| Research Article | ||
Open Vet. J.. 2024; 14(8): 1960-1967 Open Veterinary Journal, (2024), Vol. 14(8): 1960–1967 Research Article Immune response and safety of co-administered peste des petits ruminants, contagious caprine pleuropneumonia, sheep and goat pox, and Pasteurellosis vaccines in goatsTakele Tesgera Hurisa1*, Takele Abayneh Tefera1, Retta Negatu1, Teshale Sori1, Berecha Bayisa Deme1, Mirtneh Akalu Yilma2, Wondwossen Tolossa2, Abinet Legesse2, Ashetu Negewo2, Wubet W/Medhin2, Kedir Sherefa2, Getu Ayele2, Adugna Geresu3, Eyob Assefa3 and Dawit Dufera31National Veterinary Institute, Bishoftu, Ethiopia 2Luna Export Abattoir, Central Oromia, Ethiopia 3Department of Clinical Studies, Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia *Corresponding Author: Takele Tesgera Hurisa. National Veterinary Institute, Bishoftu, Ethiopia. Email: takele.tesgera [at] yahoo.com Submitted: 19/05/2024 Accepted: 11/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
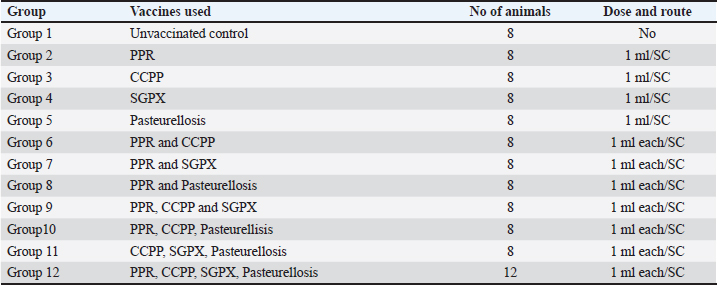
ABSTRACTBackground: Infectious diseases such as peste des petits ruminants (PPRs), contagious caprine pleuropneumonia (CCPP), sheep and goat pox (SGPX), and pasteurellosis have considerable impacts on the optimal utilization of sheep and goat resources in Ethiopia. Immunization using multiple vaccines administered simultaneously has been suggested as a cost-effective and safe approach to controlling and preventing these diseases. Aim: The aim of this study was to assess the immunogenicity and safety of multiple vaccines administered simultaneously in goats. Methods: Sero-negative PPR, CCPP, SGPX, and Pasteurellosis goats were immunized with multiple vaccines. Goats vaccinated with a single vaccine against each disease served as a positive control. The immune response of the goats was assessed using serological tests, and any adverse effects were monitored. Results: The results of the present study showed that goats vaccinated with multiple vaccines exhibited a remarkable immune response against PPR, CCPP, and pasteurellosis. In contrast, they did not produce a protective immune response against sheep or goat pox. No adverse effects were observed with any of the vaccines. Conclusion: This study suggested that combined vaccines can be effective at inducing a protective immune response in goats. However, further research is needed to fully understand the immune response to combined vaccines. Keywords: Immunogenicity, Safety, Goats, Multiple vaccines, Ethiopia. IntroductionInfectious diseases in goats are important constraints to their health and productivity. They can cause significant economic losses to farmers through reduced milk and meat production, increased mortality, and treatment costs. Proper management practices such as the use of vaccination are important for preventing the spread of infectious diseases in goat populations. Prevention and control of infectious diseases in goats are essential for maintaining the health and productivity of goat populations to achieve food security. Peste des petit ruminants (PPRs), contagious caprine pleuropneumonia (CCPP), sheep and goat pox (SGPX), and pasteurellosis are among the major infectious diseases of goats worldwide. Vaccination is an effective and feasible intervention for the control of infectious diseases. Vaccinating goats can reduce the incidence and severity of infectious diseases and the spread of the disease within and between herds. In addition, vaccination is often more cost effective than treating infected animals or dealing with the economic losses associated with disease outbreaks (Tizard, 2020). Vaccines are designed to fight diseases by introducing whole pathogens or portions of proteins called antigens into the body. These antigens stimulate different cells in the immune system, including macrophages, T cells, and B cells. When antigens enter the body, macrophages ingest and digest them into antigen fragments. These fragments are carried to the surface of cells by a molecule called the major histocompatibility complex, where they are recognized by T cells (Janeway, 2001). T cells then stimulate B cells to produce antibodies against antigens, which activate other immune defenses. The use of multivalent vaccines has long been common in human medicine. A single-injection formulation containing vaccines against diphtheria, tetanus, whooping cough, polio, and Haemophilus b meningitis has been used in children (Liu B et al., 2022). Similarly, it has been used in pet practice, such as for puppy vaccines that contain several viruses and leptospira in one product. The combination of vaccines is also common in cattle (neonatal diarrhea and bovine respiratory disease) (Martinod, 1996). Combined vaccines should have protective efficacy comparable to that of individual vaccines (Offit et al., 2002). Combining vaccines can have significant economic advantages, as it reduces the holding time of animals and vaccination time intervals, minimizing labor and management costs. In addition, it reduces stress on animals during vaccination time and may shorten the vaccination schedule. Despite several advantages, the use of combinations of vaccines has been reported to cause fever and some compromised immune responses due to undesirable interference among different antigens, although most such reactions are temporary and do not cause any long-lasting damage (Glanz et al., 2018). Hence, the use of combined vaccines or concurrent administration of different vaccines requires investigation for compatibility and effectiveness. Luna Export Abattoir and the National Veterinary Institute (NVI) conducted an experimental study to evaluate the immune effects of four common vaccines (PPR, CCPP, SGPX, and Pasteurellosis) simultaneously administered to goats. This study aimed to assess the safety and immune response of goats immunized with all four vaccines at once. The results of this study provide insights into the safety and efficacy of multiple vaccines and may inform the development of more effective vaccination strategies for small ruminants. Materials and MethodsStudy animalsA total of 100 approximately 6-month-old male indigenous goats were screened for PPR, CCPP, SGPX, and pasteurellosis at the Luna Export Abattoir. The goats that tested negative were used for vaccination in this experiment. The animals were randomly assigned to 12 groups: 11 of the groups contained 8 animals each, while one group had 12 goats. The details of the experimental layout are indicated in Table 1. The study involved the administration of different vaccines to different groups of goats, with some groups (groups 2–5) receiving individual vaccines and others (groups 6–12) receiving combinations of two or more vaccines. Preparation of vaccines and immunization of the goatsIn this study, vaccines produced against CCPP, PPR, SGPX, and pasteurellosis at the NVI, Ethiopia, were used. The CCPP vaccine was an inactivated vaccine produced from Kenyan F, 38 strains of Mycoplasma capricolum subspecies capripneumoniae. One milliliter of the vaccine contained 109 CFU of inactivated formalin that was supplemented with 0.3% saponin. The protein content in each field dose of the vaccine was 0.15 mg/ml. The vaccine used against Pasteurellosis Pasteurella multocida serotype A was formalin-killed and precipitated with 1% aluminum potassium sulfate at a concentration of 1 × 109 CFU/ml. The PPR vaccine used was a live attenuated lyophilized strain produced on VERO cells from the Nigerian strain of the PPR virus, which was designated 75/1. The field dose of the vaccine contained 2.5 TCID50 viruses/ml. The vaccine used against SGPX was a live attenuated strain produced from the Kenyan SGPX strain (0180) of Capripoxvirus. The vaccine was manufactured on VERO cells, and each field dose contained 2.5 TCID50 viruses/ml. The vaccines were administered to each animal following the manufacturer’s instructions. The experimental animals were monitored daily for any evidence of adverse reactions. Clinical examination of the animals was performed daily throughout the study period, while the rectal temperature of each animal was recorded daily for 1 week. Blood samples were collected from the goats immediately before and 7, 14, 21, and 35 days after vaccination. The animals were observed for 6 weeks after vaccination. Table 1. Experimental allocation of study animals and immunization protocol.
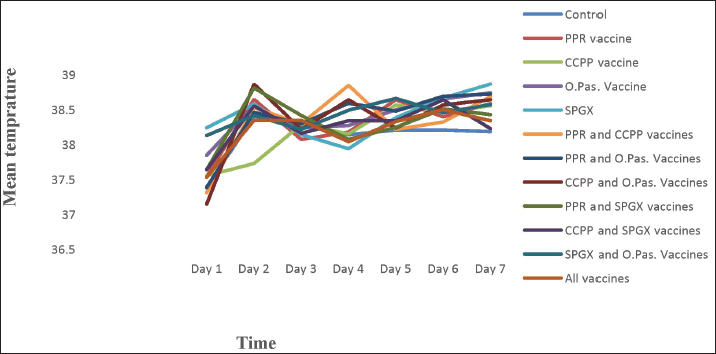
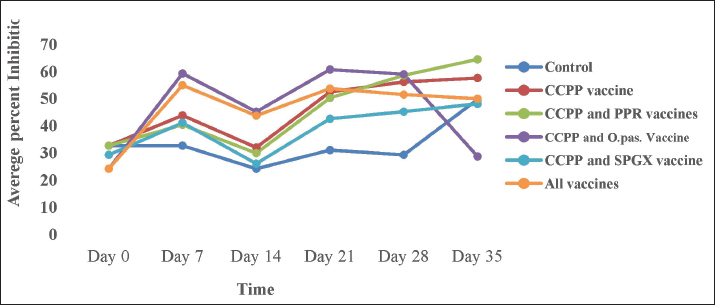
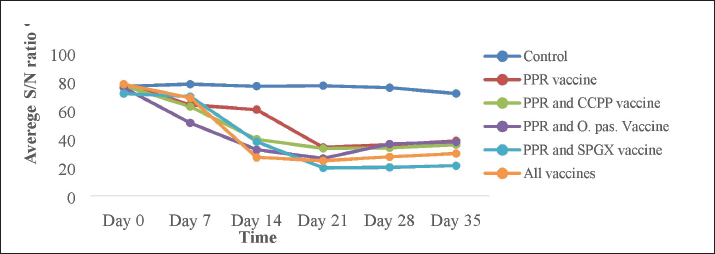
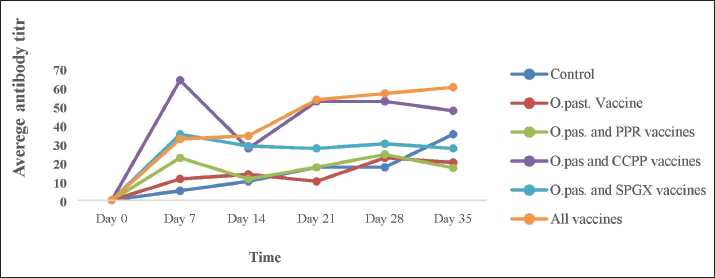
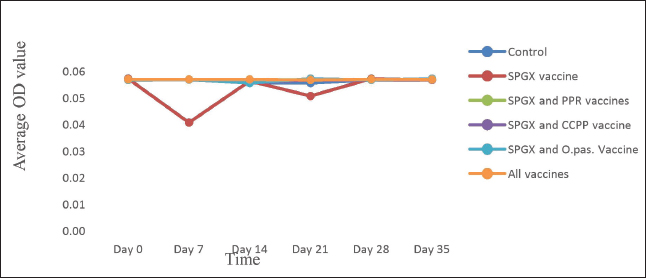
Laboratory test methodsThe antibody response to the PPR vaccine was measured using a competitive ELISA kit (Idivet, ID Screen® PPR competition, France) according to the manufacturer’s instructions. Briefly, the samples and controls were added to the ELISA wells. Next, the micro wells were filled with an anti-NP-peroxidase conjugate and incubated. After incubation, the micro wells were washed with a wash solution to remove any unbound conjugate. Then, a substrate solution was added, and a stop solution was added to each well to stop further reactions. The optical densities were measured with an EL × 800 BIOTIC ELISA Reader on a microplate photometer at a wavelength of 450 nm. The level of antibody response to the SPGX vaccine was measured using an indirect ELISA kit (IDvet, ID screen® Capripox Double Antigen Multispecies Kit, France) according to the manufacturer’s protocol. Briefly, the wells were coated with purified CPV antigen. Samples were to be tested and controls were added to the micro wells. If present, anti-CPV antibodies form an antibody-antigen complex yielding blue coloration after the addition of conjugates and substrate. A stop solution was added, and the absorbance at 450 nm was measured on a microplate reader. Antibody production against the Pasteurellosis vaccine was measured by an indirect haemaglutinattion (IHA) test according to the SOP developed at the NVI, Ethiopia. Ninety microliters of PBS were dispensed into the wells in the first row of the V-shaped microplate, and 50 μl of PBS containing sensitized RBCs was added to the remaining wells, including the negative and positive controls. Ten microliters of serum were added to the wells in the first row to obtain a 1:10 dilution. After pipetting and mixing, 50 μl was transferred serially to the next wells, and the last 50 μl was discarded. The plate was covered with a microplate, sealed, and incubated at 37°C with constant agitation for 1 hour. The IHAT antibody titers of all the samples were recorded in comparison with those of the positive and negative controls. Positive results were taken if the antibody titer was greater than 1:10. The immune response of goats to the CCPP vaccine was measured using a M. capricolum subspecies capripneumoniae antibody test kit (IDEXX CCPP, the Netherlands) following the manufacturer’s instructions. A percentage inhibition greater than 55% was considered positive for CCPP. The wells of a microtiter plate were coated with inactivated M. capricolum subsp. capripneumoniae antigens and incubated. The diluted serum samples were added to the coated wells and incubated. The plate was washed to remove any unbound serum proteins, the conjugates were added, and the plate was incubated. The plate was washed again to remove any unbound conjugate, and the substrate was added and incubated. Finally, a stopping solution was added to the wells, and the OD was recorded. Ethical approvalThe experiment was performed in accordance with the international guidelines for animal experiments. All goats in the study received all the necessary veterinary care and accepted animal welfare regulations. The ethical handling of the experimental animals was reviewed by the Animal Research Ethical Review Committee of Addis Ababa University, College of Veterinary Medicine and Agriculture with ethical reference number VM/ERC/08/02/16/2023). ResultsClinical follow-up and examination did not reveal any adverse post-vaccinal reactions in the immunized animals. Similarly, the daily rectal temperature of the experimental animals was within the normal range (between 37ºC and 39ºC). The mean daily temperature of the experimental animals is depicted in Figure 1. This study investigated the immune response of goats to individual vaccines and concurrent immunizations with different combinations of four vaccines. Figure 2 shows that goats vaccinated with the CCPP vaccine alone had a similar immune response to those concurrently vaccinated with CCPP and one or more of the other vaccines. Similarly, the seroconversion rates of goats vaccinated with the PPR vaccine alone and four of the groups vaccinated with different combinations of CCPP, SGPX, and Pasteurellosis along with the PPR were similar (Fig. 3). The immune response to the Pasteurellosis vaccine was not affected when it was administered concurrently with one or more of the other three vaccines, as shown by similar seroconversion rates in all vaccinated groups against pasteurellosis (Fig. 4). However, in the case of the immune response to the SGPX vaccine, the groups vaccinated with the SGPX vaccine alone or with the other vaccines did not show seroconversion to SGPX (Fig. 5). Except for the immune response to the SGPX vaccine, seroconversion to the other three vaccines given either alone or concurrently with the other vaccines was greater than that in the unvaccinated control group. DiscussionThe prevention and control of infectious diseases are very important for the optimal use of goats. Vaccine use is an effective and safer mechanism for preventing and controlling infectious diseases. Many vaccines are available against several infectious diseases in goats. However, the cost of vaccines and vaccination must be economically feasible (OIE and FAO, 2015). Therefore, the choice of vaccines or the type of vaccine formulation for goats is important because they are the most significant livestock species in many countries, especially in less developed or arid regions. PPR, SGPX, pasteurellosis, and CCPP are common infectious diseases that affect small ruminants in Africa, the Middle East, and Asia. These diseases have a significant economic impact on these regions due to clinical manifestations and trade restrictions (Zhang et al., 2021). Vaccination is considered the most feasible strategy to reduce their economic impact and prevent their spread (OIE and FAO, 2015).
Fig. 1. The mean daily rectal temperature of goats immunized with a single vaccine or a combination of vaccines.
Fig. 2. The antibody response against the CCPP vaccine in animals immunized with CCPP alone, CCPP and PPR, CCPP and pasteurellosis, CCPP, and SPGX vaccines, and all four vaccines simultaneously. The presence of the antibody was detected using a competitive ELISA kit. As shown in the graph, the antibody level began to increase after day 14 of vaccination and remained so high except for the group vaccinated with CCPP and Pasteurellosis, in which the antibody level declined after day 28 of vaccination. However, vaccination schedules for PPR, CCPP, SGPX, and Pasteurellosis usually do not coincide and are mostly administered at different times. Therefore, the synchronization of vaccination schedules is desired to reduce the cost of maintaining animals and vaccination. As these diseases are trans-boundary in nature, they are a major concern for most countries (Osama, 2010). PPR, SGPX, and CCPP are of particular global concern. The implementation of the Global Eradication Program with the involvement of international organizations targeting ultimate eradication is considered an effective strategy (FAO, 2014). In this study, the simultaneous administration of vaccines against infectious diseases in goats revealed good immunogenicity that is comparable to the immune response when goats are vaccinated with individual vaccines. This reduces the labor, time, and financial costs required for vaccination and should be taken into account by livestock and veterinary authorities.
Fig. 3. The antibody response against the PPR vaccine in experimental animals immunized with the PPR vaccine alone, the PPR and CCPP vaccines, the PPR and Pasteurellosis vaccines, the PPR and SPGX vaccines, and all four vaccines simultaneously. The ELISA results showed that a markedly greater antibody response was observed in animals vaccinated with various combinations of antibiotics. Interestingly, animals vaccinated with four vaccines simultaneously produced antibodies similar to those vaccinated with a single PPR vaccine and those vaccinated with combinations of two other vaccines.
Fig. 4. The HI antibody titer in response to the Pasteurellosis vaccine in experimental animals immunized with the Pasteurellosis vaccine alone, Pasteurellosis and PPR, Pasteurellosis and CCPP, Pasteurellosis and SPGX, or all four vaccines simultaneously. A greater antibody response was observed in animals vaccinated with four vaccines simultaneously and in a group vaccine with a combination of Pasteurellosis and CCPP, which remained higher after day 35 of vaccination. Concurrent administration of the four vaccines did not cause any adverse reactions during post-vaccination clinical follow-up, indicating that there is no safety concern in using a combined formulation of such vaccines. The results of the antibody assay showed that the immune response (seroconversion) was good against PPR, CCPP, and pasteurellosis. Animals vaccinated with bivalent, trivalent, or tetravalent vaccines exhibited immune responses comparable to those induced by monovalent vaccines. This indicated that concurrent vaccination with the four vaccines at any possible combination did not affect the immune response to PPR, CCPP, or pasteurellosis.
Fig. 5. The antibody response to the SPGX vaccine in animals immunized with the SPGX only, the SPGX and PPR, the SPGX and CCPP, the SPGX and Pasteurellosis, and all four vaccines administered simultaneously was assayed using an indirect ELISA kit. In all the experimental groups, a low level of antibody response to the SPGX vaccine was observed. There is limited data available on the immune response to combined vaccines in goats. However, some studies have been conducted on this topic, and the available information is summarized. A combined PPR/SGPX vaccine has been used in a vaccination campaign to control both diseases in small ruminants in Morocco, showing the potential of using a combined vaccine of PPR and SGPX as an economic vaccination strategy (Fakri et al., 2015). A similar study was also conducted in Ethiopia (Ayelet et al., 2012), revealing good antibody response and protection against challenge infection for both the PPR and the SPGX. In this study, the concurrent administration of four vaccines (PPR, CCPP, SGPX, and pasteurellosis) to goats was evaluated to assess the immune response to each vaccine and the safety of the concurrent vaccination regime. Consistent with our observations, Chaudhary and colleagues (2009) demonstrated that combined sheep pox and PPR vaccines were safe and potent, as evidenced by seroconversion and challenge studies in sheep. Similar observations were also reported by Hosamani et al. (2006), who demonstrated the safety and protective immune response in goats vaccinated with combined PPR and goat pox vaccines. The effect of the vaccine was evident in sero-conversion and challenge studies. A study published by Sun (2009) evaluated the immune response to a combined vaccine against P. multocida and Mannheimia hemolytica in sheep. The study revealed that the vaccine induced a strong humoral immune response, as evidenced by high levels of serum antibodies. Previous studies also revealed that a good level of antibody and protection was produced in cattle vaccinated for combined foot and mouth disease and hemorrhagic septicaemia (Muenthaisong et al., 2021). Completely safe and highly neutralizing antibody production was reported following the immunization of cattle with combined vaccines against lumpy skin disease and blue tongue disease, with complete protection against viremia after infection challenge (El-Sadeqy et al., 2021). A study published by Katsuhisa and colleague (2019) evaluated the immune response to a combined vaccine against P. multocida, Mannheimia hemolytica, and Haemophilus nomnui in cattle, revealing the induction of a strong immune response, as evidenced by increased antibody titers and a decreased incidence of respiratory disease. Another study published by Trotta et al. (2015) showed that simultaneous application of a Bacillus anthracis vaccine with a commercial tetravalent oil-based FMD vaccine in cattle elicited similar antibody titers to those of individual vaccines. In contrast, there was no immune response to SGPX when it was administered concurrently with the other three vaccines. It is unclear why the group vaccinated with SGPX and other vaccines did not show seroconversion to SGPX immunization. However, there could be several possible reasons for this observation. One possibility is the occurrence of immunological interference, where the presence of one vaccine may inhibit the immune response to another vaccine. Alternatively, the other vaccines given concurrently with SGPX may have stimulated the immune system in a way that suppressed the immune response to SGPX (Jatana and Nair, 2007). Another possibility is that the SGPx vaccine was not effective at inducing an immune response in goats, either due to issues with the vaccine formulation or administration. Therefore, further studies may be needed to better understand the reasons behind the lack of seroconversion after SGPX immunization in the group vaccinated with SGPX and other vaccines. The absence of an immune response to the monovalent SPGX vaccine may be because some animals are subclinically infected but appear seronegative on day 0, which may subsequently interfere with the immune response to the vaccine. In addition, Sareyyüpoğlu and colleague (2022) reported a decreased immune response to a combined vaccine against foot-and-mouth disease, the PPR, SGPX, and bluetongue virus in sheep due to interference. In conclusion, the present study demonstrated that the concurrent administration of the PPR, CCPP, SGPX, and Pasteurellosis vaccines to goats did not result in adverse reactions and did not interfere with the immune response against each vaccine. This study suggested that combined vaccines can be effective in inducing a protective immune response in goats. However, further research is needed to fully understand the immune response to combined vaccines. Limitations of the studyThis study had several limitations, including the use of few experimental animals and the lack of consideration of subclinical infections. Nevertheless, the findings of this study provide vital preliminary information for the potential development of combined vaccines comprising antigens against PPR, CCPP, SGPX, and pasteurellosis. Such vaccines would confer immense economic advantages for ranches, which are expected to contain large numbers of herds. AcknowledgmentsThe authors would like to thank the Luna Export Abattoir and the National Veterinary Institute for their overall support while performing this study. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThis research was funded by the Luna Export Abattoir. Luna Export Abattoir purchased the experimental goats and the serological kits used in the antibody assays. Data availabilityAll data included in this study are available upon request to the corresponding author. Authors’ contributionsTT performed the experiments, analyzed the data, and drafted the manuscript; TA designed the study and edited the manuscript; RN supplied the animals and serological kits; TS analyzed the data and edited the manuscript; and BB, MA, WT, AL, AN, WW, KS, GA, AG, EA, DD were involved in the experimental work and laboratory tests. ReferencesAyelet, G., Fasil, N., Jembere, S., Mekonen, G., Teshale, S. and Negussie, H. 2012. Study on the immunogenicity of combined sheep and goat pox and peste des petitis ruminants vaccines in small ruminants in Ethiopia. Afri. J. Microbiol. Res. 6(44), 7212–7217. Chaudhary, S.S., Pandey, K.D., Singh, R.P., Verma, P.C. and Gupta, P.K. 2009. A verocell-derived combined vaccine against sheep pox and peste des petits ruminants for sheep. Vaccine. 2(19), 2548–2553. Es-sadeqy, Y., Bamouh, Z., Ennahli, A., Safini, N., El Mejdoub, S., Tadlaoui, K.O., Gavrilov, B. and El Harrak, M. 2021. Development of an inactivated combined vaccine for the protection of cattle against lumpy skin disease and bluetongue viruses. Vet. Microbiol. 256, 109046. Fakri, F., Ghzal, F., Daouam, S., Elarkam, A., Douieb, L., Zouheir, Y., Tadlaoui, K. and Fassi-Fihri, O. 2015. Development and field application of a new combined vaccine against peste des petits ruminants and sheep pox. Trials Vaccinol. 4, 33–37. Fakri1, F.Z., Embarki, T., Baha, W., Tadlaoui, K.O., Fihri, O.F. and El Harrak, M. 2020. Large mass vaccination of small ruminants against peste des petits ruminants and sheep pox using a combined live attenuated vaccine. J. Vet. Med. Res. 7(5), 1200. FAO. 2014. New program to eradicate deadly livestock disease by 2030. Available via https://www.fao.org (Accessed on Jun 14, 2023). Glanz, J.M., Newcomer, S.R., Daley, M.F, DeStefano, F., Groom, H.C., Jackson, M.L., Lewin, B.J, McCarthy, N.L., McClure, D.L., Narwaney, K.J., Nordin, J.D. and Zerbo, O. 2018. Association between estimated cumulative vaccine antigen exposure through the first 23 months of life and non-vaccine-targeted infections from 24 through 47 months of age. JAMA. 319(9), 906–913. Hosamani, M., Singh, S.K., Mondal, B., Sen, A., Bhanuprakash, V., Bandyopadhyay, S.K., Yadav, M.P. and Singh, R.K. 2006. A bivalent vaccine against goat pox and Peste des petits ruminants induces a protective immune response in goats. Vaccine 24(35–36), 6058–6064. Janeway, C.A., Travers, P., Walport, M. and Shlomchik, M.J. 2001. Immunobiology: the immune system in health and disease. 5th edition, New York, NY: Garland Science; Available via https://www.ncbi.nlm.nih.gov/books/NBK27156/ Jatana, S.K. and Nair, M. 2007. Combination vaccines. Med. J. Armed Forces India 63(2), 167–171. Katsuhis, N., Konosuke, O., Rei, O., Shoko, O., Kenta, W., Yusuke, H., Yuki, I., Takaaki, A., Koji, H., Hiroto, S., Gulhabib, H. and Chikara, K. 2019. Effect of combined vaccination for Pasteurella multocida, Mannheimia hemolytica, and Histophilus somni to prevent respiratory diseases in young Japanese Black calves in the field. J. Vet. Med. Sci. 81(9), 1355–1358. Liu, B., Cao, B., Wang, C., Han, B., Sun, T., Miao, Y., Lu, Q. and Cui, F. 2022. Immunogenicity and safety of childhood combination vaccines: a systematic review and meta-analysis. Vaccines (Basel) 10(3), 472. Martinod, S. 1996. Combination vaccines for cattle: the bovine practioner-No. 30; Pfizer Central Research, 601 W Corbhusker Hwy, Lincoln, NE 68506, pp: 30–39. Muenthaisong, A., Rittipornlertrak, A., Nambooppha, B., Tankaew, P., Varinrak, T., Pumpuang, M., Muangthai, K., Atthikanyaphak, K., Singhla, T., Pringproa, K., Punyapornwithaya, V., Sawada, T. and Sthitmatee, N. 2021. Immune response in dairy cattle against combined foot and mouth disease and hemorrhagic septicemia vaccine under field conditions. BMC Vet. Res. 17, 186. Offit. P.A., Quarles, J., Gerber, M.A., Hackett, C.J., Marcuse, E.K., Kollman, T.R., Gellin, B.G. and Landry, S. 2002. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics 109, 124–129. OIE and FAO. 2015. Global strategy for the control and eradication of PPR. Paris, France: OIE; pp: 27. Osama, I.M. 2010. Studies on peste des petits ruminants, disease in the White Nile State, the Sudan. MVSc Thesis, Council of Animal Resources Research, Sudan Academy of Sciences, Khartoum, Sudan, pp: 1. Sareyyüpoğlu, B., Gülyaz, G., Saraç, F., Uzar, S., Kabaklı, O., Çokçalışkan, C., Gündüzalp, C., Uzun, E.A. and Camli, O. 2022. Antibody response of sheep to simultaneous vaccination of foot-and-mouth disease, peste des petits ruminants, sheep pox and goat pox, and bluetongue vaccines. Trop. Biomed. 39(1), 47–54. Sun, T. 2009. Evaluation of a vaccine against Mannheimia hemolytica and Pasteurella multocida in sheep. Honors Thesis; Department of Animal Science of Cornell University, pp: 1–47. Tizard, I.R. 2020. Sheep and goat vaccines. Vaccines. Vet.. 2021, 215–224. Trotta, M., Lahore, J., Cardoso, N., Melucci, O., Catena, M., Pérez-Filgueira, M., Fernández, F. and Capozzo, A.V. 2015. Simultaneous immunization of cattle with foot-and-mouth disease (FMD) and live anthrax vaccines do not interfere with FMD booster responses. Trials Vaccinol. 4, 38–42. Zhang, D., Yang, B., Zhang, T., Shi, X., Shen, C., Zheng, H., Liu, X. and Zhang, K. 2021. In vitro and in vivo analyses of coinfections with peste des petits ruminants and capripox vaccine strains. Virol. J. 18, 69. | ||
| How to Cite this Article |
| Pubmed Style Hurisa TT, Tefera TA, Negatu R, Sori T, Deme BB, Yilma MA, Tolossa W, Legesse A, Negewo A, W/medhin W, Sherefa K, Ayele G, Geresu A, Assefa E, Dufera D. Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Vet. J.. 2024; 14(8): 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 Web Style Hurisa TT, Tefera TA, Negatu R, Sori T, Deme BB, Yilma MA, Tolossa W, Legesse A, Negewo A, W/medhin W, Sherefa K, Ayele G, Geresu A, Assefa E, Dufera D. Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. https://www.openveterinaryjournal.com/?mno=201682 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.25 AMA (American Medical Association) Style Hurisa TT, Tefera TA, Negatu R, Sori T, Deme BB, Yilma MA, Tolossa W, Legesse A, Negewo A, W/medhin W, Sherefa K, Ayele G, Geresu A, Assefa E, Dufera D. Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Vet. J.. 2024; 14(8): 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 Vancouver/ICMJE Style Hurisa TT, Tefera TA, Negatu R, Sori T, Deme BB, Yilma MA, Tolossa W, Legesse A, Negewo A, W/medhin W, Sherefa K, Ayele G, Geresu A, Assefa E, Dufera D. Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 Harvard Style Hurisa, T. T., Tefera, . T. A., Negatu, . R., Sori, . T., Deme, . B. B., Yilma, . M. A., Tolossa, . W., Legesse, . A., Negewo, . A., W/medhin, . W., Sherefa, . K., Ayele, . G., Geresu, . A., Assefa, . E. & Dufera, . D. (2024) Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Vet. J., 14 (8), 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 Turabian Style Hurisa, Takele Tesgera, Takele Abayneh Tefera, Retta Negatu, Teshale Sori, Berecha Bayisa Deme, Mirtneh Akalu Yilma, Wondwossen Tolossa, Abinet Legesse, Ashetu Negewo, Wubet W/medhin, Kedir Sherefa, Getu Ayele, Adugna Geresu, Eyob Assefa, and Dawit Dufera. 2024. Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Veterinary Journal, 14 (8), 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 Chicago Style Hurisa, Takele Tesgera, Takele Abayneh Tefera, Retta Negatu, Teshale Sori, Berecha Bayisa Deme, Mirtneh Akalu Yilma, Wondwossen Tolossa, Abinet Legesse, Ashetu Negewo, Wubet W/medhin, Kedir Sherefa, Getu Ayele, Adugna Geresu, Eyob Assefa, and Dawit Dufera. "Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats." Open Veterinary Journal 14 (2024), 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 MLA (The Modern Language Association) Style Hurisa, Takele Tesgera, Takele Abayneh Tefera, Retta Negatu, Teshale Sori, Berecha Bayisa Deme, Mirtneh Akalu Yilma, Wondwossen Tolossa, Abinet Legesse, Ashetu Negewo, Wubet W/medhin, Kedir Sherefa, Getu Ayele, Adugna Geresu, Eyob Assefa, and Dawit Dufera. "Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats." Open Veterinary Journal 14.8 (2024), 1960-1967. Print. doi:10.5455/OVJ.2024.v14.i8.25 APA (American Psychological Association) Style Hurisa, T. T., Tefera, . T. A., Negatu, . R., Sori, . T., Deme, . B. B., Yilma, . M. A., Tolossa, . W., Legesse, . A., Negewo, . A., W/medhin, . W., Sherefa, . K., Ayele, . G., Geresu, . A., Assefa, . E. & Dufera, . D. (2024) Immune response and safety of co-administered Peste des Petits Ruminants, Contagious Caprine Pleuropneumonia, Sheep and Goat Pox, and Pasteurellosis vaccines in goats. Open Veterinary Journal, 14 (8), 1960-1967. doi:10.5455/OVJ.2024.v14.i8.25 |