| Research Article | ||
Open Vet. J.. 2024; 14(11): 2754-2761 Open Veterinary Journal, (2024), Vol. 14(11): 2754-2761 Research Article Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamicsHenni Vanda1, Farida Athaillah2, Wahyu Eka Sari3, Frengki Frengki1, Nurliana Nurliana3 and Muhammad Hambal2*1Pharmacology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Parasitology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Veterinary Public Health Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Muhammad Hambal. Parasitology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: hambal.m [at] usk.ac.id Submitted: 21/03/2024 Accepted: 08/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
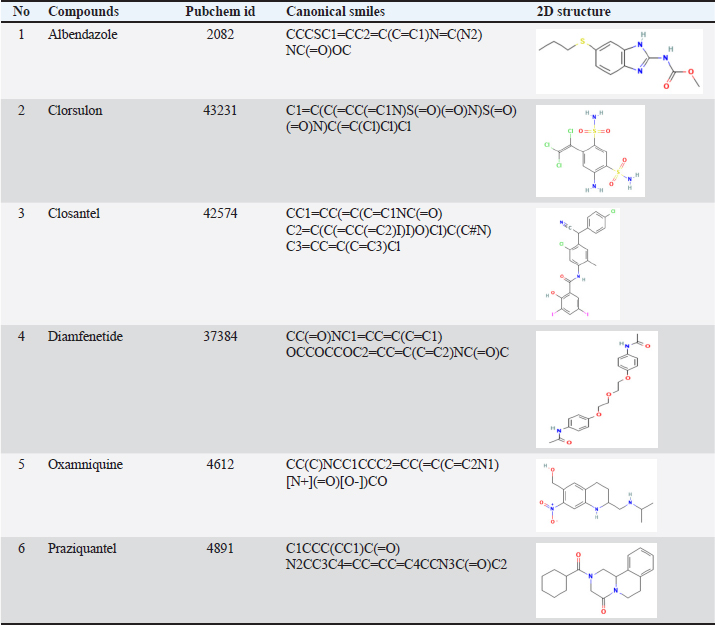
AbstractBackground: Cathepsin-L (FhCL) is a group of enzymes that most flukes express and secreted significantly in parasite-host interactions. Researches are focusing on antigens released by Fasciola gigantica as one of the keys to understanding immunologic pathways in parasite infection and targets for anthelmintics. Efforts to suppress FhCL function through vaccination or therapy using anthelmintic drugs are key factors in controlling field-level trematode infections. A molecular docking method can be used to observe the interaction between FhCL and some anthelmintic drugs to better understand the effect of these anthelmintics on FhCL. Furthermore, it is necessary to carry out molecular dynamics methods to observe the dynamic pattern of the interaction of the enzyme-ligand when the dynamics simulation is in progress. Aim: This study was carried out to screen six commercial anthelmintic drugs against cathepsin-2L (FhCL-2) and cathepsin 5L (FhCL-5) using in silico and molecular dynamics approach. Methods: The three-dimensional (3D) crystal structure of FhCL-2 and FhCL-5 enzymes were constructed based on the crystal structure of ProCathepsin 1L (pdb id. 2O6X) as a template, while six anthelmintic agents (“SMILES” format) were downloaded from PubChem and converted to 3D format using the MOE Builder tool. The enzyme modeling results were evaluated using the “Ramachandran Plot”. Results: Molecular docking results showed that all tested ligands had affinity for FhCL-2 and FhCL-5. The best ∆Gbinding value for FhCL-2 was clorsulon ligand (−15.21 kcal/mol) with pKi of 32.25 µM, which was significantly different compared to other drugs (p < 0.05). For FhCL-5, the best ∆Gbinding value was closantel ligand (−14.88 kcal/mol) with pKi of 31.51 µM, significantly different from other drugs tested (p < 0.05). The results of the molecular dynamics simulation for the two ligands showed a strong and stable interaction at their respective binding sites. Conclusion: All of the tested anti-trematode ligands had potency as inhibitors for FhCL-2 and FhCL-5 enzymes of F. gigantica, and the best candidate for FhCL-2 was clorsulon, and for FhCL-5 was closantel. This finding is useful as an approach to develop novel drugs from existing drugs as inhibitors for FhCL-2 and FhCL-5 enzymes. Keywords: Cathepsin 2L, Cathepsin 5L, Anthelmintics, In silico. IntroductionFasciola hepatica and Fasciola gigantica are causative agents that spread infectious disease (fasciolosis) in ruminants, especially in cattle and sheep. These flukes live and develop in the liver and gall bladder of animals and cause notable morbidity and mortality which is related to low productivity and fertility (Lalor et al., 2021). To overcome the losses caused by fasciolosis, proper control measures must be carried out. One of the ways to tackle this issue is by implementing an accurate anti-trematode which works as an anthelmintic agent for fasciolosis. Furthermore, a study about antigens released by F. gigantica in the form of cathepsin-L is also significant to control the disease. Cathepsin-L from F. hepatica was the first enzyme reported in flukes (Smith et al., 1993). Two different types of cathepsin-L protease that were isolated and purified from F. gigantica showed dominant activity of proteolytic enzymes (Smith et al., 1993; Dowd et al., 1994). Cathepsin-L proteases are produced by gastrodermal cells of gut trematodes and act as key factors for invasion (Barbour et al., 2021; Corrales et al., 2021). Fasciola hepatica produces diverse types of these enzymes, consisted of 17 cathepsin-L cysteine proteases, and three juveniles specific cathepsin B isotypes (Corrales et al., 2021). The predominant proteases released by adult flukes in the bile ducts are cathepsin-L peptidases including FhCL-1 and FhCL-2, while the enzymes released by juvenile flukes that penetrate the intestine are FhCL-3 and three cathepsin-B (FhCB1, 2 and 3) (Dixit et al., 2017). All these enzymes are potential candidates to study the interaction between parasite antigen and host immune response as well as targets for anthelmintics. Ryan et al. (2020) reported that adult worm parasites produce FhCL-1, FhCL-2, and FhCL-5, which are antigens in F. gigantica, while FhCL-3 and FhCL-4 are infective proteins produced in juvenile stage, which prevent the attachment of eosinophils. The proteolytic enzymes produced by mature flukes are used as targets for anthelmintic drugs, for example, FhCL-1 has been reported as a target for the development of fluorobenzoyl dipeptidyl derivative as reported by Moran et al. (2010). An inhibitor for FhCL-1 was also reported by Ferraro et al. (2016) which was chalcone, a flavonoid that has many interesting properties, including antioxidant, antidiabetic, anti-inflammatory, and possibly anthelmintics. Recently, it has been reported that the receptor target of an active compound can reach several receptors, and a receptor can also be stimulated by various molecules of different compounds with different affinities and intensities. This concept is known as “Network Pharmacology”. Tracking of receptor targets can be carried out through the molecular docking method. Molecular docking is a technique that exhibits the interaction between a micro molecule and a macromolecule indicated by the release of free energy (∆Gbinding). The ∆Gbinding value describes the bond strength that occurs between the enzyme and a specific ligand. The lower the ∆Gbinding value, the stronger the bond interaction between two ligands (Hambal et al., 2022; Jawarkar et al., 2022). Anti-trematode drugs are mainly used in the field to treat fasciolosis, including albendazole, clorsulon, diamfenetide, closantel, oxamniquine, and praziquantel. The mechanism of action of these drugs has been identified on certain receptor targets, albendazol acts on β-tubulin protein cytoskeleton which causes impaired development and locomotion of parasites (Pallotto et al., 2022; Collins et al., 2024), clorsulon works as an inhibitor of phosphoglycerate kinase and mutase enzyme (Kumar et al., 2017; Ferraroni et al., 2022). Praziquantel works well on adult worms by increasing membrane permeability towards calcium resulting in increasing calcium influx, muscle contraction, and surface modification (Summers et al., 2022), while piperazine binds to muscle membrane GABA receptors causing hyperpolarization of nerve endings (Laudisi et al., 2020). However, there is a lack of studies focusing on the molecular docking of anti-trematode drugs as inhibitors for FhCL-2 and FhCL-5. In this study, the molecular docking method was used to explore the potential of albendazole, clorsulon, diamfenetide, closantel, oxamniquine, and praziquantel as inhibitors of FhCL-2 and FhCL-5 enzymes. With the molecular docking method, the interaction of the enzyme-ligand can be observed, and further analyzed using the molecular dynamics method to obtain the dynamic pattern of enzyme-ligand interaction when the dynamics simulation is in progress. Materials and MethodsThe 3D structure of FhCL-2 and FhCL-5 enzyme macromolecules were obtained from modeling results on the ProCathepsine 1L template (PDB ID 2O6X) with a resolution of 2.65 Å. Modeling for FhCL-2 and FhCL-5 sequences was acquired from the report by Martínez-Sernández et al. (2018). The tested molecules in this study were six commercial anti-trematode agents with known modes of action (Jayawardene et al., 2021). The structure of 2D anti-trematode drugs is listed in Table 1. Building FhCL-2 and FhCL-5 modelsModel templates for FhCL-2 and FhCL-5 were selected based on PDB ID 206X database. Modeling of FhCL-2 and FhCL-5 molecules was conducted by entering FhCL-2 and FhCL-5 sequence data in the MOE sequence editor window, then continued by using the 206X template data in .pdb format in the main window. After that, “molecular homology” subprogram was run following the default tutorial. Cathepsin-L (FhCL) model was visualized using UCSF Chimera (1.13.1 software). Stereochemical evaluation based on “Ramachandran Plot” data was carried out to find out the percentage value of the preferred region. Molecular dockingMolecular docking simulation was aimed at observing and identifying the affinity and interactions that occur between macromolecules of FhCL-2 and FhCL-5 enzymes towards six anti-trematode drug ligands. Ligands and receptors were first optimized through the addition of hydrogen atoms, partial energy, and minimized energy in the most optimum conditions to obtain the most stable binding affinity. The docking process began by tracing the “binding site” of FhCL-2 and FhCL-5 receptors through the “site finder” window integrated in the MOE application. Then a “site” was selected that contained the same docking target (Ferraro et al., 2016). Furthermore, docking was carried out on all selected tested ligands. Docking results in the form of free energy (ΔGbinding) and ligand inhibition values (pKi) were achieved and visualized via LigPlot. Molecular dynamics simulationMolecular dynamics simulations were conducted to observe the stability of enzyme-ligand interactions during dynamic activities. This method was used to analyze the physical movement of molecules. The molecular dynamics simulation was run using the MOE application following the molecular dynamics protocol. Molecular dynamics simulations were carried out in several stages including minimization, heating to 310 ºK, temperature equilibrium, pressure equilibrium, and continued with a 2,000 pico second (pc) production process (Salo-Ahen et al., 2020). At the end of the simulation, the conformational deviations of the enzyme-ligand complex were analyzed through root mean square deviation (RMSD) values and hydrogen bond conditions. Table 1. Structure of “SMILES” and 2D tested ligand of six anti-trematodes.
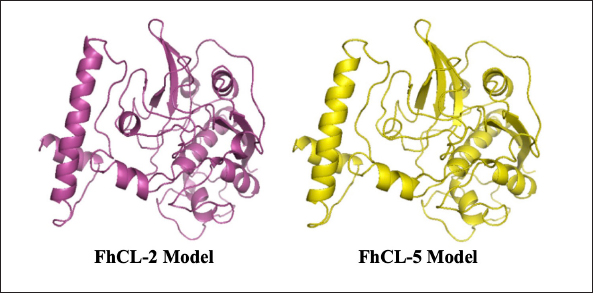
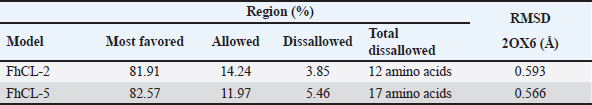
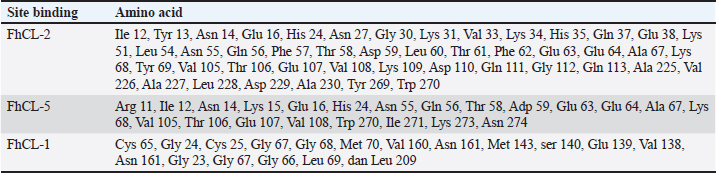
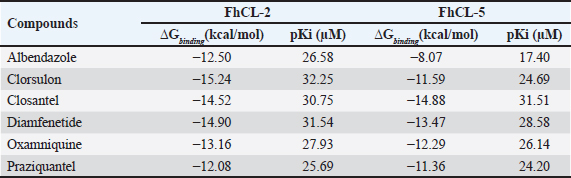
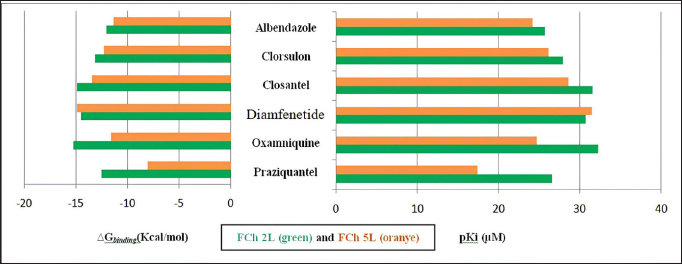
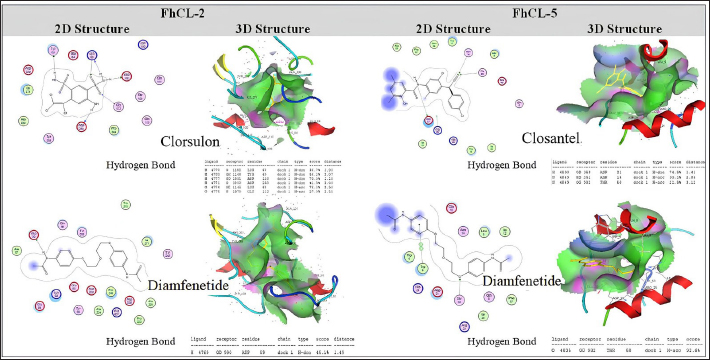
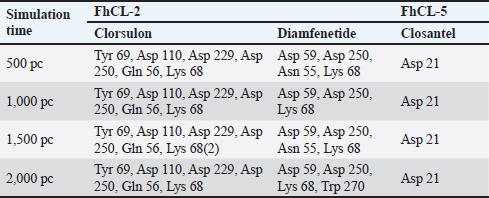
ResultsThe results of FhCL-2 modeling in the form of Ramachandran and RMSD plot analysis revealed that the best percentage residue of the protein model in the most favored region was 81.91%, in the allowed region was 14.24%, and in the disallowed region was 3.85%. RMSD value for 2OX6 was 0.593 Å. For FhCL-5, the best percentage residue of the protein model in the most favored region was 82.57%, in the allowed region was 11.97%, in the disallowed region was 5.46%, and the RMSD value was 0.566 Å (Table 2, Fig. 1). Docking was carried out using the “blind docking” method with the “site binding” target as seen in Table 3. The docking process produced two important things, i.e. orientation and position of a ligand as an inhibitor of an enzyme and predicting the affinity of a ligand for an enzyme/receptor as a docking simulation target. Both of these were in the form of a scoring function (London dG) which is estimated as the binding free energy value (∆Gbinding) in kcal/mol. The ∆Gbinding value describes the strength of the bond occurred between the enzyme and ligand. There is a relationship between ∆Gbinding value and inhibitor constant (Ki), which follows the thermodynamic equation (Fig. 2) (Bhagat et al., 2021). The results of pKi of each tested ligands and molecular docking results are provided in Table 4 and Figure 3. Visualization of 2D and 3D interactions of ligands with the best affinity on FhCL-2 (clorsulon and diamfenetide) and FhCL-5 (closantel and diamfenetide) is presented in Figure 4. A lower ∆Gbinding energy indicates a stronger interaction, suggesting that clorsulon is a potent inhibitor of FhCL-2, and closantel is a promising inhibitor of FhCL-5. The interactions of ligands in the docking results showed that the enzyme was in a rigid state, therefore it had to be evaluated in a hydrated molecular state using the molecular dynamics simulation method. Molecular dynamics simulations showed that changes in hydrogen bond residue contacts between FhCL-2 and FhCL-5 enzyme-ligands occurred during simulation. Table 5 showed ligand interaction data during molecular dynamics simulation. The hydrogen bond interaction between ligand and amino acid which was the “binding site” of the enzyme during dynamic simulation did not experience any significant differences. The hydrogen bond of FhCL-2 to clorsulon had some differences when the simulation was run at 1,500 pc count, when the formation of two hydrogen bonds with amino acid Lys 68 was present. The hydrogen bond of FhCL-2 to diamfenetide was different when the simulation was run at 1,000 pc count, there was a loss of one hydrogen bonds on amino acid Asn 55. However, one hydrogen bond was formed on amino acid Trp 270 when the simulation was run at 2,000 pc. In FhCL-5 enzyme, interaction stability with closantel ligand was seen through one hydrogen bond with amino acid Asp 21 during dynamics simulation (Fig. 5).
Fig. 1. Results of FhCL-2 and FhCL-5 modeling of the template (pdb id. 2OX6).
Fig. 2. Thermodynamic equation. Table 2. Comparison of FhCL-2 and FhCL-5 modeling on 2OX6 template based on “Ramachandran Plot”.
Table 3. “Site binding” Cathepsin enzyme model based on MOE “Site Finder” instructions.
Table 4. ∆Gbinding value and inhibitor constant (pKi) of anti-trematode ligands.
Fig. 3. Free energy (∆Gbinding (Kcal/mol)) and inhibition constant (pKi) of molecular docking results of six anti-trematode ligands.
Fig. 4. Visualization of 2D and 3D interactions of ligands with the best affinity on FhCL-2 and FhCL-5. Table 5. Ligand residue contacts during molecular dynamics simulations at 310 ºK.
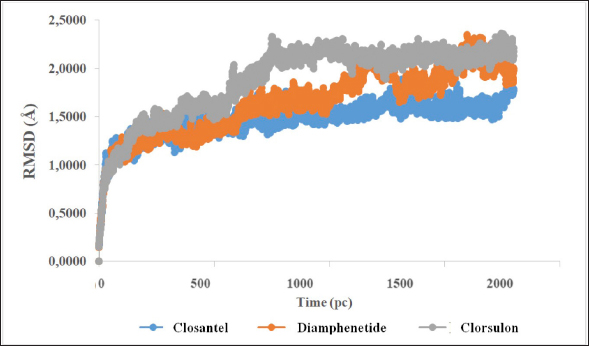
Fig. 5. RMSD of closantel ligand (blue) on FhCL-5, and clorsulon ligand (grey) and diamfenetide (light brown) on FhCL-2. DiscussionCathepsin-L also known as excretory-secretory products are released by parasites during host infiltration. These molecules are deduced to function as effector molecules efficient in modulating the host immune system, and enabling parasite survival. FhCL-2 and FhCL-5 are immunomodulatory molecules which advantageous to the flukes by manipulation of host immune processes, therefore prolonged parasitic infection (Ryan et al., 2020). In this study, the interaction of FhCL-2 and FhCL-5 ligands with anti-trematodes were discussed. Based on the thermodynamic equation, the lower ∆Gbinding value, the stronger the enzyme-ligand complex bond will be. This is due to the stability and strength of non-covalent interactions in the enzyme-ligand complex which are observed from the amount of free energy released when the interaction of the enzyme-ligand complex is formed. The docking simulation results in Table 4 showed that clorsulon and diamfenetide were two ligands that released the greatest energy when interacting with FhCL-2 enzyme. Clorsulon had the strongest affinity with a ∆Gbinding value of −15.21 kcal/mol, with an inhibition constant (pKi) value of 32.25 µM, significantly different (p < 0.05) from other ligands. The diamfenetide ligand had a ∆Gbinding value of −14.90 kcal/mol with an inhibition constant (pKi) of 31.54 µM. For FhCL-5, closantel showed a strong affinity with a ∆Gbinding value of −14.88 kcal/mol and an inhibition constant (pKi) value of 31.51 µM (p < 0.05), and diamfenetide had a ∆Gbinding value of −13.47 kcal/mol with an inhibition constant (pKi) of 28.58 µM (Fig. 3, Table 4). Evaluation of the visualization of ligand molecular complex on FhCL-2 and FhCL-5 showed the number of hydrogen bonds play a major role in increasing the affinity (Fig. 4). For FhCL-2, clorsulon ligand formed six hydrogen bonds with Lys 68(2), Tyr 69, Asp 110, Gly 112, and Asp 250. Meanwhile, the diamfenetide ligand only had one hydrogen bond with Asp 59. For FhCL-5, closantel ligand had three hydrogen bonds with amino acids Asp 21, Asn 14, and Thr 58, and diamfenetide ligand only had one hydrogen bond with Thr 58. The changes in hydrogen bonds had an impact on the deviation of molecular conformation which was observed in RMSD value. To ensure the dynamic stability of the ligand-enzyme complex, RMSD values of enzyme backbone atoms were calculated. An increased in RMSD value indicates that the enzyme structure is starting to open and the ligand is starting to find suitable binding sites or coordinates on the protein (Das et al., 2019). A stable RMSD value indicates the maximum conformation of the protein bond to the ligand and the ability to maintain its position. Interactions between residues in enzymes also impact protein structure stability (da Fonseca et al., 2024). In this study, two anti-trematode ligands, clorsulon and closantel were stable when interacting with FhCL-2 and FhCL-5 enzymes, during the simulation, indicated by the deviation in the poses of these ligands was not at ± 2 Å. In Figure 5, it was shown that the time required for these ligands to form a stable complex with FhCL-2 and FhCL-5 was relatively similar (± 100 pc). However, as the dynamics progressed, it was observed that FhCL-5 complex with closantel ligand showed the best stability with a relatively smaller RMSD value compared to other ligands, which was between 1–1.8 Å. All these results suggested that closantel and clorsulon have prospective as inhibitors of FhCL-2 and FhCL-5, therefore future studies are necessary which include in vitro and in vivo validation to confirm these findings. ConclusionAnti-trematode drugs that have been tested in this study had the ability to inhibit FhCL-2 and FhCL-5 enzymes, confirmed by the identification and evaluation of molecular interactions in silico using molecular docking and molecular dynamics methods. Molecular docking analysis showed that clorsulon ligand had the strongest affinity for FhCL-2 macromolecule, while the best affinity for FhCL-5 was closantel ligand. Both ligands demonstrated strong stability in molecular dynamics simulations, as evidenced by their low RMSD values. Consequently, closantel and clorsulon emerge as promising candidates for inhibiting FhCL-2 and FhCL-5, in addition to their previously established pharmacological effects. It has, therefore, become a powerful alternative strategy to discover and develop novel drugs from existing drugs by using a molecular docking approach. AcknowledgmentThe authors would like to express gratitude to Faculty of Veterinary Medicine, Universitas Syiah Kuala for supporting this research, and to Research Laboratory members who providing technical assistance in conducting this research. Conflict of interestAll authors declare that there is no conflict of interest. FundingThis work was supported by Universitas Syiah Kuala with grant number 60/UN11.2.1/PT.01.03/PNBP/2023. Authors’ contributionAll authors contributed to this study, and all authors read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesBarbour, T., Cwiklinski, K., Lalor, R., Dalton, J.P. and De Marco Verissimo, C. 2021. The zoonotic helminth parasite Fasciola hepatica: virulence-associated cathepsin B and cathepsin L cysteine peptidases secreted by infective newly excysted juveniles (NEJ). Animals 11, 3495. Bhagat, R.T., Butle, S.R., Khobragade, D.S., Wankhede, S.B., Prasad, C.C., Mahure, D.S. and Armarkar, A.V. 2021. Molecular docking in drug discovery. J. Pharm. Res. Int. 33, 46–58. Collins, J.B., Stone, S.A., Koury, E.J., Paredes, A.G., Shao, F., Lovato, C., Chen, M., Shi, R., Li, A.Y., Candal, I. and Al Moutaa, K. 2024. Quantitative tests of albendazole resistance in Caenorhabditis elegans beta-tubulin mutants. Int. J. Parasitol. Drugs Drug Resist. 25, 100556. Corrales, J.L., Cwiklinski, K., Verissimo, C.D.M., Dorey, A., Lalor, R., Jewhurst, H. and Dalton, J.P. 2021. Diagnosis of sheep fasciolosis caused by Fasciola hepatica using cathepsin L enzyme-linked immunosorbent assays (ELISA). Vet. Parasitol. 298, 109517. da Fonseca, A.M., Caluaco, B.J., Madureira, J.M.C., Cabongo, S.Q., Gaieta, E.M., Djata, F., Colares, R.P., Neto, M.M., Fernandes, C.F.C., Marinho, G.S. and Dos Santos, H.S. 2024. Screening of potential inhibitors targeting the main protease structure of SARS-CoV-2 via molecular docking, and approach with molecular dynamics, RMSD, RMSF, H-bond, SASA and MMGBSA. Mol. Biotechnol. 66, 1919–1933. Das, S., Shimshi, M., Raz, K., Nitoker Eliaz, N., Mhashal, A.R., Ansbacher, T. and Major, D.T. 2019. Enzydock: protein–ligand docking of multiple reactive states along a reaction coordinate in enzymes. J. Chem. Theory Comput. 15, 5116–5134. Dixit, A.K., Dixit, P. and Sharma, R.L. 2017. Cysteine proteases of parasitic helminths. In Pathophysiological aspects of proteases. Eds., Chakraborti, S. and Dhalla, N.S. pp: 657–671. Dowd, A.J., Smith, A.M., Mc Gonigle, S. and Dalton, J.P. 1994. Purification and characterisation of a second cathepsin L proteinase secreted by the parasitic trematode Fasciola hepatica. Eur. J. Biochem. 223, 91–98. Ferraro, F., Merlino, A., Dell Oca, N., Gil, J., Tort, J.F., Gonzalez, M., Cerecetto, H., Cabrera, M. and Corvo, I. 2016. Identification of chalcones as Fasciola hepatica cathepsin l inhibitors using a comprehensive experimental and computational approach. PLoS. Negl. Trop. Dis. 10, e0004834. Ferraroni, M., Angeli, A., Carradori, S. and Supuran, C.T. 2022. Inhibition of Schistosoma mansoni carbonic anhydrase by the antiparasitic drug clorsulon: X-ray crystallographic and in vitro studies. Acta Cryst. D: Struct. Biol. 78, 321–327. Hambal, M., Frengki, F., Sari, W.E. and Vanda, H. 2022. In silico prediction of flavan-3-ol as a bioactive compound of Calophyllum macrophyllum as a potential drug against angiostrongylus eosinophilic meningitis. Vet. World. 155, 1305. Jayawardene, K.L.T.D., Palombo, E.A. and Boag, P.R. 2021. Natural products are a promising source for anthelmintic drug discovery. Biomolecules 11, 1457. Jawarkar, R.D., Sharma, P., Jain, N., Gandhi, A., Mukerjee, N., Al-Mutairi, A.A., Zaki, M.E., Al-Hussain, S.A., Samad, A., Masand, V.H. and Ghosh, A. 2022. QSAR, molecular docking, MD simulation and MMGBSA calculations approaches to recognize concealed pharmacophoric features requisite for the optimization of ALK tyrosine kinase inhibitors as anticancer leads. Molecules 27, 4951. Kumar, R., Doharey, P.K., Saxena, J.K. and Rathaur, S. 2017. Molecular cloning, purification and characterization of Brugia malayi phosphoglycerate kinase. Protein Expr. Purif. 132, 152–163. Laudisi, F., Marônek, M., Di Grazia, A., Monteleone, G. and Stolfi, C. 2020. Repositioning of anthelmintic drugs for the treatment of cancers of the digestive system. Int. J. Mol. Sci. 21, 4957. Lalor, R., Cwiklinski, K., Calvani, N.E.D., Dorey, A., Hamon, S., Corrales, J.L., Dalton, J.P. and De Marco Verissimo, C. 2021. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence 12, 2839–2867. Martínez-Sernández, V., Perteguer, M.J., Hernández-González, A., Mezo, M., González-Warleta, M., Orbegozo-Medina, R.A., Romarís, F., Paniagua, E., Gárate, T. and Ubeira, F.M. 2018. Comparison of recombinant cathepsins L1, L2, and L5 as ELISA targets for serodiagnosis of bovine and ovine fascioliasis. Parasitol. Res. 117, 1521–1534. Moran, B.W., Anderson, F.P., Ruth, D.M., Fágáin, C.O., Dalton, J.P. and Kenny, P.T. 2010. Fluorobenzoyl dipeptidyl derivatives as inhibitors of the Fasciola hepatica cysteine protease cathepsin L1. J. Enzyme Inhib. Med. Chem. 25, 1–12. Pallotto, L.M., Dilks, C.M., Park, Y.J., Smit, R.B., Lu, B.T., Gopalakrishnan, C., Gilleard, J.S., Andersen, E.C. and Mains, P.E. 2022. Interactions of Caenorhabditis elegans β-tubulins with the microtubule inhibitor and anthelmintic drug albendazole. Genetics 221, iyac093. Ryan, S., Shiels, J., Taggart, C.C., Dalton, J.P. and Weldon, S. 2020. Fasciola hepatica-derived molecules as regulators of the host immune response. Front. Immunol. 11, 2182. Salo-Ahen, O.M., Alanko, I., Bhadane, R., Bonvin, A.M., Honorato, R.V., Hossain, S., Juffer, A.H., Kabedev, A., Lahtela-Kakkonen, M., Larsen, A.S. and Lescrinier, E. 2020. Molecular dynamics simulations in drug discovery and pharmaceutical development. Processes 9, 71. Smith, A.M., Dowd, A.J., Heffernan, M., Robertson, C.D. and Dalton, J.P. 1993. Fasciola hepatica: a secreted cathepsin L-like proteinase cleaves host immunoglobulin. Int. J. Parasitol. 23, 977–983. Summers, S., Bhattacharyya, T., Allan, F., Stothard, J.R., Edielu, A., Webster, B.L., Miles, M.A. and Bustinduy, A.L.2022. A review of the genetic determinants of praziquantel resistance in Schistosoma mansoni: is praziquantel and intestinal schistosomiasis a perfect match? Front. Trop. Dis. 3, 933097. | ||
| How to Cite this Article |
| Pubmed Style Vanda H, Athaillah F, Sari WE, Frengki F, Nurliana N, Hambal M. Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Vet. J.. 2024; 14(11): 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 Web Style Vanda H, Athaillah F, Sari WE, Frengki F, Nurliana N, Hambal M. Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. https://www.openveterinaryjournal.com/?mno=200686 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.4 AMA (American Medical Association) Style Vanda H, Athaillah F, Sari WE, Frengki F, Nurliana N, Hambal M. Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Vet. J.. 2024; 14(11): 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 Vancouver/ICMJE Style Vanda H, Athaillah F, Sari WE, Frengki F, Nurliana N, Hambal M. Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 Harvard Style Vanda, H., Athaillah, . F., Sari, . W. E., Frengki, . F., Nurliana, . N. & Hambal, . M. (2024) Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Vet. J., 14 (11), 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 Turabian Style Vanda, Henni, Farida Athaillah, Wahyu Eka Sari, Frengki Frengki, Nurliana Nurliana, and Muhammad Hambal. 2024. Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Veterinary Journal, 14 (11), 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 Chicago Style Vanda, Henni, Farida Athaillah, Wahyu Eka Sari, Frengki Frengki, Nurliana Nurliana, and Muhammad Hambal. "Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics." Open Veterinary Journal 14 (2024), 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 MLA (The Modern Language Association) Style Vanda, Henni, Farida Athaillah, Wahyu Eka Sari, Frengki Frengki, Nurliana Nurliana, and Muhammad Hambal. "Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics." Open Veterinary Journal 14.11 (2024), 2754-2761. Print. doi:10.5455/OVJ.2024.v14.i11.4 APA (American Psychological Association) Style Vanda, H., Athaillah, . F., Sari, . W. E., Frengki, . F., Nurliana, . N. & Hambal, . M. (2024) Potency of anti-trematode in inhibiting cathepsin-2L and cathepsin-5L of Fasciola gigantica using homology, docking, and molecular dynamics. Open Veterinary Journal, 14 (11), 2754-2761. doi:10.5455/OVJ.2024.v14.i11.4 |