| Case Report | ||
Open Vet. J.. 2024; 14(10): 2700-2706 Open Veterinary Journal, (2024), Vol. 14(10): 2700–2706 Case Report Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcomaMichihito Tagawa*, Takamasa Itoi, Ryohei Yoshitake, Kenji Kutara, Natsuki Akashi, Ikki Mitsui and Yasuhiko OkamuraFaculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan *Corresponding Author: Michihito Tagawa. Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan. Email: m-tagawa [at] ous.ac.jp Submitted: 24/06/2024 Accepted: 17/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
AbstractBackground: Intracranial histiocytic sarcoma (HS) is uncommon in dogs, and no standard treatment has yet been defined for this disease. Herein, we describe a case of intracranial HS that responded favorably to nimustine treatment. Case Description: A 9-year-old, castrated Welsh Corgi Pembroke presented with a 2-month history of quadriplegia. Intracranial disease was suspected on neurological examination, and magnetic resonance imaging (MRI) revealed a contrast-enhanced mass in the left frontal lobe. Following tissue biopsy, the patient received intravenous nimustine (ACNU) treatment. The patient's neurological symptoms partially improved, and a reduction in tumor volume was observed on MRI on day 99. After three administrations of ACNU, tumor regrowth was confirmed on day 124, and temozolomide was subsequently administered. The patient subsequently showed no major changes in clinical symptoms, but subsequently died suddenly on day 195. Conclusion: In this case, administration of ACNU provided temporary symptom improvement and tumor reduction. Therefore, ACNU monotherapy may be a therapeutic option for canine intracranial HS. Keywords: ACNU, Canine, Chemotherapy, Magnetic resonance imaging. IntroductionHistiocytic sarcoma (HS) is an uncommon malignant tumor in dogs that arises from interstitial dendritic cells in the joints, lungs, liver, and spleen (Moore, 2014). Although central nervous system involvement in HS has been reported, most cases are of the disseminated type, while the primary intracranial form is rare (Mariani et al., 2015). A combination of surgery, radiation, and chemotherapy has been reported as a useful treatment of HS. However, a standard treatment for intracerebral HS remains poorly described. Systemic therapy with lomustine (CCNU) is the standard treatment for canine HS as an adjuvant or for gross lesions (Skorupski et al., 2007). Nimustine (ACNU) is a nitrosourea alkylating agent that belongs to the same class as CCNU and is widely used to treat both human and canine neurological tumors, such as gliomas, in Japan due to its ability to cross the blood-brain barrier (Wakabayashi et al., 2001; Takahashi et al., 2023). However, there few reports on the use of ACNU for the treatment of HS are rare; in particular, the use of ACNU for intracranial HS has been extremely limited (Takahashi et al., 2014a,b; Asada et al., 2019). Tani et al. (2020) reported the effectiveness of ACNU for the treatment of 11 cases of HS, of which only two cases had intracranial HS, and both cases had no gross lesions (Tani et al., 2020). This case report describes the clinical course and imaging findings of a dog with macroscopic intracranial HS, in which ACNU was administered, and a temporary therapeutic effect was observed. Case DetailsA 9-year-old castrated Welsh Corgi Pembroke weighing 11.5 kg was referred to the Veterinary Teaching Hospital of Okayama University of Science due to quadriplegia, difficulty in urination, and increased aggression lasting for 2 months. Neurological examination on day 1 revealed a decreased postural response in the right front and rear limbs and visual impairment in the right eye. No significant abnormalities were found on hematological analysis, and serum biochemical analysis revealed high levels of alkaline phosphatase (919 IU/l; reference range, 47–254 IU/l) and lipase (221 IU/l; reference range, 10–160 IU/l). Radiography of the thorax and abdominal ultrasonography findings were unremarkable. As intracranial disease was suspected in the present case, magnetic resonance imaging (MRI) using a 1.5 Tesla superconducting unit (Vantage Elan, Canon Medical Systems, Otawara, Japan) was performed as further investigation on day 5. MRI revealed a solid mass in the left frontal lobe that the lesion was 32.4 × 29.9 × 27.7 (length × width × height) mm in size showing homogeneous contrast enhancement that shifted the brain midline to the right in contrast enhancement T1-weighted image (T1WI) (Fig. 1A–C). Continuity with the meninges was observed at the tumor margin on contrast-enhanced T1WI. High-signal intensity findings in the brain parenchyma around the tumor indicate edema and/or inflammation on T2-weighted imaging (T2WI) and fluid-attenuated inversion recovery (FLAIR) images. Cerebrospinal fluid was not collected because increased intracranial pressure was suspected based on the MRI findings of transtentorial herniation. MRI findings indicated the possibility of meningioma or HS, and the owner requested surgical volume reduction and medical treatment with anticancer drugs. Radiotherapy was refused because of the cost, and prednisolone (PREDONINE tablets 5 mg; Shionogi & Co, Osaka, Japan) was prescribed at 1 mg/kg once a day until the operation. On day 26, an MRI examination was performed again, revealing that the lesion had grown to 34.7 × 29.9 × 34.9 mm (Fig. 1D–F). The next day, a surgical biopsy due to transfrontal craniotomy was performed to reduce intracranial pressure and confirm the diagnosis. The dog was premedicated with maropitant (1 mg/kg; Cerenia, Zoetis Japan, Tokyo, Japan), fentanyl (3 μg/kg; Fentanyl Injection 0.5 mg, Terumo Corporation, Tokyo, Japan), and lidocaine (2 mg/kg; Lidocaine Intravenous Injection 2%, Terumo corporation), all administered intravenously (IV). General anesthesia was induced with propofol (Propofol Intravenous Injection 1%, Maruishi Pharmaceutical, Osaka, Japan) to facilitate IV until intubation could be performed, following which propofol-based total intravenous anesthesia accompanied by constant rate infusions of fentanyl (10 to 15 μg/kg/hr) and medetomidine (1 to 3 μg/kg/hr; Dorbene, Kyoritsu Seiyaku Corporation, Tokyo, Japan) was administered to maintain anesthesia. Methylprednisolone sodium succinate (10 mg/kg; Solu-Medrol, Pfizer Japan, Tokyo, Japan) and fructose-supplemented glycerol (GLYCEOL for I.V. Infusion, TAIYO Pharma, Tokyo, Japan) were administered IV for 30 minutes to ensure neuroprotection and intracranial pressure reduction, respectively, during the intraoperative period. Although the tumor was visible on craniotomy, it bled easily, and only a small amount of tissue was collected. MRI was performed after surgery, but no significant changes were observed in the size of the tumor (Fig. 2). After the procedures, the patient was allowed to recover from anesthesia and emerged uneventfully 1 hour after extubation. Postoperative management comprising a crystalloid fluid infusion, prednisolone (2 mg/kg once a day), and zonisamide (Epiless tablet, Kyoritsu Seiyaku Corporation, Tokyo, Japan) at 3 mg/kg twice daily was additionally prescribed.
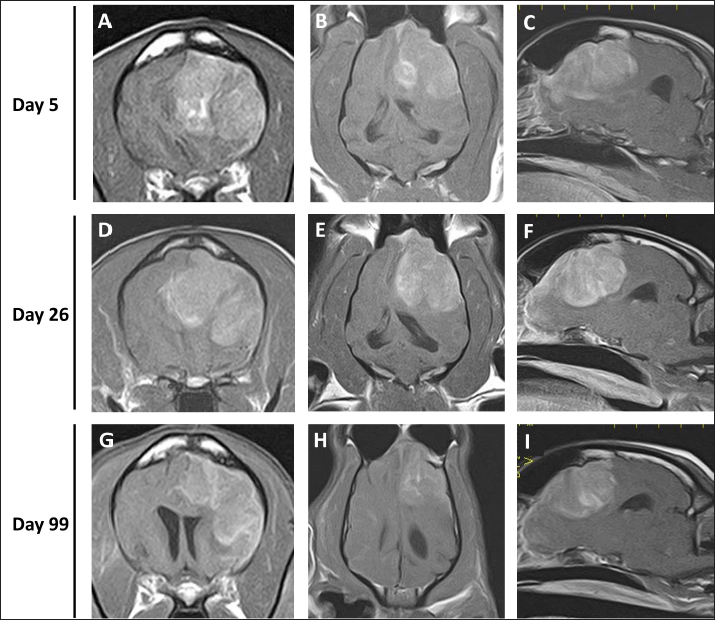
Fig. 1. Postcontrast transverse (A, D, G), dorsal (B, E, H), and sagittal (C, F, I) T1WI of the case. Each image revealed a solid mass in the left frontal lobe. Histopathological examination of the biopsied tissue was performed by a board-certified veterinary pathologist [I.M. (ACVP)] and revealed a dense sheet of slightly atypical neoplastic round cells resembling monocytes/histiocytes/macrophages. The tumor cells had abundant, pale, lacy cytoplasm, round to ovoid hypochromic nuclei with moderate anisokaryosis, and one or 2 nucleoli. The mitotic count was 2 in a 2.37 mm2 area. Some tumor cells have few or large nuclei. Rare tumor cells engulfed lipofuscin-like, pale yellow, homogeneous material. The background of the tumor cells contained a small amount of fibrovascular tissue and a small number of lymphocytes, plasma cells, and neutrophils. Ziel-Neelsen staining did not detect any acid-fast bacteria in the cytoplasm of the tumor cells. Immunohistochemistry of the tumor cells revealed membranous and cytoplasmic positive reactions to Iba-1 antibody (marker for microglia) and cytoplasmic positive reactions to the vimentin antibody (marker for mesenchymal cells). The final diagnosis of cerebral HS was made based on histomorphological and immunohistochemical findings (Fig. 3). The patient was ultimately discharged from the hospital on day 37 owing to improvement in general condition, with continued administration of prednisolone and zonisamide. On day 43, the patient returned to the hospital after several days of dysfunctional anorexia. The patient was somnolent and in lateral recumbency, and intravenous glycerol was administered because of increased intracranial pressure. On the same day, ACNU (NIDRAN for injection, Alfresa Pharma, Osaka, Japan) was administered IV at 25 mg/m2, and a 70% yield of isosorbide (ISOBIDE SYRUP 70%) at 10 ml twice daily was additionally prescribed. One week after administration of the 1st ACNU dose, the patient was able to stand with mild paresis, and the general condition of the dog improved. No adverse effects were observed, except for severe neutropenia (160 cells/µl). However, the patient developed pancreatitis on day 62, which improved after 8 days of hospitalization. After hospital discharge, the prednisolone dose was reduced to 0.5 mg/kg and continued. On day 82, a second dose of ACNU (20 mg/m2) was administered.
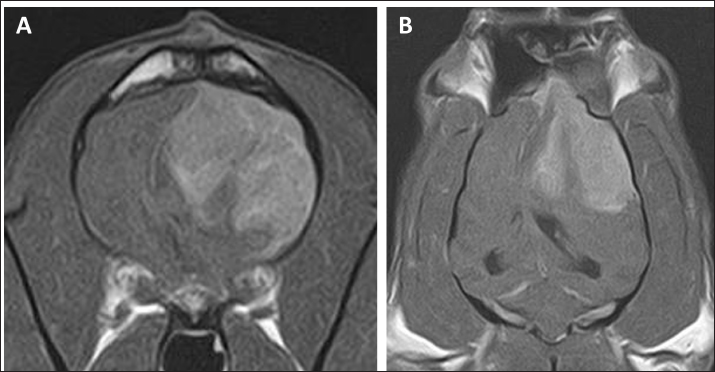
Fig. 2. Postcontrast transverse (A) and dorsal (B) T1WI of the case on day 27. No major changes in the size of the contrastenhanced tumor after surgery were observed.
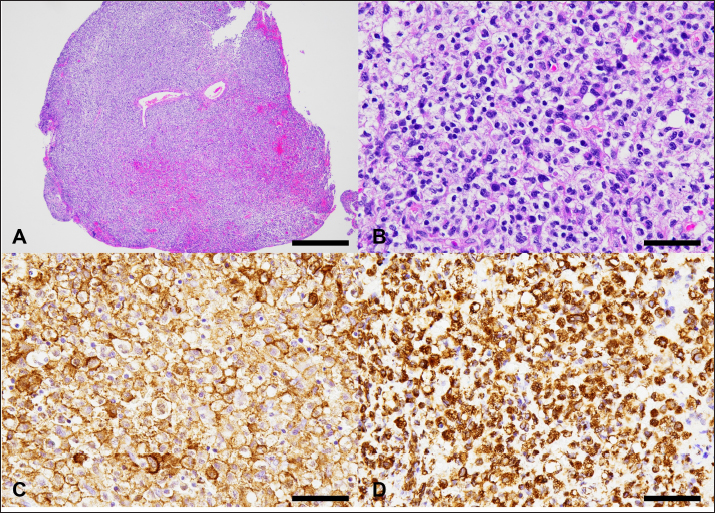
Fig. 3. Histological and immunohistochemical characteristics of the cerebral tumor. A: Low magnification of the biopsied tissue. The lesion comprises a dense sheet of neoplastic round cells, as observed on hematoxylin and eosin (HE) stain. Bar=500 μm. B: The tumor cells have abundant, pale, lacy cytoplasm, round to ovoid hypochromic nucleus with moderate anisokaryosis, and 1 or 2 prominent nucleoli. HE staining. Bar=50 μm. C: The tumor cells show a membranous as well as cytoplasmic positive reaction to Iba-1 antibody. Immunohistochemistry (IHC). Bar=50 μm. D: The tumor cells show a cytoplasmic positive reaction to Vimentin antibody. Immunohistochemistry. Bar=50 μm. On day 99, the patient presented with acute onset of somnolence and recumbency. Cerebral infarction was suspected because the entire right cerebral hemisphere and part of the thalamus showed high signal intensity on T2WI and FLAIR images, high signal intensity on diffusion weighted Imaging (DWI) images, and equal signal intensity on the ADC map (Fig. 4). MRI at this time demonstrated that the HS lesion in the left frontal lobe was 28.1 × 24.3 × 22.2 cm in size and had clearly regressed (Fig. 1G–I). The patient was also prescribed rivaroxaban (0.25 mg/kg, twice a day; Xarelto tablets 10 mg; Bayer Yakuhin, Osaka, Japan), and the owners were instructed to perform follow-up at home. The patient's neurological symptoms gradually improved, and a third dose of ACNU was administered at 20 mg/m2 on day 110. However, on day 124, an MRI was performed because a neurological examination revealed a decrease or loss of facial sensation, and trigeminal nerve injury was suspected. Although a contrast agent could not be used because the procedure was performed without anesthesia, clear re-enlargement of the HS lesions was observed (Fig. 5). ACNU was therefore changed to temozolomide (Temozolomide Tablets 20 mg “NK,” Nippon Kayaku, Tokyo, Japan), which was administered at an oral dose of 120-140 mg/m2 once daily for 5 days over a 28-day cycle on day 131, 153, and 173. During this time, there were no major changes in the patient's clinical symptoms, and the patient was able to walk with some unsteadiness. However, the patient died suddenly on day 195. A pathological autopsy was not performed at the owner's request. DiscussionThe treatment of intracranial tumors such as gliomas in dogs is driven by advanced imaging and involves a combination of treatments, including surgery, chemotherapy, and radiation therapy (José-López, 2023). However, an optimal treatment strategy for intracranial HS has yet to be developed due to its low incidence (Mariani et al., 2015). In recent years, ACNU, a nitrosourea-based anticancer drug, has been used to treat HS, and its efficacy has been reported (Tani et al., 2020). Tani et al. (2020) used this drug in two cases of intracranial HS and achieved long-term survival of more than 400 days. However, in both of these cases, macroscopic lesions were removed by surgical volume reduction, while the use of ACNU for macroscopic intracranial HS lesions has not yet been reported.
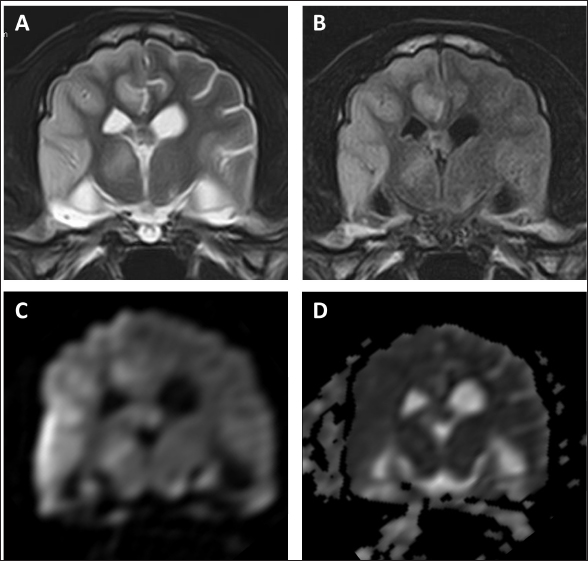
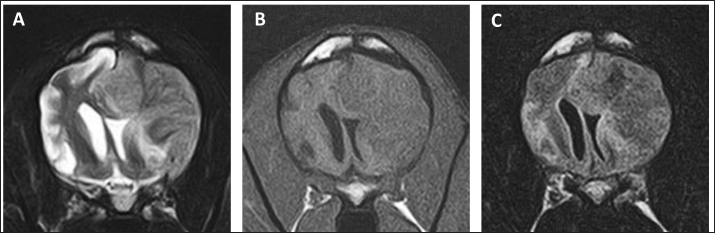
Fig. 4. MR images of the case at the level of thalamus on day 99. The entire right cerebral hemisphere and part of the thalamus showed a high signal on (A) T2-weighted and (B) FLAIR images, a high signal on (C) DWI images, and an equal signal on (D) ADC-map. In the present case, the reduction of the tumor lesion was confirmed by MRI following the second administration of ACNU. CCNU, an alkylating drug belonging to the nitrosourea family, is often used as a first-line drug therapy for the treatment of HS in dogs, with a response rate of 29% and a response duration of 96 days for gross lesions (Rassnick et al., 2010). In six dogs with HS at various sites treated with ACNU as a single agent, the response rate was 50%, with a median response duration of 48 days (Takahashi et al., 2023). In the present case, the administration of ACNU resulted in tumor reduction. The duration of the effect was short, lasting 84 days; however, the clinical symptoms improved during that time. To the best of our knowledge, this is the first report showing the macroscopic reduction effect of ACNU on intracranial HS in dogs. The patient developed pancreatitis during ACNU treatment. The major adverse events associated with ACNU in dogs include neutropenia and thrombocytopenia (Takahashi et al., 2014a,b). Mild gastrointestinal toxicity was also observed; however, it was not associated with pancreatitis. Although the causal relationship between the pancreatitis observed in this case and ACNU is unknown, no worsening of pancreatitis was observed even after subsequent administration, and it seems unlikely that ACNU was involved in the onset of pancreatitis. The subsequent cerebral infarction may have been caused by pancreatitis. Cerebral infarction is a rare complication of acute pancreatitis in humans (Hidalgo Crespo et al., 2022). With the addition of rivaroxaban, no recurrence of cerebral infarction was observed; however, the patient died suddenly on day 195. The cause of death was thought to be a recurrence of cerebral infarction or cerebral hemorrhage related to the tumor; however, the cause could not be definitively determined, because an autopsy was not performed.
Fig. 5. MR images of the case on day 124. Re-enlargement of the lesion was observed on (A) T2-weighted, (B) T1-weighted, and (C) FLAIR images. Temozolomide is an oral alkylating agent that can penetrate the blood-brain barrier and has been used to treat canine intracranial gliomas (Hidalgo Crespo et al., 2022; José-López, 2023). Temozolomide administration did not induce any worsening of the patient’s clinical symptoms and no obvious adverse events were observed. The use of temozolomide for HS in dogs has not yet been reported, and its effectiveness is unknown, as this case could not be evaluated using imaging. In human medicine, surgical resection and radiation therapy combined with temozolomide have been used to treat patients with intracranial HS with good results (Foster et al., 2015). Prospective studies are required to determine the efficacy of temozolomide for canine HS. Prednisolone has also been used as a symptomatic treatment for cerebral edema. A previous case report revealed that prednisolone had an antitumor effect on canine intracranial HS (Takahashi et al., 2021). However, another study found that prednisolone administration was associated with shorter survival times in dogs with periarticular HS (Klahn et al., 2011). As such, the efficacy of prednisolone in the treatment of HS remains unclear. In this report, we described the achievement of a macroscopic shrinkage effect of an intracranial HS following ACNU administration in a dog. ACNU monotherapy may be a therapeutic option for canine intracranial HS if radiation therapy is refused, or if surgical resection is inadequate. Further prospective studies are needed to clarify the effectiveness of ACNU therapy in treating intracranial HS with macroscopic lesions. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Authors’ contributionsMT contributed to writing the manuscript and the literature review. RY and KK contributed to the interpreting and describing the imaging findings. TI, NA, and YO performed the described surgery on the patient. IM examined the histopathology. All authors read and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAsada, H., Tomiyasu, H., Okada, K., Chambers, J.K., Goto-Koshino, Y., Uchida, K., Kagawa, Y., Ohno, K. and Tsujimoto, H. 2019. Clinical significance of the two-base insertion mutation in the TP53 gene in canine histiocytic sarcoma. Res. Vet. Sci. 124, 57–60. Foster, M., Kamaly-Asl, I., Stivaros, S., Kelsey, A., Gattamenini, R. and Kilday, J.P. 2015. Primary cerebral histiocytic sarcoma in childhood: a case report of protracted survival and review of the literature. Childs Nerv. Syst. 31, 2363–2368. Hidalgo Crespo, E., Farré Mariné, A., Pumarola I Battle, M., Borrego Massó, J.F. and Luján Feliu-Pascual, A. 2022. Survival time after surgical debulking and temozolomide adjuvant chemotherapy in canine intracranial gliomas. Vet. Sci. 9, 427. José-López, R. 2023. Chemotherapy for the treatment of intracranial glioma in dogs. Front. Vet. Sci. 10, 1273122. Klahn, S.L., Kitchell, B.E. and Dervisis, N.G. 2011. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J. Am. Vet. Med. Assoc. 239, 90–96. Mariani, C.L., Jennings, M.K., Olby, N.J., Borst, L.B., Jr Brown, J.C., Robertson, I.D., Seiler, G.S. and MacKillop, E. 2015. Histiocytic sarcoma with central nervous system involvement in dogs: 19 cases (2006–2012). J. Vet. Intern. Med. 29, 607–613. Moore, P.F. 2014. A review of histiocytic diseases of dogs and cats. Vet. Pathol. 51, 167–184. Rassnick, K.M., Moore, A.S., Russell, D.S., Northrup, N.C., Kristal, O., Bailey, D.B., Flory, A.B., Kiselow, M.A. and Intile, J.L. 2010. Phase II, open-label trial of single-agent CCNU in dogs with previously untreated histiocytic sarcoma. J. Vet. Intern. Med. 24, 1528–1531. Skorupski, K.A., Clifford, C.A., Paoloni, M.C., Lara-Garcia, A., Barber, L., Kent, M.S., LeBlanc, A.K., Sabhlok, A., Mauldin, E.A., Shofer, F.S., Couto, C.G. and Sørenmo, K.U. 2007. CCNU for the treatment of dogs with histiocytic sarcoma. J. Vet. Intern. Med. 21, 121–126. Takahashi, M., Goto-Koshino, Y., Fukushima, K., Kanemoto, H., Nakashima, K., Fujino, Y., Ohno, K., Endo, Y. and Tsujimoto, H. 2014a. Phase I dose-escalation study of nimustine in tumor-bearing dogs. J. Vet. Med. Sci. 76, 895–899. Takahashi, T., Kagawa, Y. and Ito, D. 2021. Successful use of prednisolone and radiation therapy in a dog with intracranial histiocytic sarcoma. J. Vet. Med. Sci. 83, 1782–1785. Takahashi, T., Shiozawa, H., Ishizaki, T., Okada, K. and Kondo, H. 2023. Anaplastic oligodendroglioma with nasal invasion and systemic metastasis in a dog. J. Vet. Med. Sci. 85, 1052–1056. Takahashi, M., Tomiyasu, H., Hotta, E., Asada, H., Fukushima, K., Kanemoto, H., Fujino, Y., Ohno, K., Uchida, K., Nakayama, H. and Tsujimoto, H. 2014b. Clinical characteristics and prognostic factors in dogs with histiocytic sarcomas in Japan. J. Vet. Med. Sci. 76, 661–666. Tani, H., Kurita, S., Miyamoto, R., Sawada, H., Fujiwara-Igarashi, A., Michishita, M., Azakami, D., Hasegawa, D., Tamura, K. and Bonkobara, M. 2020. Nimustine treatment of 11 cases of canine histiocytic sarcoma. J. Am. Anim. Hosp. Assoc. 56, 146. Wakabayashi, T., Yoshida, J., Mizuno, M. and Kajita, Y. 2001. Intratumoral microinfusion of nimustine (ACNU) for recurrent glioma. Brain Tumor Pathol. 18, 23–28. Articles published in Open Veterinary Journal are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License | ||
| How to Cite this Article |
| Pubmed Style Tagawa M, Itoi T, Yoshitake R, Kutara K, Akashi N, Mitsui I, Okamura Y. Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Vet. J.. 2024; 14(10): 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 Web Style Tagawa M, Itoi T, Yoshitake R, Kutara K, Akashi N, Mitsui I, Okamura Y. Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. https://www.openveterinaryjournal.com/?mno=206554 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.20 AMA (American Medical Association) Style Tagawa M, Itoi T, Yoshitake R, Kutara K, Akashi N, Mitsui I, Okamura Y. Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Vet. J.. 2024; 14(10): 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 Vancouver/ICMJE Style Tagawa M, Itoi T, Yoshitake R, Kutara K, Akashi N, Mitsui I, Okamura Y. Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 Harvard Style Tagawa, M., Itoi, . T., Yoshitake, . R., Kutara, . K., Akashi, . N., Mitsui, . I. & Okamura, . Y. (2024) Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Vet. J., 14 (10), 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 Turabian Style Tagawa, Michihito, Takamasa Itoi, Ryohei Yoshitake, Kenji Kutara, Natsuki Akashi, Ikki Mitsui, and Yasuhiko Okamura. 2024. Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Veterinary Journal, 14 (10), 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 Chicago Style Tagawa, Michihito, Takamasa Itoi, Ryohei Yoshitake, Kenji Kutara, Natsuki Akashi, Ikki Mitsui, and Yasuhiko Okamura. "Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma." Open Veterinary Journal 14 (2024), 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 MLA (The Modern Language Association) Style Tagawa, Michihito, Takamasa Itoi, Ryohei Yoshitake, Kenji Kutara, Natsuki Akashi, Ikki Mitsui, and Yasuhiko Okamura. "Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma." Open Veterinary Journal 14.10 (2024), 2700-2706. Print. doi:10.5455/OVJ.2024.v14.i10.20 APA (American Psychological Association) Style Tagawa, M., Itoi, . T., Yoshitake, . R., Kutara, . K., Akashi, . N., Mitsui, . I. & Okamura, . Y. (2024) Therapeutic effect of nimustine in a dog with intracranial histiocytic sarcoma. Open Veterinary Journal, 14 (10), 2700-2706. doi:10.5455/OVJ.2024.v14.i10.20 |