and Papadimitriou, 1998; Min et al., 2007; Nabeel et al., 2019; Nabeel et al., 2024). Although the immunocompromised group’s inflammation decreased after two months, it remained moderate to severe. Again, the cytotoxic activity of azathioprine may be responsible for the persistence of inflammation. However, the slight reduction in inflammation might be related to the body’s natural healing processes. These findings are consistent with previous research indicating that immunosuppression allows oral bacteria to enter periodontal tissues and damage supporting tissues (Sallay et al., 1984; Lopes et al., 1997). In contrast, other studies discovered that cyclosporin A did not affect the defensive systems of exposed tooth pulp in rats (Mahmoud et al., 2010). This discrepancy in results might be attributed to the varied animal models, the immunosuppressant type used, and preoperative time necessary for induction of immunosuppression, which was just 6 days in the cited study (Oh et al., 2008). Dentine bridge growth enables healthy pulps to repair themselves (Inoue and Shimono, 1992). However, bacterial infection of exposed pulps may hinder healing. As a result, infection control and keeping exposed pulp healthy and clean are vital, and they rely largely on suitable and quick sealing using pulp capping materials (Negm et al., 2016). The immunocompromised group’s absence of dentine bridging following the direct capping procedure might be attributed to azathioprine’s negative effects on various dentinogenesis-related cells and cytokines. Azathioprine suppresses TGF-β, an active component of the dentine matrix that promotes reparative dentinogenesis (Nasstrom et al., 1985; Tziafas and Papadimitriou, 1998). The study’s principal limitations were the small number of employed dogs and the short evaluation times. More studies are needed to understand the specific mechanism of action of azathioprine on pulp tissue healing, as well as to investigate its long-term effects on pulp tissue healing. ConclusionAzathioprine impairs dental pulp healing in exposed dogs after direct pulp capping with MTA, BA, or Ca(OH)2 through induction of severe inflammation and retardation of dentine bridge formation. Patients using azathioprine as an immunosuppressive medication may experience delayed healing of mechanically exposed pulps following capping with MTA, BA, or Ca(OH)2. AcknowledgmentsNone. Author contributionMAA, MMN, and AMA carried out all surgeries. AMA followed up the experimental animals. AHM and SHE evaluated the radiographs and supervised the study. ESA examined the histopathology specimens. AMA, MHI, and MAA wrote the main manuscript text and prepared figures. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflict of interestThe authors declare no conflicts of interest to report. Data availabilityThe article includes all data supporting the study’s findings. In case additional data are needed, the corresponding author can provide it upon reasonable request. ReferencesAbo El-Mal, E.O., Abu-Seida, A.M. and El Ashry, S.H. 2019. A comparative study of the physicochemical properties of hesperidin, MTA-Angelus and calcium hydroxide as pulp capping materials. Saudi Dent. J. 31, 219–227. Abo El-Mal, E.O., Abu-Seida, A.M. and El Ashry, S.H. 2021. Biological evaluation of hesperidin for direct pulp capping in dogs, teeth. Int. J. Exper. Pathol. 102, 32–44. Accorinte, M.L.R., Holland, R., Reis, A., Bortoluzzi, M.C., Murata, S.S and Dezan, JrE. 2008. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J. Endod. 34 (1), 1–6. Ahmed, M.S., Hasan, N.H., Saeed, M.G. and Abdulmajeed, A.A. 2024. Response of exposed pulp to capping with mineral trioxide aggregate mixed with hyaluronic acid as a water substitute. Al-Rafidain Dent. J. 24(1), 191–202. Al-anesi, M.A., Abu-Seida, A.M. and El Ashry, S.H. 2021. Influence of insulin on the healing of exposed dental pulp after pulp capping: an experimental study in a dog model. Spec. Care Dent. 41, 49–59. Al-Sherbiny, I.M., Abu-Seida, A.M., Farid, M.H., Motawea, I.T. and Bastawy, H.A. 2020. Histopathological pulp response of dog’s teeth capped with biosealer and biodentine: an in vivo study. Saudi Endod. J. 10, 226–233. Al-Sherbiny, I.M., Farid, M.H., Abu-Seida, A.M., Motawea, I.T. and Bastawy H.A. 2021. Chemico-physical and mechanical evaluation of three calcium silicate-based pulp capping materials. Saudi Dent. J. 33 (4), 207–214. Ayanoglou, C.M., Godeau, G., Lesty, C., Septier, D. and Goldberg, M. 1997.Cyclosporin A-induced alterations of dentinogenesis in rat molars. J. Oral Pathol. Med. 26, 129–134. Cara, C.J., Pena, A.S., Sans, M., Rodrigo, L., Guerrero-Esteo, M. and Hinojosa, J. 2004. Reviewing the mechanism of action of thiopurine drugs: towards a new paradigm in clinical practice. Med. Sci. Monit. 10, RA247–254. El-Ashry, S., Abu-Seida, A.M., Al-Boghdady, H., El-Batouty, K. and Abdel-Fattah, M. 2013. The effect of different formulations of calcium hydroxide on healing of intentionally induced periapical lesions in dogs. Pak .Vet. J. 33, 48–52. El Ashry, S.H., Abu-Seida, A.M. and Emara, R.A. 2016. Influence of addition of osteogenic supplements to mineral trioxide aggregate on the gene expression level of odontoblastic markers following pulp capping in dogs. Vet. Arhiv. 86, 685–697. Elion, G.B. 1993. The pharmacology of azathioprine. Annals NY Acad. Sci. 685, 401–407. Elkhashab, R.A., Mahran, A.H., Badr, M. and Abu-Seida, A.M. 2023. Bioactivity and pH of Nano-White MTA versus NeoMTATM Plus® and MTA Angelus® as root repair materials: An in vitro study. Int. Arab J. Dent. 14(2), 104–111. Elkhashab, R.A., Mahran, A.H., Badr, M and Abu-Seida, A.M. 2024. Histopathology and immunohistochemical reactions of Nano-White MTA versus NeoMTATM Plus® and MTA Angelus® as immediate furcation perforation repair materials in a dog model. G. Ital. Endod. 38(1), 16–27. Emara, R.A., Abu-Seida, A.M. and El Ashry, A.M. 2022. Histological evaluation of the synergistic effect of Chitosan and mineral trioxide aggregate on mechanically exposed dental pulp following pulp capping in dogs, teeth. Saudi Endod. J. 12 (1), 25–30. Evans, W.E. 2004. Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Ther. Drug Monit. 26(2), 186–191. Ewe, K., Press, A.G., Singe, C.C., Stufler, M., Ueberschaer, B., Hommel, G., et al. 1993. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn’s disease. Gastroenterology. 105(2), 367–372. Favrot, C., Reichmuth, P. and Olivry, T. 2007. Treatment of canine atopic dermatitis with azathioprine: A pilot study. Vet. Rec. 160: 520–521. Inoue, T. and Shimono, M. 1992. Repair dentinogenesis following transplantation into normal and germ-free animals. Proc. Finn. Dent. Soc. 88(Suppl 1), 183–194. Kunert, M. and Lukomska-Szymanska, M. 2020. Bio-inductive materials in direct and indirect pulp capping-a review article. Materials (Basel). 13, 1204. Lopes, M.A., Spolidorio, L.C., Line, S.R. and de Almeida, O.P. 1997. Pulpal lesions in normal and cyclosporin A treated rats. J. Endod. 23, 52–53. Mahmoud, S.H., Grawish, M.E.A., Zaher, A.R., El-Embaby, A., Karrouf, K.I. and Sobh, M.A. 2010. Influence of selective immunosuppressive drugs on the healing of exposed dogs’ dental pulp capped with mineral trioxide aggregate. J. Endod. 36, 95–99. Maltzman, J.S. and Koretzky, G.A. 2003. Azathioprine: old drug, new actions. J. Clin. Invest. 111, 1122–1124. Min, K.S., Kim, H.I., Park, H.J., Pi, S.H., Hong, C.U. and Kim, E.C. 2007. Human pulp cells response to Portland cement in vitro. J. Endod. 33(2), 163–166. Mohamed, M.F., Hashim, A.A., Obeid, M.F. and Abu-Seida, A.M.A. 2022. Solubility of three different pulp capping materials: a comparative study. Ain Shams Dent. J. 27(3), 56–60. Mohamed, M., Hashem, A.A., Obeid, M.F. and Abu-Seida, A.M. 2023. Histopathological and immunohistochemical profiles of pulp tissues in immature dogs’ teeth to two recently introduced pulpotomy materials. Clin. Oral Invest. 27(6), 3095–3103. Nabeel, M., Tawfik, H.M., Abu-Seida, A.M. and Elgendy, A.A. 2019. Sealing ability of Biodentine versus ProRoot mineral trioxide aggregate as root-end filling materials. Saudi Dent. J. 31, 16–22. Nabeel, M., Abu-Seida, A.M., Elgendy, A.A. and Tawfik, H.M. 2024. Biocompatibility of mineral trioxide aggregate and biodentine as root-end filling materials: an in vivo study. Sci. Rep. 14(1), 3568. Näsström, K., Forsberg, B., Petersson A. and Westesson, P.L. 1985. Narrowing of the dental pulp chamber in patients with renal diseases. Oral Surg. Oral Med. Oral Pathol. 59, 242–246. Naveh, N., Weissman, C., and Dottan, S.A. 1988. Azathioprine’s inhibitory effect on prostaglandin E2 production is not via cyclooxygenase inhibition. Biochem. Biophys. Res. Commun. 157(2), 727–732. Negm, A.M., Hassanien, E.E., Abu-Seida, A.M. and Nagy, M.M. 2016. Physical evaluation of a new pulp capping material developed from Portland cement. J. Clin. Exp. Dent. 8, 278-283. Negm, A.M., Hassanien, E.E., Abu-Seida, A.M. and Nagy, M.M. 2017. Biological evaluation of a new pulp capping material developed from Portland cement. Exp. Toxicol. Pathol. 69, 115–122. Oh, W.M., Hwang, I.N., Son, H.H. and Hwang, Y. 2008. Rapid periapical bone destruction during endodontic treatment of a patient with rheumatoid arthritis. J. Endod. 34(10), 1261–1263. Patel, A.A., Swerlick, R.A. and McCall, C.O. 2006. Azathioprine in dermatology: the past, the present, and the future. J. Am. Acad. Dermatol. 55(3), 369–389. Roberts, H.W., Toth, J.M., Berzins, D.W. and Charlton, D.G. 2008. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent. Mater. 24(2), 149–164. Saleh, R.S., Nagi, S.M., Khallaf, M.E., Abd El-Alim, S.H., Zaazou, M.H., Abu-Seida, A.M., Ibrahim, M.N. 2016. In-vivo assessment of dentin bridge formation after using MTA and experimental propolis paste as direct pulp capping material. Res. J. Pharm. Biol. Chem. Sci. 7, 1244–1250. Sallay, K., Listgarten, M., Sanavi, F., Ring, I. and Nowotny, A. 1984. Bacterial invasion of oral tissues of immunosuppressed rats. Infect. Immun. 43(3), 1091–1093. Seif, H., Elbanna, A., Abu-Seida, A.M. and El-Korashy, D.I. 2023. Regenerative potential of a novel Aloe vera modified tricalcium silicate cement as a pulp capping material: an animal study. Dent. Mat. J. 42(6), 868–877. Soliman, H.A., EL-Toukhy, R.I., Ebrahim, M.M., Grawish, M.E., Sobh, M.A. and Mahmoud, S.H. 2022. Influence of selective immunosuppressive drug regimens on the healing of exposed dogs’ dental pulp capped with a recent calcium silicate-based cement. Clin. Oral Invest. 26, 1417–1425. Tziafas, D. and Papadimitriou, S. 1998. Role of exogenous TGF-beta in induction of reparative dentinogenesis in vivo. Eur. J. Oral Sci. 106(Suppl. 1), 192–196. Yeo, N.K., Park, W.J., Eom, D.W., Oh, M.Y., and Lee, J.H. 2019. Effects of azathioprine and its metabolites on inflammatory cytokines in human nasal polyp organ cultures. Int. Forum Aller. Rhinol. 9(6), 648–655. | ||
| How to Cite this Article |
| Pubmed Style Al-anesi MA, Abu-seida AM, Abd-elhamid ES, Mahran AH, Ashry SHE, Issa MH, Nagy MM. Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Vet. J.. 2024; 14(7): 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 Web Style Al-anesi MA, Abu-seida AM, Abd-elhamid ES, Mahran AH, Ashry SHE, Issa MH, Nagy MM. Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. https://www.openveterinaryjournal.com/?mno=198013 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i7.11 AMA (American Medical Association) Style Al-anesi MA, Abu-seida AM, Abd-elhamid ES, Mahran AH, Ashry SHE, Issa MH, Nagy MM. Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Vet. J.. 2024; 14(7): 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 Vancouver/ICMJE Style Al-anesi MA, Abu-seida AM, Abd-elhamid ES, Mahran AH, Ashry SHE, Issa MH, Nagy MM. Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Vet. J.. (2024), [cited January 24, 2026]; 14(7): 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 Harvard Style Al-anesi, M. A., Abu-seida, . A. M., Abd-elhamid, . E. S., Mahran, . A. H., Ashry, . S. H. E., Issa, . M. H. & Nagy, . M. M. (2024) Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Vet. J., 14 (7), 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 Turabian Style Al-anesi, Mokhtar A., Ashraf M. Abu-seida, Ehab S. Abd-elhamid, Abeer H. Mahran, Salma H. El Ashry, Mohamed H. Issa, and Mohamed M. Nagy. 2024. Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Veterinary Journal, 14 (7), 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 Chicago Style Al-anesi, Mokhtar A., Ashraf M. Abu-seida, Ehab S. Abd-elhamid, Abeer H. Mahran, Salma H. El Ashry, Mohamed H. Issa, and Mohamed M. Nagy. "Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials." Open Veterinary Journal 14 (2024), 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 MLA (The Modern Language Association) Style Al-anesi, Mokhtar A., Ashraf M. Abu-seida, Ehab S. Abd-elhamid, Abeer H. Mahran, Salma H. El Ashry, Mohamed H. Issa, and Mohamed M. Nagy. "Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials." Open Veterinary Journal 14.7 (2024), 1614-1624. Print. doi:10.5455/OVJ.2024.v14.i7.11 APA (American Psychological Association) Style Al-anesi, M. A., Abu-seida, . A. M., Abd-elhamid, . E. S., Mahran, . A. H., Ashry, . S. H. E., Issa, . M. H. & Nagy, . M. M. (2024) Influence of azathioprine on healing of exposed dogs' dental pulp capped with three different materials. Open Veterinary Journal, 14 (7), 1614-1624. doi:10.5455/OVJ.2024.v14.i7.11 |