| Case Report | ||
Open Vet. J.. 2024; 14(7): 1716-1725 Open Veterinary Journal, (2024), Vol. 14(7): 1716–1725 Case Report Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literatureAlessandra Recchia, Serena Digiaro, Antonella Colella, Beatrice Greco and Paola Paradies*Department DiMePre-J, Veterinary Section, University of Bari “Aldo Moro”, Bari, Italy *Corresponding Author: Paola Paradies. Department DiMePre-J, Veterinary Section, University of Bari “Aldo Moro”, Bari, Italy. Email: paola.paradies [at] uniba.it Submitted: 02/05/2024 Accepted: 03/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
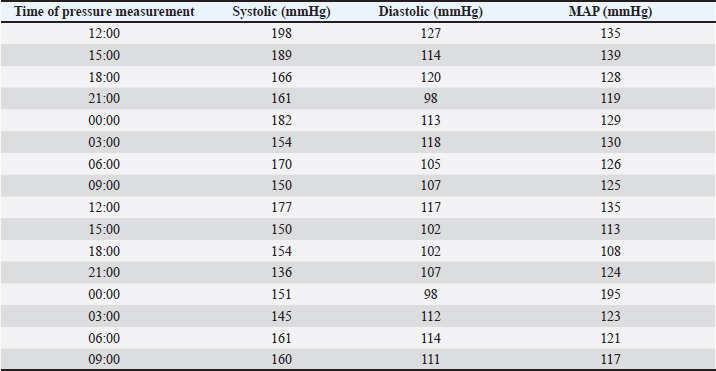
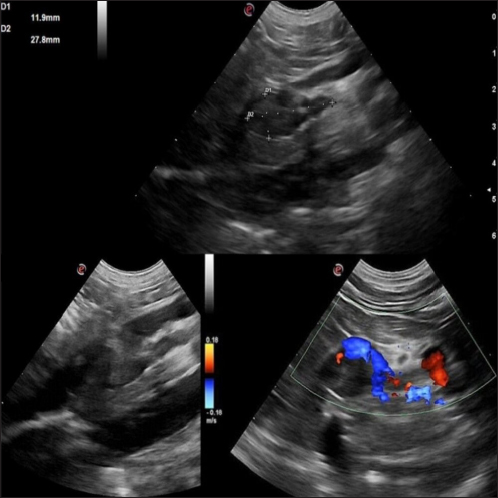
AbstractBackground: Canine pheochromocytomas (PCCs) are rare tumors of the adrenal medulla. Clinical signs are often vague, resulting in intermittent catecholamine over secretion or neoplastic invasion of adjacent structures. Case Description: A 12-year-old Epagneul Breton dog with a 1-year history of chronic kidney disease, was examined for acute onset of severe neurological signs. Based on clinical and instrumental data, hypertensive encephalopathy was suspected. Cardiac and abdominal ultrasound were performed. Severe hypertensive cardiopathy and a right adrenal gland mass with invasion of the caudal vena cava were diagnosed. Computed tomography imaging confirmed the suspect of invasive malignant neoplasia. Emergency pharmacological therapy was started to reduce systemic pressure, improve clinical signs, and stabilize the dog in view of surgical resolution. After initial improvement, patient conditions abruptly worsened, and euthanasia was elected. Histology examination confirmed a right adrenal PCC, with caval invasion. Conclusion: To the authors’ conclusions, acute hypertensive encephalopathy is a peculiar manifestation of PCCs. Ultrasound is a useful, and rapid test to suspect PCC as it can detect adrenal alterations, caval invasion, metastasis, and cardiac sequelae consistent with the condition. PCC can mimic multiple affections, and be misinterpreted, especially when a concurrent disease has already been diagnosed. Veterinarians need to be aware that comorbidities could mask clinical signs and delay diagnosis. Furthermore, this clinical case reminds us to include PCC also in the differential diagnosis of dogs with an acute onset of severe neurological signs. Keywords: Dog, Encephalopathy, Hypertension, Pheochromocytoma. IntroductionPheochromocytomas (PCCs) are catecholamine-producing neuroendocrine tumors that arise from the chromaffin cells (pheochromocytes) of the adrenal medulla or sympathetic paraganglia (Gilson et al., 1994b). Usually, this rare tumor is solitary and is located in or about the adrenal gland (Maher and McNiel, 1997). It can be benign or malignant and may be functionally active or inactive (Gilson et al., 1994a). When active, PCCs can produce and excrete the polypeptide hormones epinephrine, norepinephrine, and occasionally dopamine. When non-active they can still be capable of producing clinical signs by virtue of their space-occupying nature (Maher and McNiel, 1997). Therefore, clinical signs either result from the neoplastic production of catecholamines (e.g., episodic weakness, restlessness, tachycardia, hypertension, and collapse), or from the space-occupying nature of the tumor. PCCs affect middle-aged to older dogs with no gender or breed predilection. Because of the vague nature of their manifestations, they frequently are diagnosed as an incidental finding in dogs and in human beings (Manger and Gifford, 1993; Werbel and Ober, 1995; Barthez et al., 1997; Feldman and Nelson, 2003). Based on the rarity of the condition, the difficulties in ante mortem diagnosis, and the low number of cases reported, additional reporting to describe clinical aspects of diagnosed PCC in dogs is indicated. The aim of this study is to document a clinical case of an acute onset of hypertensive encephalopathy in a dog with right adrenal PCC and neoplastic invasion of the caudal vena cava (CVC) with a brief review of the literature. Case DetailsA 12-year-old spayed female Épagneul Breton dog, weighing 14.5 kg, was examined at the Veterinary Medical Teaching Hospital of University of Bari, Italy for acute onset of central neurological clinical signs. The owner reported that the day before consulting, the dog showed circling, loss of balance, disorientation, and nocturnal vocalizations. The dog had a 1-year history of polyuria, polydipsia, chronic kidney disease (CKD stage II IRIS), and in the late months, progressive weight loss, and tremors. On clinical examination, the animal was tachypnoeic, depressed with an altered mental status, and ataxic. It showed right head tilt, right drifting, and head pressing (Fig. 1). The patient was hospitalized, and emergency laboratory tests were run, showing an increase of urea (193 mg/dl; reference interval: 15–50 mg/dl), and creatinine (2.89 mg/dl; reference interval: 0.70–1.40 mg/dl). Glucose was in the reference interval (125 mg/dl; reference interval: 70–130 mg/dl), and the whole count blood was normal. Repeated indirect blood pressure measurements were also taken (using SunTech Vet30) (Table 1) showing severe hypertension. EKG examination showed no abnormalities. Brain computer tomography (CT) in emergency resulted in negative. A hypertensive encephalopathy was suspected. In the differential diagnosis, traumatic, toxic, and vascular central damage were also considered. Other causes, including central neoplasms, were included although considered less probable due to the rapid onset of the neurological signs. Being the dog a housed pet and based on the patient’s medical history, the hypothesis of trauma and toxic ingestion were ruled out. An echocardiographic evaluation was performed (ESAOTE Mylab Alpha). Left ventricular concentric hypertrophy along with aortic bulb dilatation, aortic regurgitation, and interventricular septal hypertrophy, in the absence of aortic stenosis, were assessed on ultrasound, suggesting a severe hypertensive cardiopathy. The abdominal ultrasound examination (Fig. 2) revealed an abnormal right adrenal gland with the presence of a not-occluding structure in the lumen of the CVC. The right adrenal gland cranial pole appeared to increase in volume (12 × 25 mm), with a mixed echogenicity, dishomogeneity, and an irregular profile with the invasion of the CVC. At color Doppler, a reduced residual flow was documented at that site in CVC.
Fig. 1. Dog presented in head pressing and with altered mental status. Table 1. Blood pressure values during the three days of hospitalization from treatment starting.
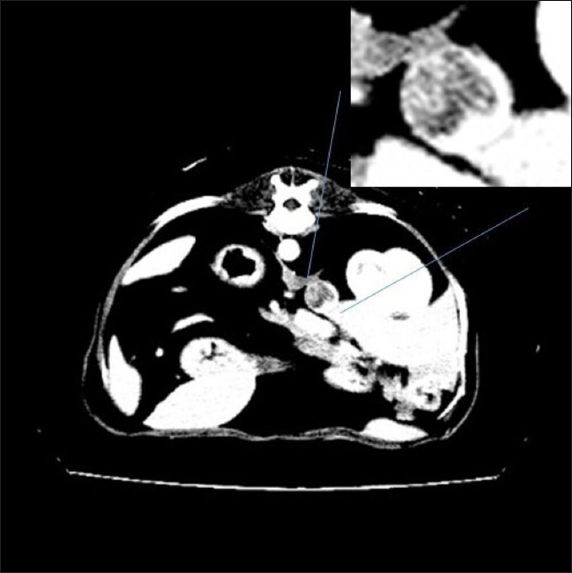
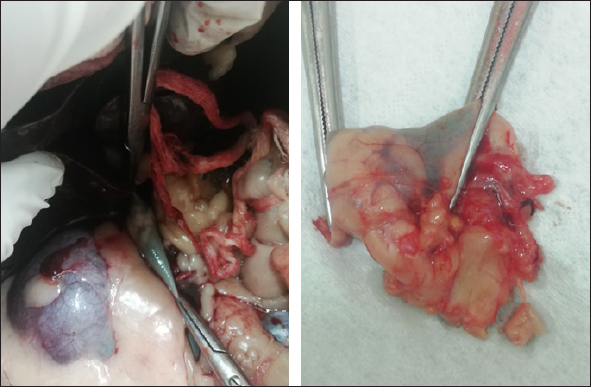
Fig. 2. Right adrenal gland showed an irregular profile and dishomogeneity and the CVC was invaded by a mass not completely occluding the vassel. A residual flow is visible with color Doppler through the vessel. An adrenal neoplasm with invasion of the CVC was suspected and it was decided to perform a total body contrast-enhanced CT investigation to better investigate the adrenal mass and the caval involvement, to evaluate other possible metastases. CT confirmed the presence of a mass at the adrenal level and a structure compatible with a neoplastic thrombus in the CVC (Fig. 3). In the meantime, the patient was hospitalized and treated with: nitroglycerin patch (10 mg/sid), labetalol (0.25 mg/kg/ev, then 25 µg/kg/minute/CRI), furosemide (0.5 mg/kg/hour/CRI), prazosin (1 mg/os), and benazepril (0,5 mg/kg/os/bid), with the aim to reduce the systemic pressure. The patient initially responded to pharmacological therapy, its general conditions improved in 3 days, and, on the owner’s request, the dog was discharged pending adrenalectomy surgery decisions. At discharging, the dog was able to walk without falling, conscious, and reactive to stimulus. Unfortunately, after a few days at home, the clinical signs deteriorated, and in sight of the patient’s general conditions, the owners elected humane euthanasia. A necropsy was performed. In the abdominal cavity, the mass of the right adrenal gland invading the CVC was visualized (Fig. 4). It appeared like a clear, yellowish-looking neoformation. The vessel was grossly distended but it was not completely obstructed. A single specimen including the adrenal mass and the invaded CVC was submitted for histologic examination. Histological diagnosis confirmed the presence of right adrenal PCC with neoplastic invasion of the CVC. A non-capsulated, not-circumscribed neoplasm that completely replaced the right adrenal medulla, compressing, and also infiltrating the adrenal cortex was described. The neoplasm was characterized by polygonal cells arranged in compact, lobules, that often were in a palisade pattern. Neoplastic cells had eosinophilic to brownish granular cytoplasm, irregularly round nuclei with finely punctuated chromatin, and distinct nucleolus. Occasionally, the neoplastic cells arranged in a palisade pattern around the blood vessels, forming sort of rosettes and they presented multifocal areas of necrosis with diffuse hemorrhagic extravasation, fibrin deposition, and rare macrophages. The specimen had moderate anisokaryosis and mild anisocytosis.
Fig. 3. Contrast-enhanced computed tomography (CT) scan; on the top, a particular of the previous image showing the structure invading CVC lumen (enhanced by contrast medium).
Fig. 4. Post-mortem findings showing the right adrenal gland invading the vena cava in situ and after excision. The portion of caval vessel invaded by the neoplastic gland is between the surgical forceps. DiscussionThis article documents a case of PCC of the right adrenal gland invading the CVC in a dog showing acute hypertensive encephalopathy. Adrenal tumors are common in dogs and may secrete an excessive amount of one or more types of hormones, causing tumor-related syndromes (Bertazzolo et al., 2014; Lee et al., 2020). They may originate in the cortex (adenoma, adenocarcinoma, and metastatic neoplasia) or medulla (PCCs, neuroblastoma, ganglioblastoma, and myelolipoma) (Drazner, 1987). In dogs, PPCs account for approximately 0.01%–0.1% of all canine tumors (Galac and Korpershoek, 2017), and affect middle-aged to older animals with no apparent sex or breed predilection (Barthez et al., 1997). They are usually solitary, slow-growing tumors that extend into the lumen of adjacent vessels, particularly the CVC (Barthez et al., 1997; Herrera et al., 2008; Gostelow et al., 2013). In dogs, PCCs should be considered malignant, due to the high incidence of neoplastic invasion into surrounding tissues (up to 56% of cases (Barthez et al., 1997; Kyles et al., 2003; Barrera et al., 2013; Galac and Korpershoek, 2017), and the metastatic behavior. The clinical signs associated with canine PCC are typically non-specific and depend on the active or inactive function of the tumor. When it is active, most of the associated clinical manifestations can be explained on the basis of the pharmacologic effects of catecholamines, resulting in hypertension (Twedt and Wheeler, 1984). They include, but are not limited to, polyuria, polydipsia, weakness, exercise intolerance, lethargy, respiratory signs (panting, dyspnea, coughing), episodic collapse, anorexia, weight loss, vomiting, anxiety, tremors, restlessness, irritability, depression, neurologic signs, retinal hemorrhage, retinal detachment, epistaxis, pulse deficits, systolic murmur, and arrhythmia (Gilson et al., 1994a; Barthez et al., 1997). Among arrhythmias, premature supraventricular and ventricular complexes and tachycardia are the most common. The conduction disturbances are due to myocardial damage, ischemia, and fibrosis, which are a result of prolonged exposure to catecholamines from the PCC (Mak and Allen, 2013; Edmondson et al., 2015). Some patients may present acute signs associated with a hypertensive crisis (shock, pulmonary edema, ventricular fibrillation, or cerebral hemorrhage) (Twedt and Wheeler, 1984). Our patient was presented for an acute onset of severe neurological signs due to hypertensive encephalopathy in the absence of cerebral hemorrhage. This report highlights that PCC should be included also in the differential diagnosis of dogs presented in emergencies with acute central neurological disorders. The pattern of secretion of PCCs can be persistent but more often is paroxysmal (Maher and McNiel, 1997). The pharmacologic effects of the catecholamines and the subsequent hypertension produced can be episodic. In fact, arterial hypertension has been documented in about 50% of affected patients during clinical examination (Barthez et al., 1997). Subsequently, a normotensive state during physical examination does not rule out the disease (Barthez et al., 1997). The concurrent presence of PCCs with other pathological conditions (e.g., diabetes mellitus, hyperadrenocorticism, hepatic disease, renal disease, and other neoplasms) has often been reported (Maher and McNiel, 1997; Feldman and Nelson, 2003). Hematologic, and biochemical abnormalities are usually unspecific in a patient with PCC and can derive from organ damage resulting from the hypertensive state. Thus, ante-mortem diagnosis is extremely challenging, requires a high index of suspicion and is often achieved postmortem. In our case, ambiguous clinical signs (polyuria, polydipsia, and weight loss) and systemic hypertension were reported in the past year’s history and were interpreted as consequences of CKD. Anyway, it is not possible to exclude the possibility that a non-manifest PCC was already present and that kidneys’ damage resulted from the hypertensive state and not vice-versa. In recent years, a biochemical diagnosis of PCC in dogs has become possible. It is based on the measurement of plasma and urinary catecholamines, in particular their metabolites: metanephrines (MTNs) such as free normetanephrine (NMN), and metanephrine (MN) (Kook et al., 2010; Gostelow et al., 2013; Green and Frank, 2013; van den Berg et al., 2023). Recently, in human medicine, the measurement of salivary MTNs has been shown to be a promising tool in the biochemical diagnosis of PCC (Eijkelenkamp et al., 2021) as well as a high value of urinary vanillylmandelic acid (Soler Arias et al., 2021). In veterinary medicine, van den Berg et al., (2023) have tried to determine reference intervals for plasma, urinary, and salivary-free MTNs, NMN, and 3-methoxytyramine in a large population of healthy dogs. They also assessed an upper reference limit for plasma-free NMN (3.56 nmol/l) which has shown good diagnostic performance in detecting PCC with high sensitivity and specificity. Differently, measurements of plasma and urine catecholamine concentrations showed poor diagnostic sensitivity and specificity for identifying PCC (Hickman et al., 2009; Green and Frank, 2013). Although PCC should stimulate high circulating catecholamine concentration in the patient blood or urine, levels may be normal because of their variable secretion. Furthermore, high levels of stress, excitement, or concurrent disease can falsely alter the catecholamine concentration in healthy patients (Maher and McNiel, 1997). Differently, because of their continuous neoplastic production and secretion, MTNs have higher diagnostic accuracy (Eisenhofer et al., 2004). In fact, free MTNs are catecholamine metabolites that enter the blood stream once they have formed. In patients with PCCs, they are derived almost entirely from catecholamine metabolism within the tumor. This process happens regardless of the variable secretion of catecholamines and the quantity of free MTNs produced (and subsequently present in the blood stream) is proportional to the chromaffin cell mass, and therefore it is proportional to the neoplastic invasion (Eisenhofer et al., 2004, 2005) without sympathetic catecholamine release influence (Gostelow et al., 2013). Currently, there is no consensus about the use of plasma or urine, but a strong preference for NMN determination (Salesov et al., 2015). In fact, either urine or plasma-free NMN concentration showed high sensitivity and specificity for diagnosis of PCC, whereas free MN concentration showed moderate sensitivity and high specificity (Gostelow et al., 2013). Furthermore, the urinary and plasma-free NMN have shown superiority in differentiating between PCC, hypercortisolism (HC), and nonadrenal disease (Salesov et al., 2015). It must be remembered that discerning between PCC and HC could be challenging in clinical practice, since adrenal medulla or cortex tumor cannot be distinguished on ultrasound, and many clinical signs of PCC and HC overlap. In our case, we did not need a biochemical diagnosis because the evidence was enough to put PCC at the top of the differential diagnosis (constant systemic hypertension, adrenal mass with caval invasion, negative cranic computed tomography, CT). In our case, ultrasound was a basic and immediate means to address to the final diagnosis documenting an adrenal mass invading the CVC. Furthermore, a severe hypertensive cardiopathy was documented at echocolordoppler further supporting the suspected diagnosis. In our case, contrast-enhanced ultrasound (CEUS) and/or fine needle aspiration (FNA) sampling were not performed. Due to the presence of caval invasion, malignancy was immediately suspected, and a CT evaluation was preferred. Once the CVC invasion was also confirmed by CT, a surgical approach was planned. Ultrasound imaging is a rapid, non-invasive, and reliable modality to evaluate suspected adrenal lesions. An adrenal mass can be detected by ultrasonography in 50%–83% of cases of canine PCC (Gilson et al., 1994a; Maher and McNiel, 1997; Feldman and Nelson, 2003), but failing at visualizing it does not rule out a possible PCCs diagnosis. Although not pathognomonic, structural features (i.e., lesion shape, size, and echotexture) are often useful diagnostic criteria. Large masses or nodules ≥ 2 cm strongly suggest malignant adrenal gland neoplasia (Cook et al., 2014; Pagani et al., 2016). At ultrasound examination, PCC can appear as a large, irregular, amorphous, encapsulated mass, associated with loss of shape and parenchymal structure. Mixed echogenicity, due to the presence of hemorrhagic/necrotic areas, is also characteristic. Most PCC are unilateral (only 10% are bilateral) (Rosenstein, 2000; Kyles et al., 2003), and the contralateral adrenal gland is normal in size and shape. PCCs in dogs are more likely to be aggressive and vascular invasion and metastases are commonly reported, respectively in up to 85% and 40% of dogs (Barthez et al., 1997; Feldman and Nelson, 2003; Cook et al., 2014; Gregori et al., 2015; Pagani et al., 2016). Tumor thrombus may extend into the phrenicoabdominal vein and CVC (Pagani et al., 2016). Even though CEUS examination of adrenal lesions is poorly reported in the veterinary literature, CEUS imaging can be a valuable tool in order to assess the malignancy of adrenal lesions. Few studies describe the features of PCC during CEUS examination (Bargellini et al., 2016; Nagumo et al., 2020). PCC seems to be characterized by a fast wash-in followed by a seemingly fast wash-out and shows both hypoperfused areas and intralesional microcirculation, as a probable result of the presence of hemorrhagic and necrotic areas and tumor neo-angiogenesis (Burti et al., 2023). CEUS can also potentially differentiate PCC from other adrenal conditions (i.e., adenocarcinoma and cortical adenoma). PCC, in fact, has a significantly lower mean transit time compared to both adenocarcinoma and cortical adenoma (Bargellini et al., 2016; Nagumo et al., 2020; Burti et al., 2023). However, cytology and histology are necessary to obtain the final diagnosis. CT has now become a routine exam for preoperative assessment of dogs with adrenal mass. It is considered more accurate than ultrasonography for the assessment of vascular invasion (Lunn and Page, 2013). When a PCC is suspected, CT scanning is the gold standard and a prerequisite before surgery is planned. It allows to conduct a screening for possible metastasis (including lymph-nodes, liver, lungs, kidneys, spleen, and bone) (Gilson et al., 1994a), and assessing the vascular invasion influences the surgical approach for the tumor resection (Kyles et al., 2003). Both humans and dogs have a heterogeneous histologic composition of adrenal neoplasm, with variable amounts of hemorrhage, necrosis, and/or mineralization occurring in both benign and malignant ones (Johnson et al., 2009). Therefore, different adrenal neoplasms may appear similar in CT (Morandi et al., 2007; Schultz et al., 2009). PCC, like other neuroendocrine tumors, has typical cytologic features: naked uniform nuclei, typical disposition of nuclei in rows and rosette-like structures, and fine chromatin with inconsistent nucleoli (Pey et al., 2020). Cytology can be an immediate, minimally invasive method to discern the origin of a primary adrenal mass (as PCC). In fact, can be a useful tool in discerning a cortical tumor from a medullary one, since discerning the two of them can be challenging if only sustained by clinical, diagnostic imaging, and laboratory findings. In dogs, FNA can be performed percutaneously under ultrasound guidance, as a minimally invasive procedure. Even though the risk of complications (bleeding, hematic contamination, and neoplastic spreading along the needle path) discourages veterinarians from performing adrenal cytologic samples, in veterinary medicine, risk assessment is only anecdotal. Evidence is based on few reports in human literature (McCorkell and Niles, 1985; Casola et al., 1986; Sood et al., 2007; Vanderveen et al., 2009), in which are reported complications after PCC aspiration (fatal hemorrhage, hypertensive crisis or paradoxical hypertensive and hypotensive crises). Some studies (Sumner et al., 2018; Pey et al., 2020) suggest that, in optimal conditions, FNA of adrenal lesions can be considered a minimally risky procedure. Pey et al., (2020), in their study, have reported that the complication rate was similar to what has recently been published about FNAs of the adrenal lesions and comparable to the complication rate of FNAs of other abdominal organs. Although for the majority of adrenal tumors, the resolution is surgical, cytological examination allows the clinician to advance a more accurate diagnostic suspicion of PCC and to implement preoperative pharmacological therapies before surgical procedures. The definitive diagnosis of PCC relies on histologic examination of the adrenal mass (Herrera et al., 2008; Gostelow, 2013). Histological PCC appearance can vary. Usually, they present a typical morphology, characterized by polygonal cells with round to oval nuclei and prominent nucleoli, granular cytoplasm arranged in small nest, separated by fibrovascular stroma. Tumor cells are often subdivided into small lobules by connective tissue septa and capillaries, creating a papillary pattern (Rosenstein, 2000). However, morphology can be atypical. In these cases, immunohistochemistry plays an important role in confirming diagnosis (Cheung et al., 2018). Pharmacological therapy is not recommended except in the case of inoperable or metastatic PCCs. It consists of the administration of alfa-blocker (phenoxybenzamine or prazosin). Calcium channel blockers may be of use in controlling hypertension, due to their blocking action on synaptic calcium channels and direct vasodilatory effects (Maher and McNiel, 1997). Also, since preoperative administration of an alpha-blocker (phenoxybenzamine or prazosin) has reduced mortality in human patients (Lucon et al., 1997; Roizen et al., 1983), and previous studies have showed a significant decrease in perioperative mortality in dogs when preoperative alpha-blocker therapy was given, pharmacological treatment with alpha-blockers is recommended before surgery (Gilson et al., 1994b; Herrera et al., 2008). A low dose should be used initially with a gradual increase to achieve normotension. Beta-blocking agents may be used to control arrhythmias or tachycardia but should never be used without concurrent alpha-blockade to avoid a state of severe hypertension with loss of vasodilatory effects (Maher and McNiel, 1997). In our dog, supportive pharmacological therapy was immediately started to reduce systemic pressure with the aim to contrast hypertensive encephalopathy and stabilize the patient as much as possible until surgery decisions were taken. The emergency protocol included labetalol, a combined alpha- and beta-adrenoceptor blocking agent that was used off label with some positive results in the first 72 hours of monitoring. Despite the scant literature in terms of dosage and effects in dogs, we choose to continue with the alpha blocker prazosin at 1 mg/os. The drug is more commonly used for urinary tract obstruction in cats. Adrenalectomy is the treatment of choice for PCC. Surgical resection of invasive PCC can be technically demanding but in the absence of local invasion, it can often be completely resected (Twedt and Wheeler, 1984). Nonresectable tumors should be debulked as much as possible to reduce the circulating catecholamine concentrations and improve the efficacy of pharmacological management (Maher and McNiel, 1997). Potential risk factors associated with poor short-term survival time in dogs undergoing adrenalectomy include size, tumor type, additional surgical procedures performed in the same anesthetic event, metastasis, and acute adrenal hemorrhage (Schwartz et al., 2008; Lang et al., 2011; Massari et al., 2011; Barrera et al., 2013). After a resection of a functional PCC, postoperative mortality can be influenced by the patient’s response to a sudden removal of the source of catecholamine release (Enright et al., 2022). When the tumor is excised, the decrease in circulating catecholamines may lead to pronounced hypotension (Maher and McNiel, 1997), which sometimes can be considered refractory, a postoperative complication with (or without) systemic consequences (Enright et al., 2022). Undiagnosed metastases may be present if blood pressure does not decline after the surgical adrenal removal (Twedt and Wheeler, 1984; Maher and McNiel, 1997; Feldman and Nelson, 2003). Short-term mortality rates after adrenalectomy have decreased in the last few years, as Enright et al., (2022), have shown in their study. They assessed a short-term surgical success rate with 44/53 dogs (83%) surviving to discharge from the hospital. Unfortunately, our patient’s clinical conditions abruptly deteriorated before surgery could have been performed. Histology examination confirmed a PCC of right adrenal gland, invading the CVC. This article describes a dog developing acute hypertensive encephalopathy due to PCC and highlights that PCC should be included in the differential diagnosis of dogs presented in emergency with acute central neurological disorders. Ultrasound is a useful and immediate test that should be included in the diagnostic protocol of dogs with acute onset of neurological signs with or without documented hypertension. In fact, a normotensive state during physical examination does not rule out the disease. Also, echocardiographic features of hypertensive cardiopathy could help to address the diagnosis even in the absence of registered hypertension. Despite the final exitus of this specific case, pharmacological therapy seems to be able to reduce systemic pressure with the initial improvement of clinical conditions. Early diagnosis is particularly relevant in view of a resolutive surgical approach. Veterinarians need to be aware that comorbidities (i.e., CKD) could mask clinical signs and delate the diagnosis. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsRecchia A. and Paradies P. conceptualized, wrote, and supervised the article. Greco B., Digiaro S., and Colella A. provided their expertise on clinical and biochemical aspects. FundingThis research received no specific grant. Data availabilityAll the data are presented within this article. ReferencesBargellini, P., Orlandi, R., Dentini, A., Paloni, C., Rubini, G., Fonti, P., Diana, A., Peterson, M. E. and Boiti, C. 2016. Use of contrast-enhanced ultrasound in the differential diagnosis of adrenal tumors in dogs. J. Am. Anim. Hosp. Assoc. 52, 132–143. Barrera, J.S., Bernard, F., Ehrhart, E.J., Withrow, S.J. and Monnet, E. 2013. Evaluation of risk factors for outcome associated with adrenal gland tumors with or without invasion of the caudal vena cava and treated via adrenalectomy in dogs: 86 cases (1993–2009). J. Am. Vet. Med. Assoc. 242, 1715–1721. Barthez, P.Y., Marks, S.L., Woo, J., Feldman, E.C. and Matteucci, M. 1997. Pheochromocytoma in dogs: 61 cases (1984-1995). J. Vet. Intern. Med. 11, 272–278. Bertazzolo, W., Didier, M., Gelain, M.E., Rossi, S., Crippa, L., Avallone, G., Roccabianca, P., Bonfanti, U., Giori, L. and Fracassi, F. 2014. Accuracy of cytology in distinguishing adrenocortical tumors from pheochromocytoma in companion animals. Vet. Clin. Pathol. 43, 453–459. Burti, S., Zotti, A., Rubini, G., Orlandi, R., Bargellini, P., Bonsembiante, F., Contiero, B., Bendazzoli, M. and Banzato, T. 2023. Contrast-enhanced ultrasound features of adrenal lesions in dogs. Vet. Rec. 193, e2949. Casola, G., Nicolet, V., vanSonnenberg, E., Withers, C., Bretagnolle, M., Saba, R.M. and Bret, P.M. 1986. Unsuspected pheochromocytoma: risk of blood pressure alterations during percutaneous adrenal biopsy. Radiology. 159, 733–735. Cheung, V.K.Y., Gill, A.J. and Chou, A. 2018. Old, new, and emerging immunohistochemical markers in pheochromocytoma and paraganglioma. Endocr. Pathol. 29, 169–175. Cook, A.K., Spaulding, K.A. and Edwards, J.F. 2014. Clinical findings in dogs with incidental adrenal gland lesions determined by ultrasonography: 151 cases (2007-2010). J. Am. Vet. Med. Assoc. 244, 1181–1185. Drazner, F.H. 1987. Small animal endocrinology. London, UK: Churchill Livingstone. Edmondson, E.F., Bright, J.M., Halsey, C.H. and Ehrhart, E.J. 2015. Pathologic and cardiovascular characterization of pheochromocytoma associated cardiomyopathy in dogs. Vet. Pathol. 52, 338–343. Eijkelenkamp, K., Osinga, T.E., van Faassen, M., Kema, I.P., Kerstens, M.N., Pacak, K., Sluiter, W.J., Links, T.P. and van der Horst-Schrivers, A.N.A. 2021. Diagnostic accuracy of salivary metanephrines in pheochromocytomas and paragangliomas. Clin. Chem. 67, 1090–1097. Eisenhofer, G., Kopin, I.J. and Goldstein, D.S. 2004. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 56, 331–349. Eisenhofer, G., Lenders, J.W.M., Goldstein, D.S., Mannelli, M., Csako, G., Walther, M.M., Brouwers, F.M. and Pacak, K. 2005. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin. Chem. 51, 735–744. Enright, D., Dickerson, V.M., Grimes, J.A., Townsend, S. and Thieman Mankin, K.M. 2022. Short and long-term survival after adrenalectomy in 53 dogs with pheochromocytomas with or without alpha-blocker therapy. Vet. Surg. 51, 438–446. Feldman, E.C. and Nelson, R.W. 2003. Canine and feline endocrinology and reproduction. Elsevier Health Sciences, St. Louis, MO: Saunder. Galac, S. and Korpershoek, E. 2017. Pheochromocytomas and paragangliomas in humans and dogs. Vet. Comp. Oncol. 15, 1158–1170. Gilson, S.D., Withrow, S.J. and Orton, E.C. 1994a. Surgical treatment of pheochromocytoma: technique, complications, and results in six dogs. Vet. Surg. VS. 23, 195–200. Gilson, S.D., Withrow, S.J., Wheeler, S.L. and Twedt, D.C. 1994b. Pheochromocytoma in 50 dogs. J. Vet. Intern. Med. 8, 228–232. Gostelow, R., Bridger, N. and Syme, H.M. 2013. Plasma-free metanephrine and free normetanephrine measurement for the diagnosis of pheochromocytoma in dogs. J. Vet. Intern. Med. 27, 83–90. Green, BA. and Frank, E.L. 2013. Comparison of plasma free metanephrines between healthy dogs and 3 dogs with pheochromocytoma. Vet. Clin. Pathol. 42, 499–503. Gregori, T., Mantis, P., Benigni, L., Priestnall, S.L. and Lamb, C.R. 2015. Comparison of computed tomographic and pathologic findings in 17 dogs with primary adrenal neoplasia. Vet. Radiol. Ultrasound. 56, 153–159. Herrera, M.A., Mehl, M.L., Kass, P.H., Pascoe, P.J., Feldman, E.C. and Nelson, R.W. 2008. Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J. Vet. Intern. Med. 22, 1333–1339. Hickman, P.E., Leong, M., Chang, J., Wilson, S.R. and McWhinney, B. 2009. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology. 41, 173–177. Johnson, P.T., Horton, K.M. and Fishman, E.K. 2009. Adrenal mass imaging with multidetector ct: pathologic conditions, pearls, and pitfalls. Radiogr. 29, 1333–1351. Kook, P.H., Grest, P., Quante, S., Boretti, F.S. and Reusch, C.E. 2010. Urinary catecholamine and metadrenaline to creatinine ratios in dogs with a phaeochromocytoma. Vet. Rec. 166, 169–174. Kyles, A.E., Feldman, E.C., De Cock, H.E.V., Kass, P.H., Mathews, K.G., Hardie, E.M., Nelson, R.W., Ilkiw, J.E. and Gregory, C.R. 2003. Surgical management of adrenal gland tumors with and without associated tumor thrombi in dogs: 40 cases (1994-2001). J. Am. Vet. Med. Assoc. 223, 654–662. Lang, J.M., Schertel, E., Kennedy, S., Wilson, D., Barnhart, M. and Danielson, B. 2011. Elective and emergency surgical management of adrenal gland tumors: 60 cases (1999-2006). J. Am. Anim. Hosp. Assoc. 47, 428–435. Lee, G.W., Yoo, C.R., Lee, D. and Park, H.M. 2020. Favorable outcome of pheochromocytoma in a dog with atypical Cushing’s syndrome and diabetes mellitus following medical treatment: a case report. BMC Vet. Res. 16, 3. Lucon, A.M., Pereira, M.A., Mendonça, B.B., Halpern, A., Wajchenbeg, B.L. and Arap, S. 1997. Pheochromocytoma: study of 50 cases. J. Urol. 157, 1208–1212. Lunn, K. and Page, R. 2013. Tumors of the endocrine system. In: Withrow and MacEwen’s small animal clinical oncology. Elsevier Health Sciences. St. Louis, MO: Saunder, pp: 504–531. Maher, E.R. and McNiel, E.A. 1997. Pheochromocytoma in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 27, 359–380. Mak, G. and Allen, J. 2013. Simultaneous pheochromocytoma and third-degree atrioventricular block in 2 dogs. J. Vet. Emerg. Crit. Care (San Antonio Tex 2001). 23, 610–614. Manger, W.M. and Gifford, R.W. 1993. Pheochromocytoma: current diagnosis and management. Cleve. Clin. J. Med. 60, 365–378. Massari, F., Nicoli, S., Romanelli, G., Buracco, P. and Zini, E. 2011. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002-2008). J. Am. Vet. Med. Assoc. 239, 216–221. McCorkell, S.J. and Niles, N.L. 1985. Fine-needle aspiration of catecholamine-producing adrenal masses: a possibly fatal mistake. AJR Am. J. Roentgenol. 145, 113–114. Morandi, F., Mays, J.L., Newman, S.J. and Adams, W.H. 2007. Imaging diagnosis- bilateral adrenal adenomas and myelolipomas in a dog. Vet. Radiol. Ultrasound. 48, 246–249. Nagumo, T., Ishigaki, K., Yoshida, O., Iizuka, K., Tamura, K., Sakurai, N., Terai, K., Seki, M., Edamura, K. and Asano, K. 2020. Utility of contrast-enhanced ultrasound in differential diagnosis of adrenal tumors in dogs. J. Vet. Med. Sci. 82, 1594–1601. Pagani, E., Tursi, M., Lorenzi, C., Tarducci, A., Bruno, B., Borgogno Mondino, E.C. and Zanatta, R. 2016. Ultrasonographic features of adrenal gland lesions in dogs can aid in diagnosis. BMC Vet. Res. 12, 267. Pey, P., Diana, A., Rossi, F., Mortier, J., Kafka, U., Veraa, S., Groth, A., MacLellan, M., Marin, C. and Fracassi, F. 2020. Safety of percutaneous ultrasound-guided fine-needle aspiration of adrenal lesions in dogs: perception of the procedure by radiologists and presentation of 50 cases. J. Vet. Intern. Med. 34, 626–635. Roizen, M.F., Hunt, T.K., Beaupre, P.N., Kremer, P., Firmin, R., Chang, C.N., Alpert, R.A., Thomas, C.J., Tyrrell, J.B. and Cahalan, M.K. 1983. The effect of alpha-adrenergic blockade on cardiac performance and tissue oxygen delivery during excision of pheochromocytoma. Surgery. 94, 941–945. Rosenstein, D.S. 2000. Diagnostic imaging in canine pheochromocytoma. Vet. Radiol. Ultrasound. 41, 499–506. Salesov, E., Boretti, F.S., Sieber-Ruckstuhl, N.S., Rentsch, K.M., Riond, B., Hofmann-Lehmann, R., Kircher, P.R., Grouzmann, E. and Reusch, C.E. 2015. Urinary and plasma catecholamines and metanephrines in dogs with pheochromocytoma, hypercortisolism, nonadrenal disease and in healthy dogs. J. Vet. Intern. Med. 29, 597–602. Schultz, R.M., Wisner, E.R., Johnson, E.G. and MacLeod, J.S. 2009. Contrast-enhanced computed tomography as a preoperative indicator of vascular invasion from adrenal masses in dogs. Vet. Radiol. Ultrasound. 50, 625–629. Schwartz, P., Kovak, J.R., Koprowski, A., Ludwig, L.L., Monette, S. and Bergman, P.J. 2008. Evaluation of prognostic factors in the surgical treatment of adrenal gland tumors in dogs: 41 cases (1999-2005). J. Am. Vet. Med. Assoc. 232, 77–84. Soler Arias, E.A., Trigo, R.H., Miceli, D.D., Vidal, P.N., Hernandez Blanco, M.F. and Castillo, V.A. 2021. Urinary vanillylmandelic acid:creatinine ratio in dogs with pheochromocytoma. Domest. Anim. Endocrinol. 74, 106559. Sood, S.K., Balasubramanian, S.P. and Harrison, B.J. 2007. percutaneous biopsy of adrenal and extra-adrenal retroperitoneal lesions: beware of catecholamine secreting tumours! Surgery. 5, 279–281. Sumner, J.A., Lacorcia, L., Rose, A.M., Woodward, A.P. and Carter, J.E. 2018. Clinical safety of percutaneous ultrasound-guided fine-needle aspiration of adrenal gland lesions in 19 dogs. J. Small Anim. Pract. 59, 357–363. Twedt, D.C. and Wheeler, S.L. 1984. Pheochromocytoma in the dog. Vet. Clin. North Am. Small Anim. Pract. 14, 767–782. van den Berg, M.F., Kooistra, H.S., Grinwis, G.C.M., van Nimwegen, S.A., van Faassen, M., Kema, I.P., Teske, E. and Galac, S. 2023. Reference intervals for plasma, urinary, and salivary concentrations of free metanephrines in dogs: relevance to the diagnosis of pheochromocytoma. J. Vet. Intern. Med. 37, 173–183. Vanderveen, K.A., Thompson, S.M., Callstrom, M.R., Young, W.F., Grant, C.S., Farley, D.R., Richards, M.L. and Thompson, G.B. 2009. Biopsy of pheochromocytomas and paragangliomas: potential for disaster. Surgery. 146, 1158–1166. Werbel, S.S. and Ober, K.P. 1995. Pheochromocytoma. Update on diagnosis, localization, and management. Med. Clin. North Am. 79, 131–153. | ||
| How to Cite this Article |
| Pubmed Style Recchia A, Digiaro S, Colella A, Greco B, Paradies P. Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Vet. J.. 2024; 14(7): 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 Web Style Recchia A, Digiaro S, Colella A, Greco B, Paradies P. Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. https://www.openveterinaryjournal.com/?mno=199699 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i7.21 AMA (American Medical Association) Style Recchia A, Digiaro S, Colella A, Greco B, Paradies P. Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Vet. J.. 2024; 14(7): 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 Vancouver/ICMJE Style Recchia A, Digiaro S, Colella A, Greco B, Paradies P. Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Vet. J.. (2024), [cited January 25, 2026]; 14(7): 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 Harvard Style Recchia, A., Digiaro, . S., Colella, . A., Greco, . B. & Paradies, . P. (2024) Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Vet. J., 14 (7), 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 Turabian Style Recchia, Alessandra, Serena Digiaro, Antonella Colella, Beatrice Greco, and Paola Paradies. 2024. Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Veterinary Journal, 14 (7), 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 Chicago Style Recchia, Alessandra, Serena Digiaro, Antonella Colella, Beatrice Greco, and Paola Paradies. "Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature." Open Veterinary Journal 14 (2024), 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 MLA (The Modern Language Association) Style Recchia, Alessandra, Serena Digiaro, Antonella Colella, Beatrice Greco, and Paola Paradies. "Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature." Open Veterinary Journal 14.7 (2024), 1716-1725. Print. doi:10.5455/OVJ.2024.v14.i7.21 APA (American Psychological Association) Style Recchia, A., Digiaro, . S., Colella, . A., Greco, . B. & Paradies, . P. (2024) Acute onset of hypertensive encephalopathy in a dog with right adrenal pheochromocytoma and neoplastic invasion of the caudal vena cava: Case report and review of the literature. Open Veterinary Journal, 14 (7), 1716-1725. doi:10.5455/OVJ.2024.v14.i7.21 |