| Case Report | ||
Open Vet. J.. 2024; 14(11): 3113-3119 Open Veterinary Journal, (2024), Vol. 14(11): 3113-3119 Case Report Ocular and perineal squamous cell carcinomas in a Holstein Friesian cowJiashi Feng1,2*, Hélène Lardé3,4, Ailbhe King3,5, Dylan Thomas3,6, Georgios Paraschou1 and Pompei Bolfa11Department of Biomedical Sciences, Ross University School of Veterinary Medicine, Basseterre, Saint Kitts and Nevis 2College of Veterinary Medicine, Kansas State University, Manhattan, KS 3Department of Clinical Sciences, Ross University School of Veterinary Medicine, Basseterre, Saint Kitts and Nevis 4Department of Veterinary Biomedical Sciences, Faculty of Veterinary Medicine, Université de Montréal, Rimouski, Canada 5Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 6School of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI *Corresponding Author: Jiashi Feng. Department of Biomedical Sciences, School of Veterinary Medicine, Ross University, Basseterre, Saint Kitts and Nevis. Email: jiashi [at] vet.k-state.edu Submitted: 01/07/2024 Accepted: 20/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
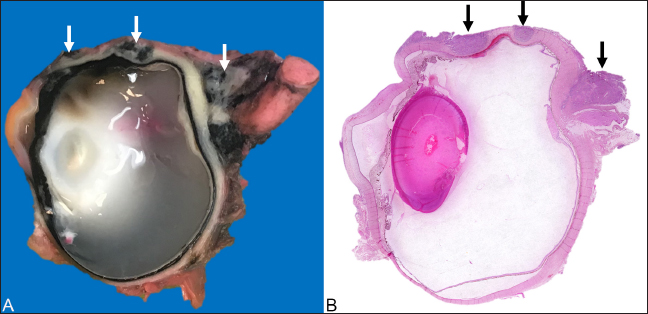
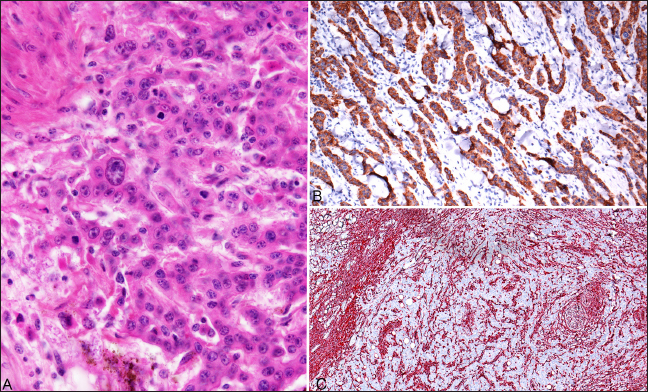
AbstractBackground: Squamous cell carcinoma (SCC) in domestic cattle is an economically significant malignant neoplasm and has been documented primarily in ocular and periocular tissues, vulva, and perineum. SCCs are often slow-growing and locally invasive, but metastasis is uncommon. Increased risk of developing SCC has been predominantly associated with high levels of sunlight exposure and hypopigmentation (skin and conjunctiva). This manuscript reports a case of ocular and perineal SCC in a Holstein Friesian cow with maxillary sinusitis secondary to tumor invasion. Case Description: An approximately 17-year-old Holstein Friesian cow was presented with a swollen, closed left eye and slow-growing perineal masses. The left eye was exenterated after showing poor response to anti-inflammatory and antimicrobial therapies, and the largest perineal mass was excised. Twelve months later, the cow developed purulent nasal discharge and dyspnea. The animal was euthanized for postmortem investigation due to health deterioration, advanced age, and suspected tumor metastasis. Conclusion: Ocular and perineal SCCs were diagnosed on biopsy and confirmed with immunohistochemistry. Postmortem gross and histological evaluations confirmed SCC invasion in the left maxillary sinus, with a secondary Trueperella pyogenes infection confirmed on aerobic culture. Histological evaluation of the enlarged lymph nodes revealed reactive lymphoid hyperplasia without evidence of tumor metastasis. Keywords: Bovine, Carcinoma, Ocular, Perineal, Immunohistochemistry. IntroductionBovine ocular Squamous cell carcinoma (SCC) is the most economically significant neoplasm of domestic cattle in North America (Russell et al., 1956; Heeney and Valli, 1985; Mauldin and Peters-Kennedy, 2016). In the US, 12.5% of all bovine carcass condemnations (Heeney and Valli, 1985) and 82% of condemnations for neoplasia (Russell et al., 1956) were caused by ocular SCC. Risk factors for ocular SCC include UV light exposure (Russell et al., 1956; Anderson and Badzioch, 1991), circumorbital hypopigmentation (Anderson, 1963; Heeney and Valli, 1985), viral infection (Dmochowski, 1967; Epstein, 1972; Ford et al., 1982; Anson et al. 1982), age (Vogt and Anderson, 1964; Heeney and Valli, 1985; Anderson and Badzioch, 1991), nutrition (Anderson et al., 1970), and genetic predisposition (Russell et al., 1976; Heeney and Valli, 1985). Out of these factors, circumorbital hypopigmentation and UV overexposure are thought to be the most significant (Tsujita and Plummer, 2010). SCC often initiates as actinic keratosis/elastosis and later progresses to carcinoma in situ and invasive carcinoma (Goldschmidt and Goldschmidt, 2016; Mauldin and Peters-Kennedy, 2016). Ocular SCC most commonly emerges on the lateral bulbar conjunctiva near the cornea, but it can also affect the lower eyelid, nictitating membrane, and medial canthus (Dubielzig, 2016). Genital and perineal SCCs in cows are relatively uncommon compared to ocular SCC (Meyers and Read, 1990; Yeruham et al., 1999; Khodakaram-Tafti et al., 2013), though they share similar risk factors (Goldschmidt and Goldschmidt, 2016; Mauldin and Peters-Kennedy, 2016). SCCs are typically slow-growing and locally invasive tumors, but metastasis is uncommon (Dubielzig, 2016; Goldschmidt and Goldschmidt, 2016; Mauldin and Peters-Kennedy, 2016). This report describes a case of ocular and perineal SCCs in a geriatric Holstein Friesian cow, and local tumor invasion in the ipsilateral maxillary sinus and secondary sinusitis after incomplete excision of the ocular SCC. Case DetailsAn approximately 17-year-old non-pregnant, non-lactating Holstein Friesian cow, from a teaching herd of Ross University School of Veterinary Medicine, was presented with swelling of both the upper and lower eyelids of the left eye, causing narrowing of the palpebral fissure. The animal lived on a pasture with uneven terrain, with other cattle of different ages, breeds, and sizes. The teaching cattle are fed a diet of primarily local fresh cut guinea grass and a 16% dairy cow ration. The animals in the teaching herd were visually assessed daily, and the narrowing of the palpebral fissure in this cow appeared within a few days before the initial presentation. The affected cow also had a history of slow-growing perineal masses, expanding to the ventral anus and causing perineal deformation. On initial examination of the affected eye, no corneal ulcer was detected on the fluorescein staining test, but the narrowed palpebral fissure and blepharospasm prevented a thorough visual evaluation of ocular structures. Ultrasonography was performed to assess the ocular structures and orbit. The eyelids and tissues in the retrobulbar space appeared hyperechoic and heterogenous; no foreign body nor fracture was detected, but the surface of the orbital bones appeared irregular. All other health parameters, including respiratory rate, heart rate, temperature, rumen contraction rate, appetite, and fecal consistency, were within normal limits during the physical exam. Differential diagnoses at the time included neoplastic infiltration or inflammation secondary to trauma or infection. Based on the clinical suspicion of panophthalmitis, the cow was treated with flunixin meglumine (1.1 mg/kg, IV, q 24 hours for 3 days), oxytetracycline dihydrate (10 mg/kg, IM, q 24 hours for 4 days) and neomycin sulfate, polymyxin B sulfate, and bacitracin zinc triple antibiotic ophthalmic ointment, USP (0.25-inch strip on left eye, q 8 hours for 7 days). The swelling of the left eye deteriorated despite the medical treatment. As a result, exenteration of the left eye was performed using a transpalpebral approach, removing the eyelids, globe, and retrobulbar soft tissues including surrounding muscles, orbital nerve, and fat pad. Excised tissues were submitted in 10% neutral buffered formalin and processed routinely for histopathological evaluation. Macroscopic and microscopic evaluations of the left globe revealed a multifocally invasive, poorly demarcated neoplasm within the bulbar conjunctiva, sclera, limbus, eyelids, and third eyelid, and invading adjacent retrobulbar skeletal muscles and connective tissue but sparing the nerve fibers (Fig. 1A and B). The neoplastic cells were poorly differentiated, showing pleomorphism and a high nucleus-to-cytoplasm ratio, and occasional atypical mitotic figures (Fig. 2A). Some neoplastic cells appeared to contain intracytoplasmic melanin granules, and melanin was also be seen within macrophages and freely in the background. Surgical margins at different sites were not free of neoplastic cells. Considering the poor cell differentiation, presence of intracytoplasmic melanin granules, and location of the tumor, several differentials were suspected, including carcinoma, melanoma, and rhabdomyosarcoma. Selected sections, including a partial globe, haired skin of eyelid, and bulbar conjunctiva, were sent to University of Guelph Animal Health Laboratory for immunolabeling for pancytokeratin, vimentin, Melan A, and desmin, to investigate the cell origin of the neoplasm. The neoplastic populations on all three sections were only positively stained for pancytokeratin (Fig. 2B), and the supporting stroma of the neoplasm stained positively for vimentin (Fig. 2C). This staining pattern was compatible with an infiltrating neoplasm of epithelial origin and a desmoplastic reaction to the tumor. Immunolabeling for pancytokeratin, Melan A, vimentin, and desmin at University of Guelph were validated in canine tissues. Internal control was used to confirm cross-reactivity of the antibody with the bovine version of each antigen.
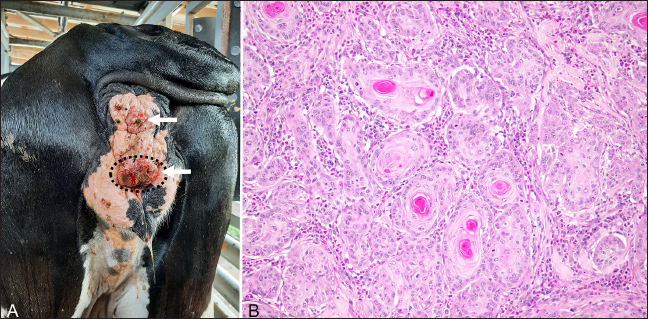
Fig. 1. Ocular squamous cell carcinoma from a 17-year-old Holstein cow with a concurrent perineal squamous cell carcinoma. (A): Photograph of a cross-section of the left eye after exenteration and formalin fixation. The poorly demarcated masses (white arrowheads) multifocally invaded corneoscleral junction, sclera, and retrobulbar skeletal muscle and soft tissues. (B): Photomicrograph of the same section shows the poorly demarcated masses (black arrows) on HandE stain. The largest perineal mass later became ulcerated and was excised (Fig. 3A) approximately 7 months after the left eye exenteration and submitted in 10% neutral buffered formalin and processed routinely for histopathological evaluations. Microscopic evaluation of the mass revealed islands and cords of well-differentiated neoplastic epithelial cells invading the dermis and subcutis. Within the neoplasm, abundant lamellated eosinophilic material was multifocally surrounded by concentric layers of epithelial cells, representing the formation of distinct keratin “pearls” associated with well-differentiated squamous cell carcinoma (SCC) (Fig. 3B). Approximately 12 months after the exenteration, the animal was presented again for copious green discharge with a putrid odor exuding from the left nostril, blocking airflow, and causing tachypnea and dyspnea. At the time, multiple lymph nodes, including left mandibular and prescapular lymph nodes, were enlarged on palpation. A fine-needle aspiration was performed on the enlarged left prescapular lymph node. The cytological evaluation demonstrated a heterogeneous population of lymphocytes and a variable number of plasma cells and macrophages, which was interpreted as reactive lymphoid hyperplasia, though tumor metastasis could not be excluded. Due to the deterioration of the health status (decreased appetite, decreased airflow, and dyspnea with putrid nasal discharge), the advanced age of the animal, and multiple suspected comorbidities (including multiple enlarged lymph nodes with suspected tumor metastasis), euthanasia was elected.
Fig. 2. Photomicrographs of an ocular squamous cell carcinoma from a 17-year-old Holstein cow with a concurrent perineal squamous cell carcinoma. (A): Neoplastic cells within sclera of the left eye were anaplastic and pleomorphic (HE). (B): Immunohistochemistry for pancytokeratin on the ocular neoplasm shows strong immunolabeling of the epithelial component of the neoplasm (IHC) (C): Immunohistochemistry for vimentin shows immunolabeling only of the intervening stromal component surrounding the pancytokeratin-positive cords of infiltrative neoplasm, consistent with a desmoplastic response to the neoplasm (IHC).
Fig. 3. Perineal squamous cell carcinoma (white arrows) from a 17-year-old Holstein cow with a previously diagnosed ocular squamous cell carcinoma. (A): Photograph of the perineal masses. The ulcerated mass (dash-line circle) was surgically excised and submitted for histopathology. (B): Histopathology revealed poorly demarcated infiltrated neoplasm composed of polygonal cells arranged in cords, trabeculae, and islands. There was moderate parakeratotic and orthokeratotic hyperkeratosis, formation of keratin ‘pearls’, consistent with a well-differentiated squamous cell carcinoma (HE). On postmortem examination, the cow was well-conditioned with a body condition score of 4/5 and weighed approximately 700 kg. The left nasal cavity contained dark green, malodorous purulent material, and the mucosa of the left maxillary sinus was expanded by an irregular, dark red, firm, ulcerated mass partially covered by fibrin strands. The perineum contained large, firm, multifocal, dark-red irregular masses, up to 2-cm in diameter. Multiple lymph nodes, including mandibular, prescapular, inguinal, and medial iliac, appeared firm and enlarged on palpation, and contained multifocal white nodules on cross sections. Tissue sections were collected from left maxillary sinus, lymph nodes (mandibular, prescapular, medial iliac, and inguinal), and representative visceral organs were fixed in neutral-buffered 10% formalin and processed routinely for histological examinations. On microscopic evaluation, the ulcerative mass from the left maxillary sinus was an infiltrative neoplasm that shared similar histological features to the ocular neoplasm, and there were multifocal areas of bone remodeling, mucosa necrosis and ulceration with neutrophilic inflammation and numerous coccobacilli. Anaerobic culture of the purulent material collected from the left maxillary sinus demonstrated the growth of Trueperella pyogenes. The lymph nodes collected contained abundant subcapsular macrophages and plasma cells and multifocal areas of hemorrhages in both the subcapsular space and medulla. No evidence of lymphatic or lymphovascular invasion of neoplastic cells was observed in the sections collected. The final diagnoses were ocular SCC with invasion to the left maxillary sinus, secondary necro-ulcerative sinusitis, and perineal SCC. DiscussionIn the present case, the animal was an approximately 17-year-old Holstein cow diagnosed with ocular and perineal SCCs, treated with exenteration of the affected eye, and survived for more than 12 months after the diagnosis. The animal lived on a tropical island and had dark pigmentation around the affected left eye and lacked pigmentation around the perineum. This cow possessed multiple risk factors for developing SCC, including advanced age, prolonged and intense solar exposure, and perineal hypopigmentation. Due to the presence of SCCs at two distant locations, metastasis or a common virus-induced origin was initially suspected. It has been reported that SCC in the eyelids shows a higher frequency of metastases than tumors of the cornea and limbus (Tsujita and Plummer, 2010). In this case, metastasis was not detected, and enlarged regional lymph nodes were found to be reactive with no evidence of tumor cells. Previous studies (Dmochowski 1967; Epstein 1972; Ford et al., 1982; Anson et al. 1982; Rutten et al., 1992) suggested the involvement of bovine papillomaviruses (BPV) and bovine herpesviruses (BHV) in the initial neoplastic transformation of bovine ocular and genital SCCs, and not necessarily the tumor maintenance. In women, human papillomaviruses (HPVs) are found to cause cervical cancer (Bosch et al., 2002) and are strongly associated with vaginal SCC (Fuste et al., 2010). In contrast, malignant transformation in bovine due to BPVs are traditionally associated with urinary bladder and upper alimentary neoplasia (Borzacchiello et al., 2003; Munday, 2014). Several herpesviruses have also demonstrated association with urogenital carcinoma in their natural hosts, such as human and sea lions (Kaufman et al., 1981; Gulland et al., 2020). To further investigate the possibility of a viral etiology in the present case, glass slides containing tissues from ocular and perineal masses were submitted to the University of Guelph Animal Health Laboratory for detection of BPVs, and the nuclear immunolabeling was negative in both sections. However, negative immunolabeling of BPVs could not rule out the possibility of BPV’s involvement. Although IHC is a useful method for detecting viral antigens in fixed tissues, false negatives can occur due to sampling. Additionally, only 13 BPVs have been characterized, while over 170 types of HPVs have been fully characterized (Rector and Van Ranst, 2013; Munday, 2014). It is likely that many BPV types have not been identified. BHV could also play a role in the etiopathogenesis of bovine SCC. A previous study (Anson et al., 1982) demonstrated the detection of BHV-5 in bovine ocular SCC tumor cells and that exposing cells derived from these tumor cells to UV light and high pH media could induce viral replication and increase the number of BHV-5 viral antigens detected by indirect immunofluorescence. These results could suggest an association between BHV infection and bovine ocular SCC since chronic UV exposure has been associated with an increased incidence of bovine ocular SCC. Mutations of tumor suppressor genes, including p53 and p16, are thought to be involved in the development of human head and neck cancers, especially SCC, and the aberration of these tumor suppression genes can be caused by somatic mutations induced by UV radiation and through inactivation by viral oncogenes such as HPVs E6/E7 (Leemans et al., 2011). These tumor suppressor genes also showed prognostic potential in human medicine. Human oropharyngeal SCC patients with p16 expressing tumors showed significantly better outcomes than those with p16 non-expressing tumors (Sedghizadeh et al. 2016), while current evidence about the prognostic value of p53 in human patients with head and neck SCC has been inconclusive (Tandon et al., 2010). Immunoexpressions of p53 and p16 have also been detected in bovine ocular SCCs. Immunodetection of p53 was reported in 30% (n=10) and 67% (n=15) of the cases (Carvalho et al 2005; Fornazari et al. 2017), and immunodetection of p16 was reported in 30% (n=10) of the cases (Fornazari et al. 2017). These results support the roles of p16 and p53 in the development of bovine ocular SCC. Further studies are needed to investigate the significance of immunoexpression of these tumor suppressor genes in bovine SCC and their potential prognostic value in this species. Trueperella pyogenes are Gram-positive, facultatively anaerobic, pleomorphic rod or coccoid bacteria, and a part of the normal microbiota of mucosal membranes of respiratory, gastrointestinal, and urogenital tracts of cattle (Rzewuska et al., 2019). This bacterium can be an opportunistic pathogen in cattle causing purulent infection, and stress or trauma often precipitates the infection (Narayanan et al., 1998; Rzewuska et al., 2019). Maxillary sinusitis in dairy cattle is often associated with infected maxillary tooth roots (Divers, 2018). Considering the anatomical proximity between the orbital cavity and the maxillary sinus, tumor invasion into the sinuses is a logical progression of ocular SCC. In the present case, the sinusitis was preceded by mucosal erosion from the ocular SCC invasion. Therefore, neoplastic conditions should be considered in unilateral maxillary sinusitis without evidence of dental diseases. Although multiple modalities exist for treating bovine ocular SCCs, enucleation or exenteration surgery remains the most used treatment with a considerable success rate (Schulz and Anderson, 2010; Gelatt, 2013). The most common post-operative complications reported are infection and tumor recurrence (Schulz and Anderson, 2010). To conclude, the present report describes a unique case of two primary SCCs, ocular and perineal, occurring on the same geriatric Holstein cow living in low latitude. The investigation of a potential viral etiology for the two tumors was inconclusive. The remnant of the incompletely excised ocular SCC regrew and invaded the left maxillary sinus causing mucosa erosion, and the animal subsequently developed sinusitis from opportunistic T. pyogenes infection. Tumor invasion should be considered when a bovine with previously documented ocular SCC developed unilateral sinusitis. Further work is needed to expand the current taxonomy of BPV and investigate the potential of viral oncogenesis in bovine SCC. AcknowledgmentThe authors thank the Ross University School of Veterinary Medicine Pathology Team (Candita Chapman and Maurice Matthew) for the assistance with the autopsy and sample collection, David Hilchie, our histology technician, for the processing of histology samples, Dr. Regina Kemper for compiling the autopsy report, Netanya Greene, our laboratory technician, for the microbiology preparation, Dr. Flavio H. Alonso for the cytological evaluation, and University of Guelph Animal Health Laboratory for the immunohistochemistry preparation. Finally, the authors thank the Animal Resources team and students of RUSVM Bovine Club (AABP) for their excellent care to cow Y49 during her lifetime as a teaching animal. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis project received no specific funding and falls under RUSVM’s Center for Integrative Mammalian Research. Authors’ contributionsJF assisted autopsy, reviewed literature, and drafted manuscript with input from all authors. PB and HL supervised the writing of the case report. PB performed pathological examination for the specimen obtained at surgery, and diagnosis. HL, AK, and DT performed medical therapy, surgical and diagnostic procedures on the live animal, provided detailed medical record, and assisted autopsy. GP performed autopsy, pathological examination for the specimen obtained at autopsy, and diagnosis. All authors have read and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. ReferencesAnderson, D.E. 1963. Genetic aspects of cancer with special reference to cancer of the eye in the bovine. Ann. N.Y. Acad. Sci. 108(3), 948–962. Anderson, D.E. and Badzioch, M. 1991. Association between solar radiation and ocular squamous cell carcinoma in cattle. Am. J. Vet. Res. 52(5), 784–788. Anderson, D.E., Pope, L.S. and Stephens, D. 1970. Nutrition and eye cancer in cattle. J. Natl. Cancer Inst. 45(4), 697–707. Anson, M.A., Benfield, D.A. and McAdaragh, J.P. 1982. Bovine herpesvirus-5 (DN599) antigens in cells derived from bovine ocular squamous cell carcinoma. Can. J. Comp. Med. 46(3), 334–337. Borzacchiello, G., Iovane, G., Marcante, M.L., Poggiali, F., Roperto, F., Roperto, S. and Venuti, A. 2003. Presence of bovine papillomavirus type 2 DNA and expression of the viral oncoprotein E5 in naturally occurring urinary bladder tumours in cows. J. Gen.Virol. 84(11), 2921–2926. Bosch, F.X., Lorincz, A., Muñoz, N., Meijer, C.J.L.M. and Shah, K.V. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55(4), 244–265. Carvalho, T., Vala, H., Pinto, C., Pinho, M. and Peleteiro, M.C. 2005. Immunohistochemical Studies of Epithelial Cell Proliferation and p53 Mutation in Bovine Ocular Squamous Cell Carcinoma. Vet. Pathol. 42(1), 66–73. Divers, T.J. 2018. Ocular diseases. In Rebhun’s Diseases of Dairy Cattle. Eds., Divers T.J. and Peek S.F. W.B. Saunders, St. Louis, United States, pp: 668–712. Dmochowski L. 1967. Electron microscope studies of the replication of a virus isolated from bovine cancer eye lesions. Proc. Am. Assoc. Cancer Res. 8, 14. Dubielzig, R.R. 2016. Tumors of the eye. In tumors in domestic animals. Ed., Meuten, D.J. Hoboken, NJ: John Wiley and Sons, Ltd., pp: 892–922 Epstein B. 1972 Isolation of bovine rhinotracheitis virus from ocular squamous cell carcinomas of cattle. Rev. Med. Vet. 53, 105–110. Ford, J.N, Jennings, P.A., Spradbrow, P.B. and Francis, J. 1982. Evidence for papillomaviruses in ocular lesions in cattle. Res. Vet. Sci. 32(2), 257–259. Fornazari, G.A., Kravetz, J., Kiupel, M., Sledge, D., Filho, I.R.D.B. and Montiani-Ferreira, F. 2017. Ocular squamous cell carcinoma in Holstein cows from the South of Brazil. Vet. World 10(12), 1413–1420. Fuste, V., del Pino, M., Perez, A., Garcia, A., Torne, A., Pahisa, J. and Ordi, J. 2010. Primary squamous cell carcinoma of the vagina: human papillomavirus detection, p16INK4A overexpression and clinicopathological correlations. Histopathol. 57(6), 907–916. Goldschmidt, M.H. and Goldschmidt, K.H. 2016. Epithelial and melanocytic tumors of the skin. In Tumors in Domestic Animals., Eds., Meuten, D.J. Hoboken, NJ: John Wiley and Sons, Ltd., pp: 88–141 Gulland, F.M.D., Hall, A.J., Ylitalo, G.M., Colegrove, K.M., Norris, T., Duignan, P.J., Halaska, B., Acevedo Whitehouse, K., Lowenstine, L.J., Deming, A.C. and Rowles, T.K. 2020. Persistent contaminants and herpesvirus OtHV1 are positively associated with cancer in wild california sea lions (Zalophus californianus). Front. Mar Sci. 7, 602565. Heeney, J.L. and Valli, V.E.O. 1985. Bovine ocular squamous cell carcinoma: an epidemiological perspective. Can. J. Comp. Med. 49, 21–26. Kaufman, R.H., Dreesman, G.R., Burek, J., Korhonen, M.O., Matson, D.O., Melnick, J.L., Powell, K.L., Purifoy, D.J.M., Courtney, R.J. and Adam, E. 1981. Herpesvirus-induced antigens in squamous-cell carcinoma in Situ of the Vulva. N. Engl. J. Med. 305(9), 483–488. Khodakaram-Tafti, A., Motaghypisheh, M. and Shirian, S. 2013. Pathological study of naturally occurring vulvar and vaginal squamous cell carcinoma (SCC) in cattle. Comp. Clin. Pathol. 22(4), 713–716. Leemans, C.R., Braakhuis, B.J.M. and Brakenhoff, R.H. 2011. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 11(1), 9–22. Mauldin, E.A. and Peters-Kennedy, J. 2016. Integumentary system. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals: Volume 1, Ed. Maxie M. G. W.B. Saunders, pp: 509–736. Meyers, S.A. and Read, W.K. 1990. Squamous cell carcinoma of the vulva in a cow. J. Am. Vet. Med. Assoc. 196(10), 1644–1646. Munday, J.S. 2014. Bovine and human papillomaviruses: a comparative review. Vet. Pathol. 51(6), 1063–1075. Narayanan, S., Nagaraja, T.G., Wallace, N., Staats, J., Chengappa, M.M. and Oberst, R.D. 1998. Biochemical and ribotypic comparison of Actinomyces pyogenes and A pyogenes-like organisms from liver abscesses, ruminal wall, and ruminal contents of cattle. Am. J. Vet. Res. 59(3), 271–276. Rector, A. and Van Ranst, M. 2013. Animal papillomaviruses. Virol. 445(1), 213–223. Russell, W C., Brinks, J.S. and Kainer, R.A. 1976. Incidence and heritability of ocular squamous cell tumors in hereford cattle. J Anim. Sci. 43(6), 1156–1162. Russell, W.O., Wynne, E.S., Loquvam, G.S. and Mehl, D.A. 1956. Studies on bovine ocular squamous carcinoma (“cancer eye”). I. Pathological anatomy and historical review. Cancer. 9(1), 1–52. Rutten, V.P., Klein, W.R., De Jong, M.A., Quint, W., Den Otter, W., Ruitenberg, E.J. and Melchers, W.J. 1992. Search for bovine papilloma virus DNA in bovine ocular squamous cell carcinomas (BOSCC) and BOSCC-derived cell lines. Am. J. Vet. Res. 53(9), 1477–1481. Rzewuska, M., Kwiecień, E., Chrobak-Chmiel, D., Kizerwetter-Świda, M., Stefańska, I. and Gieryńska, M. 2019. Pathogenicity and virulence of Trueperella pyogenes: a review. Int. J. Mol. Sci. 20(11), 2737. Schulz, K.L. and Anderson, D.E. 2010. Bovine enucleation: a retrospective study of 53 cases (1998–2006). Can. Vet. J. 51(6), 611–614. Sedghizadeh, P.P., Billington, W.D., Paxton, D., Ebeed, R., Mahabady, S., Clark, G.T. and Enciso, R. 2016. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? a systematic review and meta-analysis. Oral Oncol. 54, 15–27. Tandon, S., Tudur-Smith, C., Riley, R.D., Boyd, M.T. and Jones, T.M. 2010. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol. Biomark. Prev. 19(2), 574–587. Tsujita, H. and Plummer, C.E. 2010. Bovine ocular squamous cell carcinoma. Vet. Clin. North Am. Food Anim. Pract. 26(3), 511–529. Vogt, D.W. and Anderson, D.F. 1964. Studies on bovine ocular squamous carcinoma (“cancer eye”): XV. Heritability of Susceptibility. J. Hered. 55(3), 133–135. Yeruham, I., Perl, S., Orgad, U. and Yakobson, B. 1999. Tumours of the Vulva and Vagina in cattle—a 10-year survey. Vet. J. 158(3), 237–239. | ||
| How to Cite this Article |
| Pubmed Style Feng J, Lardé H, King A, Thomas D, Paraschou G, Bolfa P. Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Vet. J.. 2024; 14(11): 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 Web Style Feng J, Lardé H, King A, Thomas D, Paraschou G, Bolfa P. Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. https://www.openveterinaryjournal.com/?mno=205901 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i11.41 AMA (American Medical Association) Style Feng J, Lardé H, King A, Thomas D, Paraschou G, Bolfa P. Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Vet. J.. 2024; 14(11): 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 Vancouver/ICMJE Style Feng J, Lardé H, King A, Thomas D, Paraschou G, Bolfa P. Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Vet. J.. (2024), [cited January 24, 2026]; 14(11): 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 Harvard Style Feng, J., Lardé, . H., King, . A., Thomas, . D., Paraschou, . G. & Bolfa, . P. (2024) Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Vet. J., 14 (11), 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 Turabian Style Feng, Jiashi, Hélène Lardé, Ailbhe King, Dylan Thomas, Georgios Paraschou, and Pompei Bolfa. 2024. Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Veterinary Journal, 14 (11), 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 Chicago Style Feng, Jiashi, Hélène Lardé, Ailbhe King, Dylan Thomas, Georgios Paraschou, and Pompei Bolfa. "Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow." Open Veterinary Journal 14 (2024), 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 MLA (The Modern Language Association) Style Feng, Jiashi, Hélène Lardé, Ailbhe King, Dylan Thomas, Georgios Paraschou, and Pompei Bolfa. "Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow." Open Veterinary Journal 14.11 (2024), 3113-3119. Print. doi:10.5455/OVJ.2024.v14.i11.41 APA (American Psychological Association) Style Feng, J., Lardé, . H., King, . A., Thomas, . D., Paraschou, . G. & Bolfa, . P. (2024) Ocular and perineal squamous cell carcinomas in a Holstein Friesian cow. Open Veterinary Journal, 14 (11), 3113-3119. doi:10.5455/OVJ.2024.v14.i11.41 |