| Research Article | ||
Open Vet. J.. 2024; 14(8): 2057-2072 Open Veterinary Journal, (2024), Vol. 14(8): 2057–2072 Research Article Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropicsJamal Mohamed Hassan Alkhadrawy1,2, Amal Mahmoud Aboelmaaty3*, Mostafa Mohamed Abou-Ahmed1 and Abdelraouf Morsy Ghallab11Department of Theriogenology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 2National Center of Animal Health, Ministry of Agriculture, Livestock and Marine Resources, Tripoli, Libya 3Animal Reproduction and AI department, Veterinary Research Institute, National Research Centre, Giza, Egypt *Corresponding Author: Amal Mahmoud Aboelmaaty. Animal Reproduction and AI Department, Veterinary Research Institute, National Research Centre, Egypt. Email: am.aly [at] nrc.sci.eg; amalaboelmaaty1 [at] yahoo.com Submitted: 21/06/2024 Accepted: 27/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Commercial embryo flushing of horses has required hormonal management of both the donor and recipient mares throughout the breeding season. Aim: This study aimed to find out the effect of using human chorionic gonadotropin (hCG) and prostaglandin F2α (PG) on the ovarian and uterine dynamics and hemodynamics, estradiol (E2), progesterone, oxidants-antioxidants, and blood biochemicals in embryo donor mares during the hottest months of the year in a subtropical climate. Methods: Three Control estrous cycles of native mares (10–20 years; N=10) followed by two treated cycles with hCG and PGF2α were examined daily from May to August using Doppler ultrasound with blood sampling. Circulating, progesterone (P4), total cholesterol, total proteins, albumin, haptoglobin, nitric oxide (NO), catalase, alkaline phosphatase, lactate dehydrogenase (LDH), and myeloperoxidase were measured in blood serum. Results: Days during the control estrous cycle impacted the dominant follicle (DF) diameter ( p < 0.0001), antrum diameter ( p < 0.0001), area ( p < 0.0001), antral area ( p < 0.0001), and color area % (p > 0.05), and corpus luteum (CL) diameter ( p < 0.0001). PG tended to impact DF diameter (p > 0.05) but influenced its antrum diameter (p < 0.05), color area (p < 0.05), CL diameter (p < 0.01), and area (p=0.013). Days after hCG tended to impact DF antrum diameter (p > 0.05) and the antrum area (p > 0.05), but influenced CL diameter ( p < 0.0001). PGF2α and hCG increased uterine horn area (p=0.016) and color area (p=0.023), total cholesterol ( p < 0.0001), and NO ( p < 0.0001) levels but hCG increased the levels of myeloperoxidase (p < 0.005), total proteins (p < 0.001), and albumin ( p < 0.0001). Globulins achieved the highest level (p=0.054) but the Albumin/globulin ratio reached a minimum value on Day 0 of the control mares ( p < 0.0001). PGF2α increased LDH ( p < 0.0001) and sharply declined (p=0.028) progesterone. Conclusion: In conclusion, the treatment protocols of hCG and PGF2α showed minimal effects on the produced ovulating follicles and can be used during the summer season to manage embryo donor mares. Keywords: Embryo flushing, Follicular dynamics, Induction of ovulation, Lutealysis, Mares. IntroductionIn Egyptian mares, the blood flow vascularization of the ovarian follicles was estimated and evaluated after the ovulation using color or power Doppler that greatly depended on the day before the dominant and subordinate follicle’s selection until their deviation to ovulation (Abo El-Maaty and Abdelnaby, 2017). The follicle type (dominant or subordinate follicles indicated different blood vascularization from their deviation till the spontaneous ovulation (Abdelnaby and Abo El-Maaty, 2017a). Ovarian hormones and uterine and ovarian arteries PI that was measured in mares during the estrous cycle showed a characteristic change in the blood supply of both ovaries and the uterus (Bollwein et al., 2002). Several hormones are being used to manage the estrous cycle of mares, the embryo donor or embryo recipient mares. After ovulation, the administration of altrenogest (progesterone) for the subsequent 14 days and estradiol (E2) benzoate at five-day intervals (Days 0, 5, and 10) from the day of ovulation (Day 0) influenced the blood flow of the dominant ovarian and uterine arteries in mares regularly ovulated (Bollwein et al., 2004). E2 benzoate administration on the day of ovulation (Day 0) decreased the uterine PI values between Days 0 and 1 compared to the untreated cycle and increased the uterine and ovarian arteries PI during diestrus in treated mares but altrenogest increased the uterine and ovarian arteries PI values during both estrus and diestrus in treated mares (Bollwein et al., 2004). Regardless, ovsynth estrous synchronization protocol is recommended to synchronize estrous in dairy or beef cattle, it cannot be used for the synchronization of estrus in mares (Glazar et al., 2004). To minimize the number of inseminations or natural breedings of mares during their estrous phase, the ovulation could be hastened by administrating gonadotropin-releasing hormone (GnRH) after monitoring a dominant follicle (DF) with a diameter exceeding 3.0 cm (Stich et al., 2004). Another hormone was used to synchronize ovulation in mares with high efficacy in inducing and synchronizing ovulation is the human chorionic gonadotropin (hCG) was administered at the end of most synchronization protocols to synchronize ovulation (Bristol, 1993). GnRH with altrenogest and prostaglandin neither induced luteinization nor ovulation of any follicle during diestrous (Glazar et al., 2004). In pony mares, 6 hours after administrating1,500 iu of hCG and 8 days after ovulation progesterone pulse frequency, pulse amplitude, and concentrations were increased (Watson et al., 1995). During estrus, the delivery of essential components necessary for follicular maturation and steroidogenesis is largely dependent on the changes in the ovarian vascular network (Watson and Al-Zi’abi, 2002). The impact of intravenous administration of 2,500 i.u. of hCG on the follicular vascularization percentage was not observed throughout the entire experiment during the spontaneous and induced ovulations but did not change and decreased along the last 6 hours before ovulation (Boakari et al., 2017). Administrating hCG increased the thickness and echogenicity of the granulosa cells of the DFs compared to non-treated controls. The induction of ovulation using hCG or the approach of ovulation in non-treated control mares increased the granulosa and color-Doppler end-points with decreasing E2 concentrations, the color-Doppler signals, and the prominence of an anechoic band a few hours before ovulation (Gastal et al., 2006). HCG was used to induce ovulation in a dose from 2,000 to 3,000 i.u. during equine embryo flushing programs and embryos were flushed 7 and 10 days later with the recovery of 51.1% embryos per cycle and 36.1% embryos per ovulation (Panzani et al., 2014). Both hCG and prostaglandin F2α are routinely used during embryo collection programs in mares (Mahmood et al., 2024). Nitric oxide (NO) is one of the free radicles, physiological components, and vaso-regulators that play significant roles in the regulation of blood flow in the genital system (Musicki et al., 2009). To investigate its importance in the genital system, NO donors (30 mg or 60 mg of isosorbide dinitrate) were administered to mares to study their effects on the pulsatility index (PI) of both uterine arteries and of the dominant (ipsilateral to the CL) ovarian artery. Mares treated with NO donors had lower PI (high blood flow velocity) rather than the non-treated control mares. Blood flow resistance index (RI) declined (High blood flow velocity) in the dominant ovarian artery in pregnant mares treated with isosorbide dinitrate in comparison to control pregnant or cycling non-pregnant mares. Isosorbide dinitrate increases uterine and ovarian perfusion in cycling mares and ovarian perfusion in early pregnant mares (Zoller et al., 2016). Estrous cycle length, dominant preovulatory follicle diameters and ovarian hormones varied in mares spontaneously ovulated and those enrolled in embryo flushing program (Raz et al., 2009). This study aimed to investigate the effect of hormonal manipulation of embryo donor mares on ovarian and uterine dynamics and hemodynamics, progesterone (P4), E2, haptoglobin, NO, total proteins, albumin, total cholesterol, lactate dehydrogenase (LDH), catalase, alkaline phosphatase (ALP), and myeloperoxidase in Egyptian mares during the hot months of the year. Materials and MethodsAnimals, housing, and feeding Non-lactating, non-pregnant, clinically healthy, and normally cycling native mares (n=10) were used in the current study. Baladi mares were purchased from private horse studs in Giza Governorate and kept on the farm of the Theriogenology Department, Faculty of Veterinary Medicine, Cairo University 30.01056 latitude and 31.208853 Longitude. All animals were in good body status. Animals were kept individually in a closed stable at night with artificial light and in yards during the day. Animals were fed a sufficient and balanced ration containing grass supplemented with grain, hay, and mineral salt and fresh and clean water was accessible ad libitum. Mares were kept under standard preventive medicine practice according to the common equine breeding regulations. Mares were free from infectious diseases. All animals were dewormed regularly. The current work was performed in the period from May to August 2023. Reproductive Doppler examinationsThe ultrasound-Doppler scanner equipped with a12.0 MHz linear-array trans-rectal transducer (SonovetR3, Medison, Samsung, South Korea) was used for the examination of follicles or corpora lutae and uterus with their hemodynamics. Ovarian dominant and subordinate follicles and the corpus luteum (CL) were monitored using the ordinary greyscale and color Doppler modes (Fig. 1). Ovulation day (Day 0) was determined by the reach of the DF to its largest diameter and its disappearance or decrease sharply in diameter on the day after (Abo El-Maaty and Abdelnaby, 2017). The diameter and hemodynamics of the CL were determined from its first detection by ultrasound till the approach of future ovulation (Abdelnaby and Abo El-Maaty, 2017b). The diameters, the blood flow velocities, and the blood flow indices of the ovarian arteries were determined using the pulsed-wave Spectral Doppler mode (Bollwein et al., 2004). Once the diameter of the DF exceeded 30 mm a dose of 2,500 I.U. hCG (Epifasy, EIPICO, Egypt) was administered and mares were inseminated 1 and 2 days later with fresh semen. The day of ovulation (Day 0) during the non-treated estrous cycles (Control) and the day following hCG and after PGF2α.
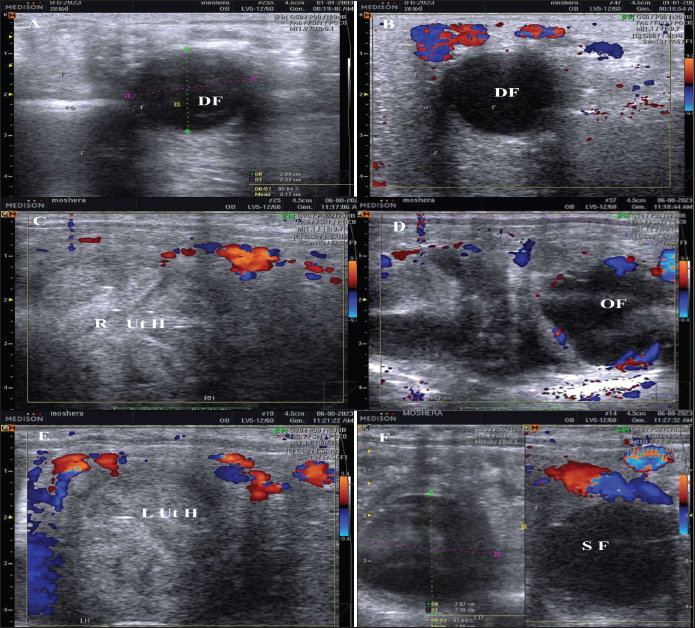
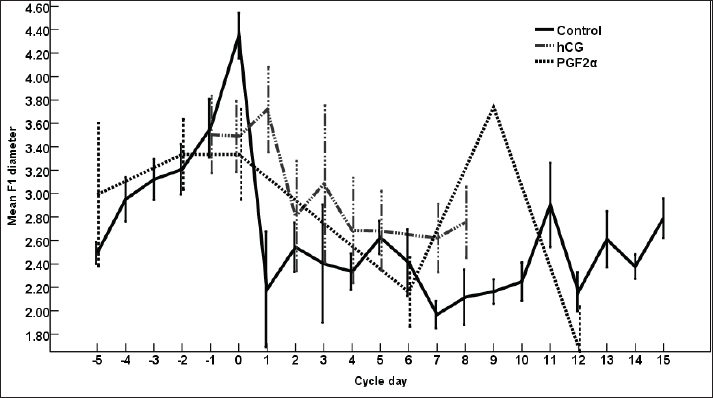
Fig. 1. The dominant follicle (DF) of 23.3 mm in diameter in grey scale (A), and color Doppler (B). The right uterine horn (R Ut H) showing the cart wheel characteristic of estrus and the uterine blood flow in color Doppler (C) and the characteristic polygonal shape of the preovulatory follicle (OV) in color Doppler (D), the left uterine horn (L Ut H) in the same mare and the same day in color Doppler, the subordinate follicle (SF) in grey scale and color Doppler (F). The ovarian follicular dynamics and hemodynamics (Abdelnaby and Abo El-Maaty, 2017a,b) were determined from Day 6 before ovulation till Day 14 in control estrous cycle and from the day of inducing ovulation (Day 1) till the day of embryo flushing Day 7 on the first round and Day 8 in the second round of embryo flushing (Abdelnaby and Abo El-Maaty, 2017a,b; Abo El-Maaty and Abdelnaby, 2017). The ovarian and uterine dynamics and hemodynamics were also recorded after terminating the pregnancy of the mares whose embryos were recovered or un-recovered embryos using prostaglandin F2α (Lutalyse, Zoetis, Belgium SA) The thickness of the granulosa area was estimated by subtracting the area of the follicle antrum area from the whole follicle area. The follicle color area/pixels were counted using software (Photoshop CC). The estimation of follicular vascularization percentage was calculated by dividing the follicle color area/ pixel by the follicle area/pixel. Moreover, the diameter and the blood flow velocities and indices were recorded for both ovarian arteries (Abdelnaby et al., 2018). The peak systolic velocity (PSV), the end-diastolic velocity, the time average mean velocity, the pulsatility index (PI), and the resistance (RI) index were determined (Bollwein et al., 2004). The disappearance of the large ovulating follicle ≥ 35mm in diameter is considered the day of ovulation (Abo El-Maaty and Abdelnaby, 2017). Mares were inseminated using an equine semen insemination catheter (IMV, France). After hCG and prostaglandin, the luteal (Abdelnaby and Abo El-Maaty, 2017b) and uterine hemodynamics (Abdelnaby et al., 2016) were recorded and the ovarian arteries blood flow indices were recorded. Embryo recoveryUterine flushing was done for all animals in Group I (young) and Group II (Senile). It was generally scheduled 7–10 days following ovulation, with Day 0 being designated the day of ovulation. Uterine flushing for embryo recovery was carried out (Alkhadrawy et al., 2024). The embryo recovery procedure included placing an equine embryo catheter size 32 (Minitube, Germany) through the cervix of the mare and into the uterine body, inflating a cuff, and then repeating the lavage of the uterus 4–5 times using 2.5–3 l of Ringer lactate (DPBS) containing 1% fetal calf serum (FCS, Cegrogen Biotech, Germany) and 1% Gentamycin (Gentavilin Fort, Vilsan Pharmaceuticals, Ankara, Turkey). The fluid was recovered through an embryo filter cup (Emsafe, Minitube, Germany) and the contents of the cup were poured into a search dish and examined under a stereomicroscope for the identification of an embryo. A rectal uterine massage was done at the last uterine flush round of each animal. After embryo flushing, flushed mares were injected 10 mg Dinoprost tromethamine (PGF2α, Lutalyse, Zoetis, Belgium SA) to resolve sticky embryos not detected on the lavage and discontinue pregnancy. Mares were examined with the US before and after embryo flush and from the following day till the next DF reached >30 mm in diameter for determining the next time for insemination and embryo flushing trial. Blood samplingParallel to each ultrasonographic and Doppler examination, blood samples were collected from the jugular vein of each mare and placed into two tubes, a tube containing EDTA as anticoagulant for assessment of hormonal profiles (Progesterone, E2) in plasma and another serum tube for NO, biochemical oxidants, and antioxidants analysis. All samples were immediately placed on ice and centrifuged (10 minutes at 3,000 r.p.m) within 2 hours after collection. Plasma and sera were stored at −20°C until assayed. Hormone assayingProgesterone and E2 hormones were assayed using a commercial ELISA kit (DRG Instruments GmbH, Germany) which was specifically dedicated to equine. For progesterone, the range of the assay was between 0.0 ng/ml to 40 ng/ml, the sensitivity was 0.045 ng/ml, and intra- and inter-assay variability was 6.81% and 7.25%, respectively. For E2, the range of the assay was between 9.7 pg/ml and 200 pg/ml, the sensitivity was 9.714 pg/ml, and intra- and inter-assay variability were 6.86% and 5.59%, respectively. Lactate dehydrogenase (LDH, NS 283001) was assayed using a colorimetric kit (Salucea, Dutch technology in life science, Haansberg19,4874 NJ Etten Leur, The Netherlands). Horse Myeloperoxidase was measured using a commercial ELISA kit (ELK6027, ELK Biotechnology, China). Total Cholesterol was assayed using a colorimetric kit (MG, Science and Technology Center “STC”, Egypt). Total proteins, albumin, total cholesterol, ALP, glutathione peroxidase (GPx) catalase, and NO were assayed using the colorimetric diagnostic kits (Biodiagnostics, Egypt). Statistical analysisData were expressed as Mean ± SEM. Analysis of variance was used for the sequential data using software (SPSS, 2016). Duncan’s Multiple Range test was used to differentiate between significant means at p < 0.05. The effect of days on the control, hCG, and PG treated cycles on DF diameter, antrum diameter, area, antrum area, color area, granulosa area, color area %, and granulosa area %, CL diameter, area, color area, uterine horns diameter, area, color area, ovarian arteries PSV, EDV, PI, RI, E2, progesterone, total cholesterol, myeloperoxidase, LDH, ALP, catalase, GPx, haptoglobin, NO, and total proteins. Ethical approval The study protocol was approved by the Animal Care and Ethical Use Committee of the Faculty of Veterinary Medicine at Cairo University (Approval ID: Vet- CU- 03162023708). ResultsThe difference in the diameter, shape, and blood flow vascularization of the dominant, pre-ovulatory, and subordinate follicles are presented in Figure 1. The characteristic uterine edema during estrus and the uterine Doppler vascularization is presented in Figure 1. The diameter of the DF increased progressively within 24 hours after administrating hCG and required 5 days after administrating PGF2α to start new ovulation and reach the maximum diameter during the first ovulation and 19 days to start the second round of hCG and induction of ovulation (Fig. 2). The diameter of the DF in the control non-treated estrous cycles increased from Day -5 to reach the maximum diameter on Day 0. However, after PG, the diameter of the dominant increased to reach its maximum on Day -2. The DF diameter of hCG-treated estrous cycles reached a minimum value on Day 2. The minimum diameter observed after ovulation was recorded on Day 1 and Day 7 in the non-treated estrous cycles but those of the PG-treated cycles were observed on Day 6 and Day 12. Days during the non-treated estrous cycle impacted the DF diameter ( p < 0.0001). Days after PG tended to impact the follicle diameter (p > 0.05).
Fig. 2. The effect of days of the control and treated estrous cycle on the dominant follicle diameter.
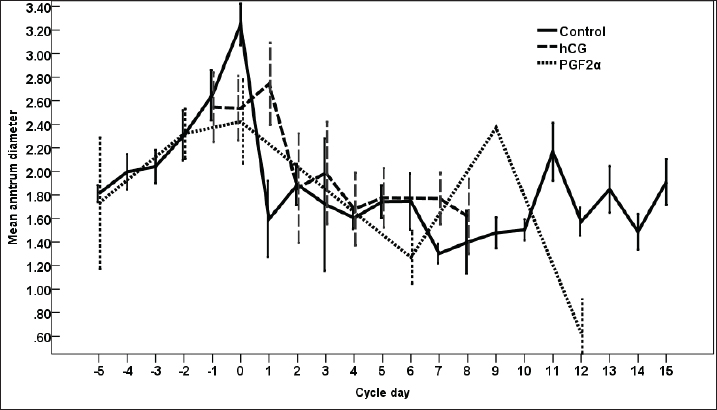
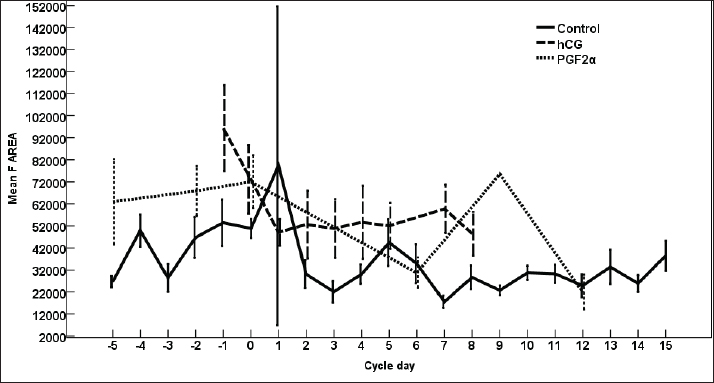
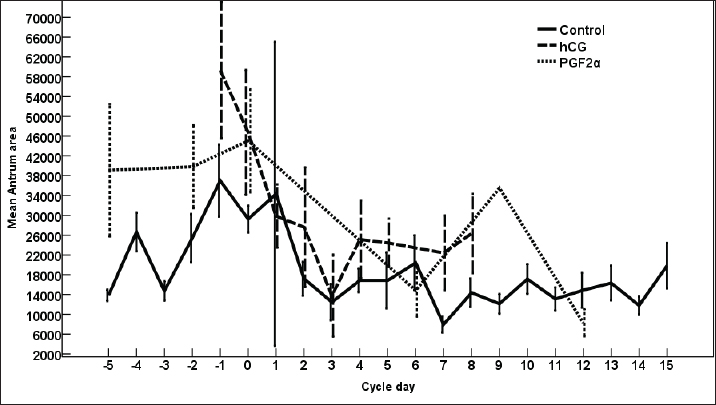
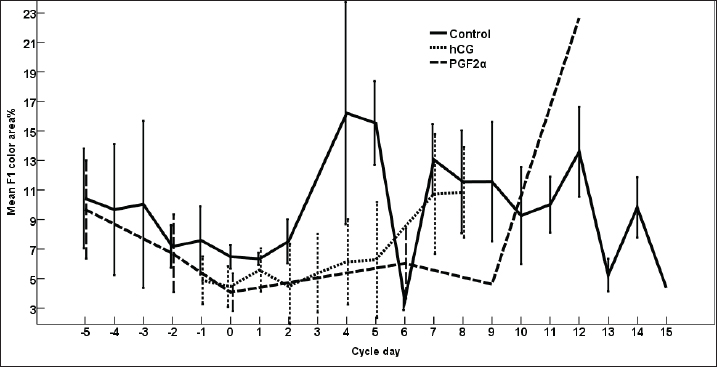
Fig. 3. The effect of days of the control and treated estrous cycle on the dominant follicle antrum diameter. The antrum diameter of the DF (Fig. 3) increased progressively within 24 hours after administrating hCG and required 5 days after administrating PGF2α to start new ovulation and reach the maximum diameter during the first ovulation and 19 days to start the second round of hCG and induction of ovulation. However, after PG, the antrum diameter of the dominant increased to reach its maximum on Day -2. The DF antrum diameter of hCG-treated estrous cycles reached a minimum value on Day 2 and Days 4–7. The minimum antrum diameter observed after ovulation was recorded on Day 1 and Day 7 in the non-treated estrous cycles but those of the PG-treated cycles were observed on Day 6 and Day 12. The days after the induction of ovulation using hCG tended to impact the antrum follicle diameter (p > 0.05). Days during the non-treated estrous cycle impacted the DF antrum diameter ( p < 0.0001). Days after PG impacted the DF antrum diameter (p < 0.05). The area of the DF (Fig. 4) increased progressively within 24 hours after administrating hCG and required 5 days after administrating PGF2α to ovulate and reach the maximum diameter during the first ovulation and 19 days to reach the second ovulation. The estrous cycle length was 21 days during the control estrous cycle and 19 days during the hCG-induced ovulation cycle. The DF area of the non-treated estrous cycles increased starting from Day 3 to reach its maximum value on Day 1 and that of PG-treated cycles increased to reach a maximum value on Day 0. The minimum area observed after ovulation was recorded on Day 3 and Day 7 in the non-treated estrous cycles but those of the PG-treated cycles were observed on Day 6 and Day 12. Whereas, the minimum area diameter of the hCG-treated cycle was observed on Day 1. Days during the non-treated estrous cycle impacted the DF area ( p < 0.0001). The antrum area of the DF (Fig. 5) increased progressively within 24 hours after administrating hCG and required 5 days after administrating PGF2α to start new ovulation and reach the maximum diameter during the first ovulation and 19 days to start the second round of hCG and induction of ovulation. The DF antrum of the not-treated control estrous cycles increased starting from Day 3 to reach its maximum value on Day 1. The minimum antrum area observed after ovulation was recorded on Day 3 and Day 7 in the non-treated estrous cycles but those of the PG-treated cycles were observed on Day 6 and Day 12. Whereas, the minimum antrum diameter of the hCG-treated cycle was observed on Day 3 (Fig. 5). The induction of ovulation using hCG tended to impact the antrum area of the DF (p > 0.05). Days during the non-treated estrous cycle impacted the DF antral area ( p < 0.0001). The DF color area (Fig. 6) decreased progressively within 24 hours after administrating hCG, followed by another decrease on Day 2 and Day 7. The color area of the DF required 3 days after administrating PGF2α to reach the lowest value and continued decreasing slowly to reach the lowest value on Day 6 then reached the highest values on Day 12 and Day 5 before either the first ovulation and the second one 19 days later before the start the second round of hCG and induction of ovulation. The DF color area of the non-treated estrous cycles attained high values on Days 2, 1, 5, 8, and 10 and reached its minimum values on Days 3, 6, and 12 (Fig. 6). Days after PG impacted the DF antrum color area (p < 0.05) The DF color area % (Fig. 7) decreased progressively within 5 days after administrating PGF2α to reach its lowest value on Day 0, then increased slowly till Day 6 followed by a transient decrease on Day 9 then reached its maximum percentage on Day 12. The DF color area % of the non-treated estrous cycles fluctuated from Day 5 till reached the lowest value on Day 0 then increased slowly till reached high values on Days 4–5, 7–9, 12, and 14 attaining the lowest value on Day 6 (Fig. 7). After hCG, the color area % of the DF reached the lowest value on Day 1 and Day 0 then increased slightly on Day 1 and continued increasing till reached the highest value on Days 7–8 (Fig. 7). Days during the non-treated estrous cycle t impacted the DF color area % (p > 0.05).
Fig. 4. The effect of days of the control and hCG and PGF2α treated estrous cycles on the dominant follicle area
Fig. 5. The effect of days of the control and hCG and PGF2α treated estrous cycles on the dominant follicle antrum area.
Fig. 6. The effect of days of the control and hCG and PGF2α treated estrous cycles on the dominant follicle color area.
Fig. 7. The effect of days of the control and hCG and PGF2α treated estrous cycles on the dominant follicle color area %.
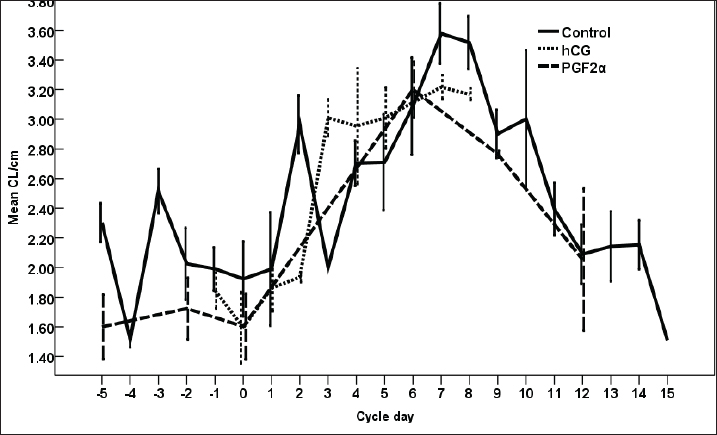
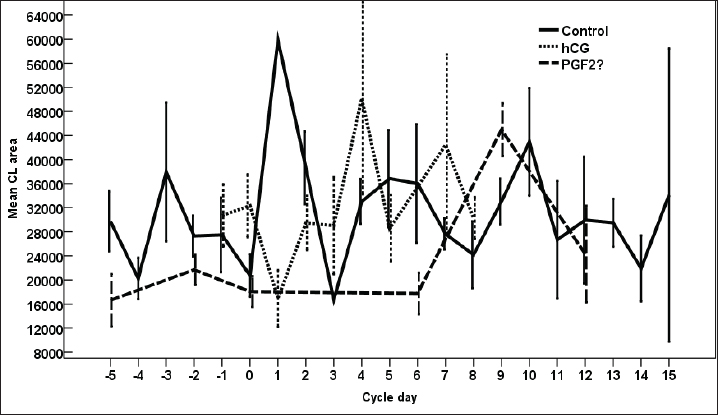
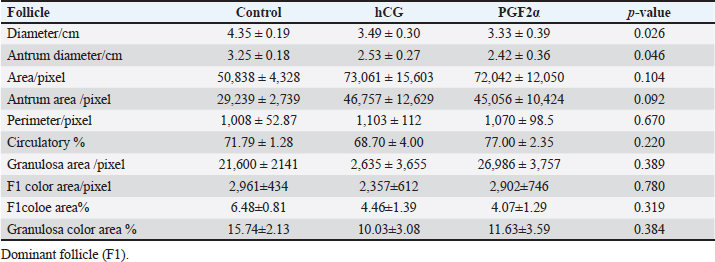
Fig. 8. The effect of days of the control and hCG and PGF2α treated estrous cycles on the corpus luteum (CL) diameter/cm. The CL diameter is influenced by the days after the non-treated control estrous cycle and those after treatment with hCG ( p < 0.0001). Four days after the non-treated estrous cycle ovulation, the CL diameter decreased sharply. CL diameter started increasing from Day 2 till reached the highest diameter on Days 7–8, then started a slow decrease till reached the minimum diameter on Day 15 with transient stabilized diameters on Day 9 for 1 day and from Day 12 to Day 14 (Fig. 8). After hCG, the CL diameter increased 2 days after ovulation till reached Day 8. After PGF2α, CL diameter decreased for 5 days then started increasing after ovulation till reached a maximum diameter on Day 6 then declined till reached the lowest diameter on Day 12 (Fig. 8). The CL diameter (p=0.003) and area (p=0.013) were influenced by the days after PGF2α treatment. In the control estrous cycle, the CL area after a transient decrease on Day 4, a significant decrease continued till reached Day 0. After ovulation, the CL area kept high values except on Days 3, 8, 11, and 14 (Fig. 9). After hCG, the CL area started increasing from Day 1 till Day 8. After, PGF2α, the CL area reached high values from Day 6 to Day 12. On the day of ovulation, the treatments of mares with either hCG or PGF2α produced ovulating follicles with lower diameter (p=0.026) and antrum (p=0.046) diameters and tended to have lower (p > 0.05) antrum area (Table 1).
Fig. 9. The effect of days of the control and hCG and PGF2α treated estrous cycles on the corpus luteum (CL) area/pixel. Table 1. The effect of treatment on the dominant follicle dynamics and blood flow on the day of ovulation (Day 0).
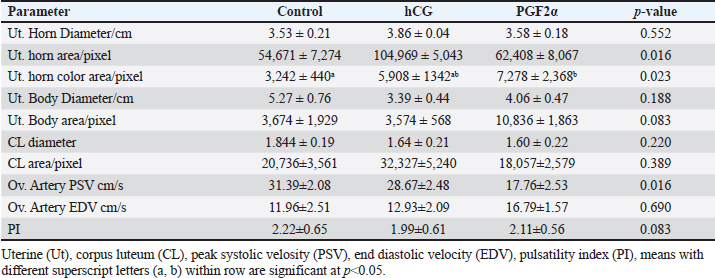
Table 2. The effect of treatment on luteal and uterine, and ovarian parameters dynamics and blood flow on the day of ovulation (Day 0).
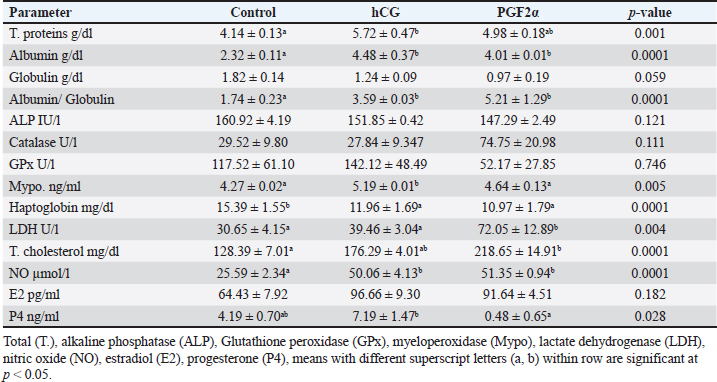
Table 3. The effect of treatment on hormonal and blood biochemicals changes on the day of ovulation (Day 0).
The treatments of mares with either hCG or PGF2α increased the uterine horn area (p=0.016) and color area (p=0.023). The uterine body area tended to increase (p > 0.05) on the ovulation day of mares treated with PGF2α (Table 2). The PSV of the ovarian artery declined on the day of ovulation after treating mares with PGF2α. The treatments of mares with either hCG tended to decrease (p > 0.05) PI on the day of ovulation (Table 2). The treatments of mares with hCG (Table 3) increased the levels of total proteins (p < 0.001) and albumin ( p < 0.0001). Globulins achieved the highest level (p=0.054) but the Albumin/globulin ratio reached the lowest value on the same day on Day 0 in the non-treated control mares ( p < 0.0001). The treatments of mares with hCG (Table 3) increased the levels of myeloperoxidase (p < 0.005). PGF2α increased LDH ( p < 0.0001). Both hCG and PGF2α increased total cholesterol ( p < 0.0001) and NO (NO; p < 0.0001) levels. PGF2α sharply declined (p=0.028) progesterone (P4) on the day of ovulation (Table 3). DiscussionIn agreement with the increase in the mean diameter of the largest preovulatory follicle in the control spontaneous estrous cycle >4.4 cm compared to that developed after hCG (3.5 cm) and that developed after administrating PGF2α, Raz et al. (2009) recorded a mean diameter of 46.2 ± 1.4 mm in the first ovulation of the breeding season and lower diameter of 41 ± 1.0 mm to that developed after administrating hCG and prostaglandin. Similar to the slight decrease in the interovulatory interval in mares treated with hCG during summer (19–20 days) compared to control (21 days), none of the mares administered hCG showed prolonged interovulatory interval (Raz et al., 2009). In agreement with Raz et al. (2009), the decrease in the interovulatory interval after administrating hCG and prostaglandin in mares subjected to embryo recovery was also observed in our mares, where the first ovulation after the administration of prostaglandin was observed 5 days later and that after administrating hCG occurred within 48 hours. The daily changes in the follicular blood flow observed in our mares spontaneously ovulated during their estrous phase, could be related to the changes in the ovarian and uterine arteries’ PI during estrus which indicated a time trend (Bollwein et al., 2002). In agreement with the non-significant change in the mean maximum CL diameter and area in the control estrous cycle, after hCG, and after prostaglandin observed in our study, after ovulation, no differences in the dominant ovarian artery PI and progesterone between the two consecutive estrous cycles, but the increase in the diameter of the CL was associated with a decrease in PI (Bollwein et al., 2002). Altrenogest administered from Days 5 to 15 post ovulation followed by prostaglandin on Day 15 increased progesterone till Day 12 then declined sharply after PG. The co-administration of GnRH on Day 8 and PG on Day 6 and altrenogest administered from Days 5 to 15 post ovulation decreased progesterone after PG and GnRH even having diestrus follicle of >30 mm in diameter. The administration of altrenogest from Days 9 to 19 with two doses of PG on day 10 and Day 19 and GnRH on Day 12 after ovulation did not luteinize follicles and declined progesterone after PG was administered on Day 10 (Glazar et al., 2004). The administration of pony mares with 1,500 i.u of hCG on Day 8 after ovulation increased progesterone pulse frequency, pulse amplitude, and concentrations 6 hours later (Watson et al., 1995). Moreover, 2,000–3,000 IU of hCG were administered before performing embryo flushing 7 and 10 days later in embryo donor mares (Panzani et al., 2014). Luteinizing hormone (LH) appeared to be secreted in an episodic fashion on Days 8 and 13, but on Day 3 in mares subjected to quarter-hourly blood sampling on these specific days (Watson et al., 1995). Progesterone (P4) showed episodic increases throughout the 12 hours on Days 3, 8, and 13 after ovulation. On Day 3, P4 increased from lower values in the morning to reach the highest values at night. Concentrations of P4 reached the highest values on Day 8 compared to Day 3 and Day 13 (Watson et al., 1995). The PI of the two ovarian arteries decreased during the estrous stage from Day 6 to Day 1 then increased for 2 days after ovulation and continued to decrease till Day 6 after ovulation followed by a steady increase till Day 15 (Bollwein et al., 2002). In this study, the induction of ovulation in embryo donner mares was followed by the induction of luteolysis on the days of embryo flushing (Day 8 and/ or Day 9). Days of embryo flushing did not influence follicle diameter but both PG and hCG treatments significantly increased the DF area and antrum area. In agreement with our results, GnRH administration on Day 8 or Day 12 following ovulation and prostaglandin on Days15; neither ovulation, change in their diameter, nor luteolysis were detected on Days 6 and 15; and 10 and 19 in all mares primly treated with altrenogest for 10-days starting from Day 5 to day 15 after ovulation (Glazar et al., 2004). Similar results to hCG were noticed when deslorelin was administered to induce ovulation in mares within 2 days when the DF diameter was >34 mmm with recording the same number of large follicles 14–15 days after ovulation compared to control non-treated mares (Stich et al., 2004). In the present study, the induction of ovulation and insemination of mares for embryo flushing was performed 8 and 9 days after hCG followed by PGF2α to induce luteolysis. Five days after PG treatment, our mares had ovulated. Contrary, mares required 7.8 ± 2.5 days after PGF2α treatment to ovulate (Zoller et al., 2016). This short interval from PG to ovulation in our study may refer to the effect of heat stress during the summer on the length of the estrous cycle or the fastened luteolysis. There is an inverse relationship between the increase in the color area and the decrease in blood flow RI and the pulsatility indices. In our study, the DF of mares spontaneously ovulated had increased blood flow color area from Days 2 to 5 during their selection for the future cycle ovulation and those treated with hCG had increased DF color area from Day 5 to Day 8 after treatment but those treated with PG showed increased DF color area on Day 14 after PGF2α treatment. In agreement with the increased blood flow RI on Day 1 and from Day 6 to Days 10 and 11 followed by a decrease from Day 2 to Days 4 to 5 that increased on Day 9 in the dominant ovarian and uterine arteries (Zoller et al., 2016). The increase in the area of the granulosa layer from Day 2 to Day 1 before spontaneous ovulation is similar to the increase in the thickness of the granulosa layer, the DF diameter or the percentage of DF color blood flow from Day 2 to Day -1 before ovulation (Ginther et al., 2009). In mares, the circulating E2 concentrations reached their maximum values nearly 2 days before ovulation (Ginther et al. 2005). The preovulatory E2 surge begins to decrease 3 days before the peak of a prolonged LH surge (about 7 days) in mares (Ginther et al. 2005). E2 concentrations in the follicular fluid have been reported to decrease (Gérard et al. 1999) and not decrease (Watson and Sertich 1991) before ovulation. Watson et al. (1995) recorded no significant difference in progesterone episodes on Days 3, 8, and 13 of the spontaneous ovulating estrous cycle. However, administrating 1,500 IU hCG on Day 8 of the estrous cycle elevated progesterone pulse amplitude and concentrations in the following 6 hours compared to the later 6 hours. During the spontaneous estrous cycle, a significant increase of equine LH was recorded during the preovulatory and luteal phases of the cycle compared to lower concentrations during the period of luteal regression. Serum levels of E2 and progesterone (P4) exhibited a reciprocal relationship. During the luteal phase of the reproductive cycle serum levels of P4 decline 10 days before ovulation and reach a nadir by Day 4 until ovulation. The activity of the newly formed corpora lutea increased P4 from the day of ovulation to reach higher levels on Day 4 (Adams et al., 1986). In agreement with our results, compared to control mares, 2,500 I.U. hCG was administered when the preovulatory follicle diameter reached 35 mm, on the day of ovulation (Day 0), and on Day 5 after ovulation did not influence the uterine morpho-echogenicity but influenced the CL area during Days 0–4 and CL vascularization, RI of the ovarian pedicle, ovarian pedicle score, and progesterone among treatments between Days 1 and 2; 7 and 8; and 11and 13 (Alonso, et al., 2019). Progesterone concentration, CL diameter and CL blood flow at any time point over time in control mares or those treated with 1,500 I.U. hCG, GnRH or their combination (Segabinazzi et al., 2021) E2 increased almost two-fold during the period of luteal regression compared to Day 3 to reach maximal concentrations 2-3 days before ovulation then significantly suppressed on Day 4 following the ovulation (Adams et al., 1986). In mares with transitional estrus, the transitional follicles had less blood flow vascularization due to the less developed and less proliferative activity of the endothelial cells in their theca and granulosa cells compared to the preovulatory follicle (Watson and Al-Ziábi, 2002). In agreement with the insignificant increase of E2 in mares treated with hCG and prostaglandin compared to the spontaneous ovulating one, E2 concentrations were lower during the first cycle of the breeding season on Day 8 as compared to Day 8 after breeding mares for embryo flush or Day 8 after treating mares with prostaglandin and Day 8 of the ovulation following embryo flush (Raz et al., 2009). Systemic or intra-luteal PG was used to induce luteolysis in mares (Weber et al., 2001) and is regularly used after embryo flushing to decrease the risks of unwanted pregnancies in the embryo donor mares (Martínez-Boví et al., 2024). In contrast to the significant decrease in progesterone after administrating prostaglandin, no differences in P4 were observed in mares on the day of administrating hCG for inseminating the mares and preparing the donor mare for embryo flushing (Raz et al., 2009). However, the decrease in the mean P4 concentrations along the spontaneous ovulation cycle compared to the induced ovulation cycle refers to the effect of days on its concentrations and that the spontaneous ovulating cycle is longer due to the inclusion of days from the estrous till ovulation (6 days), and luteal phases (Day 1–Day 15) but that following hCG took 2 days to ovulate and 8 days to embryo flushing. However, after embryo flushing and administrating prostaglandin, mares ovulated on Day 5 and Day 19 presenting a short luteal phase after prostaglandin and luteolysis, progesterone decreased sharply. According to Raz et al., (2009), the significant increase of P4 and the insignificant decrease of E2 on Day 8 of the first estrous cycle compared to that treated by hCG and that treated with prostaglandin explains the insignificant increase in the mean E2 and a significant decrease in their concentrations through our experimental interval in the treated mares with hCG and prostaglandin. The CL development and vascularization commenced after the ovulation where the mean percentage of the luteal color signals increased indicating increased blood flow that reached maximum values on Day 10 in the regular estrous cycle (Abdelnaby and Abo El-Maaty, 2017b). In mares, the days from Day 10 to Day 14 after ovulation showed a decreasing luteal color signal percentage (49.8%) was defined as the pre-luteolytic period. The CL luteolysis started after day 14 post-ovulation which was defined as the luteolytic period (Ginther et al., 2007). Meanwhile, the CL area started decreasing linearly from Day 4 to Day 19 post-ovulation, and concentrations of progesterone decreased in parallel with the CL area starting from Day 8 until the onset of luteolysis (Ginther et al., 2007). During the last phase of the CL (luteolytic period), circulating progesterone concentrations decreased 6 times faster than their greater than during the pre-luteolytic period and three times lower than the decreased CL area and CL blood-flow area (Ginther et al., 2007). Mean progesterone in the current study was not affected by hCG or PG treatments. In agreement with our results, serum progesterone on Day 13 did not vary in mares subjected to ovulation induction using hCG (Teixeira et al., 2020). However, no PG was used after embryo flushing in embryo donor mares, but progesterone concentrations declined and <10% of mares had complete luteolysis (Martínez-Boví et al., 2024). Compared to the spontaneous control oestrous cycle, the lowest progesterone was observed in mares of the current study on the day of ovulation in mares treated with PG but the highest levels were noticed in mares treated with hCG, in agreement with our results, mares showed great individual variability in their progesterone concentrations at the time of ovulation with marked fluctuation from ovulation to luteolysis (Laufkötter et al., 2024). The significant increase of cholesterol and the non-significant increase in E2 concentrations on the day of ovulation in mares treated with hCG and PG and its association with dominant ovulating follicles with the significantly lower diameter and in a significantly higher area and their ovulation within 36–48 hours after hCG and their deviation and ovulation within 5 days after inducing luteolysis using PG indicate the role of cholesterol in the synthesis of ovarian steroid hormones by the granulosa cells as cholesterol is the precursor of all steroid hormones which agree with the increase of total cholesterol in follicular fluid of the large follicles confirming its role in the increased the synthesis of steroids (Satué et al., 2019). Recent proteomic analysis of the follicular fluid agreed with the increase in the concentration of cholesterol during the day of ovulation which was referred to as the increase in the concentrations of proteins responsible for lipid metabolism in women (Zakerkish et al., 2020). During the non-breeding season in mares, total cholesterol and oxidative stress index in serum and medium follicles were strongly associated. Circulating lipid metabolites were high in blood serum compared to the follicular fluid of small- and medium-sized follicles. IL-6 and oxidative stress index were similar in serum and all follicle classes. The blood composition associated with inflammation, oxidative stress, and disturbed lipid metabolism of mares may influence the oocyte microenvironment, the oocyte quality, and subsequent embryo quality (Hedia et al., 2023). The significant increase in the concentrations of LDH on the day of ovulation after inducing luteolysis using PG indicates the progressive and dramatic decrease in the CL in association with the deviation of the next ovulating follicle since LDH is a marker of cell damage and the significant decrease in LDH levels as the DF increase in size and ovulate agree with the lowest values on the day of ovulation in the control non treated mares (Satué et al., 2019). The significant increase in the NO on the day of ovulation in mares treated with hCG and PG compared to control indicates the faster growth and maturation of the ovulating follicles after administrating hCG and luteolysis which can be referred to the role of NO in modulating the steroidogenesis of the granulosa cell in cattle (Matsumi et al., 2000) and mares (Pinto et al., 2002), follicular angiogenesis and blood flow (Basini and Grasselli, 2015), and regulate the ovulation via regulating steroid hormones production (Huang et al., 2005). Oocyte lipid content did not change over time in aged mares. When flaxseed oil was supplemented to improve oocyte function and developmental potential of maturing dominant follicles of older mare’s oocytes, it changed lipid content and reduced it in oocytes. In non-supplemented mares, the concentrations of triglycerides and free fatty acids were higher in plasma than in follicular fluid. Dietary supplementation reduced concentrations of triglycerides in follicular fluid and fatty acids in blood plasma (Catandi et al., 2022). During spontaneous ovulation, induced ovulation using hCG, induced luteolysis PGF2α the day of the cycle influenced the follicle dynamics and hemodynamics. The day of the ovulation influenced the dominant follicle diameters and the circulating hormones, enzymes, oxidants, and antioxidants. Total proteins and albumin concentrations decreased in follicular fluid than in blood serum of mares having follicle diameter >25 mm and those just ovulated or after inducing ovulation. In contrast, total cholesterol concentrations increased in the follicular fluids of follicles >25 mm compared to serum levels and in the follicular fluid of mares just ovulated or 35 hours after inducing ovulation (Collins et al., 1997). The insignificant decrease in the catalase activity associated with increased GPx activity on the day of ovulation of control mares and those treated with hCG compared to those treated with PG was also noticed when GPx 3 increased in the follicular fluid of women during their ovulation (Zakerkish et al., 2020). During the non-breeding season in mares, total cholesterol and oxidative stress index in serum and medium follicles were strongly associated. Circulating lipid metabolites were high in blood serum compared to the follicular fluid of small- and medium-sized follicles. IL-6 and oxidative stress index were similar in serum and all follicle classes. The blood composition associated with inflammation, oxidative stress, and disturbed lipid metabolism of mares may influence the oocyte microenvironment, the oocyte quality, and subsequent embryo quality (Hedia et al., 2023). Oocyte lipid content did not change over time in aged mares. When flaxseed oil was supplemented to improve oocyte function and developmental potential of maturing dominant follicles of older mare’s oocytes, it changed lipid content and reduced it in oocytes. In non-supplemented mares, the concentrations of triglycerides and free fatty acids were higher in plasma than in follicular fluid. Dietary supplementation reduced concentrations of triglycerides in follicular fluid and fatty acids in blood plasma (Catandi et al., 2022). Also, the diameter of the dominant follicle in treated mares was lower than in the control cycles of our mares. The diameter of the follicle did not relate to the follicular oxidative stress index or the biological antioxidant potential but the medium follicle follicular oxidative stress and antioxidant capacity were related to the serum levels (Hedia et al., 2023). The significant increase in myeloperoxidase (MPO) on the day of ovulation after treatment with hCG compared to control and PG treatments agrees with the constant presence of MPO in the uterine lumen of mares in the absence of inflammation and under physiological cyclic conditions (Parrilla Hernández et al., 2023). In our experiment, haptoglobin declined in mares treated with hCG and PG. Similarly, haptoglobin did not vary in the circulation of healthy mares and susceptible mares to post-mating endometritis (Tuppits et al., 2014). The increased total proteins, albumin, myeloperoxidase, and NO and the decreased haptoglobin in mares treated with hCG and PG agree with the increase in the polymorphonuclear cells and haptoglobin in control mares after artificial insemination (Wojtysiak et al., 2020). ConclusionDuring the embryo transfer program under the hot subtropical environmental conditions, the induction of ovulation using hCG or luteolysis using prostaglandin hastened ovulation of follicles with lower diameters and smaller antrum compared to spontaneously ovulating mares with no differences in their circulatory, color area % or granulosa area and granulosa color area % and increased the uterine horn area and color area and did not alter the ovarian arteries blood flow velocities or blood flow indices. The changes in the circulating biochemicals, antioxidants, acute phase proteins, ovarian hormones, and enzymes are associated with CL cells damage after inducing luteolysis of the physiological inflammation during spontaneous or induced ovulation. AcknowledgmentsThe first author wishes to thank the Libyan government for supporting this thesis with a Master’s grant from the Ministry of Agriculture, Livestock, and Aquatic Resources and Faculty of Veterinary Medicine, Cairo University for facilitating the work, the place for keeping and care of the animals, and the National Research Centre for facilitating the Doppler Ultrasound for examining mares and facilitates and tools for Embryo flushing. Conflict of interestThe authors declare that they do not have any conflict of interest. Authors ContributionJamal M. H. Alkhadrawy performed the Doppler and Ultrasound scanning, funded the hormones, and performed the image analysis; Amal M. Aboelmaaty learned the ultrasound and Doppler examination, assayed the hormones, performed the statistical analysis, wrote the manuscript and submitted it to publication; Abdelraouf M. Ghallab put the experimental design and revised the submitted version of the manuscript; Mostafa M. Abou-Ahmed revised and approved the final version of the manuscript. Data AvailabilityAll data supporting the findings of this study are available within the manuscript. Data from this article will be available upon request. ReferencesAbdelnaby, E.A. and Abo El-Maaty, A.M. 2017a. Dynamics of follicular blood flow, antrum growth and angiogenic mediators in mares from deviation to ovulation. J. Equine Vet. Sci. 55, 51–59. Abdelnaby, E.A. and Abo El-Maaty, A.M. 2017b. Luteal blood flow and growth in correlation to circulating angiogenic hormones after spontaneous ovulation in mares. Bulg. J. Vet. Med.20, 97–101. Abdelnaby, E.A., Abo El-Maaty, A.M., Ragab, R.S.A. and Seida, A.A. 2018. Dynamics of uterine and ovarian arteries flow velocity waveforms and their relation to follicular and luteal growth and blood flow vascularization during the estrous cycle in Friesian cows. Theriogenology 121, 112–121. Abdelnaby. E.A., Abo El-Maaty, A.M., Ragab, R. S.A. and Seida, A.A. 2016. Assessment of uterine vascular perfusion during the estrous cycle of mares in connection to circulating leptin, and nitric oxide concentrations. J. Equine Vet. Sci.39, 25–32. Abo El-Maaty, A.M. and Abdelnaby, E.A. 2017. Follicular blood flow, antrum growth and angiogenic mediators in mares from ovulation to deviation. Anim. Reprod. 14,1043–1056. Adams, T.E., Horton, M.B., Watson, J. and Adams B.M. 1986. Biological activity of luteinizlng hormone (LH) during the estrous cycle of mares. Domestic Anim. Endocrinol. 3, 69–77. Alkhadrawy, J.M.H., Aboelmaaty, A.M., Abou-Ahmed, M.M. and Ghallab, A.M. 2024. Effect of breeding season and age on follicular dynamics and hemodynamics in embryo donor mares subjected to luteolysis after embryo flushing. Open Vet J. 14, 852–865. Alonso, M.A., Silva, L.A., Affonso, F.J., Lemes, K.M., Celeghini, E.C.C., Lançoni, R., Carvalho, H.F. and de Arruda, R.P. 2019. Effect of hCG application at different moments of the estrous cycle on corpus luteum and uterine vascularization and serum progesterone concentration in mares. Anim. Reprod. 16, 317–327. Basini, G. and Grasselli, F. 2015. Nitric oxide in follicle development and Oocyte competence. Reproduction 150, R1–9. Barrière, P. and Deleuz,e S. 2023. Characterization of myeloperoxidase in the healthy equine endometrium. Animals (Basel) 13, 375. Boakari, Y.L., Ferreira, J.C., Canesin, H.S., Thompson, D.L. Jr, Lima, F.S., Pantoja, J.C.F. and Meira, C. 2017. Influence of two ovulation-inducing agents on the pituitary response and follicle blood flow in mares. Theriogenology 100, 95–99. Bollwein, H., Weber, F., Kolberg, B. and Stolla, R. 2002. Uterine and ovarian blood flow during the estrous cycle in mares. Theriogenology 57, 2129–2138. Bollwein, H., Kolberg, B. and Stolla, R. 2004. The effect of exogenous estradiol benzoate and altrenogest on uterine and ovarian blood flow during the estrous cycle in mares. Theriogenology 61, 1137–1146. Bristol, F. 1993. Synchronization of ovulation. In Equine reproduction. McKinnon, A.O. and Voss, J.L. Baltimore, MD: Williams and Wilkins, pp: 348–52. Catandi, G.D., LiPuma, L., Obeidat, Y.M., Maclellan, L.J., Broeckling, C.D., Chen, T., Chicco, A.J. and Carnevale, E.M. 2022. Oocyte metabolic function, lipid composition, and developmental potential are altered by diet in older mares. Reproduction 163, 183–198. Collins, A., Palmer, E., Bézard, J., Burke, J., Duchamp, G. and Buckley, T. 1997. A comparison of the biochemical composition of equine follicular fluid and serum at four different stages of the follicular cycle. Equine Vet. J. Suppl. 25, 12–6. Gastal, E.L., Gastal, M.O. and Ginther, O.J. 2006. Relationships of changes in B-mode echotexture and colour-Doppler signals in the wall of the preovulatory follicle to changes in systemic oestradiol concentrations and the effects of human chorionic gonadotrophin in mares. Reproduction 131, 699–709. Gérard, N., Duchamp, G. and Magistrini, M. 1999. Relationships between follicular fluid composition and follicular/oocyte quality in the mare. Livestock Prod. Sci. 60, 243–253. Ginther, O.J., Gastal, M.O., Gastal, E.L., Jacob, J.C., Beg, M.A. 2009. Age-related dynamics of follicles and hormones during an induced ovulatory follicular wave in mares. Theriogenology 71, 780–8. Ginther, O.J., Gastal, E.L., Gastal, M.O., Utt, M.D. and Beg, M.A. 2007. Luteal blood flow and progesterone production in mares. Anim. Reprod. Sci. 99, 213–20. Glazar, B.S., McCue, P.M., Bruemmer, J.E. and Squires, E.L. 2004. Deslorelin on Day 8 or 12 postovulation does not luteinize follicles during an artificially maintained diestrous phase in the mare. Theriogenology 62, 57–64. Hedia, M., Leroy, J.L.M.R., Govaere, J., Van Soom, A. and Smits, K. 2023. Lipid metabolites, interleukin-6 and oxidative stress markers in follicular fluid and their association with serum concentrations in mares. Vet. Res. Commun. 47, 2221–2228. Huang, H-F., Wang, B., Yang ,X-F., Luo, Q. and Sheng, J-Z. 2005. Nitric oxide mediates inhibitory effect of leptin on insulin-like growth factor I augmentation of 17β-estradiol production in human granulosa cells. Biol Reprod 72, 102–6. Laufkötter, S., Längerer, L. and Wehrend, A. 2024. Individual hormonal profiles of blood progesterone and estradiol-17β during the course of a reproductive cycle in mares]. Tierarztl Prax Ausg G Grosstiere Nutztiere. 52, 88–95. Mahmood, K., Ali, Channa, A., Ghafoor, A. and Riaz, A. 2024. Factors affecting the efficiency of equine embryo transfer (EET) in polo mares under subtropical conditions of Pakistan. PLoS One. 19, e0298066. Martínez-Boví, R., Sala-Ayala, L., Querol-Paajanen, A., Plaza-Dávila, M., Cuervo-Arango, J. 2024. Effects of repeated embryo flushing without PGF2α administration on luteal function, percentage of unwanted pregnancy and subsequent fertility in mares. Equine Vet J. 56, 796–805. Matsumi, H., Yano, T., Osuga, Y., Kugu, K., Tang, X., Xu, J.P., Yano, N., Kurashima, Y., Ogura, T., Tsutsumi, O., Koji, T., Esumi, H. and Taketani, Y. 2000. Regulation of nitric oxide synthase to promote cytostasis in ovarian follicular development. Biol Reprod 63, 141–6. Musicki, B., Liu, T., Lagoda, G.A., Bivalacqua, T.J., Strong, T.D. and Burnett, A.L. 2009. Endothelial nitric oxide synthase regulation in female genital tractstructures. J. Sex. Med. 6, 247–53. Panzani, D., Rota, A., Marmorini, P., Vannozzi, I., Camillo, F. 2014. Retrospective study of factors affecting multiple ovulations, embryo recovery, quality, and diameter in a commercial equine embryo transfer program. Theriogenology 82, 807–14. Parrilla Hernández, S., Franck, T., Munaut, C., Feyereisen, É., Piret, J., Farnir, F., Reigner, F., Pinto, C.R.F., Paccamonti, D.L., Eilts, B.E., Short, C.R., Gentry, L., Thompson, D., and Godke, R. 2002. Evidence for a nitric oxide-mediated modulation of equine granulosa cell steroidogenesis. Theriogenology 58, 579. Raz, T., Hunter, B., Carley, S. and Card, C. 2009. Reproductive performance of donor mares subsequent to eFSH treatment in early vernal transition: comparison between the first, second, and mid-season estrous cycles of the breeding season. Anim. Reprod. Sci. 116, 107–18. Satué, K., Fazio, E., Ferlazzo, A. and Medica P. 2019. Hematochemical patterns in follicular fluid and blood stream in cycling mares: a comparative note. J. Equine Vet. Sci. 80, 20–26. Segabinazzi, L.G.T.M., Oba, E. and Alvarenga, M.A. 2021. The combination of hCG and GnRH analog to hasten ovulation in mares does not change luteal function and pregnancy outcome in embryo recipient mares. J. Equine. Vet. Sci. 105, 103691. Stich, K.L., Wendt, K.M., Blanchard, T.L. and Brinsko, S.P. 2004. Effects of a new injectable short-term release deslorelin in foal-heat mares. Theriogenology 62, 831–6. Teixeira, A.C.B., Valle, G.R., Riveros, J.A.N., Diniz, J.H.W., Wenceslau, R.R., Monteiro, G.A., Leme, F.O.P. and Oliveira, L.Z. 2020. Effects of equine chorionic gonadotropin on ovulatory and luteal characteristics of mares submitted to an P4-based protocol of ovulation induction with hCG. J. Equine. Vet. Sci. 94, 103233. Tuppits, U., Orro, T., Einarsson, S., Kask, K. and Kavak, A. 2014. Influence of the uterine inflammatory response after insemination with frozen-thawed semen on serum concentrations of acute phase proteins in mares. Anim. Reprod. Sci. 146, 182–6. Watson, E. and Al-Ziábi, M. 2002. Characterization of morphology and angiogenesis in follicles of mares during spring transition and the breeding season. Reproduction 124, 227e34. Watson, E.D. and Sertich, P.L. 1991. Concentrations of arachidonate metabolites, steroids and histamine in preovulatory horse follicles after administration of human chorionic gonadotrophin and the effect of intrafollicular injection of indomethacin. J. Endocrinol. 129, 131–139. Watson, E.D., Colson, M. and Broadley, C. 1995. LH and progesterone concentrations during diestrus in the mare and the effect of hCG. Theriogenology 43, 1325–1337. Weber, J.A., Causey, R.C. and Emmans, E.E. 2001. Induction of luteolysis in mares by ultrasound-guided intraluteal treatment with PGF2alpha. Theriogenology 55, 1769–76. Wojtysiak, K., Ryszka, W., Stefaniak, T., Król, J. and Kozdrowski, R. 2020. Changes in the secretion of anti-inflammatory cytokines and acute-phase proteins in the uterus after artificial insemination in the mare. Animals (Basel) 10, 2438. Zakerkish, F., Brännström, M., Carlsohn, E., Sihlbom, C., van der Post, S. and Thoroddsen, A. 2020. Proteomic analysis of follicular fluid during human ovulation. Acta Obstet Gynecol Scand. 99, 917–924. Zoller, D., Lüttgenau, J., Steffen, S. and Bollwein, H. 2016. The effect of isosorbide dinitrate on uterine and ovarian blood flow in cycling and early pregnant mares: a pilot study. Theriogenology 85, 1562–1567. | ||
| How to Cite this Article |
| Pubmed Style Alkhadrawy JMH, Aboelmaaty AM, Abou-ahmed MM, Ghallab AM. Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Vet. J.. 2024; 14(8): 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 Web Style Alkhadrawy JMH, Aboelmaaty AM, Abou-ahmed MM, Ghallab AM. Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. https://www.openveterinaryjournal.com/?mno=204900 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.35 AMA (American Medical Association) Style Alkhadrawy JMH, Aboelmaaty AM, Abou-ahmed MM, Ghallab AM. Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Vet. J.. 2024; 14(8): 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 Vancouver/ICMJE Style Alkhadrawy JMH, Aboelmaaty AM, Abou-ahmed MM, Ghallab AM. Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 Harvard Style Alkhadrawy, J. M. H., Aboelmaaty, . A. M., Abou-ahmed, . M. M. & Ghallab, . A. M. (2024) Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Vet. J., 14 (8), 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 Turabian Style Alkhadrawy, Jamal Mohamed Hassan, Amal Mahmoud Aboelmaaty, Mostafa Mohamed Abou-ahmed, and Abdelraouf Morsy Ghallab. 2024. Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Veterinary Journal, 14 (8), 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 Chicago Style Alkhadrawy, Jamal Mohamed Hassan, Amal Mahmoud Aboelmaaty, Mostafa Mohamed Abou-ahmed, and Abdelraouf Morsy Ghallab. "Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics." Open Veterinary Journal 14 (2024), 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 MLA (The Modern Language Association) Style Alkhadrawy, Jamal Mohamed Hassan, Amal Mahmoud Aboelmaaty, Mostafa Mohamed Abou-ahmed, and Abdelraouf Morsy Ghallab. "Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics." Open Veterinary Journal 14.8 (2024), 2057-2072. Print. doi:10.5455/OVJ.2024.v14.i8.35 APA (American Psychological Association) Style Alkhadrawy, J. M. H., Aboelmaaty, . A. M., Abou-ahmed, . M. M. & Ghallab, . A. M. (2024) Effect of hCG and prostaglandin on ovarian, luteal development, and hormonal changes in embryo donor mares during the hot summer months in subtropics. Open Veterinary Journal, 14 (8), 2057-2072. doi:10.5455/OVJ.2024.v14.i8.35 |