| Research Article | ||
Open Vet. J.. 2024; 14(7): 1677-1688 Open Veterinary Journal, (2024), Vol. 14(7): 1677–1688 Research Article Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in ratsFatima S. Alaryani**Corresponding Author: Fatima S. Alaryani. Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia. Email: fsalaryani [at] uj.edu.sa Submitted: 07/06/2024 Accepted: 27/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
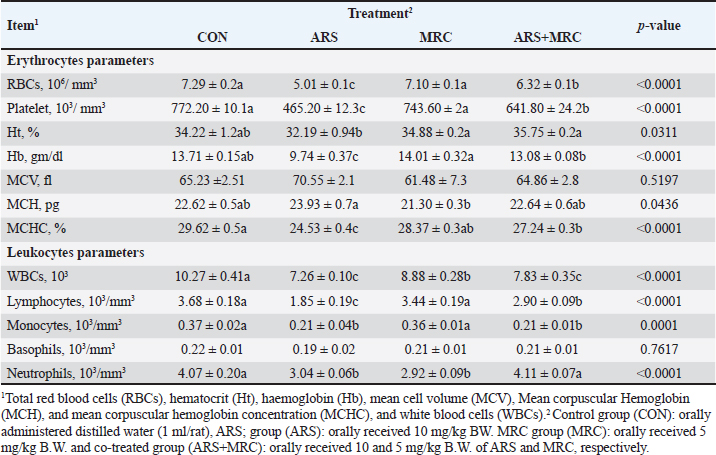
ABSTRACTBackground: Arsenic (ARS) is a toxic heavy metal that poses a significant concern for both animal and human health. Aim: The study investigated the ameliorative effect of myricetin (MRC) against arsenic-induced immune dysfunction, oxidative stress, hematological changes, hepatic and renal injuries, and inflammatory gene expression in rats. Methods: Rats were divided into 4 groups: the control group (CON) received orally administered distilled water (1 ml/rat), and the ARS group received 10 mg/kg orally, the MRC group received 5 mg of MRC/kg orally, and the co-treated group (ARS+MRC) received 10 mg/kg of ARS and 5 mg/kg b.w. of MRC orally. Results: The results showed that co-treatment of ARS-exposed rats with MRC significantly corrected erythrocyte parameters (except MCV) and leukocyte parameters (except basophils; p < 0.05). Furthermore, the ARS group significantly reduced total proteins and globulins while significantly increasing liver functions and uric acid levels (p < 0.05). Co-administration with MRC significantly mitigated the heart indices (gamma-glutamyl transferase, creatine phosphokinase, CK, lactate dehydrogenase) and lipid dysfunction caused by ARS exposure (p < 0.05). In ARS-exposed rats, there was a significant reduction in antioxidant enzymes and immunoglobulins (IgG and IgM), as well as significantly increased oxidative stress (p < 0.05). The MRC treatment effectively restored the redox status and immune variables that were disrupted by ARS exposure. Serum levels of nitric acid and lysosome were significantly lower, while levels of IL-4, TNF-α, and IFN-γ were higher in the ARS group compared to the other groups (p < 0.05). Immunohistopathology revealed that the expression of Cox2 in kidney and liver tissues varied from mild to moderate in the ARS+MRC group. Furthermore, the ARS-induced upregulation of mRNA levels of inflammatory genes such as IFN-γ, TNF-α, IL-10, and IL-6 in hepatic tissues and MRC significantly attenuated this elevation. These findings suggest that ARS has detrimental effects on blood hematology and health, triggering specific inflammatory genes and indicating the genotoxicity of ARS. However, co-treatment with MYC can mitigate these negative effects. Conclusion: MRC exhibits a significant protective effect against ARS due to its anti-inflammatory and antioxidant properties. Keywords: Arsenic, Myricetin, Inflammation, Apoptosis, Rat. IntroductionThe accumulation of toxic heavy metals in various environments is currently one of the most serious global concerns, leading to numerous detrimental effects on plants and animals (Flora et al., 2005). Arsenic (ARS) is a harmful metalloid and is considered a highly toxic metal (Goudarzi et al., 2018). According to reports from the Agency for Toxic Substances and Disease Registry and the US Environmental Protection Agency, ARS is a primary heavy metal pollutant in the USA (ATSDR., 2017). ARS is released both naturally from the Earth’s crust and through human activities, resulting in widespread environmental contamination (Bjørklund et al., 2020). Due to its high accumulation in various organs and tissues, ARS can cause harmful effects on the health of humans and animals (Fatemi et al., 2021; Bano et al., 2022). Prolonged exposure to ARS can result in various diseases, including skin cancer, cardiotoxicity, hypertension, diabetes, neurological disorders, and multiorgan impairments such as renal, hepatic, and cardiac damage (Flora et al., 2007; Turk et al., 2019; Hosseinzadeh et al., 2020; Albtoosh et al., 2022; Esfahani et al., 2022). The lungs absorb ARS into the bloodstream, where it binds with red blood cells (RBCs) and distributed to other body parts, accumulating in tissues such as the testicles, lungs, liver, kidneys, and heart (Akbari et al., 2022; Kumar Sharma et al., 2022). The liver plays a crucial role in ARS metabolism, converting it into methylated products that can bind with macromolecules and trigger liver damage (Flora et al., 2007) through oxidative stress generation (Goudarzi et al., 2018). The exact mechanism underlying ARS-induced hepatotoxicity is complex and not fully understood, but oxidative stress and inflammation are known to play significant roles (Bano et al., 2022). ARS exacerbates the production of reactive oxygen species (ROS), leading to cellular damage, lipid and protein oxidations, DNA damage, and the expression of pro-inflammatory mediators (Bano et al., 2022). Additionally, ARS can cause glucose intolerance, oxidative/inflammatory hepatic damage, antioxidant deficiencies (Esfahani et al., 2022), and immune dysfunction (Kumar Sharma et al., 2022). Excessive ROS release and inflammatory pathways work together to induce cell death through apoptosis, with inflammation strongly associated with ARS-induced hepatic failure (Akbari et al., 2022). Therefore, reducing ROS and inflammation and enhancing antioxidant capacity in the cellular system could be an effective strategy to protect against ARS-induced hepatic damage. Myricetin (3,3’,4’,5,5’,7-hexahydroxyflavone, MRC) is found in many fruits, vegetables, and herbs and is a flavonoid with potent antioxidant, anti-inflammatory, and anti-apoptotic effects (Imran et al., 2021). MRC has been used as a functional food/complementary medicine and has demonstrated promising pharmacological activities, including anticancer properties (Wang et al., 2010). It also possesses anti-tumor, analgesic, antimicrobial, and neuroprotective properties (Sun et al., 2021; Anwar et al., 2022). Studies have shown that MRC can protect against high-fat diets by modulating intestinal microbiota and preserving gut barrier function (Sun et al., 2021). It has also been identified as a potential anticancer agent in microtubule affinity-regulating kinase 4 (MARK4)-mediated malignancies and for treating atherosclerosis and vascular restenosis (Anwar et al., 2022), by suppressing inflammation pathways (Chen et al., 2020). Despite its various pharmacological properties, the protective efficacy of MRC against hepatic or renal injuries caused by ARS has not been investigated. This study aims to explore the effectiveness of MRC against oxidative stress, inflammation, and hepatic-renal injury induced by ARS, focusing on the involvement of inflammatory signaling pathways. Materials and MethodsChemicalsMRC (≥96%) was purchased from Sigma-Aldrich (M6760-25MG) with a molecular weight of 318.24 g/mol, while sodium arsenate (NaAsO2) was acquired from Merck Company (Darmstadt, Germany). Animals and experimental designThirty-two adult male rats (150 ± 5 g) were used in this experiment. The rats were housed in stainless-steel cages in a room with controlled temperature and humidity (23°C and 40%–60%, respectively) under a 12-hour light/dark cycle. They were provided with a standard basal diet and water ad libitum. After a 1-week acclimatization period, the rats were divided into four experimental groups (n=10 each). The experimental groups were as follows: the control group (CON) received orally administered distilled water (1 ml), while groups 2nd, 3rd, and 4th were orally gavage with ARS (10 mg/kg body weight; ARS group) (Turk et al., 2019; Fatemi et al., 2021; Bano et al., 2022) or rats were given 10 mg/kg of ARS +MRC at 5 mg/kg body weight (ARS+MRC group) (Dang et al., 2014; Bhatt et al., 2022), and rats were given MRC at 5 mg/kg b.w. (MRC group). The rats were treated twice weekly for 4 weeks following a protocol based on previous studies. Sampling collectionAfter 4 weeks of treatments, animals in the various investigational groups were fasted overnight and then weighed. The mice were anesthetized with ketamine/xylazine, and blood samples were collected via the retro-orbital plexus into two sterilized test tubes, one with anticoagulant and one without. The tubes containing blood with anticoagulant were used for hematological examinations. The other tubes (blood samples without anticoagulant) were left to coagulate for approximately 30 minutes and then centrifuged at 3,000 rpm for 20 minutes to separate the serum. The collected serum samples were stored at –20°C until pending biochemical examination. Rats were sacrificed and immediately dissected, and the liver and kidney tissues were excised and washed in cold phosphate-buffered saline (PBS). The specimens from the hepatic and renal tissues were preserved in neutral buffered formalin (10%) for immunohistochemical (IHC) investigations. Hematological valuationErythrogram variables, including total RBCs, hematocrit (Ht), hemoglobin (Hb), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration (MCHC), and leukogram differentiation including lymphocytes, monocytes, total leukocytes, basophils, neutrophils, and eosinophils were assessed using a Hema Screen 18 automated hematology analyzer (Hospitex Diagnostic, Sesto Fiorentino, Italy) (Thrall et al., 2012). Blood biochemistryThe serum levels of total protein and albumin were measured using commercially available kits from Biodiagnostic, Giza, Egypt. The level of globulins was determined by subtracting albumin from the total protein. The A/G ratio was calculated by dividing albumin by globulins. Liver function tests, including ALT, AST, total bilirubin, direct bilirubin, total cholesterol (TC), total triglycerides (TG), and high-density lipoprotein (HDL), were assessed using kits from Spinreact Company (Spain) following standard guidelines. Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) concentrations were determined according to the method described by Wilson et al. (1981). Kidney function was evaluated by measuring serum levels of uric acid, creatinine, gamma-glutamyl transferase (GGT), creatine phosphokinase (CPK), creatine kinase-muscle/brain (CKMB), and lactate dehydrogenase (LDH) using commercial diagnostic kits from Biodiagnostic Co., Giza, Egypt, following the manufacturer’s instructions and using the spectrophotometric method (Thrall et al., 2012). Assay of antioxidant and oxidative stressThe serum activities of SOD (Marklund, 1985), GSH (Beutler, 1963), and CAT (Goth, 1991) were measured using commercial ELISA kits (MyBioSource, San Diego, USA) following the manufacturer’s guidelines. Protein carbonyl (PC) concentrations were measured in the serum of mice according to the protocol of (Buss et al., 1997). Malondialdehyde (MDA) levels in mice serum were measured using the thiobarbituric acid method as described by (Yagi, 1982). Myeloperoxidase activity in rat serum was assessed through colorimetric assays following the method of (Sakamoto et al., 2008). Immunity and inflammatory responseThe commercial ELISA kits for determining the immunoglobulin G (IgG) and M (IgM) concentrations in mice serum were obtained from MyBioSource company (MyBioSource, San Diego, CA, USA) according to the manufacturer’s instructions. In addition, the nitric oxide level (NO) in mice serum was assessed using ELISA kits following the provided protocols (MyBioSource, San Diego, USA). The turbidimetric technique (Ito et al., 1992), using a suspension of Micrococcus lysodeikticus (Sigma-Aldrich, USA), was used to determine lysozyme activity. The levels of pro-inflammatory mediators, including TNF-α (tumor necrosis factor-alpha), IFN-γ (interferon gamma), and IL-4 (interleukin 4), were further assessed in the serum of mice in various treated groups using commercially available ELISA kits (Lifespan Biosciences, Seattle, WA, USA) according to the manufacturer’s instructions. IHC investigationIn this research, we conducted IHC staining to detect the expression of inflammatory responses by examining the staining of COX2 in hepatic and renal tissues. The rabbit monoclonal anti-COX-2 antibody (ab15191) from Abcam, United Kingdom, was used following the avidin-biotin-peroxidase complex procedure described by (Hsu et al., 1981). Additionally, sections from the untreated group were treated with PBS instead of the primary antibodies to validate the IHC test and prevent non-specific reactions and false positive results. Five fixed-size microscopic images were captured per tissue per mouse at the same magnification (×400) and exposure time using the open-source ImageJ program version 1.41. RNA isolation and quantitative real-time PCR analysisThe total hepatic RNAs were extracted using the Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s guidelines. The RNA concentrations were measured using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Delaware, USA). Subsequently, cDNA synthesis was performed using RevertAid Reverse Transcriptase (Thermo Fisher, catalog number EP0441). Relative mRNA levels of TNF-α, IFN-γ, interleukins 6 (IL-6), and 10 (IL-10) were quantified using real-time polymerase chain reaction (RT-PCR). The RT-QPCR was conducted with a SYBR Green PCR Kit (Quantitect® SYBR®, Qiagen, Valencia, CA, USA), forward and reverse primers specific to each analyzed gene, and a Rotor-Gene Q cycler (Qiagen, Valencia, CA, USA). All sample mRNA levels were normalized to the values of the GAPDH gene, and the data obtained were presented as fold changes in threshold cycle (Ct) values relative to controls using the 2^ΔΔCt method (Livak and Schmittgen, 2001). The primer sequences of the analyzed genes are listed in Table 1. Data analysisAll collected data were evaluated using one-way analysis of variance followed by Duncan’s multiple range test. Results are presented as the mean ± standard error (SE). Statistical significance was considered at p < 0.05. Ethical approvalAll procedures were approved by the Institute for Animal Ethics, following the National Institutes of Health’s guidelines for handling research animals. Every effort was made to ensure the rats were treated humanely and in accordance with ethical guidelines throughout the experiment. ResultsHematological profileThe erythrocyte indices, including RBCs, Hb, Ht, MCHC, and platelet values in the ARS group, were lower than in the other groups (p < 0.05, Table 2). However, no statistical difference was observed in the MCH values among the CON, ARS, and ARS+MRC groups (p > 0.05). Administration of MRC alone significantly enhanced all erythrocyte indices (except for MCV) compared to the other experimental groups. The values of WBCs, lymphocytes, and monocytes were lower (p < 0.05) in both the ARS and ARS +MRC groups compared to the CON and MRC groups (Table 2). Mice co-treated with MRC significantly restored the lymphocyte and neutrophil values, with non-significant effects in the CON group. Overall, treatment of ARS-exposed rats with orally administered 5 mg/kg MRC restored the erythrocyte and leukocyte values compared to the ARS group. Table 1. Primer sequences used in the present experiment.
Table 2. Effect of sodium arsenite and/or MRC oral administration on hematological profile of adult male Wistar rats.
Blood componentsMice treated with ARS exhibited a significant (p < 0.001) decrease in total protein, albumin, and globulin and a significant increase in AST, ALT, total, and direct bilirubin values compared with those in other experimental groups (Table 3). Conversely, orally treating with ARS +MRC or MRC remarkably (p < 0.05) raised total protein, albumin, and globulin. No statistical differences were observed in CON, MRC alone, or combined for total and direct bilirubin values. For kidney function, a considerable (p < 0.05) increase in the levels of uric acid in the CON and/or MRC cotreated groups as contrasted with the ARS-induced group (Table 3). The serum levels of creatinine and total glycerides did not exhibit any significant differences among all experimental groups (p > 0.05). ARS induced significantly higher levels of TC, HDL, LDL, and VLDL levels when compared with other groups (Table 3). Both treatments CON and MRC, have the lowest values of lipid profile, while the ARS +MRC exhibited intermediate values. The heart indices were also assessed; rats who received ARS had higher levels of GGT, CPK, CK-MB, and LDH concentrations when compared with the other groups (Table 3). Rats in MRC and CON groups showed significantly lower values of CPK and LDH, while the ARS +MRC group showed intermediate values with a significant difference from the SOA group. MRC ameliorated serum heart indices and lipid profile in ARS -administered mice (Table 3). Antioxidative, oxidative stress, immunity, and inflammatory responsesThe changes in indicators of redox status, immunity, and inflammatory response in the rats continuously exposed to ARS and/or MRC administration are revealed in Table 4. The SOD, GSH, and CAT activities were higher in the MRC group, while the lowest values of these indicators were detected in rats exposed to ARS (p < 0.05). Oxidative markers, including MDA, PC, and MYO, were significantly lower in the treated groups and CON groups compared to the ARS group. Co-administration of MRC in rats exposed to ARS significantly attenuated the oxidative indices (Table 4). Table 3. Effect of Sodium Arsenite and/or MRC oral administration on blood metabolites of adult male Wistar rats. .
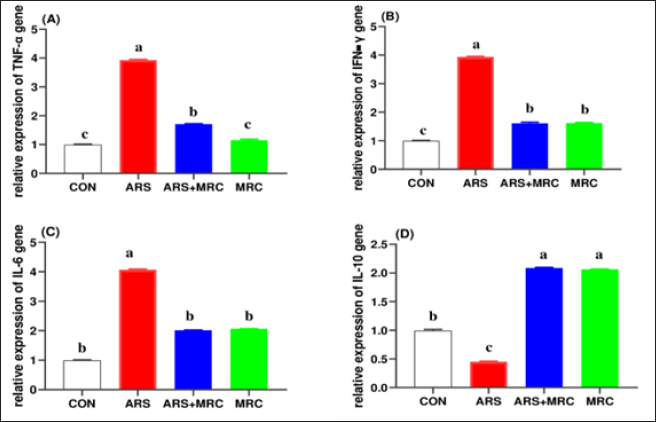
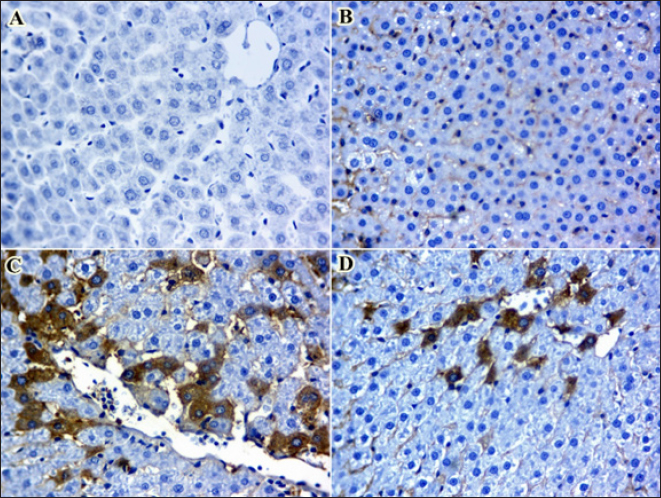
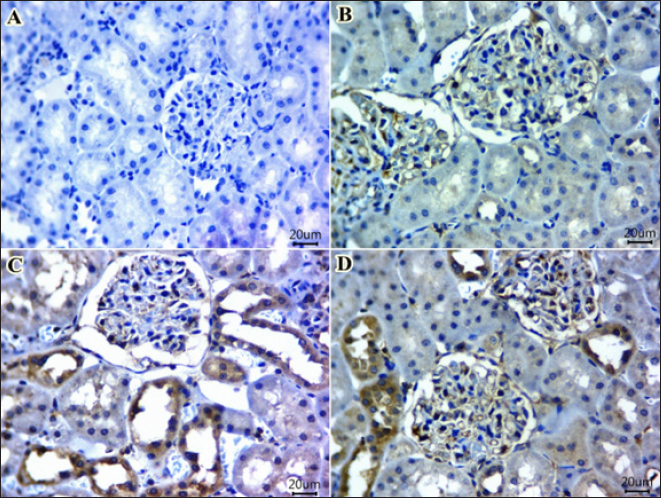
Significant increases in IgM and IgG levels were noted in the ARS +MRC group (178.3% and 191.9%, respectively) compared to the ARS group. There were no significant differences between the CON and MRC groups for IgM (Table 4), with the highest value observed in the MRC group. Rats exposed to ARS had significantly higher levels of TNF-α, IL-4, and IFN-γ compared to other experimental groups. However, NO and LZM levels were significantly lower in the ARS group, and co-treatment with MRC restored this reduction. Overall, all inflammatory responses did not show a statistically significant difference between the CON and MRC groups (p > 0.05). Inflammatory genes expressionsEffect of oral administration of arsenic and/or MRC on the mRNA expression of TNF-α (Fig. 1A), IFN-γ (Fig. 1B), IL-6 (Fig. 1C) and IL-10 (Fig. 1D) in hepatic tissues. The ARS group had higher expressions of IFN-γ, TNF-α, and IL-6 and significantly lower expression of IL-6. The co-treated group (ARS+MRC) restored the elevation of these genes when compared with the CON group (p < 0.05). No significant differences were detected between MRC and the co-treated group (ARS+MRC) (p > 0.05) for all genes (except TNF-α). IL-10 was significantly improved in the MRC and ARS+MRC groups compared to other groups (p < 0.05). Overall, MRC regulated the high expression of immune-inflammatory genes induced by ARS (Fig. 1). COX2 IHC investigationWe also investigated another inflammatory marker, COX2, in hepatic (Fig. 2 A–D) and kidney (Fig. 3 A–D) tissues because almost all arsenic effects induce an inflammatory process. Oral administration of rats with MRC alleviated ARS-induced hepatic inflammation (Fig. 2D). Additionally, mild to moderate immunostaining of Cox2 (arrow) in hepatic tissues of the ARS + MRC treated rats’ group was detected (Fig. 2D). Figure (2C) shows massive cytoplasmic immunoreactivity of Cox2 (arrow) in the liver tissues of the ARS treated rats’ group. Both the MRC group (Fig. 2B) and the CON group (Fig. 2A) exhibited negative expressions of Cox2. In Figure (3 A–D), the effects of MRC and/or ARS on COX2 expression in the kidney tissues of rats are shown. The CON (Fig. 3A) and the MRC group (Fig. 3B) show negative expressions of COX2. The SOA group (Fig. 3C) shows positive staining of COX2 in the cytoplasm of the rat’s kidney tissues. In Figure (3D), MRC+ ARS shows moderate immunostaining of COX2 expression, and MRC could mitigate this elevation of COX2 expression in the rat’s kidney tissues. Table 4. Effect of Sodium Arsenite and/or MRC oral administration on antioxidative, oxidative stress, immunity, and inflammatory responses of adult male Wistar rats.
DiscussionArsenic (ARS) is a highly toxic heavy metal that can have severe negative effects on human and animal health. Prolonged exposure to ARS has been linked to organ dysfunction and health issues in humans (Patlolla et al., 2012; Bjørklund et al., 2020). Excessive production of ROS and inflammation caused by ARS exposure can disrupt cell function by altering signaling pathways (Flora et al., 2005; Goudarzi et al., 2018). Natural antioxidants with strong antioxidant and anti-inflammatory properties may help counteract ARS-induced toxicity. This study investigated the hepatoprotective effects of MRC on ARS-induced liver and kidney damage in rats. The results showed that rats exposed to ARS exhibited liver and kidney damages, as indicated by changes in biochemical, immune, inflammation, and COX2 protein levels. Treatment with MRC alleviated these negative effects of ARS exposure. MRC, a flavonoid, showed potential pharmacological properties against ARS-induced hepato-renal toxicity in rats. Changes in blood hematological parameters can indicate exposure to toxic elements. ARS exposure led to alterations in blood parameters, such as erythrocytes and leukocytes, affecting physiological functions. Treatment with MRC restored erythrocyte and leukocyte values in ARS-exposed rats.
Fig. 1. (A-D). Effect of oral administration of arsenic and/or MRC on the mRNA expression of TNF-α (Fig. 1A), IFN-γ (Fig. 1B), IL-6 (Fig. 1C) and IL10 (Fig. 1D) in hepatic tissues. The control group (CON) received distilled water orally (1 ml/rat). The ARS group received 10 mg/kg body weight (BW) of arsenic orally. The co-treated group (ARS+MRC) received 10 mg/kg BW of arsenic and 5 mg/kg BW of MRCn orally. The MRC group received 5 mg/kg BW of MRC orally. a, b,c Means within a row without a common superscript letter differ at p<0.05. The data obtained from this study align with previous research, showing that ARS disrupts erythrocyte function in rats, leading to a decrease in RBCs, platelets, Ht, Hb, MCHC, and an increase in MCH content (Bora et al., 2022; Raeeszadeh et al., 2022). The ARS group exhibited significantly lower leukocyte counts compared to other groups, indicating the impact of ARS on microvascular integrity and blood loss (Giannetto et al., 2022). The reduction in erythrocyte-related variables in ARS-treated rats may be linked to ARS binding to RBCs, affecting oxygen transport and potentially leading to various health issues (Bora et al., 2022; Raeeszadeh et al., 2022). The increase in MCH in the ARS group suggests hemorrhagic anemia, while limited information is available on the potential impact of ARS on zinc absorption and erythrocyte function (Kuhn et al., 2017; Raeeszadeh et al., 2022). ARS-treated rats also showed leukocytopenia, which was restored by co-treatment with MRC, indicating a potential immune response modulation (Akbari et al., 2022). However, limited information is available on this interesting topic, and further exploration is needed. Additionally, ARS-treated rats exhibited leukocytopenia, characterized by lymphocytopenia, monocytopenia, and eosinopenia. In the current study, co-treatment with 5 mg of MRC orally restored these elevated levels. Similar reductions in leukocytic counts and lymphocyte percentage have been reported with other heavy metal elements (Hosseinzadeh et al., 2020; Giannetto et al., 2022). These findings highlight the importance of further research on the genotoxic effects of ARS on lymphocytes and its implications for immune function (Patlolla et al., 2012; Kuhn et al., 2017). Consequently, the interaction between phagocyte processes and humoral mediators is crucial for combating infection. Therefore, a decrease in the defensive system by leukocytes could affect the effectiveness of the cellular immune response. In mice fed a high-fat diet, (Yang et al., 2022) reported that the administration of MRC showed a potential hypoglycemic and lipid-lowering effect in both prevention and treatment. Additionally, MRC dietary inclusion decreased lipid profiles, hepatic fat accumulation, and inflammation, as well as boosted liver function in rats with a high-fat diet by modulating gut microbiota (Sun et al., 2021). ARS-triggered hepatotoxicity is associated with hepatic damage and weakened hepatic function in animals (Behairy et al., 2021; Fatemi et al., 2021). Previous studies have shown that ARS induces higher levels of AST, ALT, bilirubin, HDL, LDL, GGT, CPK, Ck-MB, and LDH, indicating hepatic and heart dysfunctions (Fatemi et al., 2021; Agraharam et al., 2022; Yang et al., 2022). The protective capacity of MRC on hepatic dysfunction has been observed in various hepatotoxic conditions (Bhatt et al., 2022; Yang et al., 2022). This research confirmed that MRC attenuated hepatic weakening by promoting antioxidative status (Barzegar, 2016).
Fig. 2. A–D. Photomicrographs of Cox2 immunohistochemistry staining in hepatic tissues of treated and control groups. A mild to moderate immunostaining of Cox2 (arrow) in hepatic tissues of ARS + MRC treated rats’ group was detected (Fig. D). Figure 2C shows massive cytoplasmic immunoreactivity of Cox2 (arrow) in liver tissues of ARS treated rats’ group. Both groups, MRC (Fig. 2B) and CON (Fig. 2A) exhibited negative expressions of Cox2 in the hepatic tissues of rats. (Magnification power X40). The antioxidative response is the primary cellular defense mechanism against various biotic and abiotic factors such as ARS toxicity. Studies have indicated that high OS synthesis might be associated with ARS toxicity (Turk et al., 2019; Akbari et al., 2022), and its hepatic and kidney toxicities occur via boosting oxidative damage (Esfahani et al., 2022). ARS could block the antioxidant defense in hepatic and kidney tissues by attacking the mitochondria pathways and causing DNA damage, as well as PC and lipid peroxidation (MDA and MYO) (Esfahani et al., 2022; Kumar Sharma et al., 2022). Mitigating or scavenging OS in the cellular system via phenolic compounds is a critical defensive strategy (Fatemi et al., 2021; Bano et al., 2022). Our findings show that rats co-treated with SOA exhibited increased levels of oxidative stress markers such as MDA, PC, and MYO, along with decreased activities of antioxidant enzymes SOD, GSH, and CAT. However, administration of MRC (5 mg) significantly improved the activities of SOD and CAT while reducing the levels of MDA, PC, and MYO. GSH, a crucial antioxidant, plays a vital role in detoxification and protection against oxidative stress induced by ARS (Flora et al., 2007; Bjørklund et al., 2020). Boosting GSH levels can help restore redox balance and mitigate ARS-induced damage. Additionally, MRC treatment enhanced the activities of ARS and CAT in ARS-intoxicated rats, indicating its antioxidant potential (Hu et al., 2018; Bora et al., 2022; Raeeszadeh et al., 2022). Previous studies have also reported similar effects of MRC on antioxidant enzymes. The antioxidative properties of MRC are attributed to its ability to facilitate H elimination, inhibit oxygen reduction, and enhance solubility (Barzegar, 2016).
Fig. 3. A–D. IHC staining of COX-2 in kidney tissues of rats exposed to sodium arsenate (Fig. 3C) or received MRC (Fig. 3B) or both administration (Fig. 3D) as compared with the control group (Fig. 3A). (A, B) kidney tissue sections from untreated rats and MRC group viewing almost no immunoexpressing for COX-2. (C) kidney tissues showing prominent COX-2 expression from the ARS group as compared with CON and MRC groups. (D) Mild to moderate immunostaining of Cox2 (arrow) in ARS + MRC treated rats’ group. (Magnification power X40). MRC is a potent inhibitor of iron-induced lipid peroxidation in rat liver (Flora et al., 2005). Nano-MRC has shown enhanced antioxidant activity and protective effects against oxidative stress in zebrafish embryos (Agraharam et al., 2022). Chronic or acute exposure to ARS toxicity has been found to deplete GSH, CAT, and SOD levels in the liver and kidney tissues of mice and rats (Hu et al., 2018; Raeeszadeh et al., 2022). Increased levels of MDA and MYO are indicative of lipid peroxidation and oxidative damage in cells. ARS-induced oxidative stress damages membrane lipids, leading to the deactivation of membrane-bound receptors and increased cellular permeability (Bjørklund et al., 2020). Previous studies have reported that ARS induces oxidative stress in renal and hepatic tissues (Bjørklund et al., 2020; Esfahani et al., 2022; Kumar Sharma et al., 2022). In this study, MRC significantly reduced MDA, PC, and MYO levels while enhancing antioxidant parameters in ARS-administered rats compared to the CON group, demonstrating its potent radical-scavenging activity. In addition to its antioxidant properties, MRC may play a role in enhancing immunoglobulin synthesis from the liver, potentially contributing to its immunomodulatory protective effects against ARS-induced hepato-renal toxicity. Exposure to ARS led to a significant reduction in humoral immunity (IgG and IgM) in rat serum, which was prevented in rats co-treated with MRC. The decrease in innate immunity elements, such as lysozyme activity and nitric oxide content, following ARS exposure, was also observed (Giannetto et al., 2022). The immunosuppressive effects of heavy metals like ARS have been reported (Ma et al., 2021), possibly due to inflammatory changes detected in rat serum. Recent studies have shown that MRC can protect innate lymphoid (natural killer) cells from ARS-induced DNA damage by reducing oxidative stress and restoring PARP1 (Poly(ADP-Ribose) Polymerase 1 activity, highlighting its potential in preventing immunotoxicity (Berköz et al., 2021). Furthermore, research suggests that MRC can counteract the immunosuppressive effects of cyclophosphamide by enhancing both adaptive and innate immune responses, indicating its immunomodulatory benefits. However, the exact mechanism underlying the immunomodulatory effects of MRC requires further investigation Cytokines are essential for regulating innate immune responses through cell-to-cell communication (Sun et al., 2021). Interferons like TNF-α and IFN-γ released by activated macrophages are closely linked to innate immune function and the inflammatory process (Goudarzi et al., 2018). Interleukins such as IL-6 and IL-10 also play crucial roles in inflammation. Inflammation is a vital part of the body’s defense mechanism against the toxic effects of various factors, including ARS toxicity (Flora et al., 2005). Studies have demonstrated that exposure to ARS increases serum levels of TNF-α, IFN-γ, and IL-4, indicating an elevation of inflammatory mediators (Flora et al., 2007) (Bano et al., 2022). TNF-α, secreted by helper cells, enhances cell-mediated immunity. Rats exposed to ARS toxicity and treated with MRC showed a significant decrease in TNF-α transcript levels in hepatic tissues compared to the ARS group. MRC administration effectively downregulated hepatic inflammation genes, including TNF-α, IFN-γ, IL-6, and IL-10 signaling in ARS-exposed rats. The mRNA levels of TNF-α, IFN-γ, IL-6, and IL-10 genes were elevated in ARS-treated animals, possibly due to increased oxidative stress. Interleukins have diverse roles in immune response and inflammation (Lee, 2016). The upregulation of genes in the ARS group exposed to ARS toxicity suggests changes in immune and inflammatory responses in rats. Serum levels of IL-4, TNF-α, and IFNγ were significantly elevated in the ARS exposure group. The study also examined the expression of the pro-inflammatory mediator COX-2 in macrophages in hepatic and kidney tissues. ARS administration increased COX-2 proteins in renal and hepatic tissues due to oxidative stress. Cyclophosphamide administration also enhanced COX-2 expression in rats (Park et al., 2021). The COX-2 enzyme is responsible for releasing prostaglandins induced by cytokines after infection or injury. In most normal cells, the expression of COX-2 is rare. MRC treatment led to a decrease in the release of inflammatory cytokines (Park et al., 2021). Our data supports previous studies suggesting that MRC administration at a dose of 5 mg/kg may reduce the expression of COX-2 proteins in the liver and kidneys of rats treated with immunosuppressive agents like cyclophosphamide (Berköz et al., 2021). The anti-inflammatory properties of MRC have been demonstrated to inhibit histamine release, decrease intracellular calcium levels, and reduce levels of IL-6 and TNF-α, consistent with our findings (Wang et al., 2010). Additionally, MRC has been shown to suppress nuclear factor kappa-B (NF-κB) pathways by inhibiting the mTOR/Akt signaling cascade, providing another mechanism for its anti-inflammatory effects (Lee, 2016). ConclusionThe data shows that ARS has toxic effects on liver and kidney tissues, but MRC can protect against this damage. Administration of MRC prevented ARS-induced harm to liver and kidney tissues, leading to improvements in blood parameters, redox status, immunity, and inflammation in rats. MRC also reduces inflammation signaling and oxidative stress, while enhancing antioxidant levels. These findings suggest that MRC, a new flavonoid could be a promising protective agent against ARS-induced liver and kidney toxicity. Further research is needed to understand its fully mechanisms. AcknowledgmentsNot applicable. Conflicts of interestThe author declares that there is no conflict of interest. FundingNot applicable. Data availabilityAll data generated or analyzed during this study are included in this published article. Author contributionsThere is one author for this study. ReferencesAgraharam, G., Girigoswami, A. and Girigoswami, K. 2022. Nanoencapsulated myricetin to improve antioxidant activity and bioavailability: a study on Zebrafish Embryos. Chemistry 4, 1–17. Akbari, S., Amiri, F.T., Naderi, M., Shaki, F. and Seyedabadi, M. 2022. Sodium arsenite accelerates D-galactose-induced aging in the testis of the rat: Evidence for mitochondrial oxidative damage, NF-kB, JNK, and apoptosis pathways. Toxicology 470, 153148. Albtoosh, A., Karawya, F., Al-Naymat, W. and Al-Qaitat, A. 2022. Potential protective effect of spirulina platensis on sodium arsenite-induced cardiotoxicity in male rats. Tissue Barriers 10, 1983330. Anwar, S., Khan, S., Anjum, F., Shamsi, A., Khan, P., Fatima, H., Shafie, A., Islam, A. and Hassan, M.I. 2022. Myricetin inhibits breast and lung cancer cells proliferation via inhibiting MARK4. J. Cell. Biochem. 123, 359–374. ATSDR., 2017. Agency for toxic substances and disease registry. Priority Substance List, US Washington, DC: Department of Health and Human Services. Bano, S., Sharif, A., Akhtar, B., Abdel-Daim, M.M., Akhtar, M.F. and Ali, F.L. 2022. Mechanistic insights on the possible protective role of polyphenols extracted from Tamarix aphylla aerial parts against sodium arsenite-induced hepatotoxicity in rats. Environ. Sci. Pollut. Res. Int. 2022, 1–14. Barzegar, A. 2016. Antioxidant activity of polyphenolic myricetin in vitro cell-free and cell-based systems. Mol. Biol. Res. Commun. 5, 87. Behairy, A., Mohamed, W.A., Ebraheim, L.L., Soliman, M.M., Abd-Elhakim, Y.M., El-Sharkawy, N.I., Saber, T.M. and El Deib, M.M. 2021. Boldenone undecylenate-mediated hepatorenal impairment by oxidative damage and dysregulation of heat shock protein 90 and androgen receptors expressions: vitamin C preventive role. Front. Pharmacol. 12, 651497. Berköz, M., Yalın, S., Özkan-Yılmaz, F., Özlüer-Hunt, A., Krośniak, M., Francik, R., Yunusoğlu, O., Adıyaman, A., Gezici, H. and Yiğit, A. 2021. Protective effect of myricetin, apigenin, and hesperidin pretreatments on cyclophosphamide-induced immunosuppression. Immunopharmacol. Immunotoxicol. 43, 353–369. Beutler, E. 1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888. Bhatt, S., Manhas, D., Kumar, V., Gour, A., Sharma, K., Dogra, A., Ojha, P.K. and Nandi, U. 2022. Effect of myricetin on cyp2c8 inhibition to assess the likelihood of drug interaction using in silico, in vitro, and in vivo approaches. ACS Omega 7, 13260–13269. Bjørklund, G., Oliinyk, P., Lysiuk, R., Rahaman, M., Antonyak, H., Lozynska, I., Lenchyk, L. and Peana, M. 2020. Arsenic intoxication: general aspects and chelating agents. Arch. Toxicol. 94, 1879–1897. Bora, S., Lakshman, M., Madhuri, D., Kalakumar, B. and Udayakumar, M. 2022. Protective effect of Lactobacillus sporogenes against arsenic-induced hematological alterations in male albino Wistar rats. Biol. Trace Elem. Res. 200, 4744–4749. Buss, H., Chan, T.P., Sluis, K.B., Domigan, N.M. and Winterbourn, C.C. 1997. Protein carbonyl measurement by a sensitive ELISA method. Free Radic. Biol. Med. 23, 361–366. Chen, M., Chen, Z., Huang, D., Sun, C., Xie, J., Chen, T., Zhao, X., Huang, Y., Li, D. and Wu, B. 2020. Myricetin inhibits TNF-α-induced inflammation in A549 cells via the SIRT1/NF-κB pathway. Pulm. Pharmacol. Ther. 65, 102000. Dang, Y., Lin, G., Xie, Y., Duan, J., Ma, P., Li, G. and Ji, G. 2014. Quantitative determination of myricetin in rat plasma by ultra-performance liquid chromatography-tandem mass spectrometry and its absolute bioavailability. Drug Res. 64, 516–522. Esfahani, P.P., Mahdavinia, M., Khorsandi, L., Rezaei, M., Nikravesh, H., and Khodayar, M.J. 2022. Betaine protects against sodium arsenite-induced diabetes and hepatotoxicity in mice. Environ. Sci. Pollut. Res. 30(4), 10880–10889. Fatemi, I., Khalili, H., Mehrzadi, S., Basir, Z., Malayeri, A., Goudarzi, M., 2021. Mechanisms involved in the possible protective effect of chrysin against sodium arsenite-induced liver toxicity in rats. Life Sci. 267, 118965. Flora, S., Bhadauria, S., Kannan, G., Singh, N., 2007. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J. Envir. Biol. 28, 333. Flora, S.J.S., Bhadauria, S., Pant, S.C. and Dhaked, R.K. 2005. Arsenic-induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci. 77, 2324–2337. Giannetto, C., Fazio, F., Nava, V., Arfuso, F., Piccione, G., Coelho, C., Gugliandolo, E. and Licata, P. 2022. Data on multiple regression analysis between boron, nickel, arsenic, antimony, and biological substrates in horses: the role of hematological biomarkers. J Biochem. Mol. Toxicol. 36, e22955. Goth, L., 1991. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta 196, 143–151. Goudarzi, M., Fatemi, I., Siahpoosh, A., Sezavar, S.H., Mansouri, E., and Mehrzadi, S., 2018. Protective effect of ellagic acid against sodium arsenite-induced cardio- and hematotoxicity in rats. Cardiovasc. Toxicol. 18, 337–345. Hosseinzadeh, A., Houshmand, G., Kalantar, M., Khalili, H.R., Mehrzadi, S. and Goudarzi, M. 2020. Neuroprotective effects of gallic acid against neurotoxicity induced by sodium arsenite in rats. Comp. Clin. Path. 29, 621–629. Hsu, S.-M., Raine, L. and Fanger, H. 1981. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 29, 577–580. Hu, Y., Yu, C., Yao, M., Wang, L., Liang, B., Zhang, B., Huang, X., Zhang, A., 2018. The PKCδ-Nrf2-ARE signalling pathway may be involved in oxidative stress in arsenic-induced liver damage in rats. Environ Toxicol Pharmacol. 62, 79–87. Imran, M., Saeed, F., Hussain, G., Imran, A., Mehmood, Z., Gondal, T.A., El-Ghorab, A., Ahmad, I., Pezzani, R. and Arshad, M.U. 2021. Myricetin: a comprehensive review on its biological potentials. Food Sci. Nutr. 9, 5854–5868. Ito, Y., Yamada, H. and Imoto, T. 1992. Colorimetric assay for lysozyme using Micrococcus luteus labeled with a blue dye, Remazol brilliant blue R, as a substrate. Chem. Pharm. Bull. 40, 1523–1526. Kuhn, V., Diederich, L., Keller IV, T.S., Kramer, C.M., Lückstädt, W., Panknin, C., Suvorava, T., Isakson, B.E., Kelm, M. and Cortese-Krott, M.M. 2017. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox Signal. 26, 718–742. Kumar Sharma, A., Kaur, A., Kaur, T., Kaur, S., Pathak, D. and Singh, A.P. 2022. Ameliorative role of inducible nitric oxide synthase inhibitors against sodium arsenite-induced renal and hepatic dysfunction in rats. Drug Chem. Toxicol. 45, 2255–2261. Lee, C.S. 2016. Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur. J. Pharmacol. 784, 164–172. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25, 402–408. Ma, H., Song, X., Huang, P., Zhang, W., Ling, X., Yang, X., Wu, W., Xu, H., Wang, W., 2021. Myricetin protects natural killer cells from arsenite-induced DNA damage by attenuating oxidative stress and retaining poly (ADP-Ribose) polymerase 1 activity. Mutat Res. Genet. Toxicol. Environ. Mutagen. 865, 503337. Marklund, S.L. 1985. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat. Res. 148, 129–134. Park, H.S., Seo, C.S., Baek, E.B., Rho, J.H., Won, Y.S. and Kwun, H.J. 2021. Gastroprotective effect of myricetin on ethanol-induced acute gastric injury in rats. J Evid. Based Complement. Altern. Med. 2021, 9968112. Patlolla, A.K., Todorov, T.I., Tchounwou, P.B., van der Voet, G. and Centeno, J.A. 2012. Arsenic-induced biochemical and genotoxic effects and distribution in tissues of Sprague–Dawley rats. Microchem. J.105, 101–107. Raeeszadeh, M., Karimi, P., Khademi, N. and Mortazavi, P. 2022. The effect of broccoli extract in arsenic-induced experimental poisoning on the hematological, biochemical, and electrophoretic parameters of the liver and kidney of rats. J. Evid. Based Complement. Altern. Med. 2022, 3509706. Sakamoto, W., Fujii, Y., Kanehira, T., Asano, K. and Izumi, H. 2008. A novel assay system for myeloperoxidase activity in whole saliva. Clin. Biochem. 41, 584–590. Sun, W.L., Li, X.Y., Dou, H.Y., Wang, X.D., Li, J.D., Shen, L. and Ji, H.F. 2021. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 36, 109641. Thrall, M.A., Weiser, G., Allison, R.W., and Campbell, T.W. 2012. Veterinary hematology and clinical chemistry. Hoboken, NJ: John Wiley & Sons. Turk, E., Kandemir, F.M., Yildirim, S., Caglayan, C., Kucukler, S. and Kuzu, M. 2019. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol. Trace Elem. Res. 189, 95–108. Wang, S.J., Tong, Y., Lu, S., Yang, R., Liao, X., Xu, Y.F. and Li, X. 2010. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Medica 76, 1492–1496. Wilson, P.W., Abbott, R.D., Garrison, R.J. and Castelli, W.P. 1981. Estimation of very-low-density lipoprotein cholesterol from data on triglyceride concentration in plasma. Clin Chem. 27, 2008–2010. Yagi, K, 1982. Assay for serum lipid peroxide level and its clinical significance. Lipid Peroxides Biol. Med. 223, 242. Yang, L., Gao, Y., Gong, J., Wang, H., Farag, M.A., Simal Gandara, J., Zhao, Y., Nie, S. and Xiao, J. 2022. Myricetin ameliorated prediabetes via immunomodulation and gut microbiota interaction. Food Front. 3(4), 749–772. | ||
| How to Cite this Article |
| Pubmed Style Fatima S. Alaryani. Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Vet. J.. 2024; 14(7): 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 Web Style Fatima S. Alaryani. Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. https://www.openveterinaryjournal.com/?mno=204761 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i7.17 AMA (American Medical Association) Style Fatima S. Alaryani. Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Vet. J.. 2024; 14(7): 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 Vancouver/ICMJE Style Fatima S. Alaryani. Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Vet. J.. (2024), [cited January 24, 2026]; 14(7): 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 Harvard Style Fatima S. Alaryani (2024) Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Vet. J., 14 (7), 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 Turabian Style Fatima S. Alaryani. 2024. Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Veterinary Journal, 14 (7), 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 Chicago Style Fatima S. Alaryani. "Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats." Open Veterinary Journal 14 (2024), 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 MLA (The Modern Language Association) Style Fatima S. Alaryani. "Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats." Open Veterinary Journal 14.7 (2024), 1677-1688. Print. doi:10.5455/OVJ.2024.v14.i7.17 APA (American Psychological Association) Style Fatima S. Alaryani (2024) Myricetin ameliorates arsenic-induced hematological changes, immune dysfunction, oxidative stress, hepatic and renal injuries and promotes inflammatory genes in rats. Open Veterinary Journal, 14 (7), 1677-1688. doi:10.5455/OVJ.2024.v14.i7.17 |