| Research Article | ||
Open Vet. J.. 2024; 14(11): 2794-2805 Open Veterinary Journal, (2024), Vol. 14(11): 2794-2805 Research Article Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit marketsManal Hadi Ghaffoori Kanaan*Department of Nursing, Technical Institute of Suwaria, Middle Technical University, Baghdad, Iraq *Corresponding Author: Manal Hadi Ghaffoori Kanaan. Department of Nursing, Technical Institute of Suwaria, Middle Technical University, Baghdad, Iraq. Email: manalhadi73 [at] yahoo.com Submitted: 04/07/2024 Accepted: 22/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
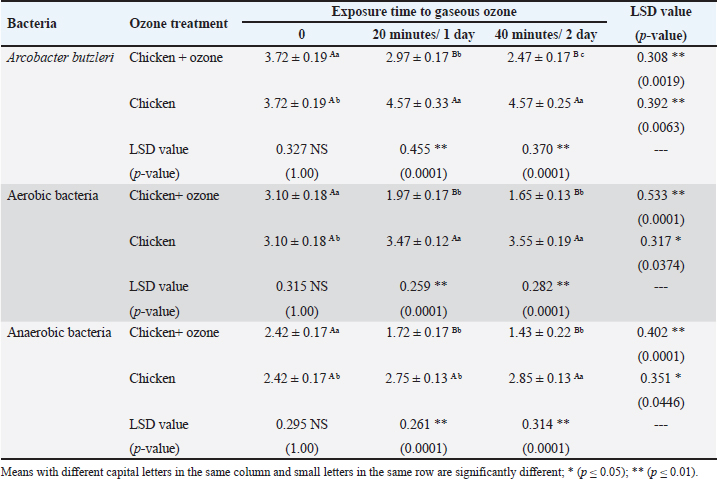
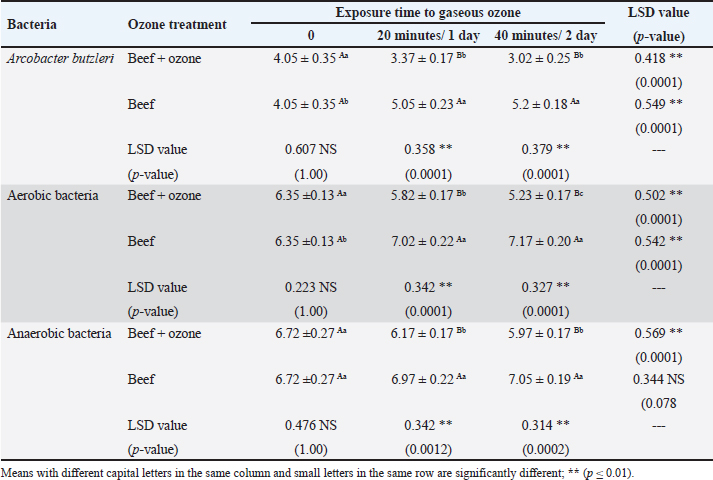
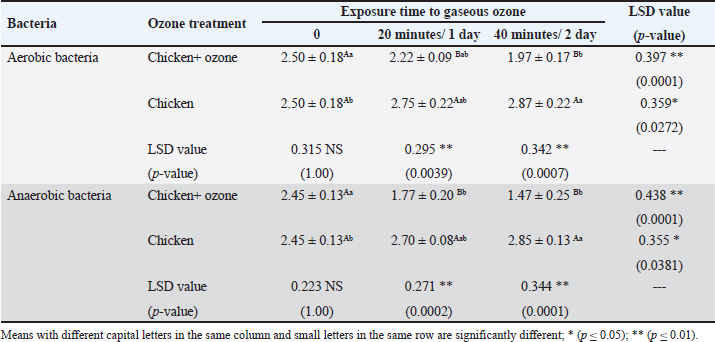
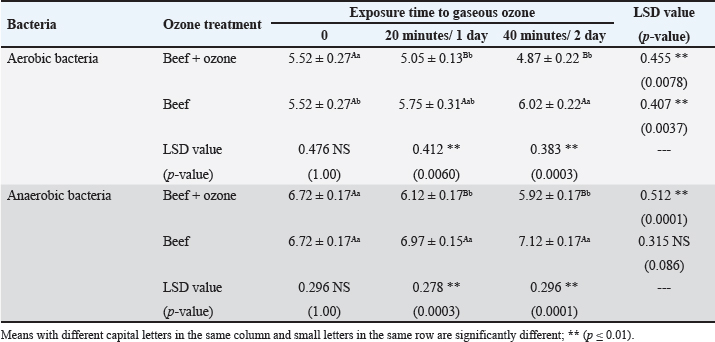
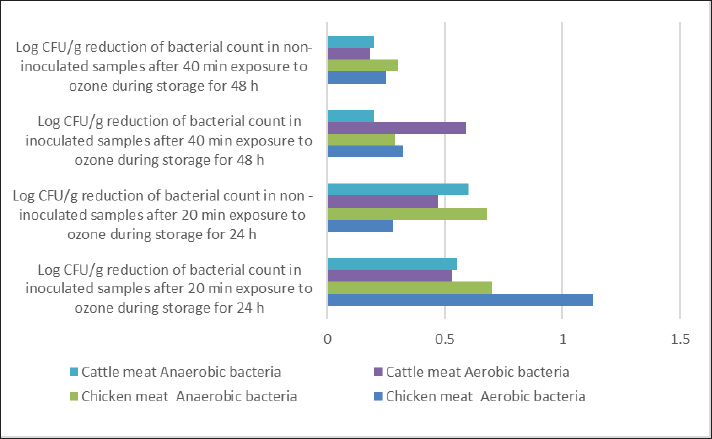
AbstractBackground: Ozone (O3) is a promising alternative antibacterial agent that has recently been used in meat processing. The understanding of the appropriate functional settings of O3 for addressing food safety problems is still insufficient. Aim: The aim of this study was, therefore, to investigate the effects of exposure to O3 on the bacteriological quality of retail meat inoculated with Arcobacter butzleri at refrigeration temperatures. Methods: Chicken and cattle meat were inoculated with 7 log CFU/g of A. butzleri previously isolated from fresh and chilled retail meat. Using an O3 generator (Ivation, Multipurpose Air Sterilizing, USA), the meat samples were treated with O3 gas (0.5 ppm) for 20 and 40 minutes at temperatures ranging from 3°C to 7°C for 48 hours. Anaerobic and aerobic bacterial growth, as well as A. butzleri were assessed. Results: The growth rates of A. butzleri, total aerobic bacteria, and anaerobic bacteria in chilled meat samples were all slowed by significant reduction by 0.75, 1.13, and 0.7 log CFU/g, respectively, when exposed to O3 gas (p ≤ 0.05). Compared to control samples kept at 3°C–7°C for a further 24 hours, the decrease was greater with an additional exposure time of 20 minutes. In contrast to the samples that did not appear to be exposed to O3, the total bacterial count of the samples exposed to O3 showed a statistically significant reduction (p ≤ 0.01) compared to the samples that were not exposed to O3. This decrease is important for the health of the population. Conclusion: This study explored the use of O3 experience as a potential antibacterial agent for meat and meat products stored in refrigerators. The results of this research may support further applications of O3 exposure, such as the installation of an O3 generator inside a refrigerator. However, further research is needed on O3 levels and exposure durations. Keywords: Arcobacter butzleri, Cattle meat, Chicken meat, Ozone, Storage conditions. IntroductionMeat is a valuable source of protein with high biological value, and the human body has become accustomed to eating meat over the course of evolution. Furthermore, the nutritional profile of meat is rich in vitamins, minerals, and other elements, and some of these nutrients are either more bioavailable or found exclusively in animal products (Font-i-Furnols, 2023). The Organization for Economic Co-operation and Development and the Food and Agriculture Organization of the United Nations forecast a 14% increase in meat consumption by 2030. The growing world population is the main reason for this increase in meat consumption (OECD/FAO, 2021). The environments in which animals have been reared up and processed, along with the properties of the end products, in which it provides ideal conditions for the development and maintenance of spoilage and harmful bacteria, making meat and meat products vulnerable to microbiological contamination (Mohammed et al., 2022; Liu et al., 2023). The microbiological safety of meat has been a public health concern for many years (Kanaan et al., 2022a; Kanaan, 2024). Different species of bacteria that cause food poisoning have been detected in meat and meat products that cause food poisoning, including Shiga-toxin-producing Escherichia coli, Campylobacter, Salmonella species, Staphylococcus aureus, Acinetobacter baumannii, and Listeria monocytogenes (Kanaan and Al-Isawi, 2019; Xu et al., 2019; Kanaan and Mohammed, 2020; Kanaan et al., 2020, 2022b; Kanaan and Khashan, 2022; Liu et al., 2023; Kanaan, 2023). Moreover, chicken meat has been recognized as the primary source of foodborne outbreaks of Arcobacter butzleri (Oliveira et al., 2018; Shange et al., 2019). Infections caused by A. butzleri provide a considerable threat to human health, resulting in severe food poisoning and sepsis (Oliveira et al. 2023). A global epidemic of A. butzleri has been documented, although the incidence of human infection is limited (Chieffi et al., 2020; Jiménez-Guerra et al., 2020). The bacterium A. butzleri has been mentioned in several studies as one of the numerous Campylobacter-like bacteria in human feces (Jiménez-Guerra et al., 2020; Oliveira et al., 2023). Moreover, any obstacles to infection with this type of bacteria are compounded by the fact that the multidrug resistant Arcobacter spreads much more widely than Campylobacter (Son et al., 2007; Riesenberg et al., 2017). Various disinfectants and some common chemical agents such as chlorine, peracetic acid, hydrogen peroxide, butylated hydroxytoluene, and tetra-butylhydroquinone were mentioned by Xue et al. (2023), for treatment and preservation to remove bacterial contamination and improve the shelf life of meat quality. Numerous of research studies have examined and assessed many food processing technologies, encompassing both thermal and non-thermal processes (Jiang et al., 2018; Stadler et al., 2020; Chacha et al., 2021; Kanaan and Tarek, 2022). Aqueous chlorine (0.5–1 ppm) is employed in the meat sector to diminish microbial load, mitigate cross-contamination risk, and maintain carcass safety. The efficacy of chlorine is contingent upon the amount employed. Nevertheless, several research indicates that chlorine by-products, including trihalomethanes, haloacetic acids, and chloramines, may possess carcinogenic properties (Gil et al., 2016; Kanaan and Abdullah, 2021; Kanaan et al., 2024a). Investigations are underway about alternatives to traditional cleaning chemicals and disinfectants that pose no risk to human and environmental health, yet possess the capability to diminish and eradicate the transmission of foodborne diseases (Kanaan et al., 2024a). O3 is classified as a Generally Recognized as Safe food processing additive that has minimal or no risk. It works considerably better for washing or sanitizing food than chemical sanitizers. O3 is a more eco-friendly technique than other antimicrobial agents currently in use as it kills microbes without leaving harmful residues on food or equipment (Pandiselvam et al., 2020; Roobab et al., 2022). Megahed et al. (2018) discovered that temperature, pH, and O3-oxidizable materials have a significant impact on O3 degradation rate and half-life. Therefore, O3 is useful in the meat processing industry when stored in cold, moist, and refrigerated environments (Bridges et al., 2018). O3 is very effective against bacteria due to its significant oxidation potential; even at doses as low as 0.01 ppm, the condition remains dangerous to microbes (Megahed et al., 2018). In addition to bacteria, molds, viruses, and protozoa are also susceptible to the antibacterial properties of O3 (Kanaan, 2018). Therefore, it has proposed O3 as a strong substitute for conventional disinfectants (Megahed et al., 2018). This study was conducted to investigate the efficacy of gaseous O3 against bacterial growth on chicken and cattle meat inoculated with A. butzleri and to evaluate its effectiveness in preserving meat at refrigeration temperature. Materials and MethodsStudy period and locationThis study was conducted from October 2023 to January 2024, in Al-Suwaira, a city in Iraq’s Wasit governorate in the middle east. Collection and processing of samplesBetween October 2023 and January 2024, a total of 32 samples of chicken (n=16) and cattle (n=16) meat were randomly collected from various supermarkets and vendor shops. The samples were packed separately in a sterile plastic bag and sent to the laboratory with ice packs within 3 hours. From the time of collection until use, all samples were stored at a temperature of −20°C in a freezer. After defrosting in the refrigerator at a temperature of 4°C for 12 hours, the wrapped meat bags were allowed to cool to room temperature for 1 hour before being inoculated. To inoculate the samples with A. butzleri, around 20 g of the material was placed on a sterile petri dish (65 × 15 mm; FB0875711C, Fisherbrand TM, UK). Two separate plastic containers, one for the inoculated and one for the non-inoculated samples, were washed with 70% ethanol to eliminate the possibility of contamination and cross-contamination before and during treatment. This study utilized a 600 mg/hour O3 generator (Ivation, Multipurpose Air Sterilising, USA) to produce O3 gas (0.5 ppm) in an O3 container, informed by previous research (Khudhir and Mahdi, 2017; Kanaan, 2018; Mohammed et al., 2022; Kanaan et al., 2024b) that employed the O3 generator 600 mg/hour to generate aqueous O3 at a concentration of 0.5 ppm over varying durations (20, 30, and 45 minutes). The O3 generator was used according to the manufacturer’s instructions. This generator has 15-time grades, so it may conduct a variety of disinfection and sterilizing duties by selecting different durations. An automatic timer plug was used to program it for 20 and 40-minute operation times, respectively. The efficacy of O3 gas (0.5 ppm) on A. butzleri and on the total bacterial population (log CFU/g) was carried out daily during this time. Calculation of ozone (O3) concentrationOzone- CHEMets Visual Kit (K-7404 O3 analysis kit, USA) (Supplementary Fig. 1) was used to measure the O3 concentration (ppm). A plastic container with 1.5 liter of tap water was covered. The container was injected with an aeration stone via the lid cavity. Four contact periods (10, 15, 30, and 45 minutes) were selected. After each exposure, the tap water was changed and the container was cleaned with fresh tap water. Five drops of A-7400 activator were added to the empty sample cup, which was filled to the 25 ml mark with ozonated water, the CHE-Met ampoule tip was inserted into the cup, the tip was broken off and ingested by the water. The ampoule was inverted several times and left for 1 minute to allow the color to change. The ampoule was placed between the color standards until a high-range comparator indicated the best color. The maximum concentration was 0.5 ppm after 15, 30, and 45 minutes. Bacterial strain preparationThe A. butzleri used in this study was obtained from a previous study (Kanaan, 2021) in which the bacterium was isolated from retail chicken and cattle meat sold in the markets of Wasit governorate in the middle east of Iraq. The bacterial strains were prepared by thawing them at 4°C and then resuscitating them on modified charcoal cefoperazone deoxycholate agar (mCCDA, Oxoid CM739, UK). They were then incubated at 30°C for 2 days. The pure colonies were cultured in Lauryl Tryptose Broth (Oxoid, CM0451, UK) (overnight at 30°C) and optical density was measured after 2 washing with buffered peptone water (BPW, Oxoid, CM0509, UK). Microbiological assessmentFor each type of sample containing both chicken and cattle meat, two different groups were formed. In the first group, an inoculum of A. butzleri at a concentration of 107 CFU per milliliter was spread evenly on the surface of meat using sterile spatulas. The second group, on the other hand, received no inoculum for comparison. Also, each group was divided into two sections, the first one was placed in a plastic chamber (L × W × H=30 × 25 × 25 cm) with normal air (21% oxygen, 78% nitrogen, and 1% other gasses) and represented the control group. The other section was placed in an airtight O3 chamber made of sturdy Tritan plastic with a lockable lid (L × W × H=30 × 25 × 25 cm), with an O3 gas intake port to inject O3 gas using an aeration stone (Diffuser) and disperse it uniformly throughout the air. An O3 generator (600 mg/hour) was used to inject gas. The O3 generator was fed with 1 liter per minute [flow rate (l pm)] of compressed air as feed gas. The gaseous O3 (0.5 ppm) was diffused into the meat samples at two different exposure times (20 and 40 minutes) at a temperature of 3°C–7°C (supplementary Fig. 2). First, O3 gas (0.5 ppm) was injected for 20 minutes at 3°C–7°C. The container was then kept at the same temperature for 24 hours before being compared with untreated samples that had also been stored at 3°C–7°C for 24 hours. In addition, the treated samples were exposed to O3 gas (0.5 ppm) at 3°C–7°C for a further 20 minutes. The chamber containing the treated samples was kept at 3°C–7°C for a further 24 hours to compare the bacterial load of the treated and untreated samples (Fig. 1). During this period, the effect of O3 gas (0.5 ppm) on A. butzleri and on the total bacterial population was constantly monitored. After every 24 hours, another meat sample was taken to assess the log CFU/g. The samples were weighed (1 g each), homogenized in a stomacher for 2 minutes, and then diluted with 9 ml of buffered peptone water. Plate Count Agar (Oxoid, CM0325B, UK) was used for the count of aerobes and anaerobes, and mCCDA-free agar was used for the total A. butzleri count. Incubation was carried out continuously at 30°C for 2 days. Dilutions were then performed according to the decimal system. Arcobacter butzleri and total aerobic bacteria were counted by incubating the plates aerobically for 48 hours at 30°C and 24 hours at 37°C, respectively. To perform the enumeration of anaerobic bacteria, Oxoid™ AnaeroGen™ 2.5 l Sachet (Oxoid, AN0025A, UK) was used in an anaerobic jar (Oxoid, AG25, UK) under anaerobic conditions. The plates were incubated at 37°C for a duration of 24 hours. The total count of aerobic and anaerobic bacteria, as well as Arcobacter butzleri, was determined using the standard plating procedure. Statistical AnalysisThe Statistical Analysis System (SAS, 2018) program, version 9, 6th edition (N.C., USA), was employed to ascertain the influence of diverse factors on the study parameters. In this research, the Least Significant Difference (LSD) test, part of the Analysis of Variance, was employed for mean comparisons (SAS, 2018). Ethical approvalThe Middle Technical University/Medical Ethics Committee/Ethics Committee Reference Number: MEC: 17 granted approval for this research on October 1, 2023. No humans or animals were employed in this investigation. Fresh meat samples were purchased from various supermarkets and vendor shops. All processes were carried out in compliance with the applicable norms and legislation. ResultsIn this study, the efficacy of gaseous O3 against the bacterial load in meat preserved at a refrigerated temperature was investigated. The results (Tables 1 and 2) showed that the initial concentrations of A. butzleri in the inoculated chicken and cattle meat samples were 3.72 and 4.05 log CFU/g, respectively. When the inoculated chicken and cattle meat samples were exposed to gaseous O3 (0.5 ppm) for 20 minutes, the number of A. butzleri decreased by 0.75 and 0.68 log CFU/g, respectively, after being kept at 3°C–7°C for 24 hours. Compared to samples not exposed to O3, high counts of A. butzleri were detected. In addition, this study showed that the total count of aerobic and anaerobic bacteria decreased in chicken and beef meat samples treated with O3 gas for 20 minutes during 24 hours of storage at 3°C–7°C compared to the untreated samples. The changes in chicken meat were also smaller: the number of aerobic and anaerobic bacteria decreased to only 1.13 log CFU/g and 0.7, respectively. The chicken meat was then kept at a refrigeration temperature of 3°C–7°C for a further day. The observations indicated a decrease in A. butzleri, aerobic and anaerobic bacteria (CFU/g) by 0.5, 0.32, and 0.29, respectively, compared to the samples used in the control group. Cattle meat showed a similar trend, with a reported lower aerobic bacterial count of 0.59 log CFU/g. Compared to the non-O3-exposed samples, a statistically significant decrease (p ≤ 0.01) in bacterial counts was observed in the O3-exposed samples. As shown in Tables 3 and 4, the non-inoculated meat samples showed no growth of A. butzleri (zero growth). Furthermore, after the non-inoculated samples were exposed to gaseous O3 at 3°C–7°C for 48 hours, there was a decrease of 0.53 log CFU/g in aerobic bacteria and 0.98 log CFU/g in anaerobic bacteria. The total count number of aerobic and anaerobic bacteria decreased by 0.65 and 0.8 log CFU/g, respectively, in the non-inoculated cattle meat samples that were exposed to O3 in the same way. These samples showed a similar trend (Fig. 2). The number of aerobic and anaerobic bacteria decreased significantly (p ≤ 0.01) in the samples that were exposed to O3 compared with the samples that were not exposed to O3 (Tables 3 and 4).
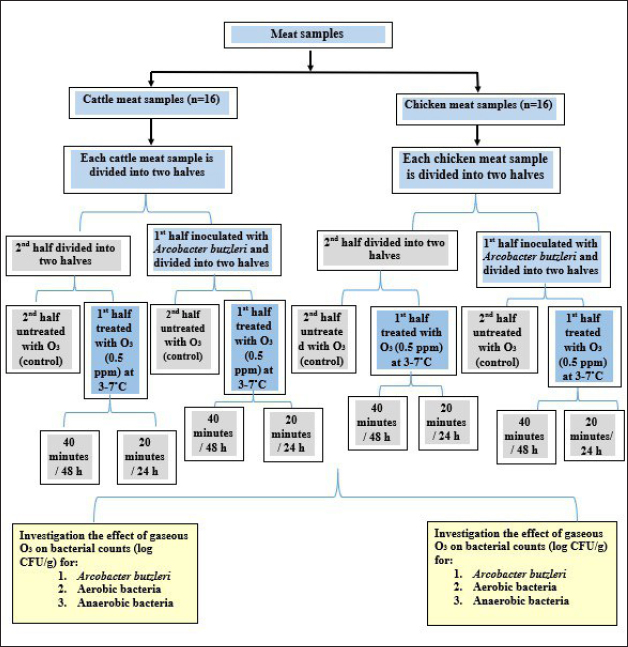
Fig. 1. A flow diagram for the study design. DiscussionResearchers have examined the effects of gaseous and liquid O3 on many microorganisms, such as viruses, protozoa, bacterial and fungal spores, and even coronaviruses, on food (Xue et al., 2023). Consumers in Iraq could be at risk of infection with A. butzleri, due to the recent detection of this bacterium in both fresh and chilled retail meat sold in the country (Jasim et al., 2021; Kanaan, 2021). Therefore, this bacterium was introduced into meat samples to evaluate the effects of O3 gas at temperatures between 3°C and 7°C over a 48-hour storage period. Table 1. Effect of O3 gas on the A. butzleri, total aerobic and anaerobic bacterial counts (log CFU/g) of inoculated chicken meat with A. butzleri during storage at 3°C–7°C.
Data in Tables (1 and 2) indicated that the exposure of meat samples infected with A. butzleri to O3 gas (0.5 ppm) for 20 minutes at 3°C–7°C resulted in a strong reduction (p ≤ 0.01) of bacterial counts. Moreover, a significant decrease in aerobic and anaerobic counts in chicken meat was observed, with aerobic counts at 1.13 log CFU/g and the anaerobic counts at 0.7 log CFU/g. This study confirmed the findings of the previously mentioned studies (Cho et al., 2014; Muhlisin et al., 2016; Khudhir and Mahdi, 2017; Kanaan, 2018; Megahed et al., 2018; Megahed et al., 2020; Giménez et al., 2021). Balamatsia et al. (2007) and Capita et al. (2013), whose research included microbial counts by type of meat at various stages of the distribution chain, found that total bacterial loads in poultry meat at the start of the storage period ranged from 4.9 to 5.66 log CFU/g. In the study by Rani et al. (2023), the most negative result was reported, the mean ± SE was only 7.7 ± 0.17 log of total bacterial count CFU/cm2 in beef during high capacity abatements. Therefore, the findings of this study were also of crucial importance for the improvement of public health and for sustainable improvement of quality and safety of food products. Due to its high reactivity and lack of toxic residues, O3 was actively studied for its bactericidal effect against foodborne pathogens. Cho et al. (2014) conducted some studies of this type and demonstrated that the growth of E. coli O157:H7 and some other anaerobic and aerobic bacteria was significantly inhibited (p ≤ 0.05) when ground beef samples were exposed to an O3 concentration of 10 × 10-6 kg O3/hour for 3 days at 4°C. Muhlisin et al. (2016) also explored the bactericidal properties of O3 in a gaseous state with regard to the total bacterial count of the chicken meat. Anaerobic and aerobic bacterial growth as well as coliform bacilli growth was inhibited, when chicken meat was treated with O3 (temperature 4°C ± 1°C) at a concentration of 10 mg O3/m3/hour over a period of 4 days. In another study conducted by Khudhir and Mahdi (2017), there was a very significant effect of antimicrobial influence on microbial counts (reduction as much as 6 logs) after aqueous O3 exposure of 0.5 parts per million for 20 minutes on bovine and ovine soft cheeses that were locally produced. Megahed et al. (2018) studied the efficacy of gaseous and aqueous O3 in eliminating pathogens derived from dairy cow dung that had infected various farm surfaces under varying operating circumstances. The researchers found that 4 minutes of gaseous O3 at 9 ppm inactivated 3.3 log of the pathogens detected in the manure. Additionally, they discovered that on rather rough surfaces, aqueous O3 at 9 ppm for 2 minutes may be an effective way to eliminate manure-based pathogens to a safe level. In contrast, on smooth surfaces, it was determined to be 4 ppm. Furthermore, Kanaan (2018) found that after half an hour of exposure to 0.5 ppm O3 in water, the bacterial count of S. aureus in infected chicken meat decreased by up to 2 logs. Table 2. Effect of O3 gas on the A. butzleri, total aerobic and anaerobic bacterial counts (log CFU/g) of inoculated cattle meat with A. butzleri during storage at 3°C–7°C.
Table 3. Effect of O3 gas on the total aerobic and anaerobic bacterial counts (log CFU/g) of non-inoculated chicken meat with A. butzleri during storage at 3°C–7°C.
Table 4. Effect of O3 gas on the total aerobic and anaerobic bacteria counts (log CFU/g) of non-inoculated cattle meat with A. butzleri during storage at 3°C–7°C.
Fig. 2. Log CFU/g reduction of bacterial counts in inoculated and non-inoculated samples after exposure to gaseous O3. Megahed et al. (2020) examined the microbial eradication capacity of aqueous mixtures of O3 and lactic acid-O3 (O3-LA) under various operation settings on chicken meat infected with a high load of Salmonella. Further studies showed that aqueous O3 (8 ppm) had a lethal effect of 1.6 log/cm2 when administered topically, while it was 1.2 log/cm2 and 1 when injected subcutaneously. Based on the observation obtained by Giménez et al. (2021), it was found that increasing the O3 concentration greatly reduced the quantity of mesophilic bacteria, lactic acid bacteria, Enterobacteriaceae, molds, and yeasts. In contrast, Botta et al. (2018) found in their study that the initial bacterial count did not change when beef samples were treated with aqueous O3. According to Xue et al. (2023), the inactivation of O3 is a multiphase process that affects various cellular structures and content elements. O3 primarily affects cell membranes, as it can filter intracellular material during stress (Botta et al., 2018). Sheng et al. (2018) noted that O3 may be able to interfere with enzymes and prevent the growth and activity of bacteria. This could be the reason for the changes reported by Aponte et al. (2018), which are due to the permeability of the cell membrane. Another research showed that O3 can cleave the DNA double bond by oxidation using of a highly reactive form of O2 (O) (Botta et al., 2018). There are a variety of internal and external variables that influence O3 when applied to microbes. According to Crowe et al. (2012), Shu et al. (2021), Liu et al. (2022), and other authors, these include various different intrinsic microbiological factors such as the type of microorganisms, characteristic of food, the physiological conditions of the microbial cells, and the microorganisms that are naturally present or artificially introduced. Additionally, the intrinsic parameters of food include the type of food, surface properties, weight, and water activity (aW) (Xue et al., 2023). Brodowska et al. (2018), Chen et al. (2020), Liu et al. (2021), Bigi et al. (2021), and Onopiuk et al. (2021) have reported that the variables for O3 therapy include temperature, contact time, O3 concentration, and the application techniques were the most common stress factors. As a result, these variations may arise from differences between studies. Tables 1 and 2 demonstrate that following preservation at 3°C–7°C for an additional 24 hours, an extension of the duration of exposure to 20 minutes resulted in a greater reduction compared with the controls. Other studies also showed that with increased exposure to O3, its efficiency against bacteria becomes higher. This was evident from the works of Kanaan (2018); Chen et al. (2020); Onopiuk et al. (2021); Aslam et al. (2021); and Kanaan et al. (2024b). However, this increase in effects seems to reach an asymptote beyond which further increases are not possible to achieve, probably due to structures within food or even due to non-viable microbial cells assumed to protect against the oxidative action of O3 (Ayranci et al., 2020). The result also showed that the bacterial inhibition rate was significantly lower when an additional day of cooling was applied. Such an effect seems to be related to the limitations of O3 exposure, as the O3 exposure could only remove superficial bacterial contamination. Tables 3 and 4, and Figure 2 confirm that, with time, the counts of total aerobic and anaerobic bacteria were lower in the case of inoculated samples than in the case of non-inoculated samples. Furthermore, the study demonstrated that exposure to O3 could in some way inhibit or retard the germs’ growth in a cold environment. According to this perspective, O3 treatment acts as an antimicrobial agent for meat products kept or stored at a cold temperature to eliminate surface bacterial infection. Cárdenas et al. (2011) previously studied the application of gaseous O3 treatment on bacterial counts on meats. Total and E. coli bacterial counts decreased by 0.5 log to attain 0 log, while the count of L. monocytogenes, injected at 102 CFU/g tissue under cold storage conditions, fell below the limit of detection. Another study conducted by Muhlisin et al. (2016) discovered that gaseous O3 applied to the beef samples did not destroy the internal form of E. coli O157:H7, but only eliminated the external contamination by this bacterium. A further piece of data supporting the effectiveness of O3 treatment during immersion chilling in improving the microbiological protection of beef comes from the finding of Kalchayanand et al. (2019) found that aqueous O3 sprays cool significantly decreased the prevalence of E. coli O157:H7 on raw beef surfaces by 1.46 log. Studies that have used O3 to reduce bacterial contamination in meat have generally shown that the surface area of ozonated meat is the primary target of O3’s antibacterial activity. The most frequent and common cause of contamination in meat is surface contamination from pathogenic microorganisms. This is an alarming situation for both health and food safety. In addition to that, O3 has been suggested as a more appropriate antibacterial agent that can improve the shelf life of meat and meat products stored in the refrigerator. ConclusionFrom this investigation, it was found that gaseous O3 of 0.5 ppm was effective in inhibiting the growth of A. butzleri in meat samples infected with A. butzleri for the first and second days of storage. In addition, gaseous O3 caused a significant reduction of bacterial populations including both aerobic and anaerobic bacteria, and greater efficacy was demonstrated for the treatment as the time of the exposure period at the same dosage was increased. In terms of public health, these results are important. The cost-effective and effective antibacterial O3-preserving treatments for food products have a number of advantages over others. Application of O3 is more efficient and energy saving in the food industry because O3 can be dissolved in much colder water, and without chemical residues, waste disposal and final rinsing are cheaper. This method improves food safety, quality, and shelf life, decreasing decomposition and spoiling losses and saving money. Additional research using different exposure times and various O3 concentrations is necessary to elucidate the impact of O3 on spoilage microorganisms and meat borne pathogens to substantiate its use and scalability in industrial environments. AcknowledgmentsThe author thanks the Technical Institute of Suwaria for letting her use its microbiology lab. The author would like to express her gratitude to Assist. Prof. Dr. Zuhair Al-Chalabi for his guidance and support. She would like to extend her gratitude to Prof. Dr. Nasr Noori Al-Anbari, College of Agriculture Engineering Sciences, University of Baghdad, for his statistical analysis support. She is also very grateful to Dr. Morteza Saki, Ahvaz Jundishapur University of Medical Sciences for his assistance in editing the English language. Conflict of interestAccording to what the author has claimed, there are no conflicting interests. FundingNo funding or contributions supported this research. Author’s ContributionsMHGK was in charge of everything involved in this work, from the research planning to the final revisions of the publication. The study’s design, sample collection, laboratory work, analysis, and writing are all included. The author evaluated and agreed to the final version of the manuscript before and during the submission of her paper. Data availabilityAll data are provided in the manuscript. ReferencesAponte, M., Anastasio, A., Marrone, R., Mercogliano, R., Peruzy, M.F. and Murru, N. 2018. Impact of gaseous ozone coupled to passive refrigeration system to maximize shelf-life and quality of four different fresh fish products. LWT. 93, 412–419. Aslam, R., Alam, M.S., Singh, S. and Kumar, S. 2021. Aqueous ozone sanitization of whole peeled onion: process optimization and evaluation of keeping quality during refrigerated storage. LWT. 151, 112183. Ayranci, U.G., Ozunlu, O., Ergezer, H. and Karaca, H. 2020. Effects of ozone treatment on microbiological quality and physicochemical properties of turkey breast meat. Ozone: Sci. Eng. 42(1), 95–103. Balamatsia, C.C., Patsias, A., Kontominas, M.G. and Savvaidis, I.N. 2007. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: correlation with microbiological and sensory attributes. Food Chem. 104(4), 1622–1628. Bigi, F., Haghighi, H., Quartieri, A., De Leo, R. and Pulvirenti, A. 2021. Impact of low-dose gaseous ozone treatment to reduce the growth of in vitro broth cultures of foodborne pathogenic/spoilage bacteria in a food storage cold chamber. J. Food Saf. 41(3), e12892. Botta, C., Ferrocino, I., Cavallero, M.C., Riva, S., Giordano, M. and Cocolin, L. 2018. Potentially active spoilage bacteria community during the storage of vacuum packaged beefsteaks treated with aqueous ozone and electrolyzed water. Int. J. Food Microbiol. 266, 337–345. Bridges, D.F., Rane, B. and Wu, V.C. 2018. The effectiveness of closed-circulation gaseous chlorine dioxide or ozone treatment against bacterial pathogens on produce. Food Control. 91, 261–267. Brodowska, A.J., Nowak, A. and Śmigielski, K. 2018. Ozone in the food industry: principles of ozone treatment, mechanisms of action, and applications: an overview. Crit. Rev. Food Sci. Nutr.58(13), 2176–2201. Capita, R., Álvarez-Fernández, E., Fernández-Buelta, E., Manteca, J. and Alonso-Calleja, C. 2013. Decontamination treatments can increase the prevalence of resistance to antibiotics of Escherichia coli naturally present on poultry. Food Microbiol. 34(1), 112–117. Cárdenas, F.C., Andrés, S., Giannuzzi, L. and Zaritzky, N. 2011. Antimicrobial action and effects on beef quality attributes of a gaseous ozone treatment at refrigeration temperatures. Food Control. 22(8), 1442–1447. Chacha, J.S., Zhang, L., Ofoedu, C.E., Suleiman, R.A., Dotto, J.M., Roobab, U., Agunbiade, A.O., Duguma, H.T., Mkojera, B.T., Hossaini, S.M. and Rasaq, W.A. 2021. Revisiting non-thermal food processing and preservation methods action mechanisms, pros and cons: a technological update (2016–2021). Foods. 10(6), 1430. Chen, C., Zhang, H., Zhang, X., Dong, C., Xue, W. and Xu, W. 2020. The effect of different doses of ozone treatments on the postharvest quality and biodiversity of cantaloupes. Postharvest Biol. Technol.163, 111124. Chieffi, D., Fanelli, F. and Fusco, V. 2020. Arcobacter butzleri: up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr Rev Food Sci Food Saf. 19, 2071–2109. Cho, Y., Choi, J.H., Hahn, T.W. and Lee, S.K. 2014. Effect of gaseous ozone exposure on the bacteria counts and oxidative properties of ground hanwoo beef at refrigeration temperature. Korean J. Food Sci. An. 34(4), 525. Crowe, K.M., Skonberg, D., Bushway, A. and Baxter, S. 2012. Application of ozone sprays as a strategy to improve the microbial safety and quality of salmon fillets. Food Control. 25(2), 464–468. Font-i-Furnols, M. 2023. Meat consumption, sustainability and alternatives: an overview of motives and barriers. Foods. 12(11), 2144. Gil, M.I. Marín, A., Andujar, S. and Allende, A. 2016. Should chlorate residues be of concern in fresh-cut salads? Food Control. 60, 416–421. Giménez, B., Graiver, N., Giannuzzi, L. and Zaritzky, N. 2021. Treatment of beef with gaseous ozone: physicochemical aspects and antimicrobial effects on heterotrophic microflora and Listeria monocytogenes. Food Control. 121, 107602. Jasim, S.A., Al-Abodi, H.R. and Ali, W.S. 2021. Resistance rate and novel virulence factor determinants of Arcobacter spp., from cattle fresh meat products from Iraq. Microb. Pathog. 152(1), 104649. Jiang, H., Liu, Z. and Wang, S. 2018. Microwave processing: effects and impacts on food components. Crit. Rev. Food Sci. Nutr. 58(14), 2476–2489. Jiménez-Guerra, G., Moreno-Torres, I.C., Moldovan, T.D., Navarro-Marí, J.M. and Gutiérrez-Fernández, J. 2020. Arcobacter butzleri and intestinal colonization. REV ESP QUIM. 33(1), 73. Kalchayanand, N., Worlie, D. and Wheeler, T. 2019. A novel aqueous ozone treatment as a spray chill intervention against Escherichia coli O157: H7 on surfaces of fresh beef. J. Food Prot. 82 (11), 1874–1878. Kanaan, M.H. 2018. Antibacterial effect of ozonated water against methicillin-resistant Staphylococcus aureus contaminating chicken meat in Wasit Province, Iraq. Vet. World. 11(10), 1445–1453. Kanaan, M.H. 2021. Prevalence, resistance to antimicrobials, and antibiotypes of Arcobacter species recovered from retail meat in Wasit marketplaces in Iraq. Int. J. One Health. 7(1), 142–150.Kanaan, M.H.G. 2023. Prevalence and antimicrobial resistance of Salmonella enterica serovars Enteritidis and Typhimurium isolated from retail chicken meat in Wasit markets, Iraq. Vet. World. 16(3), 455–463. Kanaan, M.H. 2024. Effect of biofilm formation in a hostile oxidative stress environment on the survival of Campylobacter jejuni recovered from poultry in Iraqi markets. Vet. World. 17(1), 136–142. Kanaan, M. and Abdullah, S. 2021. Evaluation of aqueous ozone as a method to combat multidrug-resistant Staphylococcus aureus tainting cattle meat sold in Wasit marketplaces. Mansoura Vet. Med. J. 22(3), 117–123. Kanaan, M.H.G. and Al-Isawi, A.J.O. 2019. Prevalence of methicillin or multiple drug-resistant Staphylococcus aureus in cattle meat marketed in Wasit province. Biochem. Cell. Arch. 19(1), 495–502. Kanaan, M.H.G., Al-Isawi, A.J.O. and Mohammed, F.A. 2022b. Antimicrobial resistance and antibiogram of thermotolerant campylobacter recovered from poultry meat in Baghdad markets, Iraq. Arch. Razi. Inst. 77(1), 249–255. Kanaan, M.H.G., Al-Shadeedi, S.M., Al-Massody, A.J. and Ghasemian, A. 2020. Drug resistance and virulence traits of Acinetobacter baumannii from Turkey and chicken raw meat. Comp. Immunol. Microbiol. Infect. Dis. 70, 101451. Kanaan, M.H.G., Khalil, Z.K., Khashan, H.T. and Ghasemian, A. 2022a. Occurrence of virulence factors and carbapenemase genes in Salmonella enterica serovar Enteritidis isolated from chicken meat and egg samples in Iraq. BMC Microbiol. 22(1), 1–8. Kanaan, M.H. G. and Khashan, H.T. 2022. Molecular typing, virulence traits and risk factors of pandrug-resistant Acinetobacter baumannii spread in intensive care unit centers of Baghdad city, Iraq. Rev. Med. Microbiol. 33(1), 51–55. Kanaan, M.H.G. and Mohammed, F.A. 2020. Antimicrobial resistance of Campylobacter jejuni from poultry meat in local markets of Iraq. Plant Arch. 20(Suppl. 1), 410–415. Kanaan, M.H.G., Mohammed, F.A. and Abdullah, S.S. 2024a. The effectiveness of aqueous ozone on residual chlorination by products in treated chicken meat. IOP Publishing. Conf. Ser. Earth Environ. Sci. 1371(6), 062036. Kanaan, M.H.G., Razak, F.S.A. and Abdullah, S.S. 2024b. Effect of ozone treatment on thermo tolerant Campylobacter contaminated poultry products retailed at Baghdad markets, Iraq. Egypt. J. Nutr. 39(2), 1–10. Kanaan, M.H.G. and Tarek, AM. 2022. Innovative modern bio-preservation module of meat by lytic bacteriophages against emergent contaminants. Open Vet. J. 12(6), 1018–1026. Khudhir, Z. and Mahdi, A.M. 2017. The efficacy of ozonated water on the microbial quality of locally produced soft cheese in Baghdad. Int. J. Sci. Res. 6(10), 338–387. Liu, C., Chen, C., Jiang, A., Zhang, Y., Zhao, Q. and Hu, W. 2021. Effects of aqueous ozone treatment on microbial growth, quality, and pesticide residue of fresh-cut cabbage. Food Sci. Nutr. 9(1), 52–61. Liu, Z., Shaposhnikov, M., Zhuang, S., Tu, T., Wang, H. and Wang, L. 2023. Growth and survival of common spoilage and pathogenic bacteria in ground beef and plant-based meat analogues. Food Res. Int. 164, 112408. Liu, Q., Zhang, R., Xue, H., Bi, Y., Li, L., Zhang, Q., Kouasseu, C.J., Nan, M. and Prusky, D. 2022. Ozone controls potato dry rot development and diacetoxyscirpenol accumulation by targeting the cell membrane and affecting the growth of Fusarium sulphureus. Pysiol. Mol. Plant 118, 101785. Megahed, A., Aldridge, B. and Lowe, J. 2018. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS One. 13(5), e0196555. Megahed, A., Aldridge, B. and Lowe, J. 2020. Antimicrobial efficacy of aqueous ozone and ozone–lactic acid blend on Salmonella-contaminated chicken drumsticks using multiple sequential soaking and spraying approaches. Front. Microbiol. 11, 593911. Mohammed, F.A., Kanaan, M.H.G. and Tarek, A.M. 2022. Assessment of the effect of aqueous ozone treatment on the sensory attributes of retail meat in the Iraqi Wasit governorate. Rev. Electron. Vet. 23(3), 28–38. Muhlisin, M., Utama, D.T., Lee, J.H., Choi, J.H. and Lee, S.K. 2016. Effects of gaseous ozone exposure on bacterial counts and oxidative properties in chicken and duck breast meat. Korean J. Food Sci. Anim. Resour. 36(3), 405. OECD/FAO. 2021. OECD-FAO Agricultural Outlook 2021–2030, Paris, France: OECD Publishing. Oliveira, M.G.X.D., Cunha, M.P.V., Moreno, L.Z., Saidenberg, A.B.S., Vieira, M.A.M., Gomes, T.A.T., Moreno, A.M. and Knöbl, T. 2023. Antimicrobial resistance and pathogenicity of Aliarcobacter butzleri isolated from poultry meat. Antibiotics. 12(2), 282. Oliveira, M.G.X.D., Gomes, V.T.D.M., Cunha, M.P.V., Moreno, L.Z., Moreno, A.M. and Knöbl, T. 2018. Genotypic characterization of Arcobacter spp. isolated from chicken meat in Brazil. Foodborne Pathog. Dis. 15(5), 293–299. Onopiuk, A., Szpicer, A., Wojtasik-Kalinowska, I., Wierzbicka, A. and Półtorak, A. 2021. Impact of ozonisation time and dose on health related and microbiological properties of rapanui tomatoes. Agriculture. 11(5), 428. Pandiselvam, R., Kaavya, R., Jayanath, Y., Veenuttranon, K., Lueprasitsakul, P., Divya, V., Kothakota, A. and Ramesh, S.V. 2020. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods–a review. Trends Food Sci. Technol. 97, 38–54. Rani, Z.T., Mhlongo, L.C. and Hugo, A. 2023. Microbial profiles of meat at different stages of the distribution chain from the abattoir to retail outlets. Int. J. Environ. Res. Public Health. 20(3), 1986. Riesenberg, A., Frömke, C., Stingl, K., Feßler, A.T., Gölz, G., Glocker, E.O., Kreienbrock, L., Klarmann, D., Werckenthin, C. and Schwarz, S. 2017. Antimicrobial susceptibility testing of Arcobacter butzleri: development and application of a new protocol for broth microdilution. J. Antimicrob. Chemother. 72(10), 2769–2774. Roobab, U., Chacha, J.S., Abida, A., Rashid, S., Muhammad Madni, G., Lorenzo, J.M., Zeng, X.A. and Aadil, R.M. 2022. Emerging trends for nonthermal decontamination of raw and processed meat: ozonation, high-hydrostatic pressure and cold plasma. Foods. 11(15), 2173. Shange, N., Gouws, P. and Hoffman, L.C. 2019. Campylobacter and Arcobacter species in food-producing animals: prevalence at primary production and during slaughter. World J. Microbiol. Biotechnol. 35, 1–16. Sheng, L., Hanrahan, I., Sun, X., Taylor, M.H., Mendoza, M. and Zhu, M.J. 2018. Survival of Listeria innocua on Fuji apples under commercial cold storage with or without low dose continuous ozone gaseous. Food Microbiol. 76, 21–28. Shu, X., Singh, M., Karampudi, N.B.R., Bridges, D.F., Kitazumi, A., Wu, VC. and De los Reyes, B. G. 2021. Responses of Escherichia coli and Listeria monocytogenes to ozone treatment on non-host tomato: efficacy of intervention and evidence of induced acclimation. PLoS One. 16(10), e0256324. Son, I., Englen, M.D., Berrang, M.E., Fedorka-Cray, P.J. and Harrison, M.A. 2007. Antimicrobial resistance of Arcobacter and Campylobacter from broiler carcasses. Int. J. Antimicrob. Agents. 29(4), 451–455. Stadler, D., Berthiller, F., Suman, M., Schuhmacher, R. and Krska, R. 2020. Novel analytical methods to study the fate of mycotoxins during thermal food processing. Anal. Bioanal. Chem. 412, 9–16. Statistical Analysis System (SAS), 2018. User’s guide. Statistical. Version 9, 6th ed. Cary, NC: SAS. Inst. Inc.. Xu, J., Zhang, P., Zhuang, L., Zhang, D., Qi, K., Dou, X., Wang, C. and Gong, J. 2019. Multiplex polymerase chain reaction to detect Salmonella serovars Indiana, Enteritidis, and Typhimurium in raw meat. J. Food Saf. 39(5), e12674. Xue, W., Macleod, J. and Blaxland, J. 2023. The use of ozone technology to control microorganism growth, enhance food safety and extend shelf life: a promising food decontamination technology. Foods. 12(4), 814. Supplementary Materials
Supplementary Fig. 1. Ozone- CHEMets Visual Kit (K-7404 ozone analysis kit).
Supplementary Fig. 2. The diagram of the gaseous O3 chamber. | ||
| How to Cite this Article |
| Pubmed Style Manal Hadi Ghaffoori Kanaan. Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Vet. J.. 2024; 14(11): 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 Web Style Manal Hadi Ghaffoori Kanaan. Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. https://www.openveterinaryjournal.com/?mno=208214 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i11.8 AMA (American Medical Association) Style Manal Hadi Ghaffoori Kanaan. Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Vet. J.. 2024; 14(11): 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 Vancouver/ICMJE Style Manal Hadi Ghaffoori Kanaan. Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Vet. J.. (2024), [cited January 24, 2026]; 14(11): 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 Harvard Style Manal Hadi Ghaffoori Kanaan (2024) Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Vet. J., 14 (11), 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 Turabian Style Manal Hadi Ghaffoori Kanaan. 2024. Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Veterinary Journal, 14 (11), 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 Chicago Style Manal Hadi Ghaffoori Kanaan. "Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets." Open Veterinary Journal 14 (2024), 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 MLA (The Modern Language Association) Style Manal Hadi Ghaffoori Kanaan. "Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets." Open Veterinary Journal 14.11 (2024), 2794-2805. Print. doi:10.5455/OVJ.2024.v14.i11.8 APA (American Psychological Association) Style Manal Hadi Ghaffoori Kanaan (2024) Effectiveness of gaseous ozone on Arcobacter butzleri and bacterial loads on retailed meat sold at Iraqi Wasit markets. Open Veterinary Journal, 14 (11), 2794-2805. doi:10.5455/OVJ.2024.v14.i11.8 |