| Research Article | ||
Open Vet. J.. 2024; 14(11): 2780-2793 Open Veterinary Journal, (2024), Vol. 14(11): 2780-2793 Research Article Bioaccumulation of lead, arsenic, and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumptionEqbal Salman Najem1, Safaa Alloul Hussein2, Ali Majhool Kane3 and Ali Ibrahim Ali Al-Ezzy1*1Department of Pathology and Poultry Disease, College of Veterinary Medicine, University of Diyala, Baqubah, Iraq 2Department of Biotechnology, College of Science, University of Diyala, Baqubah, Iraq 3Department of Pathology, College of Veterinary Medicine, University of Kufa, Kufa, Iraq *Corresponding Author: Ali Ibrahim Ali Al-Ezzy. Department of Pathology and Poultry Disease, College of Veterinary Medicine, University of Diyala, Baqubah, Iraq. Email: ali.ib [at] uodiyala.edu.iq Submitted: 27/06/2024 Accepted: 08/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Pollution of aquatic environments with heavy metals causes severe adverse effects on fish, invertebrates, and human. The importance of this study lies in the fact that long-term ingestion of heavy metal-contaminated fish can result in the accumulation of harmful metals in numerous organs and pose a major risk to human health. Aim: The current study was designed to investigate the concentrations of toxic arsenic (As), lead (Pb), and mercury (Hg) in the liver, gills, and muscles of highly consumed aqua cultured common carp (Cyprinus carpio L.) in Baqubah city, to evaluate the toxicopathological bioaccumulation of As, Pb, and Hg in consumed fish and the potential human health risk after consumption and give clear indication for a status of heavy metal contamination for water used in aquaculture of common carp (C. carpio L.). Methods: A total of 10 Fresh fish of common carp (C. carpio L.) were randomly selected from local Baqubah markets/Diyala province/Iraq in different interval from September 2022 to January 2023. The source of fish in Baqubah’s local markets is fish aquacultured in earthen ponds as well as cages in the Tigris River in Diyala province. Flame atomic absorption spectrometer was used to estimate the level of As, Pb, and Hg in liver, gills, and muscles of collected fish. Histopathological sections were prepared for evaluation of toxic pathological effects of metals on C. carpio organs. Results: Bioaccumulation of arsenic and lead in liver, gill, and muscle samples was within and less than the permissible limit set by WHO while the bioaccumulation of mercury exceeds the permissible limits set by WHO. Histopathological findings of the gill section showed telangictatisis and epithelial lifting in secondary lamellae with hemorrhage and blood congestion and central venous dilation and epithelium hyperplasia with complete fusion of the secondary lamellae and edema in the filamentary epithelium in addition to mononuclear cells infiltration. Histopathological findings of liver revealed degenerative and necrotic changes in liver tissue distinguished by pyknosis with the existence of necrosis in cells and dilation of the sinusoids with cytoplasmic vacuolation. Conclusion: The bioaccumulation of mercury is higher than permitted levels in common carp (C. carpio) which indicates high contamination level of main sources for fresh waters in Diyala province mainly from Diyala and Tigris rivers. Keywords: Bioaccumulation, Heavy metals, Cyprinus carpio, Human health, Pathological effects. IntroductionBecause of the fast growth of industry and agriculture heavy metal pollution of aquatic habitats becomes serious. Pollution of aquatic environments with heavy metals causes severe adverse effects on fish, invertebrates, and people (Uluturhan and Kucuksezgin, 2007). Heavy metal may exert beneficial or harmful effects on animal, plant, and human according to their level (Forstner and Wittman, 1981). Many heavy metals are toxic to living creatures even at low quantities, although some are necessary for biological processes and only become hazardous at comparatively large concentrations. Fish and other living things are easily exposed to heavy metal accumulation due to their non-biodegradable nature (Akter et al., 2014). Consequently, eating fish exposes people to heavy metal exposure through the food chain. Both fish and humans are negatively impacted by heavy metals in various ways. According to Vitek et al. (2007), heavy metals in fish disrupt growth and reproduction in addition to causing histological alterations in the liver, spleen, kidney, and gills. Heavy metal accumulated in humans affects the kidneys, liver, brain, lungs, and muscles negatively (Petera and Viraraghavanb, 2005). Such as Pb results in cardiovascular disease and elevated blood pressure in adults (Al-Hossainy et al., 2012). Hg toxicity includes, neurotoxicity, gastrointestinal toxicity, and nephrotoxicity (Tchounwou et al., 2003). Also, exposure to As can affect practically all organ systems including the cardiovascular, gastro-intestinal, renal, respiratory systems, hepatobilliary, dermatologic, and nervous systems (Tchounwou et al., 2003). According to Youn-Joo (2003), heavy metals enter the aquatic ecosystem from both natural and man-made sources, including domestic and industrial activities and they caused adverse effects on human health, aquatic ecosystems, food chain. Recently, because of the nutritional and medicinal advantages of fish, their consumption has increased globally (El-Moselhy et al., 2014). Fish is significant in human diet because it contains high-quality protein and omega-3 polyunsaturated fatty acids. Omega-3 fatty acids lower cholesterol and the risk of heart disease and are essential for healthy growth (Al bader, 2008). Vitamins and minerals found in fish flesh are vital to human health. Fish are frequently regarded as the most appropriate object among the bioindicators of aquatic ecosystems since they are necessary part of human sustenance (Abdel-Baki et al., 2011). Fish may contain significant levels of metals from the water and are frequently at the top of the aquatic food chain (Mansour and Sidky, 2002). As a result, the amount of metals in fish reflects the accumulation of these elements in the food chain (Pintaeva et al., 2011). Furthermore, consuming heavy metal-contaminated fish over an extended period of time can cause dangerous metals to accumulate in various organs, which are extremely dangerous for human health. (Renieri et al., 2014). The effects of pollutants on aquatic habitats are currently being extensively documented and measured using several physiological and histopathological biomarkers of fish, such as Melanomacrophagic centers, heat shock proteins, follicular atresia, metallothionein, vitellogenin in males, apoptosis, hormonal abnormalities, fibrosis, and steatosis in teleosts (Prado et al., 2011). As a result of chemical contaminants fish develop toxicopathic lesions in their organs. These abnormalities in gills include lamellar fusion, and necrosis. Epithelial cell lifting, hyperplasia, hypertrophy, and damage of the lamellar epithelium, as well as increased mucous secretion, and chloride cell proliferation while abnormalities in livers include necrosis, vacuolar and fatty degeneration, nucleus pyknosis, and karyorhexis, as well as engorgement and congestion of blood vessel (Hinton and Lauren, 1990). The importance of this study lies in the fact that long-term ingestion of heavy metal-contaminated fish can result in the accumulation of harmful metals in numerous organs and pose a major risk to human health. The goal of this study was to estimate the concentrations of toxic As, Pb, and Hg in the liver, gills, and muscles of highly consumed cultured fish species common carp (Cyprinus carpio) in Baqubah city to assess their accumulation and the risk to human health through their consumption. Materials and MethodsSample collectionFresh fish samples (n=10) of common carp (C. carpio L.) were randomly selected from local Baqubah markets/Diyala province/Iraq in different intervals from September 2022 to January 2023. The source of fish in Baqubah’s local markets is fish aqua cultured in earthen ponds as well as cages in the Tigris River in Diyala province. The samples were transported to the pathology laboratory on ice box. After that fish were dissected and pieces of the dorsal muscle, liver, and gills were gathered, washed with distilled water, packed in polyethylene bags, and stored at –20°C until chemical analysis. As, Pb and Hg levels in fish samples were expressed as ppm. For histopathological examination gills and liver were carefully gathered and were fixed in 10 % formaldehyde solution and then prepared for examination. Chemical analysisThe samples of liver, gills, and muscles were taken out of the freezer and left to thaw at room temperature 25°C then prepared for atomic absorption spectrometer measurement according to the method described by (Ang and Lee, 2005). Estimation of As level in fish samplesAs shown in Figure 1, the As level was measured in fish samples at Ministry of Industry and Minerals, Ibn Sina Center, through the use of the Flame atomic absorption spectrometer (FAAS). The standard solutions used to make the calibration curve for As are: 0.025 ppm—0.05 ppm–0.1 ppm—0.2 ppm (Fig. 1). Estimation of Pb level in fish samplesAs shown in Figure 2, the Pb level was measured in fish samples at Ministry of Industry and Minerals, Ibn Sina Center, through the use of the FAAS. The standard solutions used to make the calibration curve for Pb are: 0.1 ppm—0.3 ppm—0.6 ppm—0.9 ppm (Fig. 2). Estimation of Hg level in fish samplesAs shown in Figure 3, the Hg level was measured in fish samples at Ministry of Industry and Minerals, Ibn Sina Center, through the use of the FAAS. The standard solutions used to make the calibration curve for Hg are: 0.2 ppm—0.4 ppm—0.6 ppm—0.8 ppm (Fig. 3). Histopathological analysisSamples of the gills and liver were rinsed in tap water then prepared for histopathological examination according to the method described by Drury et al. (1976). Statistical analysisData analysis performed using SPSS for windows TM version 17.0. All variables are expressed as (Mean± Standard Error). The significance level for the ANOVA test, which was used to analyze categorical data, was 0.05 (two-tail).
Fig. 1. As calibration curve.
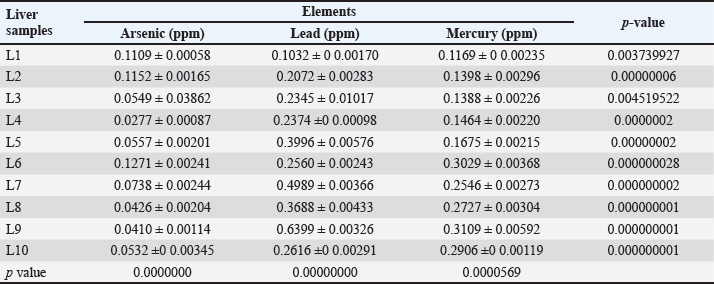
Fig. 2. Pb calibration curve. Ethical approvalThe authors adhered to all relevant international, national, and institutional guidelines for the care and use of animals. ResultsThe values obtained from the present study for selected heavy metals in liver of C. carpio are given in Table 1 and Figure 4. The results of the current study showed that all liver samples of C. carpio in 100% had a concentration of As and Pb less than the permissible limit of the WHO. The permissible limits for As and Pb set by WHO (1990) are (0.5 ppm) and (1.5 ppm), respectively. While Hg concentration in four liver samples (L1, L2, L3, L4) in 40% was within the permissible limit of WHO and in six liver samples in 60% was higher than the permissible limit of WHO (1990). The permissible limit for Hg set by WHO (1990) is (0.14 ppm).
Fig. 3. Hg calibration curve. Table 1. Heavy metals concentration in liver of C. carpio (ppm). Values are expressed as mean ± SE.
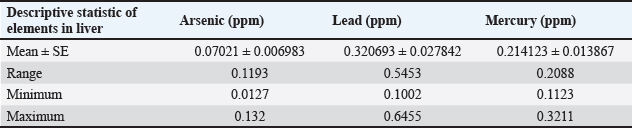
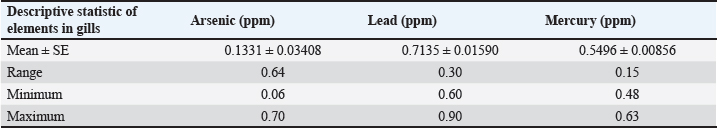
Table 2. Mean ± SE, range, minimum, and maximum value of heavy metal content in liver samples of C. carpio.
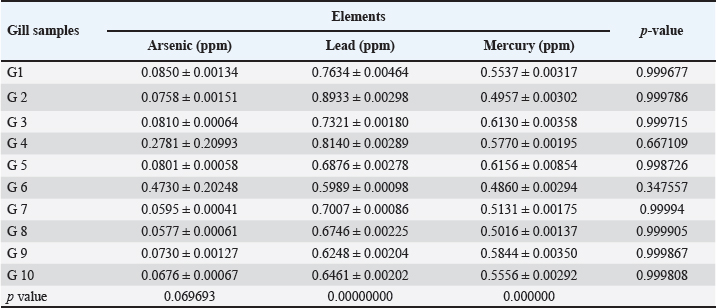
Fig. 4. Concentration of Arsenic, Lead, and Mercury in liver of C. carpio. Table 3. Heavy metals concentration in gill of C. carpio (ppm). Values are expressed as mean ± SE.
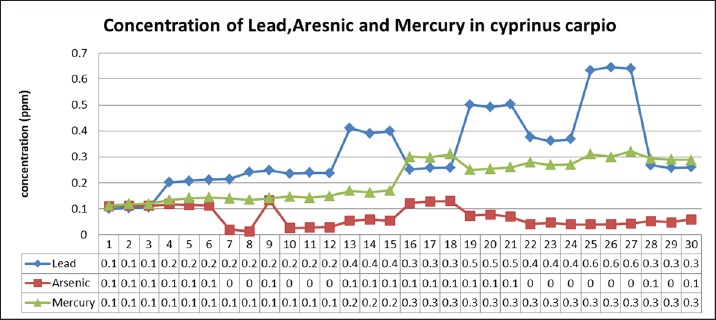
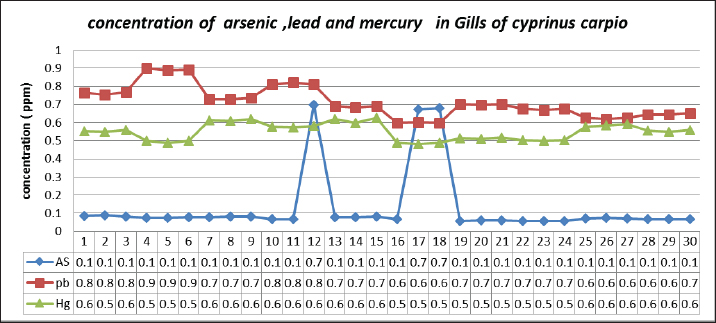
Table 2 depicts the mean ± SE, Range, minimum, and maximum value of heavy metal content in liver samples of C. carpio. The study also showed that the average As and Pb concentration was less than the permissible limit of the WHO, while the average Hg concentration was higher than the permissible limit of the WHO (1990) (Table 2). The concentrations of selected heavy metals in the gill of C. carpio are given in Table 3 and Figure 5. Our study results showed that all gill samples of C. carpio in 100% had the As and Pb concentrations less than the permissible limit of the WHO (1990) which is (0.5 ppm) and (1.5 ppm) respectively but the concentration of Hg in 100% was higher than the permissible limit of WHO (1990) which is (0.14 ppm). Table 4 represents the mean ± SE, Range, minimum, and maximum value of heavy metal content in gill samples of C. carpio. Furthermore, average concentration of As and Pb was less than the permissible limit of the WHO, while it was higher than the permissible limit of the WHO for Hg Table 4. The selected heavy metals concentration in muscle of C. carpio are given in Table 5 and Figure 6. Heavy metals analysis revealed that the concentration of As and Pb in muscle of C. carpio in 100% were less than the permissible limit of the WHO (1990) which is (0.5 ppm) and (1.5 ppm) respectively while Hg concentration in one muscle samples (M3) in 10% was within the permissible limit of WHO and in nine muscle samples in 90% was higher than the permissible limit of WHO (1990) which is (0.14 ppm). Table 4. Mean ± SE, range, minimum, and maximum value of heavy metal content in gill samples of C. carpio.
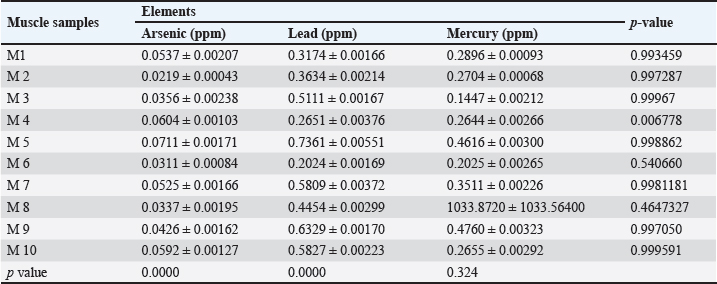
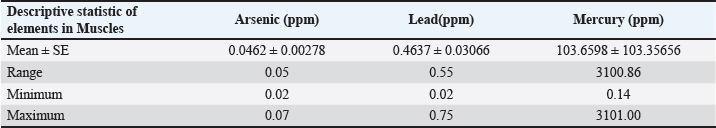
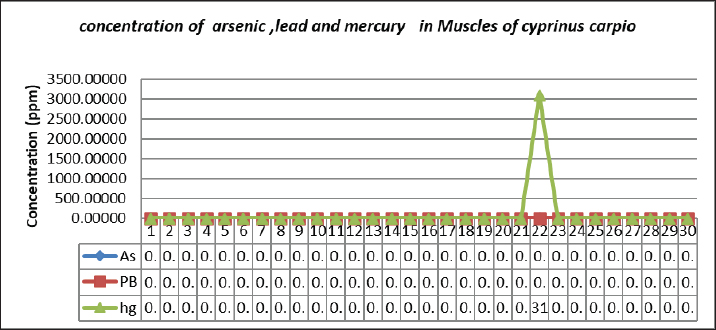
Fig. 5. Concentration of Arsenic, Lead, and Mercury in gills of C. carpio. Table 6 represents the mean ± SE, Range, minimum, and maximum value of heavy metal content in muscle samples of C. carpio. In addition, As and Pb average concentration was less than the permissible limit of the WHO, while it was higher than the permissible limit of the WHO for Hg Table 6. Histopathological findings of gill section showed telangictatisis and epithelial lifting in secondary lamellae (Fig. 7) with hemorrhage and blood congestion (Fig. 8), also, the result showed the central venous dilation with blood congestion and epithelium hyperplasia with complete fusion of the secondary lamellae (Figs. 9 and 10) and edema in the filamentary epithelium (Fig. 11) in addition to mononuclear cells infiltration (Figs. 12 and 13). The result of histopathological alteration of the liver revealed degenerative and necrotic changes in liver tissue distinguished by pyknosis with the existence of necrosis in cells (Figs. 14 and 15) also, the result revealed dilation of the sinusoids with cytoplasmic vacuolation (Fig. 16). DiscussionEnvironmental pollution with heavy metals can seriously harm to all living things, and the bioaccumulation of heavy metals in the food chain can pose a serious health risk to humans (Adimalla and Wang, 2018). As a strategy for current work, authors depend on open access data sources available in Google Scholar from 1990 to 2023 from Iraq and around to evaluate the results and estimates the outcomes. As is one of the most potentially harmful heavy metals in the environment, and is a non-essential metal that enters the aquatic ecosystem through both natural and numerous human-caused processes (Saha et al.2016). According to USFDA (1993) human exposure of As in 90% originates from fish and other marine organisms. The current study’s findings revealed the presence of As in liver, gill, and muscle samples of C. carpio collected from the local Baqubah markets. It revealed that the bioaccumulation of arsenic in C. carpio has reached below the permissible limits of the WHO (1990) for human consumption which is (0.5 ppm). Table 5. Heavy metals concentration in muscle of C. carpio (ppm). Values are expressed as mean ± SE.
Table 6. Mean ± SE, range, minimum, and maximum value of heavy metal content in muscle samples of C. carpio.
Fig. 6. Concentration of Arsenic, Lead, and Mercury in muscle of C. carpio. This results agree with Carlos et al. (2020) who found that heavy metal concentrations (Hg, Pb, Cu, As, Cd, and Zn) in fish muscle of (Mugil incilis, Cathorops mapale, Eugerres plumieri, Centropomus undecimalis, and Elops smithi) were low compared to the maximum levels for fish consumption set by national and international monitoring organizations, while disagree with Robeena et al. (2019) who found that bioaccumulation of heavy metal (As), Cadmium (Cd), Iron (Fe), and (Pb) in Channa punctatus in Ramganga river in India has surpassed the levels that are safe for human consumption.
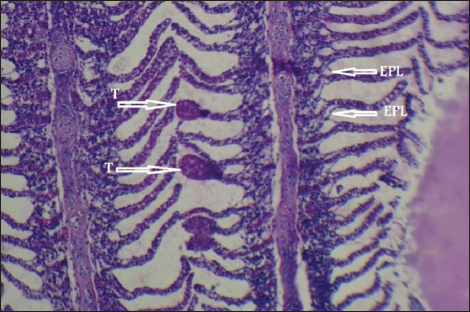
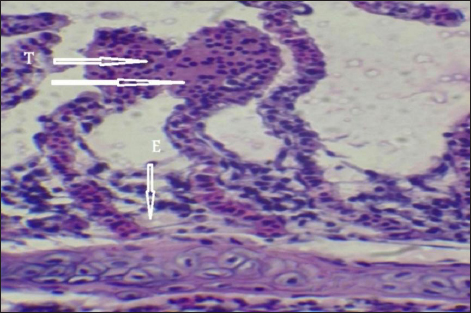
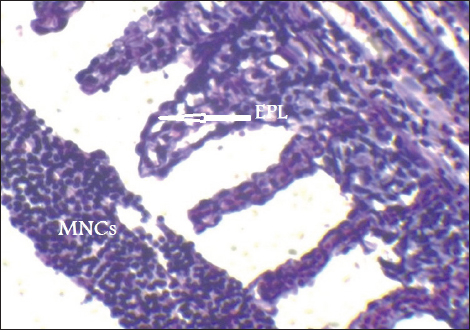
Fig. 7. Histopathological sections of gill of C. carpio showing telangictatisis (T) and epithelial lifting (EPL) (H&E X10).
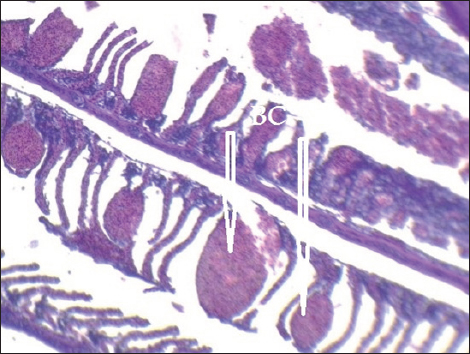
Fig. 8. Histopathological sections of gill of C. carpio showing severe telangiectasia in secondary lamellae with hemorrhage and blood congestion (BC) (H&E X10). Pb has detrimental effects on both human health and the environment. Pb’s toxicity varies according to its concentration, oxidation state, and chemical composition (Khan et al., 2016). According to Gercia-Leston et al. (2010), lead has a number of harmful health effects, including neurotoxicity and nephrotoxicity. The current study’s findings showed that Pb was detected in C. carpio liver, gill, and muscle samples that were taken from the local Baqubah markets. Furthermore, the bioaccumulation of Pb in these samples had fallen below the WHO’s (1990) permitted limits for human consumption, which are 1.5 ppm. These results are also observed by Zarith and Mohd (2015) who found that the heavy metals concentrations of (Zn, Cu, Ni, Pb, Cd, and Mn) in water samples and six species of freshwater fish (Barbonymus schwanenfeldii, Hampala microlepidota, Hemibagrus nemurus, Mystacoleucus marginatus, Oreochromis niloticus, and Cyclocheilicthys apogon) collected from Galas River and pool of Beranang mining were lower than the permissible limit set by Malaysian Food Regulations 1983 and Food Act 2003 and agree with Carlos et al. (2020). While disagree with Alsarraj et al. (2014) who studied the concentration of lead and cadmium metals in the organs and tissues of different fish species in Tigris river near the city of Mosul, the highest value of lead was in the gills of C. carpio and it was 4.01 ± 8.78 μg/g which was higher than the permissible limit of WHO and disagree with Robeena et al. (2019).
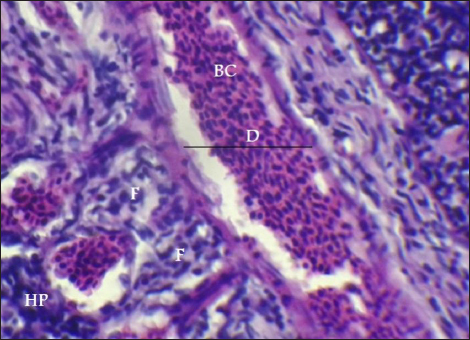
Fig. 9. Histopathological sections of gill of C. carpio showing central venous dilation (D) with blood congestion (BC), epithelium hyperplasia (HP) with complete fusion of the secondary lamellae (F) (H&E X40).
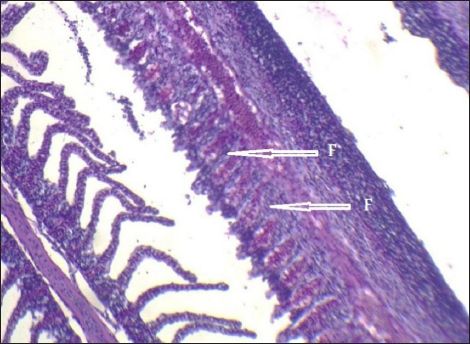
Fig. 10. Histopathological sections of gill of C. carpio showing complete fusion in the secondary lamellae (F) (H&E X40). Mercury is a hazardous heavy metal that is not necessary for life and is hard to eliminate. Long-term storage in the tissues may result in lesions, neurological dysfunction, and changes in behavior and cognition (Authman et al., 2015). Additionally, during pregnancy, mercury can pass through the placenta and reach the fetus, which may have an impact on the central nervous system’s development (Renieri et al., 2014). The current study’s findings demonstrated that mercury was present in C. carpio liver, gill, and muscle samples that were taken from the local Baqubah markets, and that the mercury’s bioaccumulation had above the WHO’s (1990) allowable level of 0.14 ppm.
Fig. 11. Histopathological sections of gill of C. carpio showing edema in the filamentary epithelium (E) (H&E X40).
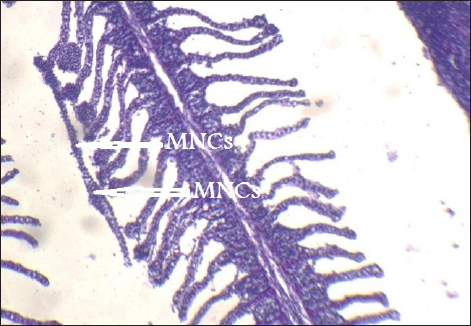
Fig. 12. Histopathological sections of gill of C. carpio showing Mononuclear cells infiltration (MNCS) (H&E X10). The present results are disagree with Fabio et al. (2016) who observed the bioaccumulation of Cd, Hg, Cr, Pb, and Zn in muscle of Pseudoplatystoma corruscans collected from Paraopeba River, Brazil, do not go over what is considered safe for human consumption. It is well known that differences in a variety of factors, including aquatic environments and the kind and degree of water pollution, feeding habits, and the amount of fish—demersal or pelagic—that inhabit the water make it difficult to compare mineral concentrations, even within the same tissue in different species (Muzyed, 2011). Kamaruzzaman et al. (2010) revealed a correlation between the concentration of minerals and several intrinsic fish traits, such as age, size, and genetic makeup.
Fig. 13. Histopathological sections of gill of C. carpio showing Mononuclear cells infiltration (MNCS) (H&E X40).
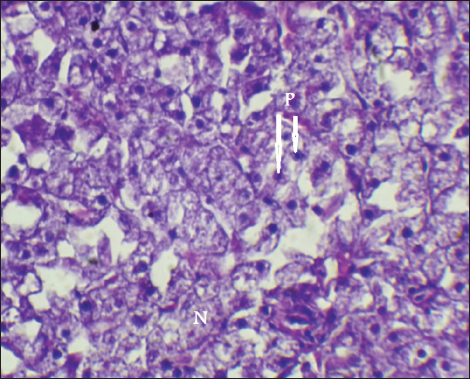
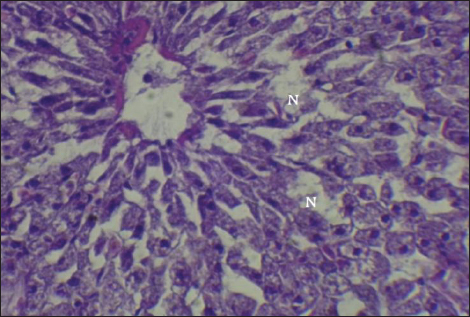
Fig. 14. Histopathological sections of liver of C. carpio showing necrosis(N) and Pyknosis (P) (H&E X40). This study showed that C. carpio contains different concentrations of the selected heavy metals in the liver, gill, and muscle, the causes of this differences is due to the collection of samples at different intervals and, therefore, the level of contamination in water and food and sediment varies depending on the time period also differences in fish’s internal uptake and water temperature. Kalay et al. (1999) revealed that the amounts of minerals that different fish species accumulate in their tissues vary noticeably. Furthermore, Canli and Atli (2003) revealed that heavy metals levels in fish vary between species and different aquatic environments. Conversely, Farkas et al. (2000) linked variations in mineral concentrations among fish to their feeding habits, the ability of each species to bioconcentrate, and the mineral’s biochemical characteristics. Roméo et al. (1999) also state that the environment, metabolism, needs, level of contamination in water, sediment, and food, as well as water temperature and salinity, all affect a fish’s ability to accumulate heavy metals. Also, Brraich and Kaur (2017) revealed that the levels of heavy metals in different fish organs are directly impacted by the contaminating of the aquatic environment and by the fish’s internal uptake, control, and removal of heavy metals.
Fig. 15. Histopathological sections of liver of C. carpio showing necrosis (N) (H&E X40).
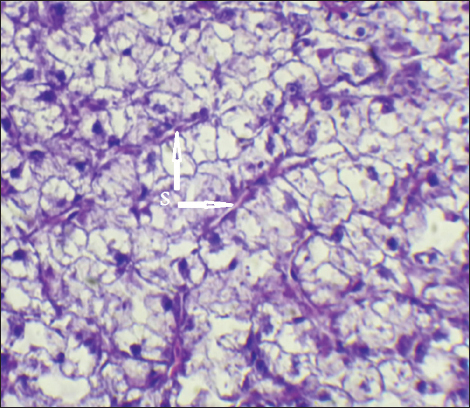
Fig. 16. Histopathological sections of liver of C. carpio showing dilation of the sinusoids (S) with cytoplasmic vacuolation (H&E X40). The changes in gill tissue are not exclusive to As, Pb, and Hg toxicity (e.g., telangiectasis, epithelial lifting, hemorrhage, congestion, etc.) which have been previously reported in other stressful situations, like pesticide exposure (Fanta et al., 2003). All of these changes may be defense mechanisms meant to lengthen the time it takes for waterborne contaminants to diffuse and enter the bloodstream (Arellano et al., 1999). With particular reference to exposure to metals, Mustafa et al. (2012) observed telangiectasis, necrosis, in mirror carp C. carpio exposed to Cu, with the epithelium lifted away from the basement membrane. Similar results were also noted in Sole senegalensis, the Senegalese sole, after it was exposed to a sub-lethal concentration of Cu for 7 days (Arellano et al., 1999). It is well known that exposure to heavy metals can cause a variety of histopathological changes (Mustafa et al., 2012). These changes may be related to the harmful effects of heavy metals on hepatocytes, as the liver is involved in the biotransformation and detoxification of various pollutants and toxicants (Arellano et al., 1999). The observed necrosis in certain areas of the liver tissue may have been caused by the fish’s overwork in eliminating the toxins from its body during the detoxification process; this observation is consistent with the findings of Rhaman et al. (2002). ConclusionThe bioaccumulation of mercury is higher than permitted levels in common carp (C. carpio) which indicates a high contamination level of fresh waters in Diyala province. The illegal dumping of waste from various small-scale industries and the illegal burning of waste close to freshwater sources are the main causes of this contamination. Water is therefore unfit for human and fish consumption. Therefore, it’s essential to regularly check the quality of the water, and more research is required to identify an affordable, environmentally friendly alternative to heavy metal contamination. It is recommended to increase awareness for the effect of water contamination on health status and quality of fishes fit for human consumption. Further studies for the evaluation of bioaccumulation of other heavy metals in local fishes. AcknowledgmentsSpecial thanks to Department of Pathology, College of Veterinary Medicine, University of Diyala for making available all the necessary facilities for this work. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo funding agencies. Authors’ contributionsAll authors are equally contributing in planning, laboratory work, manuscript preparation, and statistical analysis. Data availabilityAll related data presented in text. ReferencesAbdel-Baki, A.S., Dkhil, M.A. and Al-Quraishi, S. 2011. Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia, Afr. J. Biotechnol. 10, 2541–2547. Adimalla, N. and Wang, H. 2018. Distribution, contamination, and health risk assessment of heavy metals in surface soils from northern Telangana India. Arabian J. Geosci. 11, 684. Akter, M., Sikder, T. and Ullah, A.K.M.A. 2014. Water quality assessment of an industrial zone polluted aquatic body in dhaka, Bangladesh. Am. J. Environ. Protec. 3, 232–237. Al bader, N. 2008. Heavy metal levels in most common available fish species in Saudi market. J. Food Technol. 6(4), 173–177. Al-Hossainy, A.F., Mohamed, A.E., Hassan, F.S.M. and Abd Allah, M.M. 2012. Determination of cadmium and lead in perch fish samples by different pulse anodic stripping voltammetry and furnace atomic absorption spectrometry. Arab. J. Chem. 10, S347–S354. Al-Sarraj, E., Janker, M. and Al-Rawi, S. 2014. Bioaccumulation study of some heavy metals in tissues and organs of three collected fish species in Tigris River within Mosul City. Rafidain J. Sci. 25(4), 43–55. Ang, H.H. and Lee, K.L. 2005. Analysis of mercury in Malaysian herbal preparations. JMBR. 4(1), 31–36. Arellano, J.M., Storch, V. and Sarasquete, C. 1999. Histological changes and copper accumulation in liver and gills of the Senegales Sole, Solea senegalensis. Ecotoxicol. Environ. Safe 44, 62–72. Authman, M.M.N., Zaki, M.S., Khallaf, E.A. and Abbas, H.H. 2015. Use of fish as bio-indicator of the effects of heavy metals Pollution. J. Aquac. Res. Dev. 6, 328. Brraich, O.S. and Kaur, M. 2017. Histopathological alterations in the gills of Labeo rohita (Hamilton-Buchanan) due to lead toxicity. Indian J. Exp. Biol. 55(8), 576–583. Canli, M. and Atli, G. 2003. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Poll. 121(1), 129–136. Carlos, H., Pinzón-Bedoya, M., Pinzón-Bedoya, L., Pinedo-Hernández, J., Urango-Cardenas, I. and Marrugo-Negrete, J. 2020. Assessment of potential health risks associated with the intake of heavy metals in fish harvested from the largest Estuary in Colombia, Int. J. Environ. Res. Public Health, 17, 2921. Drury, R., Wallington, E. and Cancerson, R. 1976. Carletons histological technique. 4th ed. London, UK: Oxford University Press. El-Moselhy, K.M., Othman, A.I., El-Azem, H.A. and El-Metwally, M.E.A. 2014. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 1, 97–105. Fabio, P.A., Lourenco, A.S., Helio, B.S., Marcos, V.T.G. and Nilo, B. 2016. Bioaccumulation of mercury, cadmium, zinc, chromium, and lead in muscle, liver, and spleen tissues of a large commercially valuable catfish species from Brazil. An. Acad. Bras. Cienc. 88(1), 137–147. Fanta, E., Rios, F.S.A., Romão, S., Vianna, A.C.C. and Freiberger, S. 2003. Histopathology of the fish Corydoras paleatus contaminated with sublethall levels of organophosphorus in water and food. Ecotoxicol. Environ. Safe. 54, 119–130. Farkas, A., Salanki, J. and Varanka, I. 2000. Heavy metal concentrations in fish of Lake Balaton. Lakes Reservoirs Res. Manag. 5(4), 271–279. Forstner, U. and Wittman, W. 1981. Metal pollution in the aquatic environment. Berlin, Germany: Springer-Verlag, 272, p. 486. Hinton, D.E. and Laurén, D.J. 1990. Integrative histopathological approaches to detecting effects of environmental stressors on fishes. Am Fish Soc Symp 8, 145–166. Gercia-Leston, J., Mendez, J., Pasaro, E. and Laffon, B. 2010. Genotoxic effects of lead: an updated review. Environ. Int. 36, 623–636. Kalay, M., Ay, Ö. and Canli, M. 1999. Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull. Environ. Contam. Toxicol. 63(5), 673–681. Kamaruzzaman, B.Y., Ong, M.C. and Rina, S.Z. 2010. Concentration of Zn, Cu and Pb in some selected marine fishes of the Pahang coastal waters, Malaysia. Am. J. Appl. Sci. 7(3), 309–314. Khan, S., Rauf, R., Muhammad, S., Qasim, M. and Din, I. 2016. Arsenic and heavy metals health risk assessment through drinking water consumption in the Peshawar District, Pakistan. Hum. Ecol. Risk Assess. Int. J. 22, 581–596. Mansour, S.A. and Sidky, M.M. 2002. Ecotoxicological studies. 3: heavy metals contaminating water and fish from Fayoum Govemorate, Egypt. Food Chem. 78, 15–22. Mustafa, S.A., Davies, S.J. and Jha, A.N. 2012. Determination of hypoxia and dietary copper mediated sub-lethal toxicity in carp, Cypinus carpio, at different levels of biological organization. Chemosphere 87, 413–420. Muzyed, S.K.I. 2011. Heavy metal concentrations in commercially available fishes in Gaza strip markets. M.Sc. Thesis, Islamic University of Gaza, Gaza Strip, Palestine, pp: 64. Petera, J.A.L. and Viraraghavanb, T. 2005. A review of public health and environmental concerns. Environ. Int. 31, 493–501. Pintaeva, E.T., Bazarsadueva, S.V., Radnaeva, L.D., Pertov, E.A. and Smirnova, O.G. 2011. Content and character of metal accumulation in fish of the Kichera River (a tributary of Lake of Baikal), Contemp. Prob. Ecol. 4, 64–68. Prado, P.S., Souza, C.C., Bazzoli, N. and Rizzo, E. 2011. Reproductive disruption in lambari Astyanax fasciatus from a Southeastern Brazilian reservoir. Ecotox. Environ. Safe 74, 1879–1887. Renieri, E.A., Alegakis, A.K., Kiriakakis, M., Ninceti, M., Ozcagli, E., Wilks, M.F. and Tsatsakis, A.M. 2014. Cd, Pb and Hg biomonitoring in fish of the Mediterranean region and risk estimations on fish consumption. Toxics 2, 417–442. Rhaman, M.Z., Hossain, Z., Melleh, M.F.A. and Ahmed, G.U. 2002. Effect of diazinon 60EC on Anabus testudineus, channa punctatus and Barbades gomonotus Naga. ICLARM Quart. 25, 8–11. Robeena, S., Baby, T., Nida, I., Abeer, H. and Elsayed, F.A. 2019. Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (U.P.), India. Saudi J. Biol. Sci. 26, 979–984. Roméo, M., Siau, Y., Sidoumou, Z. and Gnassia-Barelli, M. 1999. Heavy metal distribution in different fish species from the Mauritania coast. Sci. Total Environ. 232(3), 169–175. Saha, N., Mollah, M.Z.I., Alam, M.F. and M.S. Rahman, M.S. 2016. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 70, 110–118. Tchounwou, P.B., Patlolla, A.K. and Centeno, J.A. 2003. Carcinogenic and systemic health effects associated with arsenic exposure-a critical review. Toxicol. Pathol. 31, 575–588. Uluturhan, E. and Kucuksezgin, F. 2007. Heavy metal contaminants in Red Pandora (Pagellus erythrinus) tissues from the Eastern Aegean Sea, Turkey. Water Res. 41, 1185–1192. USFDA (United States Food and Drug Administration). 1993. Guidance Document for Arsenic in Shellfish. Washington, DC: US Food and Drug Administration. Vitek, T., Spurny, P., Mares, J. and Zikova, A. 2007. Heavy metal contamination of the Loucka River water ecosystem. Acta Vet. Brno. 76, 149–154. WHO (World Health Organization). 1990. Environmental Health Criteria 101:Methyl Mercury, WHO/IPCS. Geneva, Switzerland: WHO. Youn Joo, A. 2003. Total, dissolved, and bioaccumulation in freshwater fish with emphasis on the dietary influence. Water Res. 34, 4234–4242. Zarith, S.B. and Mohd, Y.I. 2015. Determination of heavy metal accumulation in fish species in Galas River, Kelantan and Beranang mining pool, Selangor. Procedia Environ. Sci. 30, 320–325. | ||
| How to Cite this Article |
| Pubmed Style Najem ES, Hussein SA, Kane AM, Al-ezzy AIA. Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Vet. J.. 2024; 14(11): 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 Web Style Najem ES, Hussein SA, Kane AM, Al-ezzy AIA. Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. https://www.openveterinaryjournal.com/?mno=207041 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.7 AMA (American Medical Association) Style Najem ES, Hussein SA, Kane AM, Al-ezzy AIA. Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Vet. J.. 2024; 14(11): 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 Vancouver/ICMJE Style Najem ES, Hussein SA, Kane AM, Al-ezzy AIA. Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 Harvard Style Najem, E. S., Hussein, . S. A., Kane, . A. M. & Al-ezzy, . A. I. A. (2024) Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Vet. J., 14 (11), 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 Turabian Style Najem, Eqbal Salman, Safaa Alloul Hussein, Ali Majhool Kane, and Ali Ibrahim Ali Al-ezzy. 2024. Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Veterinary Journal, 14 (11), 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 Chicago Style Najem, Eqbal Salman, Safaa Alloul Hussein, Ali Majhool Kane, and Ali Ibrahim Ali Al-ezzy. "Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption." Open Veterinary Journal 14 (2024), 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 MLA (The Modern Language Association) Style Najem, Eqbal Salman, Safaa Alloul Hussein, Ali Majhool Kane, and Ali Ibrahim Ali Al-ezzy. "Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption." Open Veterinary Journal 14.11 (2024), 2780-2793. Print. doi:10.5455/OVJ.2024.v14.i11.7 APA (American Psychological Association) Style Najem, E. S., Hussein, . S. A., Kane, . A. M. & Al-ezzy, . A. I. A. (2024) Bioaccumulation of lead, arsenic and mercury in vital organs of common carp (Cyprinus carpio L.): Assessment of pathological effects and possible hazards associated with human consumption. Open Veterinary Journal, 14 (11), 2780-2793. doi:10.5455/OVJ.2024.v14.i11.7 |