| Short Communication | ||
Open Vet. J.. 2024; 14(11): 3089-3099 Open Veterinary Journal, (2024), Vol. 14(11): 3089-3099 Short Communication Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary studyCristian Mihăiță Crecan1, Valeria Ciulu-Angelescu1*, Iancu Adrian Morar2, Alexandru Florin Lupșan1, Mirela Alexandra Tripon2, Denisa Bungărdean3, Zsofia Daradics4 and Cosmin Petru Peștean11Department of Veterinary Anesthesiology and Surgery, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine Cluj Napoca, Cluj Napoca, Romania 2Department of Veterinary Reproduction and Obstetrics, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine Cluj Napoca, Cluj Napoca, Romania 3Department of Veterinary Physiology and Pathophysiology, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine Cluj Napoca, Cluj Napoca, Romania 4Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine Cluj Napoca, Cluj Napoca, Romania *Corresponding Author: Valeria Ciulu-Angelescu. Department of Veterinary Anesthesiology and Surgery, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine Cluj Napoca, Cluj Napoca, Romania. Email: valeria.angelescu [at] usamvcluj.ro Submitted: 11/06/2024 Accepted: 25/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Global technological advancements have shifted equine lameness diagnostics from purely subjective assessment toward more objective, quantitative methods. Traditional gait evaluations are increasingly being supplemented by innovative technologies to enhance diagnostic accuracy. Aim: This study aimed to determine if traditional gait assessment could be effectively complemented by quantitative measurements using an affordable, self-constructed device, the Lameness Detector 0.1, which incorporates inertial motion sensors. Methods: A total of 42 adult sport horses diagnosed with hindleg lameness due to osteochondritis dissecans were assessed pre- and post-intra-articular anesthesia using both subjective evaluation and the Lameness Detector 0.1. Quantitative data were collected by recording electric impulses on the device’s accelerometer across three axes (X, Y, Z). Statistical analysis was performed to compare pre- and post-joint block values, stratified by lameness degree, and to analyze correlations between quantitative measurements and subjective evaluations. Results: Significant differences were observed on the X-axis (p < 0.001) between pre- and post-joint block assessments, with a decrease in impulse counts post-block. For horses scoring 2/5 and 3/5 on the AEEP scale, Y-axis data also showed significant variation (p < 0.05), with impulse numbers increasing as lameness scores decreased. Z-axis data demonstrated low specificity. Overall, the mean X-axis impulse counts correlated strongly with subjective lameness scores (p ≤ 0.006 for significant correlations). Conclusion: The Lameness Detector 0.1 provides a reliable and cost-effective means to complement subjective gait analysis. The quantitative data, particularly X-axis impulses, correlate well with traditional lameness scores, suggesting potential value in integrating this device for enhanced diagnostic precision in equine lameness assessment. Keywords: Accelerometer-generated impulses, Adult sport-horses, Equine lameness, Kinematic locomotion exam, Sensors. IntroductionRegardless of their age, breed, gender, or use, lameness is the most common problem impairing horses around the whole globe (Kane et al., 2000), not only by limiting their usability, but also impacting their general welfare status and health (Atkins et al., 2019), especially when it becomes a chronic ailment. Thus, early recognition, proper diagnosis, and appropriate treatment are paramount for each lame horse. Despite being a classical standard procedure, and regardless of the clinician’s experience, the visual appraisal of equine lameness inherently bears the limitations of the human visual perception of motion (Bragança et al., 2018). Acknowledging this, and given the constantly improving technological possibilities for medical diagnostics, the need for affordable and easy-to-use measurement systems to quantify and examine the characteristics of equine gait yielded lately objective techniques, with good potential for practical daily use in a clinical setting (Bragança et al., 2018). As the kinetic methods (i.e., those that analyze the forces resulting from movement) rely on a complex instrument system and a complicated data collection procedure, the kinematic methods (which analyze the movement of different body segments during locomotion) are preferred. For kinematic gait analysis, the optical motion capture systems are considered the “gold standard”, but because these involve a significant number of cameras and supporting infrastructure, inertial measurement units (IMUs) gained more popularity as a promising cost-effective alternative (Bosch et al., 2018). Moreover, technological innovations allow the usage of wireless devices, without the need for physical connection (cables) between the sensors and the data processing unit. The IMU sensors are built using a gyroscope, an accelerometer, and a magnetometer, and they measure acceleration, angular velocity, and the Earth’s magnetic field in three dimensions (Hardeman et al., 2021). Using the data provided by such sensors and special signal-processing algorithms, asymmetry measures can be calculated for vertical head and pelvis movements and their stride-by-stride differences, to demonstrate lameness (Keegan et al., 2011). The number of marketed devices that use IMUs to detect equine lameness is high; however, there are differences among the various systems and brands with regard to their complexity, ease of use, positioning, and tracked parameters (Crecan and Pestean, 2023). Several studies evaluated the potential of IMUs to be used as supporting tools during the clinical examination of equine patients, with generally promising results (McCracken et al., 2012, Keegan et al., 2013; Leelamankong et al., 2020). The utility of IMU-based devices in detecting gait asymmetries suggestive of several pathologies has also been demonstrated (McCracken et al., 2012, Keegan et al., 2013; Donell et al., 2015; Bell et al., 2016; Leelamankong et al., 2020). Nevertheless, currently available IMUs are usually costly (Crecan and Pestean, 2023). For adequate data acquisition, care must be taken to ensure that all sensors are correctly placed; in addition, some horses might perceive the attachment of sensors to their body as a stress factor, which could interfere with their natural gait. However, as described by Hardeman et al. (2021), the opinions and expectances of equine orthopedic clinicians regarding the use of qualitative gait analysis devices both by veterinarians and non-veterinarians are divided, and for certain locomotion ailments of the horse, the consecrated diagnostic methods continue to hold ground. For example, in lameness caused by osteochondritis (OC) the strictly cartilaginous modifications or subtle bony lesions may be easily missed, yet the gold standard in the diagnostic of both OC and osteochondritis dissecans (OCD) is still radiology (van Weeren, 2019). Depending on the joint and its accessibility, ultrasonography is a good alternative, by itself, or in combination with radiography, with additional benefits in cartilage damage visualization and exact determination of the osteochondral fragments’ position (Whitton, 2015). Both for confirmation of the radiologic and/or ultrasonographic diagnosis, and as a treatment modality of choice in OCD, arthroscopy is used. An instance in which many of the OC/OCD cases are diagnosed is the interpretation of prepurchase examination radiographs, many times for clinically sound animals. Especially in patients with only OC (no cartilage fragmentation), regardless of the existence or nonexistence of joint effusions, lameness can be absent or so discrete that it can elude a classical subjective lameness evaluation. Because of this reason, the use of quantitative, objective lameness assessment methodologies can bring benefits by either a more sensitive diagnostic provided by a specific device, or by increasing the clinician’s attention to applying a more thorough examination because of the warning of the device. The present study focused on the lameness diagnostic in adult sport-horses, suffering from hindleg lameness caused by tarsal OC or OCD. Our objective was to evaluate whether an inexpensive, easy-to-use self-constructed device, the Lameness detector 0.1 (Crecan et al., 2022) is suitable for collecting quantitative measurements that could complete the classical subjective lameness assessment, and to assess agreement between the results collected with this device and those obtained by subjective gait analysis. Rather than replacing the classical lameness diagnostic methods, our tool was meant to complete them, and to indicate, early during the horse’s examination, if more attention and extending the standard assessment protocol would be needed for a proper diagnosis. Materials and MethodsThis study was conducted in an equine hospital and enrolled adult sport-horse patients diagnosed with tarsal OCD. The animals had been brought to the facility either because of various lameness grades and hock effusions or following the interpretation of their pre-purchase examination radiographs showing intra-articular fragmentation. Regardless of the situation, the study inclusion criteria had been (1) clinically manifested lameness and (2) the existence of tarsal OCD. At hospitalization, all horses were subjected to a general health examination, followed by an orthopedic assessment, performed by five experienced evaluators [experience: 10 years (2 evaluators), 12 years (1 evaluator), 16 years (1 evaluator), and 25 years (1 evaluator)]. For the latter, the patients were inspected first while standing, then while walking and trotting in a straight line and in circles on a flat terrain covered with asphalt, then palpation was performed followed by two-staged flexion tests (distal limb flexion to test the joints of the coffin, pastern, and fetlock in the first stage, then proximal limb flexion for the joints of the hock, stifle, and hip in the second stage) for both hindlegs. All examinations were conducted during dry weather, at different timepoints during the day; all efforts were made to avoid, eliminate, or minimize any factors that might have resulted in the horses being distracted. During the inspection, the American Association of Equine Practitioners (AAEP, 2024) lameness scale was used, awarding scores from zero (no perceptible lameness) to five (most extreme lameness) as described by the AAEP guidelines. The subjective lameness evaluation was performed independently by each of the five evaluators; the final AAEP score was decided by agreement. Next, an original wireless lameness detector system (Lameness detector 0.1) was used to assess the patients gait, for five consecutive strides. The system was formed by four identical devices, each of these being attached to a pastern of the horses with adjustable flexible banderoles, in a setup similar to protective leg equipment (Fig. 1). Each device consisted of a Lithium-Ion Polymer accumulator (1,400 mAh) on top of which a three-axis accelerometer (ADXL345) was mounted to measure the static and dynamic acceleration on each of its three axes (with a data-returning power of 13 bits), an Arduino board with 14 digital and eight analog pins (charged at 3.3 V, with a constant functioning at 8 MHz to transmit the data for processing) and a Bluetooth device (with a 106 m action range). These parts of the device were protected and held together by a system of jointed capsules projected by using the Open Scad software and made of polylactic acid (Plastic 2) on a 3D printer. In order to process the Bluetooth-transmitted data from the lameness detector, a laptop was equipped with the Arduino software and a specific PC software (named Lameness detector 0.1 software). Additionally, the laptop had installed the GraphPad Prism software (version 5.0 for Windows, San Diego CA) too, for statistical data processing. The specific Lameness detector 0.1 software had been used for the graphical representation of the data collected by the Arduino software (represented by 1 impulse per 10 ms on each of the 3 axes), in a screen divided into four, with one quarter for the data from each leg of the examined horse. Figure 2 illustrates how the graphical representation of the gait analysis changes during the different phases of the stride. Before data were recorded, a proper fitting was ensured by measuring static (gravitational) acceleration while the horse was standing; the positioning of the device was readjusted until the displayed values were close to 0 m/s2 on the X and Z axes, and 9.8 m/s2. on the Y axis. Based on these values, the positioning of the devices was readjusted as needed. Visual markers (lines drawn on the device and the horse’s hoofs) were also used to ensure correct alignment. The process of fitting the device was relatively short, with a duration of about 1–1.5 minutes. More details on the components of the Lameness detector 0.1 device, its proper fitting, and the type of data that can be collected were previously described by Crecan et al. (2022).
Fig. 1. The Lameness detector 0.1 system, fitted with adjustable straps to the dorsal aspect of a horse’s pasterns.
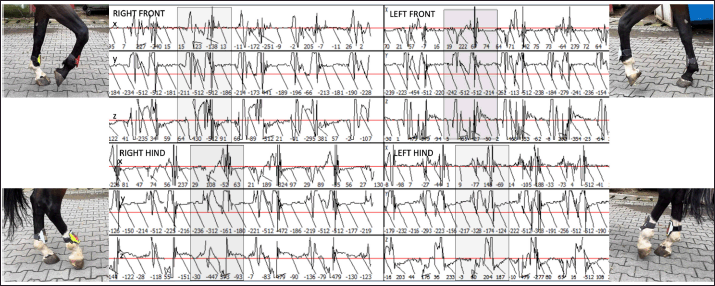
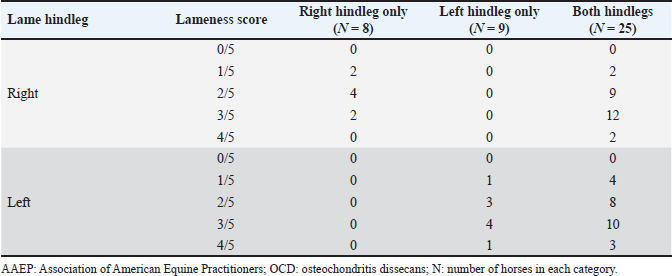
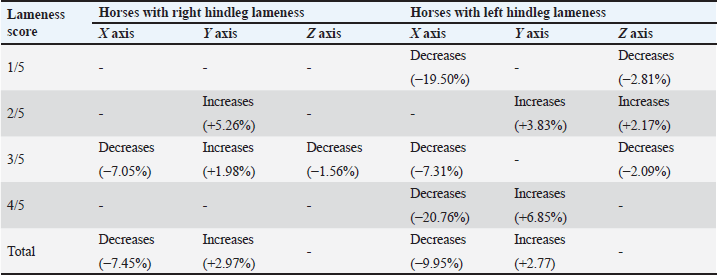
Fig. 2. Sample of the data collected using the Lameness detector 0.1 and processed with the Arduino software. Shaded areas mark the extent of one step. During the objective lameness assessment with the Lameness detector 0.1 device, all the impulses transmitted by the sensors for each of the three axes (X, Y, and Z) have been recorded. Both the subjective lameness assessment and the qualitative gait assessment using the Lameness detector 0.1 device were performed twice: first at baseline, and a second time later in the orthopedic examination, following flexion tests and at 8 minutes after the intra-articular anesthesia. The diagnostic protocol continued with radiographs, ultrasonographic investigation, and finally the diagnostic and treatment, by arthroscopy. The statistical analysis of the data included the calculation of descriptive statistical parameters (mean and median values for the number of impulses on each axis, as well as the standard deviation of the mean) on Microsoft Excel spreadsheets. All values were included, without the exclusion of outliers. The obtained values have been compared for each of the three axes between the two assessments (before and after the intra-articular anesthesia), taking into account the initial AAEP lameness score (at baseline, before the joint block). For data comparison, the paired samples t-test (Raspa et al., 2022a,b) was used, after testing the normality distribution by the Kolmogorov-Smirnov test (Spadari et al., 2023). To verify the existence of correlations, Spearman’s rank correlation coefficients (for non-parametric data) have been calculated. The level of statistical significance was set at p < 0.05. The more complex statistical analyses were carried out using the SPSS (version 17, 2010, www.spss.com) statistical software. All procedures had been performed as part of consecrated treatment protocols, with the informed consent of the animals’ owners and at pre-established costs. All horse owners have signed the mandatory informed consent documents. Ethical approval (No. 12/2014) was granted by the Bioethics Commitee of the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (Romania). Results and DiscussionThe study population included 42 horses. Besides 10 horses without registered pedigree, the sample comprised the following breeds: Hungarian sport horse (n=13), Oldenburg (n=6), Trotter (n=5), Holsteiner (n=2), Friesian (n=2), Andalusian (n=1), Dutch warmblood (n=1), Thoroughbred (n=1), and Romanian sport horse (n=1). The mean age of the studied animals was 9 years (ranging from 4 to 14 years) with the highest number of horses in the 6-years-old age group (n=8), and six animals in each of the 7-, 8-, and 9-years-old groups, respectively. Out of the 42 horses, 20 were mares and 8of the 22 stallions were gelded. According to the consecrated AAEP lameness scale, all the horses were found to be lame in at least one hindleg in the classical visual lameness assessment, with scores ranging from 1/5 to 4/5 (AAEP lameness scale). Out of the 42 animals, 8 were lame in their right hindleg, 9 in their left hindleg, and 25 were lame in both their hindlegs. The lameness scores before intra-articular anesthesia are presented in Table 1. Before the intra-articular anesthesia, a lameness score of 4/5 (the most severe grade detected in our study) was recorded for 2/42 (4.76%) of the studied horses in their right hindlegs, and for 4/42 (9.52%) had a 4/5 lameness score in their left hindleg. After the joint blocks, no horses exhibited 4/5 lameness, but the percentage of horses with a mild lameness score (1/5) increased: from 4/42 (9.52%) at baseline to 24/42 (57.14%) after intra-articular anesthesia. These results suggest that in many cases, the lameness decreased in intensity following the joint block but did not disappear completely, as demonstrated by the percentual increase of the lower lameness scores. Table 1. Lameness scores (according to AAEP guidelines) before intra-articular anesthesia in the 42 studied horses suffering from hock OCD.
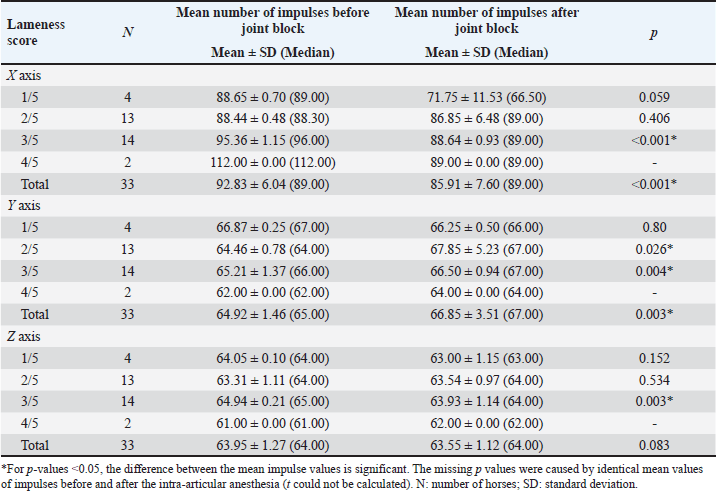
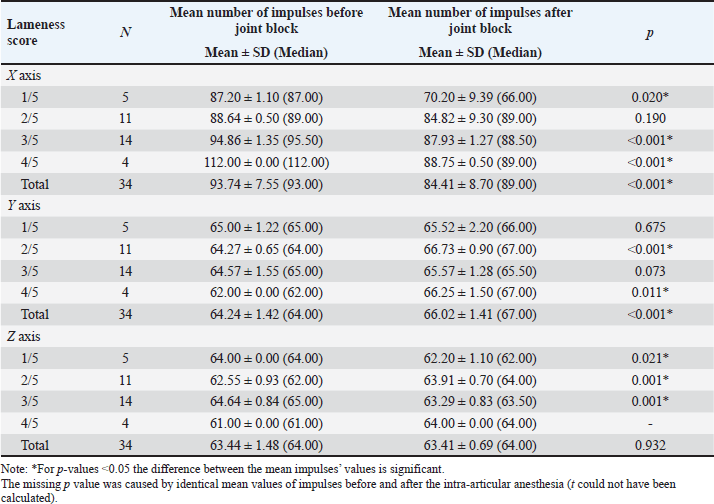
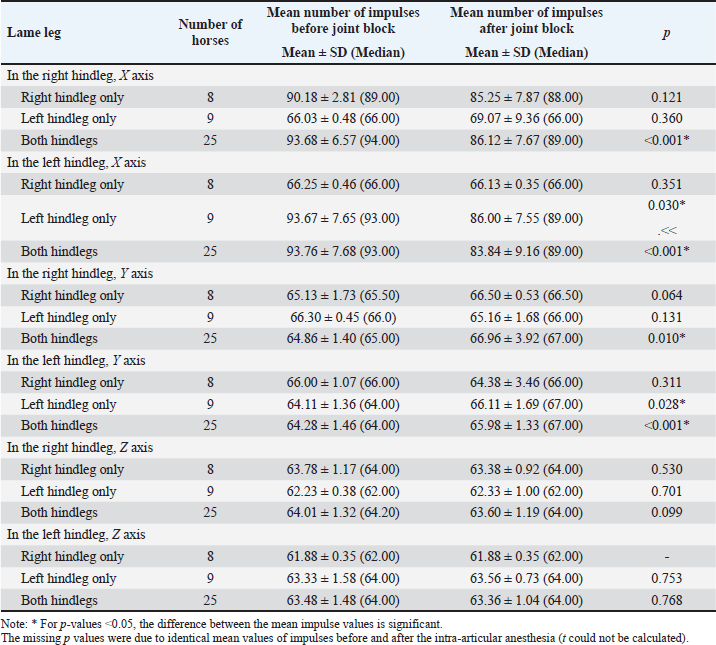
Comparisons between the mean number of impulses recorded before and after the joint block in horses with lameness of the right hindleg are presented in Table 2. The mean number of impulses on the X axis showed a significant decrease (p < 0.001) between the pre- and post-joint anesthesia values for the overall group and for horses with scores of 3/5 on the AAEP scale, a sign of gait improvement. On the Y axis, the differences were significant (p < 0.05) overall and for the horses scoring 2/5 and 3/5 on the AAEP scale, but in these instances, the number of the impulses increased as the lameness decreased in the assessed (right) leg. The Z axis showed less specificity with regard to the significance of the joint-block effect. The mean number of the recorded impulses decreased when the gait improved, showing statistical significance (p < 0.01) only for the horses with 3/5 scores on the AAEP lameness scale in their right hindleg. Table 3 shows the comparisons between the mean number of impulses recorded before and after the joint block in the horses with lameness of the left hindleg. After the joint block, horses with left hindleg lameness presented a statistically significant (p < 0.05) decrease of the mean number of impulses recorded on the X axis; this change was observed in the overall group and in the horses scoring 1/5, 3/5, and 4/5 on the AAEP lameness scale (Table 3). On the Y axis, the number of impulses increased post-anesthesia, compared with the assessment before the joint block. For the horses with a 4/5 score for left hindleg lameness the difference was significant at a p < 0.05, while for the overall group and the horses scoring 2/5, the difference was even more significant (p < 0.001). The analysis of the Z axis values showed decreased values for the mean number of impulses after joint anesthesia for the horses scoring 1/5 and 3/5 on the lameness scale but increasing values in the assessment of the horses having a score of 2/5. The p-values were highly significant (p=0.001) when the impulses were compared between pre- and post-joint anesthesia in the horses scoring 2/5 and 3/5. The standard deviations reported in Tables 2 and 3 show that the recoded values were clustered relatively tightly around the means, suggesting that the precision of the device is high. Although subjective lameness assessment is widely performed all around the world, it is increasingly considered less reliable than objective movement evaluation, especially for detecting subtle movement asymmetries; in addition, the latter method also avoids interobserver disagreement (Müller-Quirin et al., 2020). Among the kinematic methods, the IMUs appear to be a promising cost-effective, and practical solution in clinical practices. In our study, the results obtained with the Lameness detector 0.1 were in line with the lameness scores established according to the AAEP guidelines in the studied OCD patient horses. The post-joint block evolution of the values recorded with the Lameness detector 0.1 device on the X axis was also generally aligned with the changes in the AAEP lameness scores. As the first three tables show, the difference between the pre- and post-anesthetic mean number of impulses on all axes tended to be higher as the lameness grade increased, suggesting that the Lameness detector 0.1 could be more useful in assessing more severe lameness. In such cases, the subjective lameness evaluation is usually more accurate as well, even when performed by less experienced veterinarians. The mean number of impulses on the X axis of the Lameness detector 0.1 proved to be the most in line with the lameness score established using subjective evaluation. The values recorded on the Y and Z axes are important in the diagnostic too, but only in correlation with the values observed on the X axis. The mean number of the impulses recorded on each axis of the accelerometer was found to be the more certain element in establishing the lameness degree. These values and their change under the influence of intra-articular an-esthesia followed the tendency shown by the subjective gait-assessment scores. In all cases, the mean number of impulses recorded on the X axis decreased after the joint block and those on the Y axis increased (Tables 2–5). The explanation for this finding was related to the function of the accelerometer incorporated in our device. A three-axis accelerometer measures the acceleration in relation to the three Cartesian coordinate axes. These axes are linked to themselves and fixed, thus the reference system for the tool is itself, meaning that if the accelerometer is rotated, the orientation of the axes changes. Because of the position of the Lameness detector 0.1, the impulses on the X axis were recorded in the swing phase of the foot, during its forward movement. The increase recorded on the Y axis showed a longer vertical movement of the lame leg, the horses avoided the touch of the foot to the ground, and the more that the pain felt was more intense. On the Z axis, the lateral movements were recorded, and the mean number of impulses on this axis had not been consistent with the lameness degree (Tables 2 and 3). Table 2. Mean (± SD) and median values of the impulses recorded by the Lameness detector 0.1 in the right hindleg of the horses presenting lameness in that leg (N=33), assessed before and after intra-articular anesthesia.
Table 4 synthetizes the direction of variation in the mean numbers of Lameness detector 0.1 impulses on its three axes in the horses for which statistically significant differences were observed between the pre- and post-joint block gait assessments. In order to better understand the direction of these variances and the cause of the mean impulse numbers increasing after the joint-anesthesia compared with the mean numbers obtained during the pre-anesthesia assessment, we compared pre- and post-joint block values separately for horses that were lame only in their right hindleg, only in their left hindleg, or in both their hindlegs, regardless of their AAEP score (Table 5). In the horses presenting lameness exclusively in their right hindleg, the mean number of the impulses did not differ significantly when we compared the pre-joint block and the post-joint block values, on either of the axes. In the horses presenting lameness exclusively in their left hindleg, these values varied significantly (p < 0.05) after the joint block compared with their initial values on both the X and Y axes, decreasing on the first and increasing on the latter, respectively. The horses with bilateral hindleg lameness presented a similar tendency to those with left hindleg lameness, with a significant decrease of the mean number of impulses on the X axis (p < 0.001) and an increase on the Y axis (p=0.01), recorded after the joint block. Table 3. Mean (± SD) and median values of the Lameness detector 0.1 recorded impulses in the left hindleg of the horses presenting lameness in that leg (N=34), assessed before and after intra-articular anesthesia.
Table 4. Variation of the Lameness detector 0.1 impulses (mean numbers) in the horses with significant (p < 0.05) pre- and postjoint anesthesia differences.
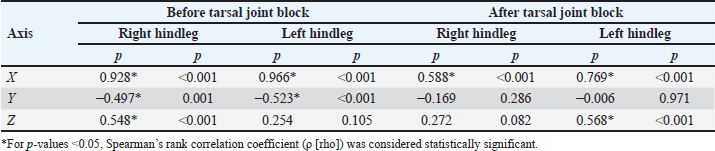
In order to explore the relationship between the mean number of impulses recorded by the Lameness detector 0.1 and the AAEP lameness score of the studied horses, correlations were calculated for each hindleg, before and after performing the intra-articular anesthesia of the tarsal joint (Table 6). Table 5. Comparisons of the mean values of the impulses recorded by the Lameness detector 0.1 on its three axes according to the lame hindleg of the assessed horses, regardless of their lameness scores, before and after intra-articular anesthesia.
Both before and after the tarsal joint anesthesia, the lameness score of the assessed horses showed a significant (p < 0.001) correlation with the mean number of impulses recorded on the X axis of the Lameness detector 0.1. On the Y axis, however, the observed negative correlation was significant (p=0.001) only for the evaluation before the joint block in both legs. For the impulses recorded on the Z axis, the correlations were positive; statistical significance (p < 0.001) was observed for the right hindleg only before and in the left hindleg only after intra-articular anesthesia. When the correlation calculation was performed taking into account the number of lame legs (right hindleg only, left hindleg only, or both hindlegs), the mean number of impulses correlated significantly with the AAEP lameness score as follows: When only the left hindleg was lame: only prior to the joint block on the X axis (ρ=0.952, p < 0.001); When both hindlegs were lame: in both legs, before the joint block on the X axis (ρ=0.910, p < 0.001 in the right hindleg and ρ=0.933, p < 0.001 in the left hindleg, respectively); only in the left hindleg after the joint block both on the X (ρ=0.533, p=0.006) and the Y axis (ρ=−0.549, p=0.005). For the horses with exclusively right hindleg lameness, no significant correlation was found for the mean number of impulses recorded by the Lameness detector 0.1 device on any of its axes with the AAEP lameness scale score, either before the joint block or after it. We hypothesize that this finding might be due to the low number of horses in this situation (N=8, the smallest group in our study) in conjunction with less severe lameness in this group compared horses with exclusively left hindleg lamenss (exclusively right hindleg lameness: 2/8 with AAEP score ≥ 3/5; exclusively left hindleg lameness: 5/9 with AAEP scores ≥3/5). Table 6. Correlation between the results obtained with the Lameness detector 0.1 (mean number of impulses) and the AAEP scores established during subjective evaluation, before and after joint block.
Among all orthopedic developmental diseases of horses, OC has the highest economic impact (Arrantes Baccarin et al., 2012), being the most prevalent cause of orthopedic impairment (Vos, 2008; Whitton, 2015). The process is triggered by early vascular damage leading to ischemic chondronecrosis and consequently disturbing the endochondral ossification (no new lesion can form after its completion), almost always at certain predilection sites within a joint (van Weeren, 2019). Even though the lesions develop focally, often more than a single joint is affected in the same animal. Thus, OC is considered today a developmental disease that occurs multifocally at specific predilection sites (Olstad et al., 2015). However, OC is a highly dynamic condition, and many of the lesions can heal as long as the extracellular matrix of the articular cartilage is still remodeling (van Weeren, 2019), with the ‘age of no return’ depending on the affected joint (Arrantes Baccarin et al., 2012) and the foal’s breed. According to Naccache et al. (2018), the most commonly affected articulations include the metacarpophalangeal or metatarsophalangeal (fetlock), tarsocrural (hock) and femoropatellar (stifle) joints, and the predilection sites are the dorsoproximal aspect of the sagittal ridge of the third metacarpal/metatarsal bone in the fetlock, the sagittal ridge of the distal tibia, the lateral and medial malleolus of the tibia, the lateral and medial trochlea and the basis of the talus in the hock, and in stifle joints the lateral and medial trochlea of the femur, and the sulcus intertrochlearis of the patella. The impact of physical factors on the occurrence of cartilage fragmentation (OCD) and other characteristic clinical signs (joint effusion with or without lameness) is in-contestable, as the typical OC/OCD patients are yearlings (at different ages according to breed) after the beginning of their training period. However, many cases fall outside this pattern, and diagnosing at any age, from young foals to horses over 10 years of age, is described (van Weeren, 2019). In our reference population of horses, the OCD lesions became clinically manifest at a later age of the patients than in many research reports, highlighting the impact of physical effort in triggering the clinical signs even later in the competitional career of the sport horse. The results of this preliminary study suggest that in horses with lameness caused by OCD, the data obtained with the Lameness detector 0.1 correlates well with the outcome of the subjective gait assessment performed by highly experienced evaluators. Considering its low cost and relative ease of use, the Lameness detector 0.1 could be useful especially for veterinarians at the beginning of their career, providing them with a method of complementing subjective gait assessment with objective data. The Lameness detector 0.1, a simple, relatively inexpensive, and easy-to-set-up quantitative lameness evaluation device, could thus be used as a screening tool at the beginning of the horse’s lameness examination, indicating if a more detailed assessment is needed. This preliminary study has a few limitations, the most important of which is the lack of comparison between the results obtained with the Lameness detector 0.1 and another, validated, objective gait assessment method. Based on the encouraging results reported here, we plan to further pursue the development of the Lameness detector 0.1 in studies that also include such comparisons. Another limitation is represented by the inclusion of measurements taken during five strides only; while in theory, a higher number of strides might be useful for decreasing inter-stride variability, we found that in the current setup, including more strides increased the chance of errors in data acquisition. In future studies, we also plan to improve data processing, allowing us to evaluate the number of strides associated with the best performance of the device. Lastly, we plan to explore the potential of using artificial intelligence to corroborate data gathered using the Lameness detector 0.1 and a video recording of the subjective lameness assessment procedure. ConclusionThe quantitative results obtained using the Lameness detector 0.1 device proved to be well correlated with lameness severity established according to AAEP guidelines in horses with tarsal OCD, with the best relevance obtained when considering the mean numbers of impulses measured on the X axis of the accelerometer. Considering its relatively low costs and ease of use, the Lameness detector 0.1 device can be used to complement subjective gait assessment and could prove especially useful for less experienced evaluators. AcknowledgmentsEditorial services were provided by Timea Kiss (c/o Cristian M. Crecan). Conflicts of interestThe authors declare no conflict of interest. FundingThis research received no external funding. Authors’ contributionsCristian Mihăiță Crecan: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Writing—Original Draft. Valeria Ciulu-Anghelescu: Conceptualization, Project Administration, Supervision, Validation, Writing—Review & Editing (Corresponding Author). Iancu Adrian Morar: Data Curation, Investigation, Resources, Visualization. Alexandru Florin Lupșan: Formal Analysis, Investigation, Software, Visualization. Mirela Alexandra Tripon: Investigation, Methodology, Writing—Original Draft. Denisa Bungărdean: Data Curation, Formal Analysis, Writing—Review & Editing. Zsofia Daradics: Data Curation, Supervision, Investigation. Cosmin Petru Peștean: Conceptualization, Investigation, Methodology, Supervision, Writing—Review & Editing. Data availabilityAll data generated or analyzed during this study are included in this published article. ReferencesAAEP. 2024. Lameness exams: evaluating the lame horse. Lexington, KY: American Association of Equine Practitioners [Internet]. Available via https://aaep.org/horsehealth/lameness-exams-evaluating-lame-horse Arrantes Baccarin, R.Y., Pereira, M.A., Roncati, N.V., Bergamaschi, R.R. and Hagen, S.C. 2012. Development of osteochondrosis in Lusitano foals: a radiographic study. Can. Vet. J. 53(10), 1079–1084 Atkins, C.A., Pond, K.R., Madsen, C.K., Moorman, V.J., Roman-Muniz, I.N., Archibeque, S.L. and Grandin, T. 2019. Sensor analysis and initial assessment of detectable first hoof contacts and last break-overs as unique signal fluctuations for equine gait analysis. Transl. Anim. Sci. 3(4), 1389–1398. Bell, R.P., Reed, S.K., Schoonover, M.J., Whitfield, C.T., Yonezawa, Y., Maki, H., Pai, P.F. and Keegan, K.G. 2016. Associations of force plate and body-mounted inertial sensor measurements for identification of hind limb lameness in horses. Am. J. Vet. Res. 77, 337–345. Bosch, S., Bragança, F.M.S., Marin-Perianu, M., Marin-Perianu, R., van der Zwaag, B.J., Voskamp, J., Back, W., van Weeren, P.R. and Havinga, P. 2018. EquiMoves: a wireless networked inertial measurement system for objective examination of horse gait. Sensors 18(3), 850. Bragança, F.M.S., Rhodin, M. and van Weeren, P.R. 2018. On the brink of daily clinical application of objective gait analysis: what evidence do we have so far from studies using an induced lameness model? Vet. J. 234, 11–23. Crecan, C.M., Morar, I.A., Lupsan, A.F., Repciuc, C.C., Rus, M.A. and Pestean, C.P. 2022. Development of a novel approach for detection of equine lameness based on inertial sensors: a preliminary study. Sensors 22(18), 7082. Crecan, C.M. and Pestean, C.P. 2023. Inertial sensor technologies—their role in equine gait analysis, a review. Sensors 23(14), 6301. Donell, J.R., Frisbie, D.D., King, M.R., Goodrich, L.R. and Haussler, K.K. 2015. Comparison of subjective lameness evaluation, force platforms and an inertial-sensor system to identify mild lameness in an equine osteoarthritis model. Vet. J. 206, 136–142. Hardeman, A.M., van Weeren, P.R., Barança, F.M.S., Warmerdam, H. and Bok, H.G.J. 2021. A first exploration of perceived pros and cons of quantitative gait analysis in equine clinical practice. BEVA Equine Vet. Educ. 34(10), e438–e444. Kane, A., Traub-Dargatz, J., Losinger, W.C. and Garber, L.P. 2000. The occurrence and causes of lameness and laminitis in the US horse population. Proc. Am. Assoc. Equine Pract. 46, 277–280 Keegan, K.G., Kramer, J., Yonezawa, Y., Maki, H., Pai, P.F., Dent, E.V., Kellerman, T.E., Wilson, D.A. and Reed, S.K. 2011. Assessment of repeatability of a wireless, inertial sensor-based lameness evaluation system for horses. Am. J. Vet. Res. 72(9), 1156–1163. Keegan, K.G., Wilson, D.A., Kramer, J., Reed, S.K., Yonezawa, Y., Maki, H., Pai, P.F. and Lopes, M.A. 2013. Comparison of a body-mounted inertial sensor system-based method with subjective evaluation for detection of lameness in horses. Am. J. Vet. Res. 74, 17–24. Leelamankong, P., Estrada, R., Mählmann, K., Rungsri, P. and Lischer, C. 2020. Agreement among equine veterinarians and between equine veterinarians and inertial sensor system during clinical examination of hindlimb lameness in horses. Equine Vet. J. 52, 326–331. McCracken, M.J., Kramer, J., Keegan, K.G., Lopes, M., Wilson, D.A., Reed, S.K., LaCarrubba, A. and Rasch, M. 2012. Comparison of an inertial sensor system of lameness quantification with subjective lameness evaluation. Equine Vet. J. 44, 652–656. Müller-Quirin, J., Dittmann, M.T., Roepstorff, C., Arpagaus, S., Latif, S.N. and Weishaupt, M.A. 2020. Riding soundness—comparison of subjective with objective lameness assessments of owner-sound horses at trot on a treadmill. J. Equine Vet. Sci. 95, 103314. Naccache, F., Metzger, J. and Distl, O. 2018. Genetic risk factors for osteochondrosis in various horse breeds. Equine Vet. J. 50(5), 556–563. Olstad, K., Ekman, S. and Carlson, C.S. 2015. An update on the pathogenesis of osteochondrosis. Vet. Pathol. 52(5), 785–802. Raspa, F., Tarantola, M., Muca, E., Bergero, D., Soglia, D., Cavallini, D., Vervuert, I., Bordin, C., De Palo, P. and Valle, E. 2022a. Does feeding management make a difference to behavioural activities and welfare of horses reared for meat production? Animals (Basel) 12(14), 1740. Raspa, F., Vervuert, I., Capucchio, M.T., Colombino, E., Bergero, D., Forte, C., Greppi, M., Cavallarin, L., Giribaldi, M., Antoniazzi, S., Cavallini, D., Valvassori, E. and Valle, E. 2022b. A high-starch vs. high-fibre diet: effects on the gut environment of the different in-testinal compartments of the horse digestive tract. BMC Vet. Res. 18, 187. Spadari, A., Gialletti, R., Gandini, M., Valle, E., Cerullo, A., Cavallini, D., Bertoletti, A., Rinnovati, R., Forni, G., Scilimati, N and, Giusto, G. 2023. Short-term survival and postoperative complications rates in horses undergoing colic surgery: a multicentre study. Animals (Basel) 13(6), 1107. van Weeren, P.R. 2019. Chapter 89—osteochondritis dissecans. In Equine surgery, 5th ed. Eds., Auer, J. and Stick, J.M. Philadelphia, PA: W.B. Saunders, pp: 1509–1528. Vos, N.J. 2008. Incidence of osteochondrosis (dissecans) in Dutch warmblood horses presented for pre-purchase examination. Ir. Vet. J. 61(1), 33–37. Whitton, C. 2015. Osteochondrosis in horses (Osteochondritis dissecans, Dyschondroplasia). In The merck veterinary manual, 20th ed. Ed., O’Neil, M.J. Rahway, NJ: Merck & Co., Inc., [Online edition]. Available via https://www.msdvetmanual.com/musculoskeletal-system/lameness-in-horses/osteochondrosis-in-horses. | ||
| How to Cite this Article |
| Pubmed Style Crecan CM, Ciulu-angelescu V, Morar IA, Lupșan AF, Tripon MA, Bungărdean D, Daradics Z, Peștean CP. Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Vet. J.. 2024; 14(11): 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 Web Style Crecan CM, Ciulu-angelescu V, Morar IA, Lupșan AF, Tripon MA, Bungărdean D, Daradics Z, Peștean CP. Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. https://www.openveterinaryjournal.com/?mno=205356 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i11.38 AMA (American Medical Association) Style Crecan CM, Ciulu-angelescu V, Morar IA, Lupșan AF, Tripon MA, Bungărdean D, Daradics Z, Peștean CP. Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Vet. J.. 2024; 14(11): 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 Vancouver/ICMJE Style Crecan CM, Ciulu-angelescu V, Morar IA, Lupșan AF, Tripon MA, Bungărdean D, Daradics Z, Peștean CP. Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Vet. J.. (2024), [cited January 24, 2026]; 14(11): 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 Harvard Style Crecan, C. M., Ciulu-angelescu, . V., Morar, . I. A., Lupșan, . A. F., Tripon, . M. A., Bungărdean, . D., Daradics, . Z. & Peștean, . C. P. (2024) Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Vet. J., 14 (11), 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 Turabian Style Crecan, Cristian Mihăiță, Valeria Ciulu-angelescu, Iancu Adrian Morar, Alexandru Florin Lupșan, Mirela Alexandra Tripon, Denisa Bungărdean, Zsofia Daradics, and Cosmin Petru Peștean. 2024. Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Veterinary Journal, 14 (11), 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 Chicago Style Crecan, Cristian Mihăiță, Valeria Ciulu-angelescu, Iancu Adrian Morar, Alexandru Florin Lupșan, Mirela Alexandra Tripon, Denisa Bungărdean, Zsofia Daradics, and Cosmin Petru Peștean. "Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study." Open Veterinary Journal 14 (2024), 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 MLA (The Modern Language Association) Style Crecan, Cristian Mihăiță, Valeria Ciulu-angelescu, Iancu Adrian Morar, Alexandru Florin Lupșan, Mirela Alexandra Tripon, Denisa Bungărdean, Zsofia Daradics, and Cosmin Petru Peștean. "Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study." Open Veterinary Journal 14.11 (2024), 3089-3099. Print. doi:10.5455/OVJ.2024.v14.i11.38 APA (American Psychological Association) Style Crecan, C. M., Ciulu-angelescu, . V., Morar, . I. A., Lupșan, . A. F., Tripon, . M. A., Bungărdean, . D., Daradics, . Z. & Peștean, . C. P. (2024) Quantitative lameness assessment in horses by using an accelerometer-based simple device: A preliminary study. Open Veterinary Journal, 14 (11), 3089-3099. doi:10.5455/OVJ.2024.v14.i11.38 |