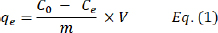| Research Article | ||
Open Vet. J.. 2025; 15(3): 1468-1479 Open Veterinary Journal, (2025), Vol. 15(3): 1468-1479 Research Article Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutionsKadhim Hassan Abbas1, Ishtar Adnan Mohammed Alethari2, Esraa Taher Muslim1 and Orooba Meteab Faja1*1Department of Public Health, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq 2Department of Pharmacology, Physiology, and Biochemistry, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah, Iraq *Corresponding Author: Orooba Meteab Faja. Department of Public Health, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah City, Iraq. Email: orooba.faja [at] qu.edu.iq Submitted: 16/11/2024 Accepted: 11/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: Antibiotic resistance is a challenging worldwide health issue. Cadmium (Cd) contamination in aqueous environments poses a significant threat to human health and ecosystem integrity because of its toxic and nonbiodegradable nature. Aim: The current study aimed to understand the antibacterial activity of green synthesized and surface-modified silver nanoparticles (AgNPs) with silica and dopamine (DA) for efficient Cd removal from aqueous solutions. Methods: A green and ecofriendly nanosorbent, AgNPs [at] TEOS [at] DA, was synthesized to study its antibacterial activity and efficient removal of Cd (II) ions from aqueous solutions. The nanosorbent synthesis process adheres to green chemistry principles, utilizing biologically derived reducing agents. AgNPs [at] TEOS [at] DA exhibit a uniform spherical morphology, providing a high surface area for interactions with target Cd (II) ions. Results: SP nanoparticles exhibited the highest inhibition zones for various bacteria and were most effective. The study established a 15-minutes incubation time as optimal for rapid and consistent Cd (II) ion absorption. The adsorption efficiency was pH-dependent, with the maximum absorption observed at a pH of approximately 6.0. The concentration-dependent adsorption behavior was also observed in accordance with the established adsorption kinetics. The environmentally friendly synthesis process and efficient Cd (II) ion removal capability of AgNPs [at] TEOS [at] DA make them promising candidates for addressing environmental and water quality challenges. This study contributes to the advancement of ecofriendly materials for heavy metal removal while supporting sustainable and environmentally conscious practices. Conclusion: This study paves the way for the utilization of ecofriendly materials for heavy metal removal, fostering a more sustainable and environmentally conscious approach to wastewater treatment. Keywords: Antibiotic resistance, Aqueous Solutions, Cadmium, Dopamine, Green Synthesis, Silica, Silver nanoparticles. IntroductionThe greatest public health concern for society is still antibiotic resistance. Because it is so hard to create and find new natural compounds, new strategies are urgently demanded (Janabi, 2021). Heavy metal pollution of natural water supplies is a global concern due to its impact on ecosystems and human health impacts (Mishra et al., 2019). Of these heavy metals, one that is a corrosive and nonbiodegradable element, cadmium (Cd) is a high threat in water-based environments (Khalef et al., 2022). It enters waterways mostly through industrial discharges, farm runoff, and industrial wastewater. It persists in water bodies and can build up in organisms, making Cd a disturbing pollutant. Decades of chronic inhalation of even very low levels of Cd can result in a variety of diseases, such as kidney failure, bone loss, and cancer (Fatima et al., 2019). Cd contamination is an environmental catastrophe in its own right. Cd that enters waterways can also be taken up by aquatic organisms, which makes its way into the food chain (Orata and Sifuna, 2023). It is not only a direct threat to the oceans but also a direct danger to human species, whose food depends on such habitats. Moreover, Cd-polluted water supplies can damage terrestrial ecosystems when used for irrigation, and Cd can also accumulate in crops, threatening food safety. Because Cd lingers in the environment and is very harmful, effective techniques for purging Cd from water are essential (Suhani et al., 2021). In the fight against Cd contamination, several techniques have been developed for extracting metals from water (Duan et al., 2020). Chemical precipitation, ion exchange, and membrane filtration are typical old methods. Such techniques can work, but they can involve toxic chemicals, secondary pollutants, and a lot of energy. As a result of these issues, the development of sustainable and ecological removal methods has increased (Qureshi et al., 2023). These procedures were designed to reduce the environmental footprint of Cd extraction while being highly efficient and selective. Nanotechnology—especially nanoparticles—in environmental remediation is becoming a popular solution because they promise both high efficacy and sustainability. Our work in this respect introduces a new and clean Cd (II) extraction method from water. Thus, we propose a nanocomposite with silver nanoparticles (AgNPs) bonded to silica (tetraethyl orthosilicate; TEOS) and modified with dopamine (DA), named AgNPs [at] TEOS [at] DA. This nanocomposite has several desirable properties, making it a promising candidate for the effective removal of Cd. For example, AgNPs have an abundance of surface area and catalytic activity and thus can be highly sensitive to Cd (II) ions. The TEOS surface not only fixes the AgNPs but also provides an extra surface area for adsorption. The DA modification increases the nanocomposite’s Cd (II) ion specificity of the nanocomposite. Significantly, the entire AgNPs [at] TEOS [at] DA synthesis is “green chemistry”, and no toxic reagents or organic solvents are used in this process, with the focus on the ecofriendliness of the nanocomposite. We have been working on the synthesis, characterization, and use of this nanocomposite for effective Cd removal, which promises to be an environmentally friendly, effective heavy metal remediation option for marine waters. Materials and MaterialsSilver nitrate (AgNO3, 7761-88-8; Sigma Co.), (TEOS, 131903; Sigma Co.), DA HCl (DA, 62-31-7; Sigma Co.), and all reagents and solvent were obtained from Emertat chim (Emertat Co., Iran). Green synthesis of silica- and DA-coated AgNPs as adsorption nanocompositeTo synthesize silver nitrate, 4% w/v BSA was dissolved in ultrapure water under vigorous stirring. Then, 0.002% w/v of AgNO3 was added to the solution. After 20 minutes 300 µl of NaOH 11 N was gently added and the temperature suddenly increased to 90°C. The solution was left to react for 2 hours, during which the color of the solution changed from colorless to orange–yellow (Gharbavi et al., 2023). AgNPs [at] TEOS were prepared following a previously reported method with some modifications (Sui et al., 2012). First, 2 ml of TEOS (10% v/v in ethanol) was added to the AgNP mixture, and the mixture was vigorously stirred for another 4 hours to ensure the successful preparation of AgNP [at] TEOS. The resulting nanostructure was collected by centrifugation at 10,000 rpm for 10 minutes and freeze-dried. Subsequently, a round-bottom flask was filled with ultraclean distilled water, 0.5% v/v aqueous ammonia, and 0.2% w/v AgNPs [at] TEOS. Subsequently, 20 mg of DA was added to the solution above, and the solution was agitated for 24 hours at 65 °C to ensure the production of DA-treated AgNPs [at] TEOS (AgNPs [at] TEOS [at] DA). Finally, the obtained product was centrifuged and washed with ultrapure water until the supernatant was colorless (Xiang et al., 2020). Characterization of the nanocompositeFT-IR spectroscopy The chemistry of all potential nanostructures was determined by Fourier-transform infrared spectroscopy (FTIR; Tensor 2, Biotage, Bruker, Germany). The FT-IR standard pills were prepared by mixing 200 mg of KBr white powder with 2 mg of samples and pressing under a 12-ton press (Gharbavi et al., 2021). The FT-IR results for all samples were obtained at 400–4,000 cm 1 spectral resolution. Dynamic light scattering analysis The hydrodynamic diameter average size (nm), polydispersity index (PDI), and zeta-potential (mV) of the nanostructures were determined by dynamic light scattering (DLS) with a Nano-Zeta sizer (Malvern Instruments, Worcestershire, UK, model Nano ZS) device. We diluted 0.1 ml of each sample with 2 ml of deionized water in a fresh Malvern sample vial to calculate the hydrodynamic diameter (Z-average), PDI, and zeta potential (Jafari et al., 2022). FESEM analysis The size and shape of AgNPs [at] TEOS [at] DA were calculated from FESEM (MIRA TESCAN, Czech Republic) images, and the element distributions of the prepared NPs were also estimated using the EDS technique. They covered the samples with platinum and viewed them at 15 kV and 100,000 magnification (Gharbavi et al., 2022). Cadmium adsorption by AgNPs [at] TEOS [at] DA AgNPs [at] TEOS [at] DA conducted batch experiments on the adsorption of Cd (II) from water. These variables were the effects of various useful factors such as incubation time, pH, and starting concentration. All vessels were shaken at 150 rpm and room temperature (Li et al., 2020). The pH values of the solutions were maintained within the correct levels of HCl and NaOH using a pH meter (Felenji et al., 2022). The experiments were performed at pH=6 with constant AgNPs [at] TEOS [at] DA dose (0.1 g) for the time period (5, 15, 30, and 60 minutes). For the Cd (II) adsorption experiments, 100 mg AgNPs [at] TEOS [at] DA were dissolved in a jar containing 100 ml of Cd (II) solutions of the following concentrations: 0.05, 0.1, 0.2, 0.4, 0.5, and 1 mg. ml1) at pH=6 for 10 minutes until equilibrium was achieved (Awwad et al., 2020). The nano-adsorbent was then magnetically lysed from the solutions, and the remaining Cd (II) concentration was determined using an atomic absorption spectrophotometer. Then, the removal capacity, qe (mg.g−1), of AgNPs [at] TEOS [at] DA to Cd (II) was determined using Eq. (1) (10):
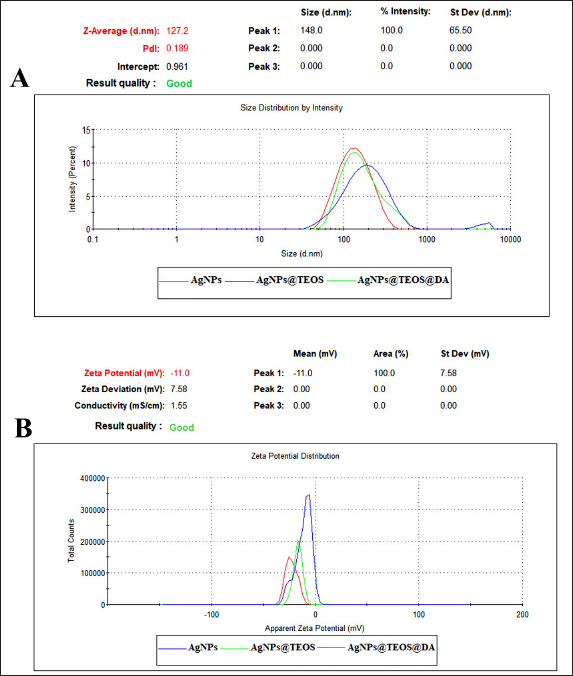
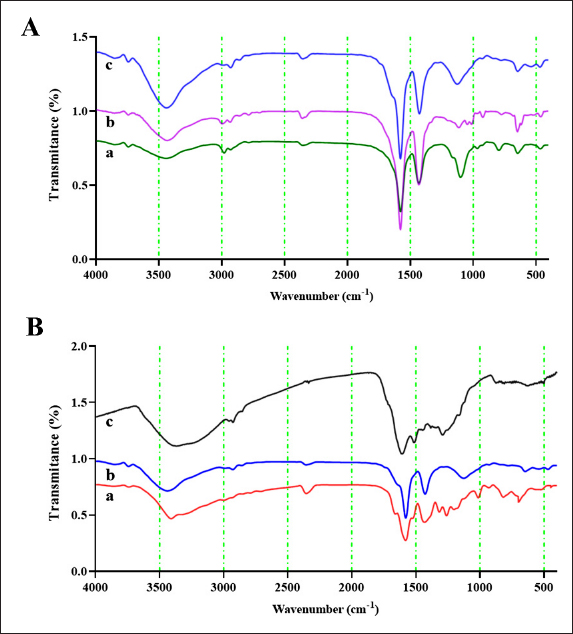
Here, C0 (mg/l) is the initial concentration of Cd (II) solutions, Ce(mg/l) is the equilibrium concentration of Cd (II) solutions, m (g) is the mass of AgNPs [at] TEOS [at] DA, and V (L) is the volume of Cd (II) solutions. Antibacterial activity of the nanoparticles They assessed the antibacterial activity of bacteria in fish using the Miller–Hinton procedure. Bacteria Isolates (E. coli, S. aureus, V. cholerae, and so on) were cultured on Mueller–Hinton agar, a common susceptibility test material. Different concentrations of nanoparticles were suspended on discs of sterile filter paper and placed on bacterially inoculated agar plates. After 24 hours at 37°C, the plates were incubated, and the inhibition zone was determined (mm). ResultsThe reason why AgNPs [at] TEOS [at] DA was chosen as an adsorption nanocomposite to efficiently deactivate the Cd (II) ions in water comes down to some of the following reasons observed during this experiment. AgNPs [at] TEOS [at] DA’s super-adsorbent performance is a product of its particular material combination. AgNPs have a high surface area and catalytic capacity, so they interact well with Cd (II) ions. TEOS is a surface stabilizing and adsorbent agent, which keeps the nanocomposite intact and adsorbent. In addition, the DA modification also makes the composite more attractive to Cd (II) ions by complexation and chelation. These factors give the nanocomposite an impressive adsorption capacity, as shown by the results. These findings indicate that environmental responsibility and effective adsorption do not have to be antagonistic, which could prove a solution to the heavy metal removal challenge for ecosensitive applications. This experimental observation revealed the extraordinary adsorption capacity of AgNPs [at] TEOS [at] DA. Even with low Cd (II) and very short contact times, the nanocomposite exhibited outstanding adsorption. This explains why it is perfect for situations in which heavy metal removal is needed quickly and effectively. Zeta potential, hydrodynamic average size, and PDI calculationWe must know their zeta potential to understand their physicochemical properties. We used DLS to study the zeta properties of AgNPs, AgNPs [at] TEOS, and AgNPs [at] TEOS [at] DA very precisely and obtained important insights. Results indicate different surface charge properties of these nanoparticles. In particular, the zeta potentials of AgNPs were 10.320.97, 14.981.36, and 20.891.62 mV for AgNPs [at] TEOS, and 20.891.62 mV for AgNPs [at] TEOS [at] DA (Fig. 1B). Such observations illustrate the asymmetrical nature of surface charges on these nanocomposites. Zeta potential values were negative for AgNPs at AgNP [at] TEOS and then AgNPs [at] TEOS [at] DA. The lower the zeta potential, the higher the electrostatic attraction between the nanoparticles. This is normally translated into increased stability and less aggregation. Thus, AgNPs [at] TEOS is the evolution of AgNPs from AgNPs to TEOS, which gives rise to a TEOS coating and an increased and negative zeta potential, which means greater stability (Dayana et al., 2019). The DA modification in AgNPs [at] TEOS [at] DA increased the zeta potential further, indicating better stability and the possibility of effective nanoparticle dispersion (Liu et al., 2020). The increase in zeta potential also correlates with the higher electrostatic attraction between nanoparticles, which helps to avoid agglomeration and therefore the stability of the nanocomposite. The hydrodynamic dimension of nanoparticles determines how they behave and interact in different environments. DLS is a powerful method for probing the size distributions and dispersion properties of nanostructures. The hydrodynamic sizes of AgNPs, AgNPs [at] TEOS, and AgNPs [at] TEOS [at] DA were calculated using the average hydrodynamic sizes (from the DLS measurements) to perfection. The obtained values of 130.633.55 nm, 167.753.05 nm, and 227.672.35 nm are very close to the dimensions of these nanoparticles when they are in solution. The hydrodynamic increase from AgNPs to AgNPs [at] TEOS and finally AgNPs [at] TEOS [at] DA was due to the changes introduced in the synthesis, as shown in Figure 1A. Nanoparticle suspension uniformity is important for applications requiring uniform nanoparticle distributions. To measure the homogeneity of the nanoparticle suspensions, we used PDI. The PDI values for all the studied nanostructures indicate that the status is positive in terms of reaching homogeneous nanoparticle distributions. FTIR spectroscopy analysisFT-IR spectroscopy is one of the most widely used techniques for the validation of silver nanoparticle synthesis and monitoring of surface modification. FT-IR spectrum (Fig. 2A, B) takes you visually through the evolution of nanoparticles. Figure 2A gives us a rather fascinating example where AgNPs, TEOS, and AgNPs [at] TEOS FT-IR spectra are carefully examined to verify surface modification by tetraethyl TEOS. AgNPs [at] TEOS FT-IR emission captures a gorgeous vibrational concerto in the 3850–380 cm-1 wavelength range.
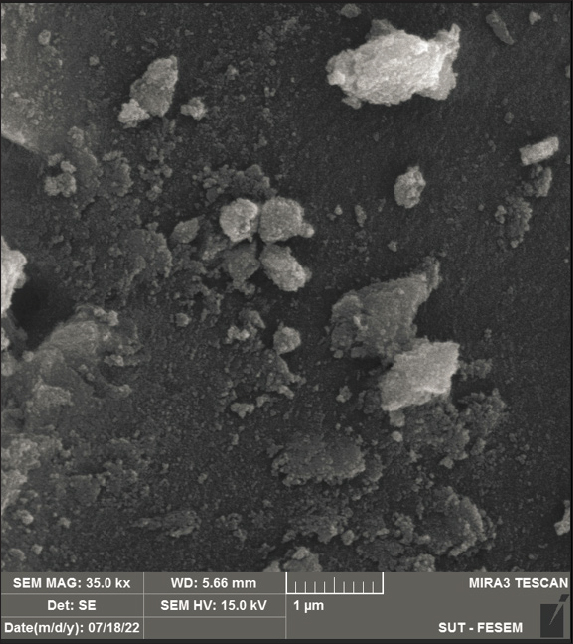
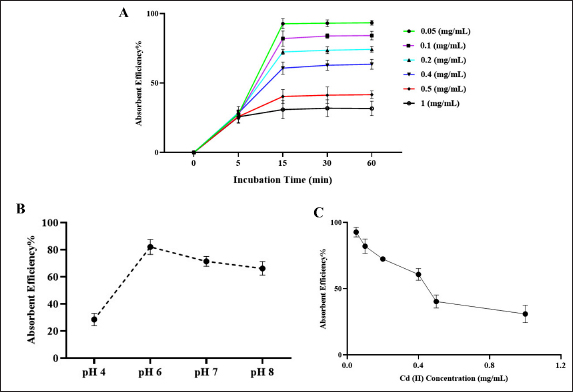
Fig. 1. Dynamic Light Scattering (DLS) analysis. (A) Hydrodynamic diameters (Z-average (nm) of all nanostructures. (B) Zeta potentials (mV) of all nanostructures. Morphology analysis using FESEMThe shape and morphology of the synthesized nanoparticles were conducted through field emission scanning electron microscopy (FESEM). FESEM images provide compelling visual insights into the structural characteristics of AgNPs [at] TEOS [at] DA. Effect of incubation timeTo assess the absorption efficiency of Cd (II) using the proposed nano sorbent (AgNPs [at] TEOS [at] DA), we examined the impact of various incubation times (5, 15, 30, and 60 minutes) under different initial concentrations of Cd (II) solution (0.05, 0.1, 0.2, 0.4, 0.5, and 1 mg/ml). The results presented in Figure 4A provide valuable insights into the absorption kinetics of Cd (II). During the initial 5-minutes incubation period with varying Cd (II) solution concentrations, no significant increase in absorption efficiency was observed. The absorption efficiency ranged from 27.87% for the lowest concentration (0.05 mg/ml) to 25.66% for the highest concentration (1 mg/ml). These results indicate that at this early stage, the absorption process is still developing and that equilibrium has not yet been reached. When the incubation time was extended to 15 minutes, a remarkable shift in the absorption efficiency was noted across different Cd (II) concentrations. The absorption efficiency ranged from 92.74% for the lowest concentration (0.05 mg/ml) to 30.90% for the highest concentration (1 mg/ml). Notably, what makes this observation particularly significant is the consistent and stable absorption efficiency observed at this time point across all Cd (II) solution concentrations.
Fig. 2. FT-IR spectroscopy. (A) FT-IR spectra of TEOS (a), AgNPs (b), and AgNPs [at] TEOS (c). (B) FTIR spectra of DA (a), AgNPs [at] TEOS (b), and AgNPs [at] TEOS [at] DA (c). Effect of pHThe acidity of a solution plays a pivotal role in the adsorption process, as it significantly affects the adsorption capacity (Dil et al., 2017). To gauge the influence of pH on removal efficiency, a series of tests were conducted within a pH range of 4–8. The results are shown in Figure 4B. In solutions with lower pH values, an abundance of hydrogen ions is present. According to the principles of surface complex formation, hydrogen ions are preferentially absorbed rather than metal ions. Effect of initial Cd (II) ions concentrationThe batch adsorption experiments were systematically conducted over a range of initial Cd (II) concentrations, specifically 0.05, 0.1, 0.2, 0.4, 0.5, and 1 mg/ml, while maintaining a constant pH of 6. In each adsorption test, 1 mg/ml AgNPs [at] TEOS [at] DA was employed, and the contact time was standardized to 15 minutes. The results in Figure 4C provide valuable insights into the impact of the initial Cd (II) concentration on the adsorption process. As the initial Cd (II) concentration increased from 0.05 to 1 mg/ml, a notable trend emerged in the quantity of Cd (II) adsorption, which exhibited a decline. The removal efficiency was particularly high at low initial Cd (II) concentrations, primarily due to the availability of unoccupied binding sites on the adsorbent. In contrast, at higher Cd (II) concentrations, these binding sites were nearly entirely covered, resulting in a decrease in the removal efficiency as the metal concentration increased.
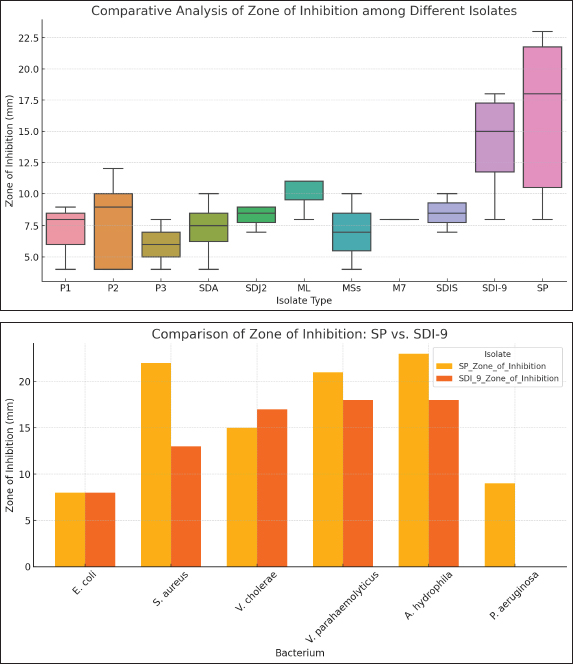
Fig. 3. Analysis of AgNPs [at] TEOS [at] DA Morphology using FESEM technique. Antibacterial activity of the nanoparticlesThe statistics showed that nanoparticles and isolates worked well in combating different bacteria species. ANOVA showed that inhibition zones varied between isolates and that some treatments were much better (p < 0.05). SP had the highest inhibition zones for various bacteria and was the most effective. These findings underscore the ability of specific nanoparticles such as SP to have better antibacterial properties and the need to tailor formulations to bacterial specificity (Fig. 5). DiscussionThe antibacterial activity of the nanoparticles against fish-borne bacteria is consistent with previously reported values. AgNPs have shown, for example, that they are bactericidal against the fish pathogen Aeromonas sp. and can thus be applied to aquaculture (Shaalan et al., 2016). The same can be said of zinc oxide nanoparticles that exhibit broad-spectrum antimicrobial activity, destroying bacteria such as E. coli and S. aureus (Krishnamoorthy et al., 2022). These studies confirm what we found: nanoparticles could potentially be an effective alternative to conventional antibiotics for the treatment of bacterial infections in aquaculture (Karim et al., 2018; Yaseen et al., 2020; Abd-Alhassen et al., 2021; Ghazi et al., 2024). However, nanoparticle concentration, environmental impact, and resistance formation are essential for their long-term use.
Fig. 4. (A) Effect of incubation time on Cd (II) absorbance percentage. Experimental conditions: pH=6, adsorbent dose=1 mg/ml, temperature 25 °C. (B) Effect of pH on Cd (II) absorbance%. Experimental conditions: incubation time 15 minutes, initial Cd (II) ions concentration 0.1 mg/mL, adsorbent dose 1 mg/ml, and temperature 25 °C. (C) Effect of initial Cd (II) ions concentration on Cd (II) absorbent efficiency%. Experimental conditions: incubation time, 15 minutes; pH, 6; adsorbent dose, 1 mg/ml; temperature, 25 °C. The green synthesis strategy of AgNPs [at] TEOS [at] DA is the unique feature of this system (Gharbavi et al., 2020a). This is why synthesis is performed without the need to use toxic reagents and organic solvents (hence “green chemistry”). Not only does this help prove the environmental quality of the nanocomposite, but it also shows that it is a good option for environmental protection. The surface charge properties can be applied to such nanostructures for environmental cleanup and other purposes because they affect how the nanoparticles behave in solution and how they interact with the target species, such as heavy metal ions (Gharbavi et al., 2019). The AgNP [at] TEOS TEOS coating is larger because it creates a shell around the nanoparticles. The second step (DA) modification of AgNPs [at] TEOS [at] DA doubled the size of the nanocomposite, largely because organic molecules were adsorbed on the nanocomposite surface. These hydrodynamic changes in size matter how these nanoparticles disperse in the solution. They are also larger, suggesting a tendency toward sedimentation and decreased movement. But the price is the more surface area for adsorption and reactivity, the higher the surface area, which is extremely useful in the heavy metal removal industry, where a larger surface area can increase the adsorption capability (Gharbavi et al., 2020b). This property is important, especially in applications that require predictable and well-scattered nanoparticle networks (Gharbavi et al., 2020c). There is also a curious bending resonance at approximately 1,100 cm1. These ghostly signatures speak loudly to the existence of Si-OH bonds and Si-O-Si motif and speak loudly of the emergence of AgNPs [at] TEOS. Notably, the fact that Ag NPs are not left with distinct footprints in the FT-IR spectra indicates that they are bound to TEOS, revealing the emergence of AgNPs [at] TEOS (Geng et al., 2022). A highlight in the FT-IR spectrum of AgNPs [at] TEOS [at] DA shows a bright peak at approximately 3,400 cm1. This peak is due to the stretching vibrations of O-H and N-H bonds, which are common functional groups of DA. This peak can be observed in Figure 2B. It is especially interesting to observe how DA interacts with the TEOS molecule in the nanocomposite. Perhaps the most dramatic result is a clear peak around 1,650 cm1 in the FTIR band of AgNPs [at] TEOS [at] DA (Rajeshwari et al., 2022). This vibrational resonance is due to the bowing vibrations of the N-H bond, which is the amide (NH2) functional group. The occurrence of this peak represents amide bonds between DA’s amino groups and the TEOS group, which in turn gives rise to the amine-functionalized AgNPs [at] TEOS [at] DA. Moreover, the region of the spectral field around 1,100 cm1 is also key to explaining the DA/TEOS coupling. In this area, the Si-O-Si bonds are observed to indicate the TEOS component. The Si-O-Si bonds along with the amide bonds occur at the same time, which indicates a close and coordinated relationship between DA and the TEOS-based silica layer. The FT-IR spectrum of AgNPs [at] TEOS [at] DA confirms surface modification with DA, and the peaks are typical of amide bonds between DA and TEOS-derived silica, and Si-O-Si bond. Such results provide options for custom-designed surface functionalization to improve the strength and performance of nanocomposite. AgNPs [at] TEOS [at] DA is a promising platform for applications that require controlled surface interactions (e.g., effective heavy metal extraction from solutions and controlled drug delivery) (Fig. 2). As seen in Figure 3, an overwhelming and significant point emerges: the nanoparticles are uniformly spherical and near-uniform. Their form is particularly homogenous, with little difference in size and shape. The spherical structure and regularity of these FESEM images represent the accuracy and control of the synthesis procedure (Mousazadeh et al., 2022).
Fig. 5. Antibacterial activity of nanoparticles against isolated fish bacteria Spherical nanoparticles are also typically very large in surface area-to-volume ratio, meaning that a large proportion of the structure is visible to the environment. This surface area allows for more active spaces for chemical reactions such as metal ions to be adsorbed. When it comes to metal absorbents, a greater surface area means a greater ability to trap and extract metal contaminants from solutions. Aerodynamic nanoparticles easily transport and disperse metal ions into the adsorption locations on their surface. The shape of the ball lowers the diffusion coefficient and allows metal ions to easily pass through the sites of action. This is especially useful for fast adsorption. Moreover, spherical nanoparticles do not clump or settle like asymmetrical nanoparticles. Such stability keeps the active surface area available, and the nanostructure spreads out in the solution, which retains its adsorption capacity for a longer time (Rajalakshmi et al., 2022). In addition, spherical nanoparticles give you a sharp and facile to functional surface. There are functional groups, coatings, or ligands that can be directly attached to the sphere and used to tailor the interaction with specific metal ions or contaminants. Such multifunctional surface modifications make metal adsorption more selective and efficient (Molina et al., 2020). In summary, spherical nanostructures are very suitable for metal adsorption because of their high surface area, uniformity, mass transfer efficiency, stability, functionalization ease, scalability, and decreased risk of channeling or clogging. These properties make spherical nanostructures promising candidates for environmental and industrial applications such as the removal of heavy metals from water (Fig. 3). This consistency also means that after 15 minutes, the absorption is already in equilibrium, and more time incubation does not yield much-improved absorption. Taking all this into account, 15 minutes of incubation is the most time for the nano-absorbent to achieve optimum and consistent absorption. This important result provides the keys to many real-world scenarios in which efficient and time-sensitive metal ion removal is needed, for example, in water treatment and environmental remediation (Wu et al., 2022). In all Cd (II) solution concentrations tested, the absorption efficiency was low for the first 5 minutes of incubation. This is something we see a lot about adsorption because initially, the surface of the adsorbent may not be fully available or primed for adsorption. These results are in line with the view that there will be an initial phase of adsorption in which the adsorbent surface is “wetting” up with adsorbate molecules. However, perhaps most importantly, after 15 minutes of incubation, the absorption efficiencies shift markedly. The absorption efficiency reaches equilibrium regardless of the initial Cd (II) solution concentration. This means that 15 minutes is the time at which absorption becomes most efficient, and incubation longer will not improve much. Moreover, the quick arrival of equilibrium at 15 minutes is useful. For situations in which Cd (II) needs to be withdrawn rapidly from aqueous solutions, such as in water treatment and environmental cleanup, the AgNPs [at] TEOS [at] DA nanosorbent is of note. Because it reaches equilibrium in a short period of time and retains its absorption rate over time, metal ion removal is rapid and accurate. Conclusion: The yields derived from the time-dependence of incubation indicate that equilibrium was achieved quickly at 15 minutes. AgNPs [at] TEOS [at] DA can be considered a promising, effective Cd (II) sorbent nanosorbent. These results are consistent with general adsorption dynamics and with other research on the adsorption of heavy metal ions (Elkhatib et al., 2019). The acidity of a solution also affects the adsorption ability of the solution (Dil et al., 2017). As depicted in Figure 4B, one can see that the excretion of Cd (II) under low pH is significantly less effective than that above it. The amount of Cd (II) adsorbed was also much higher as the solution pH increased from 4 to approximately 6.0. It is caused by precipitation of Cd (II) as a precipitate of insoluble Cd (OH)2. In other words, when these pH values are higher than 6, the ions of Cd (II) start hydrolyzing to create microscopic amounts of cadmium hydroxyl species (Omidvar-Hosseini and Moeinpour, 2016). Further elaboration is required to explain the lack of metal adsorption observed in pH6 solutions, which can be explained by protonated AgNPs [at] TEOS [at] DA surfaces and electron repulsion and H+-Cd (II) competition for active sites. Throughout the pH range of 4 to 6, equilibrium Cd (II) ions absorbs are vastly improved because protonation decreases with increasing pH. This decrease also lowers the H+-Cd (II) competition for active sites; therefore, Cd (II) ions can be more readily taken up. However, as the pH of the solution increases above 7, the precipitation of Cd (II) ions, in the form of cadmium hydroxide, are precipitated (Da Conceição et al., 2021). Compared with Cd (II) ions, the newly generated species are less adsorbable and exhibit only a marginal decline in removal efficiency and adsorption. Therefore, the pH at which maximum absorption occurs was approximately 6.0. Thus, pH 6.0 was chosen as the reference condition for all of the following absorption experiments. It behaves in this way because it fits the Langmuir adsorption model and is normal for adsorption. At lower starting concentrations, there are plenty of empty adsorption zones for metal ions to hit the adsorbent. As the metal ions become concentrated, these sites become over occupied until saturated, which reduces the removal capacity (de Castro Alves et al., 2019). This is a reminder that choosing the right initial concentration of Cd (II) ions for the best removal efficiency is critical in real-world applications to ensure that binding sites are efficiently exploited without saturation (Fig. 4). We have produced AgNPs [at] TEOS [at] DA—a sustainable and green nanosorbent—and tested its performance in removing Cd (II) ions from aqueous solutions. The nanoparticles are antibacterial against isolated fish bacteria. Green synthesis based on biologically derived reducing agents goes well with principles of environmentally sustainable material development. This underscores the need for green chemistry in nanomaterial synthesis. We introduce a new nanosorbent AgNPs [at] TEOS [at] DA, which is a green synthesis combined with Cd (II) removal. The results inform the adsorption kinetics and equilibrium factors, with practical application potential. AgNPs [at] TEOS [at] DA can be the answer for ecological cleanup and water purification following the concepts of sustainable material synthesis. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingNo institutional funding is present, but only self-funded funds are. Authors’ contributionsAll authors contributed to the manuscript. Data availabilityAll data can be provided by the corresponding author upon reasonable request. ReferencesAbd-Alhassen, J.K., Janabi, A.H.D. and Aboktifa, M.A. 2021. Antioxidant and antimicrobial evaluation of lycopene isolated from watermelon. Biochem. Cell. Arch. 21, 2905–2910. Awwad, A., Amer, M. and Al-Aqarbeh, M. 2020. TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: adsorption and thermodynamics of Pb (II) and Cd (II) ions in aqueous solution. Chem. Int. 4(4), 168–178. Da Conceição, F.T., da Silva, M.S.G., Menegário, A.A., Antunes, M.L.P., Navarro, G.R.B., Fernandes, AM, 2021. Precipitation as the main mechanism for Cd (II), Pb (II), and Zn (II) removal from aqueous solutions using natural and activated forms of red mud. Environ. Adv. 4, 100056. Dayana, I., Sembiring, T., Tetuko, A.P., Sembiring, K., Maulida, N., Cahyarani, Z. 2019. The effect of tetraethyl orthosilicate (TEOS) additions as silica precursors on the magnetite nanoparticles (Fe3O4) properties for ferro-lubricant application. J. Mol. Liq. 294, 111557. De Castro Alves, L., Yáñez-Vilar, S., Piñeiro-Redondo, Y., Rivas, J. 2019. Novel magnetic nanostructured beads for cadmium (II) removal. Nanomaterials 9(3), 356. Dil, E.A., Ghaedi, M. and Asfaram, A. 2017. The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 34, 792–802. Duan, C., Ma, T., Wang, J. and Zhou, Y. 2020. Removal of heavy metals from aqueous solution using carbon-based adsorbents: a review. J. Water Process Eng. 37, 101339. Elkhatib, E., Mahdy, A., Mahmoud, A. and Moharem, M. 2019. Efficient removal of Cd (II) from contaminated water and soils using nanoparticles from nitrogen fertilizer industry waste. J. Environ. Health Sci. Eng. 17, 1153–1161. Fatima, G., Raza, AM, Hadi, N., Nigam, N. and Mahdi, A.A. 2019. Cadmium in human diseases: it is more than a mere metal. Indian J. Clin. Biochem. 34, 371–378. Felenji, H., Johari, B., Moradi, M., Gharbavi, M. and Danafar, H. 2022. Folic acid-conjugated iron oxide magnetic nanoparticles based on bovine serum albumin for targeted delivery of curcumin to suppress liver cancer cells. Chem. Afr. 5(5), 1627–1639. Geng, A.Y., Ding, G.Q., She, H.H., Wang, H.X., Li, X.Q. and Zhu, Y.L. 2022. Effect of hydrolysis of tetraethyl orthosilicate (TEOS) on titanium distribution of TS-1 zeolite. J. Fuel Chem. Technol. 50(11), 1471–1479. Gharbavi, M., Danafar, H. and Sharafi, A. 2020a. Microemulsion and bovine serum albumin nanoparticles as a novel hybrid nanocarrier system for efficient multifunctional drug delivery. J. Biomed. Mater. Res. Part A. 108(8), 1688–1702. Gharbavi, M., Johari, B., Mousazadeh, N., Rahimi, B., Leilan, M.P., Eslami, S.S. and Sharafi, A. 2020b. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep., 47, 6517–6529. Gharbavi, M., Johari, B., Rismani, E., Mousazadeh, N., Taromchi, A.H. and Sharafi, A. 2020c. NANOG decoy oligodeoxynucleotide–encapsulated niosomes nanocarriers: a promising approach to suppress the metastatic properties of U87 human glioblastoma multiforme cells. ACS Chem. Neurosci. 11(24), 4499–4515. Gharbavi, M., Johari, B., Ghorbani, R., Madanchi, H. and Sharafi, A. 2023. Green synthesis of Zn nanoparticles and in situ hybridized with BSA nanoparticles for Baicalein targeted delivery mediated with glutamate receptors to U87-MG cancer cell lines. Appl. Organomet. Chem. 37(1), e6926. Gharbavi, M., Manjili, H.K., Amani, J., Sharafi, A. and Danafar, H. 2019. In vivo and in vitro biocompatibility study of novel microemulsion hybridized with bovine serum albumin as nanocarrier for drug delivery. Heliyon, 5, e01858. Gharbavi, M., Mousavi, M., Pour-Karim, M., Tavakolizadeh, M. and Sharafi, A. 2022. Biogenic and facile synthesis of selenium nanoparticles using Vaccinium arctostaphylos L. fruit extract and anticancer activity against an in vitro model of breast cancer. Cell Biol. Int. 46(10), 1612–1624. Gharbavi, M., Sharafi, A., Motamed Fath, P., Oruji, S., Pakzad, H. and Kheiri Manjili, H. 2021. Formulation and biocompatibility of microemulsion-based PMBN as an efficient system for paclitaxel delivery. J. Appl. Biotechnol. Rep. 8, 114985. Ghazi, A.M., Ali Al-Bayati, M.A. and Janabi, A.H. 2024. Metabolomics-detected alterations generated by phytosomal propolis and phytosomal Lycopene in male rats with induced benign prostatic hyperplasia. Iraqi J. Vet. Sci. 38(Suppl. I–IV), 7–15. Jafari, B., Gharbavi, M., Baghdadchi, Y., Manjili, H.K., Mahmoudi, J., Jafari-Anarkoli, I. and Amini, F. 2022. Mitigated oxidative stress and cognitive impairments in transient global ischemia using niosomal selegiline-NBP delivery. Behav. Neurol. 2022, 4825472. Janabi, A.H.D. 2021. Molecular docking analysis of anti-severe acute respiratory syndrome-coronavirus 2 ligands against spike glycoprotein and the 3-chymotrypsin-like protease. J. Med. Signals Sens. 11(1), 31–36. Karim, S.M., Mansour, K.A., Janabi, A.H.D. and Al-Nakeeb, N.K.M. 2018. First phylogenetic characterization of pseudocowpox virus from cattle in Al-Qadisiyah province, Iraq. Iraqi J. Vet. Sci. 33(1), 123–126. Khalef, R.N., Hassan, A.I. and Saleh, H.M. 2022. Heavy metal’s environmental impact. In Environmental Impact and Remediation of Heavy Metals. Eds., Saleh, H.M and Hassan, A.I. London, England: IntechOpen. Krishnamoorthy, R., Athinarayanan, J., Periyasamy, V. S., Alshuniaber, M. A., Alshammari, G., Hakeem, M.J., Ahmed, M.A. and Alshatwi, A.A. 2022. Antibacterial mechanisms of zinc oxide nanoparticle against bacterial food pathogens resistant to beta-lactam antibiotics. Molecules 27(8), 2489–2498. Li, S.S., Wang, X.L., An, Q.D., Xiao, Z.Y., Zhai, S.R., Cui, L. and Ma, Y. 2020. Designing carboxyl methylcellulose and chitosan-derived nanostructured sorbents for efficient removal of Cd (II) and Cr (VI) from water. Int. J. Biol. Macromol. 143, 640–650. Liu, Y., Gan, D., Chen, M., Ma, L., Yang, B. and Li, L 2020. Bioinspired dopamine modulating graphene oxide nanocomposite membrane interposed by super-hydrophilic UiO-66 with enhanced water permeability. Sep. Purif. Technol. 253, 117552. Mishra, S., Bharagava, R.N., More, N., Yadav, A., Zainith, S., Mani, S. and Zaman, M. 2019. Heavy metal contamination: an alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future. Eds., Sobti, R., Arora, N. and Kothari, R. Singapore: Springer. Molina, A., Patil, North, Ventosa, East, Liras, M., Palma, J. and Marcilla, R. 2020. New anthraquinone-based conjugated microporous polymer cathode with ultrahigh specific surface area for high-performance lithium-ion batteries. Adv. Funct. Mater. 30(6), 1908074. Mousazadeh, N., Gharbavi, M., Rashidzadeh, H., Nosrati, H., Danafar, H. and Johari, B. 2022. Anticancer evaluation of methotrexate and curcumin-coencapsulated niosomes against colorectal cancer cell lines. Nanomedicine 17(4), 201–217. Omidvar-Hosseini, F. and Moeinpour, F. 2016. Removal of Pb (II) from aqueous solutions using Acacia Nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. J. Water Reuse Desal. 6(4), 562–573. Orata, F. and Sifuna, F. 2023. Uptake, bioaccumulation, partitioning of lead (Pb) and cadmium (Cd) in aquatic organisms in contaminated environments. Lead Mercury Cadmium Aquat. Environ. 166, 181. Qureshi, F., Yusuf, M., Ibrahim, H., Kamyab, H., Chelliapan, S., Pham, C.Q. and Nguyen, D.H. 2023. Contemporary avenues of the hydrogen industry: opportunities and challenges in the eco-friendly approach. Environ. Res. 229, 115963. Rajalakshmi, R., Rebekah, A., Viswanathan, C. and Ponpandian, N. 2022. Evolution of intrinsic 1–3D WO3 nanostructures: tailoring their phase structure and morphology for robust hydrogen evolution reaction. Chem. Eng. J. 428, 132013. Rajeshwari, V., Vedhi, C. and Fernando, J. 2022. Dopamine sensor based on core-shell poly-paraphenylene diamine/titanium dioxide/multiwalled carbon nanotube nanocomposite. Mater. Today Proc. 68, 287–293. Shaalan, M., Saleh, M., El-Mahdy, M. and El-Matbouli, M. 2016. Recent progress in applications of nanoparticles in fish medicine: a review. Nanomedicine 12(3), 701–710. Suhani, I., Sahab, S., Srivastava, V. and Singh, R.P. 2021. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 27, 1–7. Sui, N., Monnier, V., Yang, Z., Chevolot, Y., Laurenceau, E., Souteyrand, E. and Perrin, F. 2012. Preparation of core-shell silver/silica nanoparticles and their application for enhancement of cyanine 3 fluorescence. Int. J. Nanosci. 11(4), 1240020. Wu, W., Liu, Z., Azeem, M., Guo, Z., Li, R. and Li, Y. 2022. Hydroxyapatite-tailored hierarchical porous biochar composite immobilized Cd (II) and Pb (II) and mitigated their hazardous effects in contaminated water and soil. J. Hazard. Mater. 437, 129330. Xiang, L., Lin, J., Yang, Q., Lin, S., Chen, S. and Yan, B. 2020. Facile preparation of hierarchical porous polydopamine microspheres for rapid removal of chromate from the wastewater. J. Leather Sci. Eng. 2(1), 1–10. Yaseen, M.M., Karawan, A.C., Alfatlawi, M.A.A. and Janabi, A.H.D. 2020. The role of gut bacterial cytochrome-P450 of mosquito larvae in degradation of temephos insecticide. Ann. Trop. Med. Public Health. 23(1), S412. | ||
| How to Cite this Article |
| Pubmed Style Abbas KH, Alethari IAM, Muslim ET, Faja OM. Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Vet. J.. 2025; 15(3): 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 Web Style Abbas KH, Alethari IAM, Muslim ET, Faja OM. Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. https://www.openveterinaryjournal.com/?mno=198458 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.36 AMA (American Medical Association) Style Abbas KH, Alethari IAM, Muslim ET, Faja OM. Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Vet. J.. 2025; 15(3): 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 Vancouver/ICMJE Style Abbas KH, Alethari IAM, Muslim ET, Faja OM. Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 Harvard Style Abbas, K. H., Alethari, . I. A. M., Muslim, . E. T. & Faja, . O. M. (2025) Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Vet. J., 15 (3), 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 Turabian Style Abbas, Kadhim Hassan, Ishtar Adnan Mohammed Alethari, Esraa Taher Muslim, and Orooba Meteab Faja. 2025. Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Veterinary Journal, 15 (3), 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 Chicago Style Abbas, Kadhim Hassan, Ishtar Adnan Mohammed Alethari, Esraa Taher Muslim, and Orooba Meteab Faja. "Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions." Open Veterinary Journal 15 (2025), 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 MLA (The Modern Language Association) Style Abbas, Kadhim Hassan, Ishtar Adnan Mohammed Alethari, Esraa Taher Muslim, and Orooba Meteab Faja. "Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions." Open Veterinary Journal 15.3 (2025), 1468-1479. Print. doi:10.5455/OVJ.2025.v15.i3.36 APA (American Psychological Association) Style Abbas, K. H., Alethari, . I. A. M., Muslim, . E. T. & Faja, . O. M. (2025) Anti-bacterial activity of green synthesized and surface modified silver nanoparticles with silica and dopamine for efficient cadmium removal from aqueous solutions. Open Veterinary Journal, 15 (3), 1468-1479. doi:10.5455/OVJ.2025.v15.i3.36 |