| Research Article | ||
Open Vet. J.. 2024; 14(7): 1634-1643 Open Veterinary Journal, (2024), Vol. 14(7): 1634–1643 Research Article Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potentialSeyedeh Mina Masoumi1, Mohammad Reza Youssefi2* and Seyed Shapoor Reza Shojaei11Department of Veterinary Parasitology, Karaj Branch, Islamic Azad University, Karaj, Iran 2Department of Veterinary Parasitology, Babol Branch, Islamic Azad University, Karaj, Iran *Corresponding Author: Mohammad Reza Youssefi. Department of Veterinary Parasitology, Babol Branch, Islamic Azad University, Babol, Iran. Email: youssefi929 [at] hotmail.com Submitted: 21/04/2024 Accepted: 21/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
AbstractBackground: Chronic toxoplasmosis has been strongly implicated in the development of psychosis and schizophrenia. Additionally, the understanding of schizophrenia has been significantly reshaped by insights into N-methyl-D-aspartate receptor (NMDAR) hypofunction. Aim: This study aimed to compare the behavioral, antioxidant, and NMDAR changes in mice subjected to Toxoplasma gondii infection and those treated with ketamine to induce schizophrenia-like symptoms. Methods: Sixty male BALB/c mice were divided into six groups: toxoplasmosis (TOXO) (infected), ketamine-induced schizophrenia (KET), TOXO+KET, TOXO+sulfadiazine-trimethoprim treatment (SDT), TOXO+KET+SDT, and control (CON) (uninfected). After 10 weeks post-infection, behavioral tests were conducted, brain antioxidant status and lipid peroxidation were analyzed, and NMDA-NR1/NR2A expressions were assessed. TOXO and KET induced distinct behaviors: hyperlocomotion, anxiety, and memory impairment. Results: Antioxidant enzyme levels decreased, and lipid peroxidation increased in TOXO and schizophrenic mice brains. NMDAR downregulation, especially NR-1 and NR2A, was evident due to T. gondii and ketamine. Sulfadiazine-trimethoprim ameliorated NMDAR downregulation, but not all of the behavioral alterations. Conclusion: Further studies are needed to elucidate specific NMDAR subunit roles in toxoplasmosis-induced pathophysiology, offering potential therapeutic insights. This investigation highlights the intricate relationship between chronic toxoplasmosis, NMDAR dysfunction, and schizophrenia-like behaviors. Insights gained could pave the way for innovative interventions targeting both cognitive and neurological impairments associated with these conditions. Keywords: Antioxidant status, Behavioral alterations, Ketamine, Toxoplasma gondii. IntroductionToxoplasma gondii, a protozoan parasite belonging to the Apicomplexa phylum, stands as a remarkable example of parasitic success, adept at infecting a wide array of warm-blooded animals. Although its intermediate hosts vary extensively, cats uniquely serve as the definitive hosts, supporting the sexual stage of the parasite’s lifecycle and acting as the source of infective oocysts (Mendez and Koshy, 2017). In humans, T. gondii is primarily acquired through the ingestion of oocyst-contaminated soil and water, consumption of undercooked meat harboring tissue cysts, or congenitally from mother to fetus. Once within a host, the parasite’s rapid replicative tachyzoite stage gives way to a chronic phase featuring slowly replicating bradyzoites, encysted within host tissues (Youssefi et al., 2007; Youssefi et al., 2009; Elmore et al., 2010). These cysts, housing bradyzoites, establish lifelong infections in various tissues, with a notable predilection for the central nervous system (CNS) (Matta et al., 2021). Chronic T. gondii infection in rodents has been linked to CNS dysfunction, encompassing alterations in neurotransmitter levels, protein expression, and behavioral shifts such as hyperactivity, cognitive impairments, and aversion to predatory urine odor (Parlog et al., 2015; Alsaady et al., 2019). Furthermore, in humans, chronic toxoplasmosis has exhibited correlations with bipolar disorder, epilepsy, psychosis, and schizophrenia (Elsheikha et al., 2016). Perturbations in diverse neurotransmitters and signaling pathways, including dopamine and γ-aminobutyric acid (GABA), have been observed in T. gondii-infected mice (Mendez and Koshy, 2017). However, incongruent findings from certain studies probing the dopaminergic and GABAergic pathways have introduced controversy regarding the precise role of these neurotransmitters. In recent decades, the emergence of N-methyl-D-aspartate receptor (NMDAR) hypofunction has profoundly influenced our comprehension of schizophrenia’s pathophysiology and treatment (Coyle, 2012). It has been demonstrated that NMDAR antagonists, such as ketamine, can replicate the complete spectrum of psychotic, negative symptom, cognitive, and physiological features of schizophrenia in individuals without the condition. The NMDAR is composed of subunits from two gene families, NR1 (comprising eight distinct spliced forms) and NR2 (A, B, C, and D). Functional NMDARs consist of both NR1 and NR2 subunits, with different combinations of NR2 subunits resulting in NMDARs possessing distinct pharmacological and physiological characteristics. Research involving postmortem brain studies of individuals with schizophrenia has indicated alterations in the expression of various NMDAR subtypes (Weickert et al., 2013). Additionally, it has been proposed that the disruption of normal developmental changes in NMDARs may contribute to the onset of schizophrenia (Snyder and Gao, 2013). Interestingly, studies have reported that T. gondii infection in mice is associated with elevated NMDAR autoantibodies, which play a pivotal role in the development of NMDAR hypofunction (Li et al., 2018). Given the central role of NMDARs in the behavioral alterations and neuropathological features of CNS dysfunction, this study aims to comprehensively compare the behavioral effects, brain antioxidant status, lipid peroxidation levels, and the expressions of NMDA-NR1 and NMDA-NR2A receptors in experimental T. gondii infection with those induced by ketamine, a model of schizophrenia, in mice. Through this investigation, we seek to enhance our understanding of the intricate interplay between parasitic infections, neurotransmitter systems, and neuropsychiatric conditions. Materials and MethodsChemicalsKetamine was purchased from (Trittau-Rotexmedica) Germany. Sulfadiazine and trimethoprim were obtained from Aras Bazar Pharmaceutical Company (Amol, Iran). Phosphate-buffered saline (PBS) was acquired from Art-med (Tehran, Iran). All other chemicals used were of analytical grade and commercially available. ParasitesThe Type II Prugniad strain of T. gondii parasite (Pru) was graciously provided by Professor Ahmad Daryani from the Toxoplasmosis Research Center, Mazandaran University of Medical Sciences, Iran. This strain was collected from the brain cysts of chronically infected BALB/c mice. AnimalsA total of 60 male BALB/c mice weighing between 25 and 30 g were sourced from the Pasteur Institute of Iran, North Research Center, Amol, Iran. Following a week of acclimatization, the mice were randomly divided into six groups (10 mice each) as follows. TOXO: Mice were infected with T. gondii through intraperitoneal injection of 103 tachyzoites suspended in 0.1 ml sterile (PBS) (Martynowicz et al., 2020). KET: Schizophrenia was induced by intraperitoneal injection of ketamine at a dosage of 20 mg/kg starting 8 weeks post-infection for 14 days (Monte et al., 2013). TOXO+KET: T. gondii-infected mice received schizophrenia induction using ketamine at a dosage of 20 mg/kg starting 8 weeks post-infection for 14 days. TOXO+SDT: T. gondii-infected mice received oral sulfadiazine and trimethoprim at dosages of 100 and 20 mg/kg, respectively, starting 8 weeks post-infection for 14 days (Mehlhorn et al., 1995). TOXO+KET+SDT: T. gondii-infected mice received intraperitoneal ketamine, oral sulfadiazine, and trimethoprim, at the aforementioned doses, starting 8 weeks post-infection for 14 days. CON: Mice received normal saline instead of any administration and served as the control group (uninfected group). Eight weeks after T. gondii infection, blood was collected from the tails of TOX, TOXO+KET, and TOX+KET+SDT mice to confirm parasite infection through serological analysis of collected serum samples by latex aglutanision kit manufactured by Zist Faravaran Pars (ZFP). At the end of the study, 10th week post-infection, following behavioral tests, the mice were euthanized by cervical dislocation, and their brains were extracted for antioxidant status analysis, as well as the assessment of NMDA-NR1 and NR2A expressions. The schematic study protocol and injections are illustrated in Figure S1. Behavioral TestsOpen-field test (OFT) The OFT was conducted to evaluate anxiety behavior and exploratory activity in rodents. The OFT apparatus consisted of an acrylic glass area (30 × 30 × 15 cm), divided into nine squares. Parameters such as the total number of squares crossed with all paws, crossings and time spent in the border and center of the apparatus, grooming frequency, stretching, flat back approaches, and rearing were recorded for each mouse over a 5-minutes period (Hajizadeh Moghaddam et al., 2023). Elevated plus maze (EPM) The EPM is a reliable behavioral test to assess anxiety-like behavior in mice. The EPM includes two open arms (30 × 5 cm) and two closed arms (30 × 5 × 25 cm) connected by a central platform (5 × 5 cm) raised 30 cm above the floor. Based on innate fear and anxiety tendencies, mice avoid open arms and prefer enclosed ones. Anxiety-related behavior is quantified over a 5-minutes interval by recording the total number of arm entries and the percentage of time spent in open and closed arms (Xie et al., 2020). Y-mazeThe Y-maze is composed of three arms set at a 120° angle, constructed from black plexiglass with dimensions of 39.5 × 8.5 × 13 cm (Prieur and Jadavji, 2019). This maze evaluates short-term spatial working memory in mice by leveraging their natural curiosity to explore unvisited areas. After an introduction to the maze’s center, the mouse is free to explore all three arms. The number of arm entries and returns to each arm is recorded to calculate parameters such as spontaneous alternation performance, which includes visiting three different arms consecutively (alternate arm return) or repeatedly visiting the same arm (same arm return).
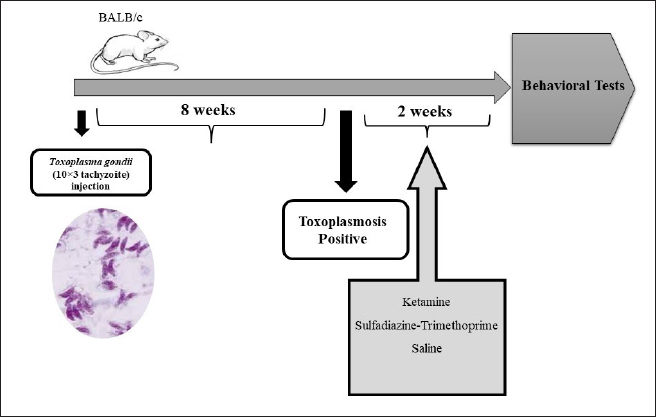
Fig. S1. Schematic diagram of the experimental protocol and injections. Table 1. Measurement and determination of NMDA-NR1 and NMDA-NR2 gene expression levels using specific primers.
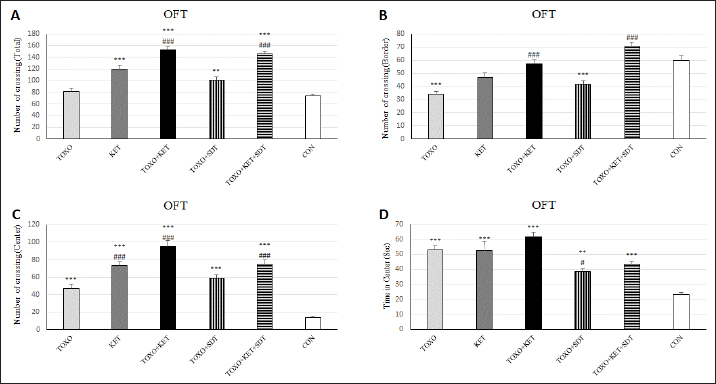
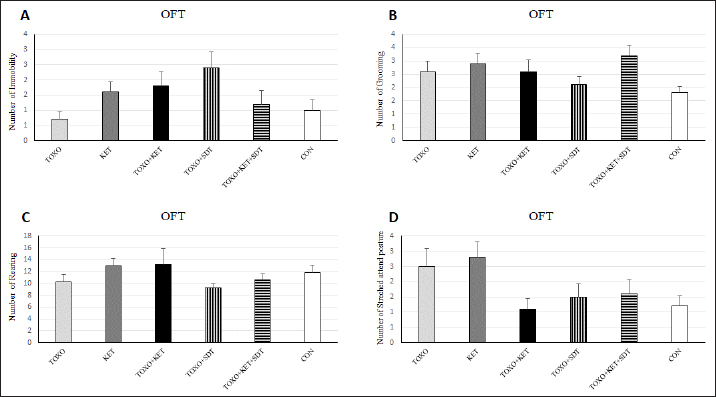
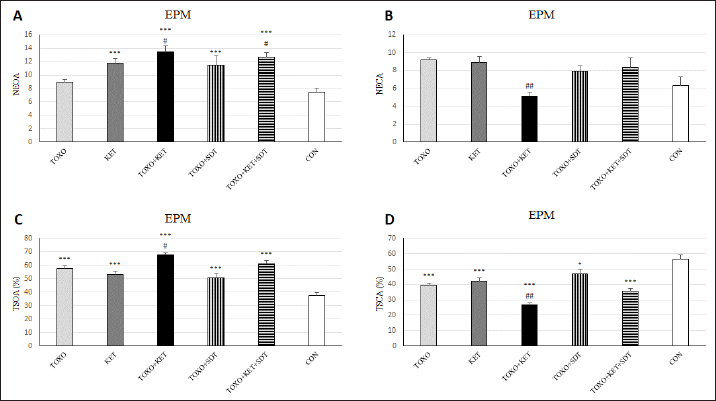
Brain antioxidant status and lipid peroxidationAntioxidant enzyme activities catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) content (a marker of lipid peroxidation) were spectrophotometrically measured in brain homogenates from five mice in each experimental group using commercial kits (Navandsalamat, Iran). NMDA-NR1 and NR2A expressionReal-time reverse transcription PCR was performed as previously described (Sun et al., 2021). Brain samples were homogenized in RNAiso (Takara) using a sonicator. Total RNA was isolated using RNAiso following the manufacturer’s instructions. The isolated 1 μg of RNA was reverse-transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). PCR was carried out using the Thermal Cycler Dice Real-Time System TP800 (Takara) with SYBR Premix Ex Taq II (Takara). The primer sequences are listed (Table 1). Quantitation was done using the crossing point method and normalized to 18S rRNA. The effect of gravity changes on 18S rRNA expression was verified and found to be insignificant. Statistical analysisBehavioral data underwent one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc testing for multiple comparisons between experimental groups. The mRNA expression differences among the groups were analyzed using Tukey’s test. A p-value below 0.05 was considered statistically significant, and all statistical analyses were conducted using SPSS version 26. Ethical approvalThe study was approved by the institutional Research Ethics Committees of Islamic Azad University-Karaj Branch (IR.IAU.K.REC.1401.137). ResultsEffect of treatments on the OFTTo investigate the potential impacts of toxoplasmosis and schizophrenia on the exploratory behavior of mice, we evaluated parameters including the total number of crossings, center crossings, and border crossings in the OFT. As depicted in Figure 1A, the total number of crossings was significantly higher in the TOXO+KET and TOXO + KET + SDT groups compared to CON and TOXO groups (F (5, 54)=36.66, p < 0.001). Notably, the number of border crossings was lower in the TOXO and TOXO+SDT groups relative to the CON (F (5, 54)=19.60, p < 0.001) (Fig. 1B). Moreover, center crossings were elevated in all treated groups compared to CON, with KET, TOXO+KET, and TOXO+KET+SDT displaying significantly higher values than TOXO mice (F (5, 54)=16.48, p < 0.001) (Fig. 1C). However, the time spent in the center was generally higher in all treated groups compared to CON (F (5, 54)=38.77, p < 0.001). Notably, in the TOXO+SDT group, this time was lower than TOXO (F (5, 54)=38.77, p < 0.05) (Fig. 1D). Experimental induction of toxoplasmosis and schizophrenia did not significantly affect the immobility count (F (5, 54)=4.17, p > 0.05) (Fig. 2A), grooming behavior (F (5, 54)=8.74, p > 0.05) (Fig. 2B), rearing activity (F (5, 54)=5.70, p > 0.05) (Fig. 2C), or the number of stretched attend postures (F (5, 54)=2.43, p > 0.05) (Fig. 2D) among the treated mice. Effects of treatments on the EPMTo explore possible anxiolytic-like effects of T. gondii and ketamine in mice, we examined the number of entries into open and closed arms, as well as the time spent in these arms in the EPM. The treated groups, except TOXO, exhibited higher entries into open arms compared to the CON (F (5, 54)=8.40, p < 0.001). Notably, entries into open arms in the TOXO+KET and TOXO+KET+SDT groups were significantly higher than in the TOXO group (F (5, 54)=22.59, p < 0.01) (Fig. 3A). Moreover, the percentage of time spent in open arms was elevated in all treated groups relative to the CON (F (5, 54)=10.17, p < 0.001) (Fig. 3B), while the percentage of time spent in closed arms was reduced in all treated groups compared to CON (F (5, 54)=19.69, p < 0.001) (Fig. 3B). Importantly, the TOXO+KET group displayed the lowest time spent in closed arms, significantly differing from the TOXO group (F (5, 54)=19.69, p < 0.01) (Fig. 3D).
Fig. 1. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET). (TOXO+KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the number of total squares crossed (A), border (B), center crossing (C), and time in the center (D) relative to control (CON) in the open field test (OFT). Values are presented as mean ± SEM for the number of 10 mice per group. *p < 0.05 versus CON, **p < 0.01 versus CON, ***p < 0.001 versus CON, #p < 0.05 versus TOXO, ###p < 0.05 versus TOXO (ANOVA followed by Student–Neuman–Keuls test).
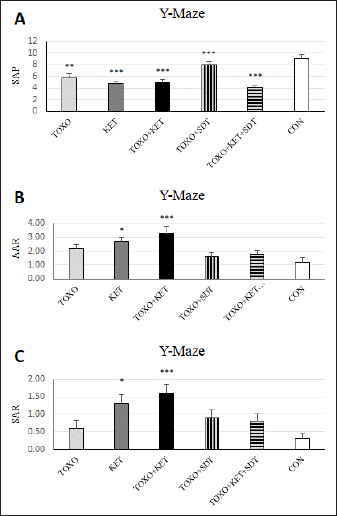
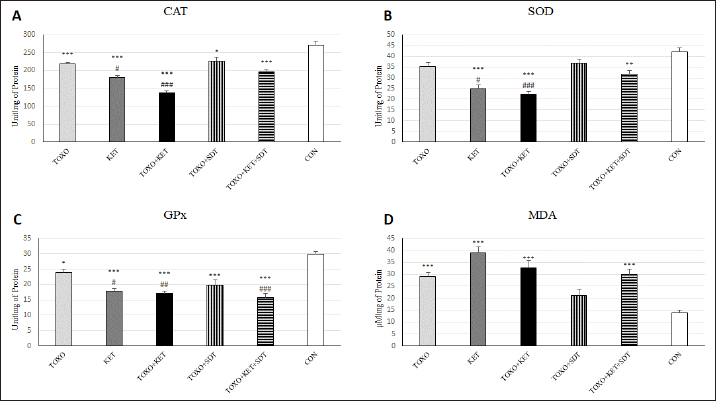
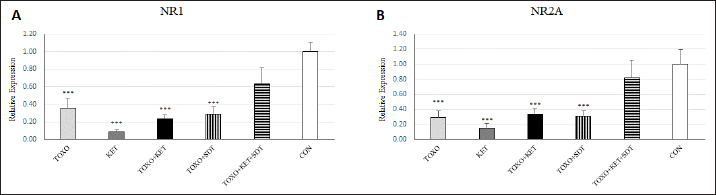
Fig. 2. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the number of immobility (A), grooming (B), rearing (C), and stretched attend posture (D) relative to the control (CON) in the open field test (OFT). Values are presented as mean ± SEM for the number of 10 mice per group (ANOVA followed by Student–Neuman–Keuls test). Effect of treatments on the Y-mazeTo assess potential aberrations in exploratory behavior induced by T. gondii and ketamine, we utilized the Y-maze to calculate measures such as spontaneous alternation performance, alternate arm returns, and same arm returns. Spontaneous alternation performance values were decreased in all treated groups compared to the CON (F (5, 54)=13.07, p < 0.001) (Fig. 4A). However, both alternate arm returns (F (5, 54)=5.55, p < 0.001) (Fig. 4B) and same arm returns (F (5, 54)=4.35, p < 0.01) (Fig. 4C) were elevated in the KET and TOXO+KET groups relative to the CON. Effect of treatments on the brain antioxidant enzymes and lipid peroxidationExperimental toxoplasmosis and schizophrenia led to detrimental effects on brain antioxidant defenses. CAT activity was reduced in all treated groups compared to the CON (F (5, 24)=33.67, p < 0.001) (Fig. 5A). Additionally, CAT levels were lower in the KET and TOXO+KET groups relative to TOXO. SOD activity was lower in the KET, TOXO+KET, and TOXO+KET+SDT groups compared to CON and TOXO (F (5, 24)=18.78, p < 0.001) (Fig. 5B). Similarly, GPx activity was diminished in all treated groups compared to the CON (F (5, 24)=21.61, p < 0.001) (Fig. 5C). Conversely, MDA levels, indicative of lipid peroxidation, were elevated in all treated groups (except TOXO+SDT) compared to the CON (F (5, 24)=14.63, p < 0.001) (Fig. 5D). Effect of treatments on the brain NMDAR-NR1 and NR2A mRNA expressionTo probe the roles of T. gondii and ketamine in NMDAR modulation, we analyzed NR1 and NR2A mRNA expressions in mouse brains. Experimental toxoplasmosis and schizophrenia downregulated NR1 mRNA expression in the whole brain samples of TOXO, KET, TOXO+KET, TOXO+SDT, and TOXO+KET+SDT groups compared to CON (F (5, 24)=12.53, p < 0.001) (Fig. 6A). Similarly, NR2A expression was diminished in all treated groups, except TOXO+KET+SDT, relative to the CON (F (5, 24)=18.48, p < 0.001) (Fig. 6B). DiscussionA growing body of evidence has uncovered intriguing links between toxoplasmosis and schizophrenia. Numerous research endeavors, including large-scale meta-analyses, have proposed T. gondii as a potential causative agent behind a range of neurological and psychiatric symptoms. The consensus from these studies underscores the undeniable connection between toxoplasmosis and a considerable number of schizophrenia cases in humans (Schwarcz and Hunter, 2007; Elsheikha et al., 2016; Fuglewicz et al., 2017). Insights from animal models have further revealed that T. gondii has the capacity to manipulate the natural behaviors of its host, a strategic adaptation aimed at enhancing its distribution (Afonso et al., 2012).
Fig. 3. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the number of entries in open arm (NEOA) (A), entries in closed arms (NECA) (B), time spent in open arms (TSOA%) (C), and time spent in closed arms (TSCA%) (D) relative to the control (CON) in the EPM. Values are presented as mean ± SEM for the number of 10 mice per group. *p < 0.05 versus CON, ***p < 0.001 versus CON, #p < 0.05 versus TOXO, ##p < 0.01 versus TOXO (ANOVA followed by Student–Neuman–Keuls test). In the study by Gatkowska et al. (2012), T. gondii infection was found to curtail exploratory activity in mice, dampening their climbing and rearing tendencies during the OFT. Infected mice exhibited a diminished preference for the central zone, displaying increased time spent in the border areas of the OFT. Additionally, grooming behavior was reduced compared to uninfected counterparts (Gatkowska et al., 2012). In our investigation, the induction of toxoplasmosis did not impact total crossings, yet a significant elevation in center crossings and time spent in the central zone of the OFT was observed in TOXO mice. However, grooming, immobility, and rearing behaviors remained unaffected by toxoplasmosis. Conversely, the administration of ketamine resulted in heightened exploratory activity, evidenced by increased total crossings, center crossings, and time spent in the central area among KET mice. This aligns with previous studies highlighting ketamine-induced hyperlocomotion (Onaolapo et al., 2017; Kokkinou et al., 2021; Moghaddam et al., 2021). When schizophrenia and toxoplasmosis were simultaneously induced in TOXO+KET and TOXO+KET+SDT mice, a notable increase in total, border, and center crossings was observed within these groups. The neurotropic protozoan parasite, T. gondii, has been shown to induce specific behavioral shifts in rodents, including a reduction in predator aversion and a decrease in general anxiety (Boillat et al., 2020). Notably, however, a study by Evans et al. did not yield significant findings regarding T. gondii’s impact on anxiety-related parameters in rats subjected to the EPM test (Evans et al., 2014). In our investigation, although TOXO mice did not exhibit a heightened number of entries into open or closed arms compared to CON mice, they displayed an elevated percentage of time in open arms and a reduced percentage of time in closed arms relative to CON mice. These findings suggest that TOXO reduces anxiety and fear behavior in infected mice. In contrast, KET mice exhibited a greater number of entries into open arms and a higher percentage of time spent in open arms compared to CON mice. This phenomenon aligns with the emerging evidence highlighting the pivotal role of glutamatergic neurotransmission in the underlying biological mechanisms of stress responses and anxiety-related disorders (Bermudo-Soriano et al., 2012). Consequently, drugs targeting glutamatergic neurotransmission, including NMDAR antagonists, show promise as future candidates for pharmacological interventions in anxiety-related behaviors. In our study, the simultaneous induction of schizophrenia and toxoplasmosis in TOXO+KET mice remarkably exhibited anxiolytic-like effects in the behavioral tests. The glutamatergic system’s involvement in fear-conditioning acquisition and extinction is well documented (Walker and Davis, 2002). Furthermore, it has been reported that T. gondii-infected mice struggle with consolidating fear, which resonates with our findings of lower anxiety-related behaviors in TOXO+KET and TOXO+KET+SDT mice. This suggests that ketamine might potentiate the neurological impact of T. gondii on anxiety, and the sulfadiazine-trimethoprim treatment for toxoplasmosis did not effectively counter these effects (Ihara et al., 2016). The same has been observed in our study, in mice of TOXO+KET and TOXO+KET+SDT which showed lower anxiety-related behaviors. Probably, ketamine exacerbates neurological effects of T. gondii on anxiety, and treatment of toxoplasmosis by sulfadiazine-trimethoprim was not able to hamper its effects.
Fig. 4. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the number of spontaneous alteration performance (SAP) (A), alternate arm return (AAR) (B), and same arm return (SAR) (C) relative to the control (CON) in Y-maze. Values are presented as mean ± SEM for the number of 10 mice per group. *p < 0.05 versus CON, **p < 0.01 versus CON, ***p < 0.001 versus CON, #p < 0.05 versus TOXO, ###p < 0.001 versus TOXO (ANOVA followed by Student–Neuman–Keuls test). Our results from the Y-maze test underscore the disruption of short-term memory and cognitive performance, particularly in KET and TOXO+KET mice. This observation mirrors previous reports highlighting spatial and recognition memory deficits induced by NMDAR antagonists (Sun et al., 2021). T. gondii infection has been demonstrated to significantly downregulate genes linked to synaptic transmission and cognitive behavior in the prefrontal cortex of mice (Wu et al., 2023). While the cognitive impairment resulting from parasitic infection is well recognized in animals and humans, there currently exists no effective therapy for the cognitive deficits associated with T. gondii infection. However, in our study, treatment with sulfadiazine-trimethoprim showed some promise in ameliorating memory deficits in treated mice. The brain antioxidant defense mechanisms play a pivotal role in safeguarding against neuronal damage. The neurodegeneration induced by the protozoan parasite T. gondii has been attributed to oxidative damage in the brains of infected mice (Bottari et al., 2016). Ketamine administration in mice disrupted brain antioxidant status and triggered increased lipid peroxidation, a finding corroborated by our observations of decreased levels of CAT, SOD, and GPx, coupled with elevated (MDA levels in TOXO, KET, and especially TOXO+KET mice). This indicates that the compounded adverse effects of combined toxoplasmosis infection and ketamine administration exacerbate the negative impact on brain antioxidant status. While the dopamine theory of schizophrenia has long been a cornerstone in understanding its etiology, recent attention has shifted towards the significant role of NMDARs. In contrast to the dopaminergic model, the complex array of symptoms and neuropsychological dysfunctions in schizophrenia can be more cogently explained through a glutamate/NMDA framework (Kantrowitz & Javitt, 2010). Recent research has unveiled a connection between T. gondii and schizophrenia through cross-reactive mechanisms in the host’s immune responses, which impact NMDAR dysfunction. It’s been suggested that anti-T. gondii immune responses developed during active protozoan infection may cross-react with host NMDARs, ultimately disrupting neural circuits and leading to cognitive deficits (Lucchese, 2017). In our study, a downregulation of NMDAR-NR1 and NR2A was evident in mice subjected to schizophrenia and toxoplasmosis, except for TOXO+KET+SDT mice. This raises the possibility that NR1 and NR2A subunits might play a role in some of the neurological effects induced by T. gondii and ketamine. Nevertheless, despite significant behavioral alterations, no significant shift was noted in NR1 and NR2A expressions among TOXO+KET+SDT mice.
Fig. 5. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the brain activities of CAT (A), SOD (B), GPx (C), and MDA content (D) relative to the control (CON). Values are presented as mean ± SEM for the number of 5 mice per group. *p < 0.05 versus CON, **p < 0.01 versus CON, ***p < 0.001 versus CON, #p < 0.05 versus TOXO, ##p < 0.05 versus TOXO, ###p < 0.001 versus TOXO (ANOVA followed by Tukey’s test).
Fig. 6. Effects of experimental toxoplasmosis (TOXO), ketamine-induced schizophrenia (KET), simultaneous toxoplasmosis + Ketamine-induced schizophrenia (TOXO + KET), and sulfadiazine-trimethoprim as the treatment for toxoplasmosis (TOXO+SDT) and toxoplasmosis + ketamine-induced schizophrenia (TOXO+SDT+KET) on the brain mRNA expression of N-methyl-D-aspartate-NR1 (A) and NR2A (B) relative to the control (CON). Values are presented as mean ± SEM for the number of 5 mice per group. ***p < 0.001 versus CON (ANOVA followed by Tukey’s test). ConclusionIn summary, our study demonstrates significant behavioral changes, including hyperlocomotion, anxiety-related behaviors, and memory deficits, induced by both T. gondii infection and ketamine administration in mice. Additionally, a marked decrease in antioxidant enzyme levels and increased lipid peroxidation were observed in the brain samples of mice experiencing toxoplasmosis and schizophrenia-like symptoms. The downregulation of NMDAR subunits, specifically NR-1 and NR2A, likely contributes to the neurological effects observed with T. gondii and ketamine. Treatment with sulfadiazine-trimethoprim showed a mitigating effect on these changes. AcknowledgmentAuthors would like to express their gratitude toward Samad Nouri for his technical support during this study. Authors’ contributionMRY and SH.SH designed the study and wrote the manuscript. SMM contributed to the interpretation and editing of the manuscript. MRY and SH.SH were responsible for data collection and manuscript submission. All authors reviewed the article and approved the final version of the manuscript. FundingThe study was funded by the Islamic Azad University, Karaj Branch. The funder has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Conflict of interestThe authors declare that they have no conflict of interest. Data availabilityAccess to all relevant data is free upon request directed to the corresponding author. ReferencesAfonso, C., Paixao, V.B. and Costa, R.M. 2012. Chronic toxoplasma infection modifies the structure and the risk of host behavior. PLoS One 7(3), e32489. Alsaady, I., Tedford, E., Alsaad, M., Bristow, G., Kohli, S., Murray, M., Reeves, M., Vijayabaskar, M., Clapcote, S.J. and Wastling, J. 2019. Downregulation of the central noradrenergic system by Toxoplasma gondii infection. Infect. Immun. 87(2), e00789–e00718. Bermudo-Soriano, C.R., Perez-Rodriguez, M.M., Vaquero-Lorenzo, C. and Baca-Garcia, E. 2012. New perspectives in glutamate and anxiety. Pharmacol. Biochem. Behav. 100(4), 752–774. Boillat, M., Hammoudi, P.-M., Dogga, S.K., Pages, S., Goubran, M., Rodriguez, I. and Soldati-Favre, D. 2020. Neuroinflammation-associated aspecific manipulation of mouse predator fear by Toxoplasma gondii. Cell Rep. 30(2), 320–334. Bottari, N.B., Baldissera, M.D., Tonin, A.A., Rech, V.C., Alves, C.B., D’Avila, F., Thome, G.R., Guarda, N.S., Moresco, R.N. and Camillo, G. 2016. Synergistic effects of resveratrol (free and inclusion complex) and sulfamethoxazole-trimetropim treatment on pathology, oxidant/antioxidant status and behavior of mice infected with Toxoplasma gondii. Microb. Pathog. 95, 166–174. Coyle, J.T. 2012. NMDA receptor and schizophrenia: a brief history. Schizophrenia Bull. 38(5), 920–926. Elmore, S.A., Jones, J.L., Conrad, P.A., Patton, S., Lindsay, D.S. and Dubey, J. 2010. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 26(4), 190–196. Elsheikha, H.M., Büsselberg, D. and Zhu, X.-Q. 2016. The known and missing links between Toxoplasma gondii and schizophrenia. Metabol. Brain Dis. 31, 749–759. Evans, A.K., Strassmann, P.S., Lee, I.-P. and Sapolsky, R.M. 2014. Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long–Evans rats. Brain Behav. Immun. 37, 122–133. Fuglewicz, A.J., Piotrowski, P. and Stodolak, A. 2017. Relationship between toxoplasmosis and schizophrenia: a review. Adv. Clin. Exp. Med. 26(6), 1031–1036. Gatkowska, J., Wieczorek, M., Dziadek, B., Dzitko, K. and Dlugonska, H. 2012. Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasitol. Res. 111, 53–58. Hajizadeh Moghaddam, A., Mashayekhpour, M.A. and Tabari, M.A. 2023. Anxiolytic-like effects of citral in the mouse elevated plus maze: involvement of GABAergic and serotonergic transmissions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 396(2), 301–309. Ihara, F., Nishimura, M., Muroi, Y., Mahmoud, M.E., Yokoyama, N., Nagamune, K. and Nishikawa, Y. 2016. Toxoplasma gondii infection in mice impairs long-term fear memory consolidation through dysfunction of the cortex and amygdala. Infect. Immun. 84(10), 2861–2870. Kantrowitz, J.T. and Javitt, D.C. 2010. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 83(3–4), 108–121. Kokkinou, M., Irvine, E.E., Bonsall, D.R., Natesan, S., Wells, L.A., Smith, M., Glegola, J., Paul, E.J., Tossell, K. and Veronese, M. 2021. Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: a translational imaging study with ketamine. Mol. Psychiat. 26(6), 2562–2576. Li, Y., Viscidi, R.P., Kannan, G., McFarland, R., Pletnikov, M.V., Severance, E.G., Yolken, R.H. and Xiao, J. 2018. Chronic Toxoplasma gondii infection induces anti-N-methyl-d-aspartate receptor autoantibodies and associated behavioral changes and neuropathology. Infect. Immun. 86(10), e00398–e00318. Lucchese, G. 2017. From toxoplasmosis to schizophrenia via NMDA dysfunction: peptide overlap between Toxoplasma gondii and N-methyl-d-aspartate receptors as a potential mechanistic link. Front. Psychiat. 8, 37. Martynowicz, J., Doggett, J.S. and Sullivan Jr, W.J. 2020. Efficacy of guanabenz combination therapy against chronic toxoplasmosis across multiple mouse strains. Antimicrob. Agents Chemother. 64(9), e00539–e00520. Matta, S.K., Rinkenberger, N., Dunay, I.R. and Sibley, L.D. 2021. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol. 19(7), 467–480. Mehlhorn, H., Dankert, W., Hartman, P. and Then, R. 1995. A pilot study on the efficacy of epiroprim against developmental stages of Toxoplasma gondii and Pneumocystis carinii in animal models. Parasitol. Res. 81, 296–301. Mendez, O.A. and Koshy, A.A. 2017. Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathogens 13(7), e1006351. Moghaddam, A.H., Maboudi, K., Bavaghar, B., Sangdehi, S.R.M. and Zare, M. 2021. Neuroprotective effects of curcumin-loaded nanophytosome on ketamine-induced schizophrenia-like behaviors and oxidative damage in male mice. Neurosci. Lett. 765, 136249. Monte, A.S., de Souza, G.C., McIntyre, R.S., Soczynska, J.K., dos Santos, J.V., Cordeiro, R.C., Ribeiro, B.M.M., de Lucena, D.F., Vasconcelos, S.M.M. and de Sousa, F.C.F. 2013. Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: possible involvement of antioxidant and nitrergic pathways. J. Psychopharmacol. 27(11), 1032–1043. Onaolapo, A.Y., Aina, O.A. and Onaolapo, O.J. 2017. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed. Pharmacother. 92, 373–383. Parlog, A., Schlüter, D. and Dunay, I.R. 2015. Toxoplasma gondii-induced neuronal alterations. Parasit. Immunol. 37(3), 159–170. Prieur, E.A. and Jadavji, N.M. 2019. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio-protocol 9(3), e3162–e3162. Schwarcz, R. and Hunter, C.A. 2007. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia Bull. 33(3), 652–653. Snyder, M.A. and Gao, W.-J. 2013. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell. Neurosci. 7, 31. Sun, Z.-Y., Gu, L.-H., Ma, D.-L., Wang, M.-Y., Yang, C.-C., Zhang, L., Li, X.-M., Zhang, J.-W. and Li, L. 2021. Behavioral and neurobiological changes in a novel mouse model of schizophrenia induced by the combination of cuprizone and MK-801. Brain Res. Bull. 174, 141–152. Walker, D.L. and Davis, M. 2002. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol. Biochem. Behav. 71(3), 379–392. Weickert, C.S., Fung, S., Catts, V., Schofield, P., Allen, K., Moore, L., Newell, K.A., Pellen, D., Huang, X.-F. and Catts, S. 2013. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiat. 18(11), 1185–1192. Wu, Y., Xu, D., He, Y., Yan, Z., Liu, R., Liu, Z., He, C., Liu, X., Yu, Y. and Yang, X. 2023. Dimethyl itaconate ameliorates the deficits of goal-directed behavior in Toxoplasma gondii infected mice. PLoS Neglec. Trop. Dis. 17(5), e0011350. Xie, L., Liu, Y., Hu, Y., Wang, B., Zhu, Z., Jiang, Y., Suo, Y., Hu, M., Gao, J. and Ullah, R. 2020. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice. Translational Psych 10(1), 202. Youssefi, M., Arabkhazaeli, F. and Hassan, A.T.M. 2009. Seroprevalence of Neospora caninum infection in rural and industrial cattle in northern Iran. Iran. J. Parasitol. 4(1), 15–18. Youssefi, M., Sefidgar, A., Mostafazadeh, A. and Omran, S.M. 2007. Serologic evaluation of toxoplasmosis in matrimonial women in Babol, Iran. Pakistan J. Biol. Sci. 10(9), 1550–1552. | ||
| How to Cite this Article |
| Pubmed Style Masoumi SM, Youssefi MR, Shojaei SSR. Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Vet. J.. 2024; 14(7): 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 Web Style Masoumi SM, Youssefi MR, Shojaei SSR. Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. https://www.openveterinaryjournal.com/?mno=198580 [Access: August 31, 2025]. doi:10.5455/OVJ.2024.v14.i7.13 AMA (American Medical Association) Style Masoumi SM, Youssefi MR, Shojaei SSR. Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Vet. J.. 2024; 14(7): 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 Vancouver/ICMJE Style Masoumi SM, Youssefi MR, Shojaei SSR. Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Vet. J.. (2024), [cited August 31, 2025]; 14(7): 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 Harvard Style Masoumi, S. M., Youssefi, . M. R. & Shojaei, . S. S. R. (2024) Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Vet. J., 14 (7), 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 Turabian Style Masoumi, Seyedeh Mina, Mohammad Reza Youssefi, and Seyed Shapoor Reza Shojaei. 2024. Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Veterinary Journal, 14 (7), 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 Chicago Style Masoumi, Seyedeh Mina, Mohammad Reza Youssefi, and Seyed Shapoor Reza Shojaei. "Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential." Open Veterinary Journal 14 (2024), 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 MLA (The Modern Language Association) Style Masoumi, Seyedeh Mina, Mohammad Reza Youssefi, and Seyed Shapoor Reza Shojaei. "Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential." Open Veterinary Journal 14.7 (2024), 1634-1643. Print. doi:10.5455/OVJ.2024.v14.i7.13 APA (American Psychological Association) Style Masoumi, S. M., Youssefi, . M. R. & Shojaei, . S. S. R. (2024) Exploring the interplay of chronic toxoplasmosis and NMDAR dysfunction: Insights into schizophrenia-like behaviors and therapeutic potential. Open Veterinary Journal, 14 (7), 1634-1643. doi:10.5455/OVJ.2024.v14.i7.13 |