| Research Article | ||
Open Vet. J.. 2024; 14(8): 1942-1951 Open Veterinary Journal, (2024), Vol. 14(8): 1942–1951 Research Article Sudden death due to enterotoxemia among Arabian camels (Camelus dromedaries) and associated risk factorsAsmaa G. Mubarak1, Fatma A. Khalifa2, Yumna Elsobky3, Ahmed Abdel-Rady4,5, Wael Felefel6, Adel Hassan Saad7, Ehab Y. Abdelhiee8, Abdullah M. Alhassan9, Hisham Awny10, Eman M. Elghazaly11, Ashraf M. Abu-Seida12*, Abdulrahman Abdulkarim13 and Asmaa G. Youseef141Department of Zoonoses, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 2Department of Infectious Diseases, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 3Department of Hygiene and Zoonosis, Faculty of Veterinary Medicine, University of Sadat City, Menofia, Sadat City, Egypt 4Department of Animal Medicine (Infectious Diseases), Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 5Consultant of Infectious Diseases in Animal Health Laboratory, WEQAA-Center, Dammam, Kingdom of Saudi Arabia 6Department of Parasitology, Faculty of Veterinary Medicine, Matrouh University, Matrouh, Egypt 7Nutrition and Clinical Nutrition Department, Faculty of Veterinary Medicine, Matrouh University, Matrouh, Egypt 8Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Matrouh University, Matrouh, Egypt 9Director of Laboratories in Eastern Region, WEQAA-Center, National Center for the Prevention and Control of Plants Pests and Animal Diseases, Dammam, Kingdom of Saudi Arabia 10High Institute of Public Health, Alexandria University, Alexandria, Egypt 11Department of Microbiology, Faculty of Veterinary Medicine, Matrouh University, Matrouh, Egypt 12Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 13Faculty of Veterinary Medicine, Omar Almukhtar University, Bayda, Libya 14Department of Zoonoses, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt *Corresponding Author: Ashraf M. Abu-Seida. Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Email: ashrafseida [at] cu.edu.eg Submitted: 14/05/2024 Accepted: 08/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Sudden death is defined as an unexpected death occurring with no observed antecedent clinical signs. Aim: The current study was performed to notice the tangible causes of sudden death among 51 out of 340 she-camels on a private farm in the eastern region of El Khafgi, Saudi Arabia. Methods: A retrospective cohort study design was conducted to investigate the sudden death of camels through microscopic examination of fecal matter to identify the gastrointestinal parasites, analysis of whole blood thin films to diagnose blood parasites, blood culturing to recognize bacterial infection as Pasteurella multicida, and macroscopic postmortem examination to identify the gastrointestinal adult worm. The quantity and composition of feed were also analyzed. Afterward, a commercial multiscreen Ag-ELISA kit technique determined the toxins of Clostridium perfringens (C. perfringens). Results: The results revealed that the incidence rate of sudden death was 15%. The sudden death occurred due to C. perfringens enterotoxins detected in the rumen, intestinal content, and intestinal wall. The enterotoxins and Alpha toxins were noticed, but the other toxin types, including Beta and Epsilon, could not be detected. All C. perfringens toxins were discovered to be negative in fecal matter. A significant association was reported between sudden death, she-camels age, and feeding habits as risk factors (p=0.020 and 0.028, respectively). Risk factor assessment by relative risk (RR) revealed that the odds of RR of sudden death occurring among she-camels aged over two years were higher than those less than two years (2.24 CI 95%, 1.093–4.591). Furthermore, the odds RR of sudden death occurring due to exposure of she-camels to a concentrated ration of 18% were higher twice than those not exposed (2.346 CI 95%, 1.039–5.296). Conclusion: Clostridium perfringens enterotoxaemia should be listed as a cause of sudden death in camels and the alteration in diet with 18% concentration feed changes the intestinal environment, which leads to C. perfringens proliferating and yielding potent toxins. More observations and interferences like regular immunization are recommended to reduce the disease and increase the awareness of the farmers of the importance of risk factors. Keywords: Camels, Clostridium perfringens, Enterotoxins, Risk factors, Sudden death. IntroductionThe desert is considered impoverished, so one of the animals that have adapted to live there is the desert ship one-humped camel (Camelus dromedarius). These camels are considered the primary means of transportation in Saharan and sub-Saharan countries and the primary source of leather production for humans. Moreover, their meat and milk contain less cholesterol and fat than other animal milk and meat, making them safe for people allergic to bovine milk (Sheet et al., 2021). Camels are reservoir hosts for blood parasites such as Trypanosoma evansi and gastrointestinal helminths such as trematodes, tapeworms, or nematodes (El-Seify et al., 2021). Moreover, the camels in Asia and Africa harbor several hemophagous ectoparasites, such as ticks and fleas, which eventually may transmit zoonotic viral and bacterial pathogens (Sazmand et al., 2019). Multiple causes lead to unexplained mortalities in camels, including endoparasite infection, either blood or gastrointestinal parasites, which cause catarrhal and hemorrhagic gastro-enteritis, followed by clostridial diseases including enterotoxaemia, then monensin poisoning, chronic copper poisoning, pneumonia, and bloat (Bouragba et al., 2020; Esmaeel et al., 2021). The most common collective reason for death recorded in the camel farm is “Heyam” syndrome (53.3%) (Kaaboub et al., 2021). The most significant Gram-positive anaerobe bacterium is Clostridium perfringens (C. perfringens). The sporulation of C. perfringens allows it to persist in fecal matter, soil, and under different environmental conditions for many years, so controlling disease transmission becomes difficult. This organism is ranked as a biosafety level II organism. Therefore, careful handling according to appropriate biosafety standards of suspected infected samples with C. perfringens is a potential protective factor through personal protective equipment (Naureen et al., 2022). Clostridium perfringens is a natural intestinal inhabitant among humans and animals, including sheep, goats, cattle, and camels. Whenever a low bacterial count produces a minor quantity of toxins removed rapidly from the gut by normal peristalsis, which leads to no damage or illness. A large number of toxins produced by C. perfringens are considered food-borne gastrointestinal food poisoning and edaphic zoonotic diseases due to the corruption of meat products with the intestinal matter and fecal matter of infected hosts (Ghoneim et al., 2017). Clostridium perfringens is classified into seven toxin types (A, B, C, D, E, F, and G) according to the production of four significant toxins, namely alpha (CPA), beta (CPB), epsilon (ETX), and iota (ITX) (Kiu et al., 2018). The CPA is released from all seven- toxin types, the CPB is produced from both B and C types, ETX from C and D types, and type E yields ITX (Alsaab et al., 2021). The prevalence in Saudi Arabia by using the ELISA procedure to notice Clostridium perfringens enterotoxaemia types is 67.2% for the main types: C. perfringens type A, followed by type D (16.4%), then type B (13.4%), and type C (3%). Still, the prevalence of enterotoxaemia among different hosts is included that; cows (66.7%), small ruminate goats, and sheep (44.6%) and (39.5%), respectively, but camels (33.3%) (Omer et al., 2020). The pathogenesis of C. perfringens enterotoxaemia is the production of toxins through the enormous explosion of this occupant bacterium in the intestinal milieu (Finnie et al., 2020). Subsequently, these toxins penetrate the systemic circulation due to increased intestinal permeability, leading to the entero-toxemic phase. This phase causes harmful effects on blood vessels in many organs, including the brain. Consequently, there is an elevation in plasma protein and fluid levels within affected blood vessels, specifically in the subintimal wall and perivascular space, resulting in increased intracranial pressure and neurological syndrome due to enhanced vascular permeability (Uzal et al., 2018). Finally, whenever global parenchymal edema is primarily vasogenic, death occurs due to a mix of vasogenic and cytotoxic factors (Finnie et al., 2022). The main symptoms of C. perfringens infections are hemorrhagic enteritis with ulceration of the mucosa, convulsions, hydrothorax, and neurological abnormalities that lead to significant economic loss due to sudden death with a high mortality rate. In addition, postmortem inspection revealed that there is an accumulation of gas in the intestines, the lungs are dense and fluid-filled, and the heart has pericardial fluid with small hemorrhages; still, the liver is light brown (Elhelw et al., 2022). There are many predisposing factors, particularly modified risk factors, including sudden changes in diet with overfeeding of green fodder rich in proteins or carbohydrates, deworming, overcrowding, and handling of animals. On the other hand, the non-modified risk factors, including seasonality deviations and age, alter the intestinal environment, which leads to C. perfringens proliferating and producing potent toxins (Hussain et al., 2022). Clostridium perfringens enterotoxaemia on the farm is diagnosed based on indirect serological methods, including ELISA, PCR, and real-time PCR, which depend on coproantigen detection, and direct diagnosis, including the case history, symptoms, risk factors, and postmortem lesions, which is considered a tentative diagnosis (Pawaiya et al., 2020; Felefel et al., 2023). In addition, freshly dead animal tissues are examined immediately using one drop of chloroform for each 10 ml of intestinal content, which at 4˚C leads to the stability of toxins (Mohiuddin et al., 2016). The most effective ways of preventing clostridial infection are vaccines, typically containing one or more clostridial bacterins or toxoids, and antibiotics. However, antibiotics can sometimes be ineffective and cause the development of bacterial resistance (Mahmood et al., 2021; Elhelw et al., 2022). The hypothesis of this study was that a change in diet with a concentration feed of 18% would alter the intestinal environment, promoting the proliferation of C. perfringens and the production of potent toxins. This phenomenon was believed to have led to the sudden deaths of camels (Camelus dromedarius) on a private farm in El Khafgi, Eastern Region, Saudi Arabia. Materials and MethodsStudy design The current study was conducted through a retrospective cohort design. The following practical steps and guidelines were applied for the epidemic investigation: Step 1: The epidemiological description of cases regarding animals, place, and time Study animals A total of 51 out of 340 one-humped camels (Camelus dromedarius) suffered from sudden death. Study setting The current study was performed in El Khafgi, Eastern Region, Saudi Arabia, on a private camel farm (48°30′E, 28°25′N). Study duration The study extended from August to September 2022. Step 2: Confirmation of diagnosis by laboratory investigations and postmortem examination Laboratory investigations Blood sample examination Blood samples of 289 clinically healthy she-camels inside the farm were collected from the jugular vein and then examined by thin Giemsa stain blood films to detect the blood parasites. Furthermore, the blood samples were cultured on blood agar media and then incubated at 37°C for 24 hours to identify the bacterial infection as Pasteurella multocida. Fecal matter examination Three hundred forty fecal samples were collected from the 51 she-camels that suddenly died. Additionally, 289 clinically healthy she-camels were examined using direct fecal smear and flotation techniques to detect light protozoa oocysts or helminths. Furthermore, the hot Ziehl-Neelsen staining technique was employed to distinguish intestinal apicomplexan protozoa parasites or helminths (Felefel et al., 2023). Detection of C. perfringens toxins by ELISA test To identify toxins and cellular antigens in C. perfringens, a commercial multiscreen Ag-ELISA kit (Bio-X Diagnostics, Belgium) was employed according to Moustafa et al. (2022). Feed regulation The quantity and composition of feed were analyzed. Postmortem examination The suddenly dead she-camels were subjected to a postmortem lesion examination. Samples were collected from internal organs, including intestinal contents, intestinal wall, rumen contents, and feces. These samples were sent to a diagnostic veterinary laboratory in Dammam for analysis. Subsequently, all samples were mixed in phosphate-buffered saline at a ratio of 1:9. They were cultivated in brain-heart infusion broth and incubated at 37°C for 24 hours. Afterward, the cultures were filtered using a bacterial filter, and 1 ml of the filtrate was extracted for use in the ELISA test to detect Alpha, Beta, Epsilon, and C. perfringens enterotoxins. All handled samples were frozen at −80°C until the ELISA test was conducted. Step 3: Identification of affected animals and their characteristics During sampling, a questionnaire was designed and completed to collect data about risk factors and the number of sudden-death animals. This clinical and epidemiological data included clinical signs, time of onset of symptoms, age, history of exposure to a particular source of infection, and diet consumption. Step 4: Formulate a hypothesis Based on time, location, and animals to explain potential sources, suspected causative agents, modes of transmission, and environmental factors favoring the infection and subsequent sudden death were hypothesized. Step 5: Testing hypothesis The hypothesis was tested through analytic epidemiologic calculations to confirm the diagnosis through the following parameters: The attack ratio The attack ratio was calculated according to Rockhill et al. (1998) and Rothman and Greenland (1998) as follows:
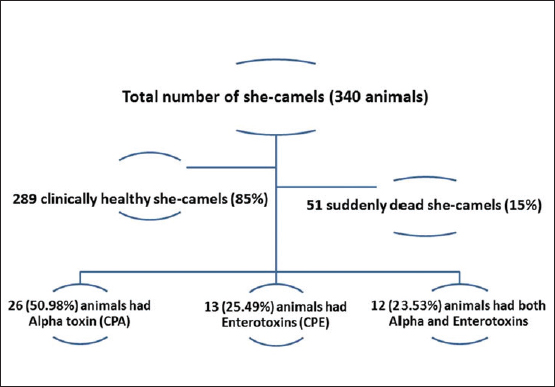
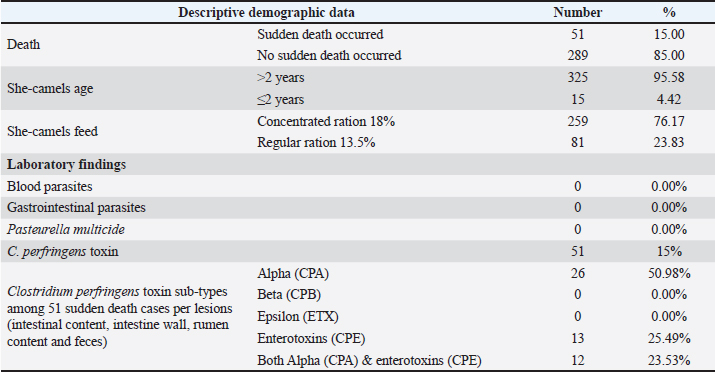
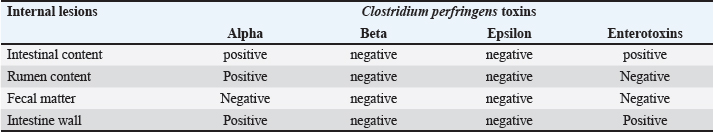
Relative risk (RR) When its value was higher than 1.0, it indicated an increased outbreak risk. It was calculated according to Rockhill et al. (1998) and Rothman and Greenland (1998) as follows: RR=[The number of sudden deaths among those exposed to risk factors in specific animals (Ie)] / [The number of sudden deaths among unexposed to risk factors in specific animals (Io)]. Attributable risk (AR) It was measured by sudden death attributed to particular exposure risk factors. It was calculated according to Rockhill et al. (1998) and Rothman and Greenland (1998) using the following formula: AQ=Ie – Io. The number needed to treat (NNT) This measure was reflected by the number of animals required to be treated to prevent one additional adverse outcome. It was calculated according to Rockhill et al. (1998) and Rothman and Greenland (1998) using the following formula: NNT=1/AR. A single intramuscular dosage of long-acting Amoxicillin trihydrate (Clamoxyl LA, Zoetis, USA) at a dose of 1 ml/10 kg was given to the apparently healthy female camels as a preventive dose against C. perfringens. Moreover, the apparently healthy she-camelss were vaccinated with a combination vaccine adjuvanted by Montanide gel including strains of BVDV and C. perfringens type A toxoid to ensure safe and efficient protection against clostridial infections (Elhelw et al., 2022). Relative risk reduction (RRR) It could be calculated according to Rockhill et al. (1998) and Rothman and Greenland (1998) using the following formula: RRR=1 − RR. Statistical analysis Data were collected in Excel spreadsheets and analyzed using SPSS version 20. Fisher’s exact test and Pearson chi-square tests were used at a significance level of 95% to assess the association between exposed and non-exposed animals regarding risk factors. Receiver operating characteristic (ROC) curve analysis was utilized to identify the most significant risk factors influencing sudden death cases. Risk factors, with an area under the curve greater than 0.5, were considered significant. Additionally, binary logistic regression was conducted to predict the odds ratio of risk factors. The probability of risk factors was calculated using the following equation: X100 (Exp (b0+b1 (risk factor))/(1+Exp (b0 + b1 (risk factor) × 100. Ethical approval All particular animal procedures were revised and approved by the State Ethics Commission and the Ethics Committee of Alexandria University, Egypt (serial number 0305894 on December 15, 2022; FWA no. 00018699; and IRB no. 00012098). Camels were included in the study after obtaining oral permission from the owners to identify the leading causes of sudden death on the farm. The study complied with the legislation on animals practicing veterinary medicine in Saudi Arabia. The study was not an animal experiment but epidemiological research using standard sampling methods for diagnostic purposes and trials to determine the leading cause of sudden death in camels. All procedures were performed according to institutional animal care guidelines and approved by the Ministry of Environment, Water, and Agriculture Committee in Dammam, Saudi Arabia. ResultsThe total camels in the farm and suddenly dead she-camels as well as the causative C. perfringens toxins are shown in a flow diagram (Fig. 1). Descriptive demographic data and laboratory findings of the examined she-camels are shown in Table 1. The case history indicated that all camels in the farm had not been vaccinated against C. perfringens, there had been an abrupt change in ration, and fatalities had occurred. The sudden death incidence rate was 15% among the examined she-camels, predominately in those aged over 2 years. Parasites were not detected in blood and fecal matter samples. On the other hand, C. perfringens toxins were detected. Alpha-toxin was detected in 50.98% of the suddenly dead animals, particularly in all postmortem lesions except fecal matter. Also, enterotoxins were detected in 25.49% of the suddenly dead she-camels, particularly in both intestinal content and the intestinal wall. While the other toxins tested were negative (Table 2).
Fig. 1. Flow chart showing the numbers of total examined she-camels, suddenly dead she-camels, and types of C. perfringens toxins detected in the dead animals. Table 1. Descriptive demographic data and laboratory findings of the examined she-camels.
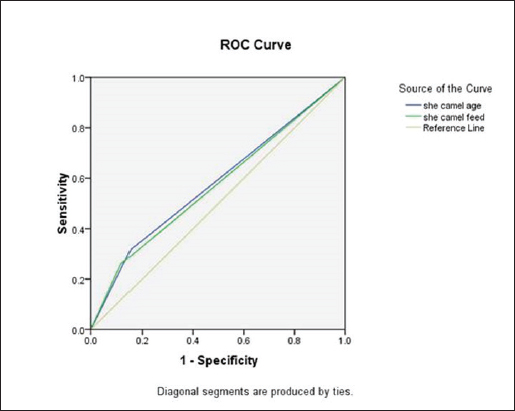
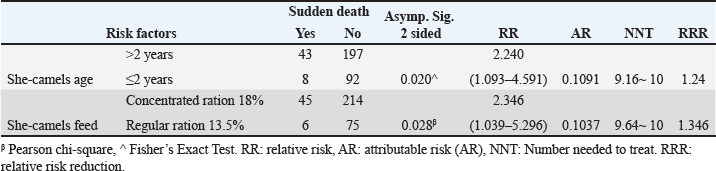
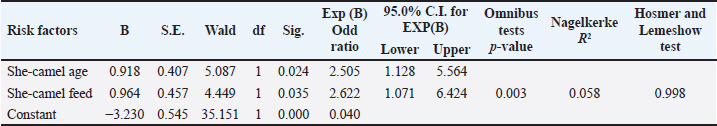
The live body weight of the examined camels ranged between 250 kg and 400 kg. These animals were fed on 3–4.5 kg concentrated ratios 13.5% or 18%/head/day and roughage feed ad libitum 1%–2% of live body weight. Out of 340 she-camels, 259 (76.17%) consumed a concentrated ration of 18%. Tables (3, 4, and 5) and Figure 2 demonstrated a significant association between sudden death, she-camels age, and feeding habits as risk factors (p=0.020 and 0.028, respectively). Risk factor assessment by RR revealed that the odds of RR of sudden death occurring among she-camels aged over 2 years were higher than those less than 2 years (2.24 CI 95%, 1.093–4.591). Furthermore, the odds RR of sudden death occurring due to exposure of she-camels to a concentrated ratio of 18% were higher twice than those not exposed (2.346 CI 95%, 1.039–5.296). As regards sudden death attributed to both risk factors, AR was 10.91% and 10.37%, respectively. When the ration was changed from a concentrated ration of 18% to a regular ration of 13.5% in only ten she-camels, the risk of sudden death decreased by 134.6 times. On the other hand, despite the RR reduction decreasing by 124 times when treating 10 camels, the camel age was a non-modified risk factor. Additionally, the camel’s age and feeding habits had ROC area under curve values above 0.5, indicating a significant association with C. perfringens infection. Table 5 predicted the risk factors using binary logistic regression. Both risk factors are twice as risky for she-camel age cases as the control group, with a she-camel age odds ratio of 2.505% 95.0% CI (1.128–5.564). Moreover, with a she-camel feeding odds ratio of 2.622 (1.071–6.424), both risk factors were significant (p=0.024 and 0.035, respectively). Still, the feeding was riskier with modified risk factors than camel age with non-modified risk factors. Overall, the model included both significant risk factors: the omnibus tests were significant (p=0.003), Nagelkerke R2 was preferred to the model, accounting for almost 5.8% of the variance of sudden death cases, and the Hosmer and Lemeshow test assessed whether the predicted probabilities matched the observed probabilities, so p=0.998, there was no difference between observed probabilities and predicted probabilities. That means a set of risk factors, both she-camel age and she-camels feeding, is needed to predict the actual sudden death probabilities that occur accurately. Finally, the probability of sudden death due to the she-camel feed was higher than the she-camel age (9.39% and 9.01%, respectively). DiscussionIn Saudi Arabia, the prevalence of harsh desert regions necessitates that one-humped camels (Camelus dromedaries) endure extreme environmental conditions like privation of water and scarcity of food, which explains the difference in the anatomical characteristics of camels from other animals to face ecological conditions that are not suitable for healthy living for different animals (Moselhy et al., 2023). This study detected sudden death due to enterotoxemia among Arabian camels (Camelus dromedaries) and associated risk factors which included the she-camel age and feeding.
Fig. 2. The area under the ROC curve of the related risk factors. Table 2. Distribution of C. perfringens toxins in different internal lesions from dead she-camels.
Table 3. The association between sudden death and different risk factors in the examined she-camels.
The antigens included endoparasites and bacterial infections, such as enterotoxins from Pasteurella multocida and C. perfringens. These enterotoxins significantly impact she-camels by lowering all vital processes, including milk production, fertility, calving rates, and working efficiency. In addition, the most noteworthy impact is the sudden death of camels (Al-Megrin et al., 2015). Table 4. The area under the curve of risk factors.
Table 5. The binary logistic regression model to estimate the odd ratio of risk factors.
In the current study, conducted in Dammam, Eastern Saudi Arabia, the ambient temperature was 41°C from August to September 2022; this environmental condition contributed to the relatively low occurrence of parasitic infections. On the other hand, Wafa et al. (2015) reported that the summer is the peak season for intestinal parasitic infection among camels, with a 34.2% prevalence of the disease in adult camels and 59.6% prevalence in the Riyadh region (the capital of Saudi Arabia). The present study revealed a sudden death attack rate of 15%, which exceeds the rate of 3% documented in Wajir County. In the Riba area, 6 out of 200 adult camels at risk succumbed to death, with postmortem inspection reports revealing no visible lesions in any organ (Gitonga et al., 2016). The case fatality rate for camels in Saudi Arabia ranges between 25% and 80% (52.3% ± 38%) (Hussain et al., 2014). The suitable environmental factors, including the low temperature and different geographical location areas, are significant factors that permitted C. perfringens to survive, so the highest prevalence of enterotoxaemia originated in the northern part of Saudi Arabia. This area is characterized by colder weather (Kathie et al., 2008). This study reported the incidence of sudden death among camels over 2 years old, aligning with Swelum et al. (2014) findings. They noted that sudden death regularly occurs in adult camels (>5 years) in good physical condition with no previous signs of illness, especially in the morning (Swelum et al., 2014). Intestinal disease in camels stems from bacterial causes, particularly C. perfringens, which produce enterotoxins leading to fatal enteric disease (Li et al., 2016). Its pathogenicity depends on the type of toxins absorbed into the bloodstream, which affect many organs (Kiu et al., 2018). In the present study, it was detected that the leading risk factor was the change that occurred in the ratio; a high concentric ratio of 18% led to the release of the Alpha toxin and the C. perfringens enterotoxin in sudden death cases. This is in agreement with the results of earlier authors who noted that the predisposing factors as non-nutritive products encourage intestinal establishment, growth, and toxin production by C. perfringens (Allaart et al., 2013). Furthermore, reduction of the intestinal transit leads to maintenance of C. perfringens and their toxins, as detected in affected camels, consistent with the previous findings (Hussain et al., 2022). Similarly, a current study in Saudi Arabia by Sawsan et al. revealed that the prevalence of enterotoxaemia in camels is 21.5%, with significant risk factors related to camel age, and the highest frequency of infection is in September (50%) (Sawsan et al., 2020). Clostridium perfringens enterotoxin is responsible for food poisoning and was primarily discovered in the USA (Lindström et al., 2011; Grass et al., 2013). Infection typically arises from consuming inadequately packaged food within 8–12 minutes, with symptoms of toxicity lasting less than 24 hours (Freedman et al., 2016; Zaragoza et al., 2019). The cause of death due to C. perfringens enterotoxins is the creation of pores in the cell membrane, permitting the unregulated influx of calcium and causing necrosis and gastrointestinal disease in adults (Uzal et al., 2010). Alpha-toxin is the most common type of C. perfringens, which leads to hydrolyzed cell membrane phospholipids and cell necrosis, contributing significantly to gas gangrene. Indeed, there are three mechanisms for gas gangrene. First, it decreases pathogen clearance at infected sites. Second, it reduces the blood flow to tissues, creating a micro-aerophilic environment conducive to C. perfringens proliferation. Third, it activates arachidonic acid and protein kinase C in host cell metabolism, potentially leading to immune-mediated pathologies of organs (Takehara et al., 2016). Finally, one injectable dosage of long-acting Amoxicillin trihydrate is adequate as a preventive dose against C. perfringens in apparently healthy female camels. Camels should be vaccinated with a combination vaccine adjuvanted by Montanide gel including strains of BVDV and C. perfringens type A toxoid every 6 months to guarantee safe and efficient protection against infections in the field (Elhelw et al., 2022). The main limitations of this study included the lack of individual camel medical records on the farm, which made it difficult for the research team to pinpoint the reason for the sudden camel deaths. This has complicated and delayed their work. Furthermore, the considerable distance between the camel farm and the big laboratories in the Dammam area slowed the diagnosis and contributed to unexpected deaths. Finally, the preservation of feces and blood samples across such great distances may have an impact on the accuracy and timeliness of the diagnosis. ConclusionBased on the evidence presented, it can be concluded that the sudden death of she-camels was caused by the change from a regular silage ration of 13.5% to a higher concentration of 18% and a modified risk factor in subjecting she-camels to alterations in intestinal flow and the production of toxins by C. perfringens. A single intramuscular dosage of long-acting Amoxicillin trihydrate is sufficient as a prophylactic dose against C. perfringens in the apparently healthy she-camels. Regular immunization at 6-month intervals is an efficient way to prevent camels from C. perfringens infections in the field. AcknowledgmentsThe authors would like to thank the staff of the Diagnostic Veterinary Laboratory at the Dammam Veterinary Diagnostic Laboratory, Ministry of Environment, Water, and Agriculture, Saudi Arabia. Additionally, they thank Prof. Dr. Sabreen Fadl, Professor of Biochemistry, for her assistance in editing the current manuscript. Authors’ contributionAGM, AGY, and FAK wrote the original manuscript text. YE and AMAS prepared the figures. AAR and AMA diagnosed the cases. WF, AMAS, and AA prepared the tables of results. AHS created the nutrition and clinical nutrition results. EYA checked the manuscript text for plagiarism. HAAE and AA prepared the tables of results and calculated the sample size. EME was consulted regarding C. perfringens toxin. AMAS and AA wrote the final manuscript. All authors wrote and revised the final manuscript. FundingThis study was self-funded and did not receive any funding from any agency. Conflict of interestThe authors declare that there are no conflicts of interest regarding the publication of this manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAllaart, J.G., van Asten, A.J. and Gröne, A. 2013. Predisposing factors and prevention of Clostridium perfringens-associated enteritis. Comp. Immunol. Microbiol. Infect. Dis. 36(5), 449–464. Al-Megrin, W. 2015. Prevalence rate of intestinal parasites in camels in Riyadh, Saudi Arabia. Intern. J. Zool. Res. 11(2), 65–70. Alsaab, F., Wahdan, A. and Saeed, E.A. 2021. Phenotypic detection and genotyping of Clostridium perfringens associated with enterotoxemia in sheep in the Qassim Region of Saudi Arabia. Vet. World. 14(3), 578–584. Bouragba, M., Laatamna, A.K., Cheddad, F.E., Baroudi, D., Houali, K. and Hakem, A. 2020. Gastrointestinal parasites of dromedary camel (Camelus dromedarius) in Algeria. Vet. World 13(8), 1635–1640. Elhelw, H.A., El Fadeel, M.A., El-Sergany, E., Allam, A., Elbayoumy, M.K., El-Kattan, A.M. and El-Kholy, A.A. 2022. Preparation and field study of combined vaccine against Clostridium perfringens type A and bovine viral diarrhea virus in camels. Clin. Exp. Vaccine Res. 11(1), 30–42. El-Seify, M., Elshahawy, S., Ibrahim, O. and Ahamed, Z. 2021. An Abattoir-based study on helminths of slaughtered camels (Camelus dromedarius) in Aswan Province, Egypt. International. J. Vet. Sci. 4(3), 119–129. Esmaeel, S.A., Hussain, K.J. and Al-Taliby, M.A. 2021. Seroprevalence of crimean congo hemorrhagic fever in cows by ELISA in Mosul city. Iraqi J. Vet. Sci. 35(4), 803–807. Felefel, W., Abdel-Rady, A., Abd El-Rahim, I., Elkamshishi, M.M. and Mostafa, W. 2023. Detection of Cryptosporidium parvum in calf feces using microscopical, serological, and molecular methods. Iraqi J. Vet. Sci. 37(2), 383–389. Felefel, W., Shaaban, A., Eassa, S.M. and Loutfy, N.F. 2023. The prevalence of parasitic infections among slaughtered animals in mechanical abattoirs. Iraqi J. Vet. Sci. 37(2), 469–477. Finnie, J.W. and Uzal, F.A. 2022. Pathology and pathogenesis of brain lesions produced by Clostridium perfringens type D epsilon toxin. Int. J. Mol. Sci. 23, 9050. Finnie, J.W., Navarro, M.A. and Uzal, F.A. 2020. Pathogenesis and diagnostic features of brain and ophthalmic damage produced by Clostridium perfringens type D epsilon toxin. J. Vet. Diagn. Invest. 32(2), 282–286. Freedman, J.C., Shrestha, A. and McClane, B.A. 2016. Clostridium perfringens enterotoxin: Action, genetics, and translational applications. Toxins 8(3), 73. Ghoneim, N.H. and Hamza, D.A. 2017. Epidemiological studies on Clostridium perfringens food poisoning in retail foods. Rev. Sci. Tech. 36(3), 1025–1032. Gitonga, N. and Kamau, J. 2016. Camel sudden death syndrome: outbreak investigation in Wajir County. Afr. Drylands Inst. Sust. (UoN-ADIS) 5, 3–15. Grass, J.E., Gould, L.H. and Mahon, B.E. 2013. Epidemiology of foodborne disease cutbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 10, 131–136. Hussain, R., Guangbin, Z., Abbas, R.Z., Siddique, A.B., Mohiuddin, M., Khan, I., Rehman, T.U. and Khan, A. 2022. Clostridium perfringens types A and D involved in peracute deaths in goats kept in the Charleston ecosystem during winter season. Front. Vet. Sci. 9, 849856. Hussain, R., Javed, M.T., Mahmood, F., Hussain, T., Chaudhry, H.R., Aslam, M.S. and Ghori, M.T. 2014. Clinicopathologic findings of enterotoxaemia in Chinkara deer (Gazella bennettii) under desert conditions in Pakistan. Pak. Vet. J. 34, 400–402. Kaaboub, E.A., Ouchene, N., Ouchene, N.A., Dahmani, A., Ouchtati, I., Haif, A. and Khelef, D. 2021. Investigation of the principal vectors of abortive diseases in one-humped camels (Camelus dromedarius). Iraqi J. Vet. Sci. 35(3), 411–415. Kathie, A.G., Sarah, K., Ijeoma, E., June, P., Charles, O., Robin, H., Michael, W.P. and Jim, M. 2008. The Identification and characterization of Clostridium perfringens by real-time PCR, location of enterotoxin gene, and heat resistance. Foodb. Path. Dis. 5(5), 629–639. Kiu, R. and Hall, L.J. 2018. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 7(1), 141. Li, J., Uzal, F.A. and McClane, B.A. 2016. Clostridium perfringens sialidases: potential contributors to intestinal pathogenesis and therapeutic targets. Toxins 8, 341. Lindström, M., Heikinheimo, A., Lahti, P. and Korkeala, H. 2011. Novel insights into the epidemiology of Clostridium perfringens type A food poisoning. Food Microbiol. 28, 192–198. Mahmood, M.A. and Essa, M.A. 2021. Antimicrobial activity of peptides extracted from camels’ blood neutrophils against some pathogenic bacteria. Iraqi J. Vet. Sci. 35(1), 33–37. Mohiuddin, M., Iqbal, Z. and Ur Rahman, S. 2016. Prevalence of Clostridium perfringens β2-toxin in sheep and goat population in Punjab, Pakistan. Thai J. Vet. Med. 46(3), 491–496. Moselhy, A.A. and El-Ghazali, H.M. 2023. Gross histological and electron microscopical features of the hard palate mucosa in the one-humped camel (Camelus dromedarius). Iraqi J. Vet. Sci. 37(3), 651–657. Moustafa, S., Zakaria, I., Moustafa, A., AboSakaya, R. and Selim, A. 2022. Bacteriological and serological investigation of Clostridium perfringens in lambs. Sci. Rep. 12 (1),19715. Naureen, S., Rabbani, M., Sheikh, A.A., Hashmi, A.S. and Jayaro, B.M. 2022. Prevalence and risk factors analysis of soil-borne clostridium perfringens in selected districts of Punjab province. Pak. J. Anim. Plant Sci. 32(2), 362–369. Omer, S.A., Babiker, S.H., Aljulaifi, M.N., Al-Olayan, E.M., Alagaili, A.N. and Mohammed, O.B. 2020. Epidemiology of enterotoxaemia in livestock in the Kingdom of Saudi Arabia. J. King Saud Univ. Sci. 32, 2662–2668. Pawaiya, R., Gururaj, K., Gangwar, N., Singh, D., Kumar, R. and Kumar, A. 2020. The challenges of diagnosis and control of enterotoxaemia caused by Clostridium perfringens in small ruminants. Adva. Microbiol. 10, 238–273. Rockhill, B., Newman, B. and Weinberg, C. 1998. Use and misuse of population attributable fractions. Am. J. Public Health. 88(1),15–19. Rothman, K.J. and Greenland, S. 1998. Modern epidemiology. 2nd Edition. Philadelphia, PA: Lippincott Williams and Wilkins. Sawsan, A., Omer, S.E., Babiker, H., Mohammed, Z.N., Ebtesam, M.A., Abdulaziz, N.A. and Osama, B.M. 2020. Epidemiology of enterotoxaemia in livestock in the Kingdom of Saudi Arabia. J. King Saud Univ. Sci. 32(5), 2662–2668. Sazmand, A., Joachim, A. and Otranto, D. 2019. Zoonotic parasites of dromedary camels: so important, so ignored. Parasit. Vector 12, 610. Sheet, O.H., Jwher, D.M., Al-Sanjary, R.A. and Alajami, A.D. 2021. Direct detection of Staphylococcus aureus in camel milk in the Nineveh governorate by using the PCR technique. Iraqi J. Vet. Sci. 35(4), 669–672. Swelum, A.A., Ismael, A.B., Khalaf, A.F. and Abouheif, M.A. 2014. Clinical and laboratory findings associated with naturally occurring babesiosis in dromedary camels. Bull. Vet. Inst. Pulawy. 58, 229–233. Takehara, M, Takagishi, T. and Seike, S. 2016. Clostridium perfringens α-toxin impairs innate immunity via inhibition of neutrophil differentiation. Sci. Rep. 6, 28192. Uzal, F.A., Vidal, J.E., McClane, B.A. and Gurjar, A.A. 2010. Clostridium Perfringens toxins involved in mammalian veterinary diseases. Open Toxicol. J. 2, 24–42. Uzal, F.A., Navarro, M.A., Li, J., Freedman, J.C., Shrestha, A. and McClane, B.A. 2018. Comparative pathogenesis of enteric clostridial infections in humans and animals. Anaerobe. 53, 11–20. Wafa, A.I. 2015. Prevalence rate of intestinal parasites in camels in Riyadh, Saudi Arabia. Int. J. Zool. Res. 11, 65–70. Zaragoza, N.E., Orellana, C.A., Moonen, G.A., Moutafis, G. and Marcellin, E. 2019. Vaccine production to protect animals against pathogenic clostridia. Toxins 11, 525. | ||
| How to Cite this Article |
| Pubmed Style Mubarak AG, Khalifa FA, Elsobky Y, Abdel-rady A, Felefel W, Saad AH, Abdelhiee EY, Alhassan AM, Awny H, Elghazaly EM, Abu-seida AM, Abdulkarim A, Youseef AG. Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Vet. J.. 2024; 14(8): 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 Web Style Mubarak AG, Khalifa FA, Elsobky Y, Abdel-rady A, Felefel W, Saad AH, Abdelhiee EY, Alhassan AM, Awny H, Elghazaly EM, Abu-seida AM, Abdulkarim A, Youseef AG. Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. https://www.openveterinaryjournal.com/?mno=201491 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.23 AMA (American Medical Association) Style Mubarak AG, Khalifa FA, Elsobky Y, Abdel-rady A, Felefel W, Saad AH, Abdelhiee EY, Alhassan AM, Awny H, Elghazaly EM, Abu-seida AM, Abdulkarim A, Youseef AG. Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Vet. J.. 2024; 14(8): 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 Vancouver/ICMJE Style Mubarak AG, Khalifa FA, Elsobky Y, Abdel-rady A, Felefel W, Saad AH, Abdelhiee EY, Alhassan AM, Awny H, Elghazaly EM, Abu-seida AM, Abdulkarim A, Youseef AG. Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 Harvard Style Mubarak, A. G., Khalifa, . F. A., Elsobky, . Y., Abdel-rady, . A., Felefel, . W., Saad, . A. H., Abdelhiee, . E. Y., Alhassan, . A. M., Awny, . H., Elghazaly, . E. M., Abu-seida, . A. M., Abdulkarim, . A. & Youseef, . A. G. (2024) Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Vet. J., 14 (8), 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 Turabian Style Mubarak, Asmaa G., Fatma A. Khalifa, Yumna Elsobky, Ahmed Abdel-rady, Wael Felefel, Adel Hassan Saad, Ehab Y. Abdelhiee, Abdullah Mohamed Alhassan, Hisham Awny, Eman M. Elghazaly, Ashraf M. Abu-seida, Abdulrahman Abdulkarim, and Asmaa G. Youseef. 2024. Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Veterinary Journal, 14 (8), 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 Chicago Style Mubarak, Asmaa G., Fatma A. Khalifa, Yumna Elsobky, Ahmed Abdel-rady, Wael Felefel, Adel Hassan Saad, Ehab Y. Abdelhiee, Abdullah Mohamed Alhassan, Hisham Awny, Eman M. Elghazaly, Ashraf M. Abu-seida, Abdulrahman Abdulkarim, and Asmaa G. Youseef. "Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors." Open Veterinary Journal 14 (2024), 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 MLA (The Modern Language Association) Style Mubarak, Asmaa G., Fatma A. Khalifa, Yumna Elsobky, Ahmed Abdel-rady, Wael Felefel, Adel Hassan Saad, Ehab Y. Abdelhiee, Abdullah Mohamed Alhassan, Hisham Awny, Eman M. Elghazaly, Ashraf M. Abu-seida, Abdulrahman Abdulkarim, and Asmaa G. Youseef. "Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors." Open Veterinary Journal 14.8 (2024), 1942-1951. Print. doi:10.5455/OVJ.2024.v14.i8.23 APA (American Psychological Association) Style Mubarak, A. G., Khalifa, . F. A., Elsobky, . Y., Abdel-rady, . A., Felefel, . W., Saad, . A. H., Abdelhiee, . E. Y., Alhassan, . A. M., Awny, . H., Elghazaly, . E. M., Abu-seida, . A. M., Abdulkarim, . A. & Youseef, . A. G. (2024) Sudden death due to enterotoxemia amongst Arabian camels (Camelus dromedaries) and associated risk factors. Open Veterinary Journal, 14 (8), 1942-1951. doi:10.5455/OVJ.2024.v14.i8.23 |