| Research Article | ||
Open Vet. J.. 2024; 14(11): 2806-2816 Open Veterinary Journal, (2024), Vol. 14(11): 2806-2816 Research Article Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approachSriram Vamshi Krishna1, Aniket Sarkar2, Suchandan Banerjee2 and Anindya Sundar Panja2,3*1Department of Veterinary Microbiology, College of Veterinary Science, P.V. Narismha Rao Telangana Veterinary University, Warangal, India 2Department of Biotechnology, Molecular Informatics Laboratory, Oriental Institute of Science and Technology, Vidyasagar University, Midnapore, India 3Department of Biotechnology, Burdwan Institute and Management and Computer Science, Burdwan, India *Corresponding Author: Anindya Sundar Panja. Department of Biotechnology, Molecular Informatics Laboratory, Oriental Institute of Science and Technology, Vidyasagar University, Midnapore, India. Email: biotech2ani [at] gmail.com; anindyapanja [at] oist.edu.in Submitted: 29/07/2024 Accepted: 19/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Lumpy skin disease (LSD) is a transboundary virus disease that mostly affects cattle. It has recently been reported all over the world, which highlights the need for efficient control methods. LSD poses serious economic dangers worldwide. Aim: The aim of this study was to screen novel antiviral compounds for the control of LSD. Methods: By using in silico approach, ADMET, docking, and molecular simulations, this work was designed to investigate 13 active compounds for antiviral effects against Lumpy skin disease virus (LSDV). Results: ADMET study of the selected 13 compounds revealed that Apigenin-4’-glucoside and Vidarabine did not show any critical hazards. The docking study identified potential antiviral compounds against LSDV, with Apigenin-4’-glucoside (ΔG=-6.6 ± 1.1) and Vidarabine (ΔG=-5.53 ± 0.73) showing promising interactions with key viral proteins. Molecular dynamics simulations confirmed the stability and robustness of these interactions, suggesting their potential as effective antiviral agents. Conclusion: Molecular analyses verify the strong antiviral activity of apigenin-4’-glucoside against LSDV among the selected compounds. This work sheds light on the way to explore potent anti-LSDV molecule. Moreover, the outcome of the study should screen after more extensive clinical studies. Keywords: Lumpy skin disease, ADMET, Antiviral, Molecular simulations. IntroductionLumpy skin disease (LSD), an important transboundary disease listed under threatens. LSD is listed due to its potential for rapid spread and for causing significant economic losses in the dairy sector worldwide (Tuppurainen and Oura, 2012; Akther et al., 2023). Lumpy skin disease virus (LSDV) is an enveloped DNA virus containing around 150 kilobase pairs genome with relatively large sizes (230–260 nm). The LSDV belongs to genus Capripoxvirus, which is genetically related to the sheep pox virus (SPPV) and goat pox virus (GTPV) viruses (Buller et al., 2005; Bhanuprakash et al., 2006; Givens, 2018). LSDV infects all ages and breeds of cattle, although the young and cattle in the peak of lactation are highly susceptible (Tuppurainen et al., 2011). Until 1989, LSD was reported in the African continent only. However, the disease is moved outside Africa to Madagascar and the Middle East and caused serious economic loss to the livestock industry (Brenner et al., 2006; Tuppurainen and Oura, 2012). Recently LSD is reported in several disease-free countries, including India. Dairy industries should be concerned about the importance of its transmission, control, and eradication (Sprygin et al., 2019). The disease was first reported in India in 2013, and since then, it has spread rapidly across the country, causing significant losses to the dairy industry (Batra et al., 2015; Allam et al., 2018). Capripoxviruses is typically host-specific, can cross-infect, with SPPV and GTPV causing disease in both species. Diagnosis relies on clinical signs: pain, limb swelling, appetite loss, fever, and nodular skin, especially in cattle. These clinical manifestations aid LSD diagnosis and treatment monitoring (Bhadauria et al., 2023). Confirmatory diagnosis can be made based on Electron microscopy, immunoperoxidase staining, Enzyme-linked immunosorbent assay, including polymerase chain reaction test. Preventive measures for LSD include restricting the movements of the sick cattle, quarantine, and sacrificing the infected cattle are highly recommended (Wolff et al., 2020). Control and prevention of LSD in countries like Albania, Bulgaria, Greece, Montenegro, Serbia, and Ethiopia already concentrate on vaccination strategies (Gari et al., 2015; Klement et al., 2020). Because the LSDV has an intricate immune escape mechanism, no safe and efficient vaccines have been developed for this disease till now. SPPV and GTPV have antigenic homology and cross-protection with LSDV. Therefore, the vaccines of these two viruses can be used to prevent the LSD. Although, the above two vaccines may have some potential risks because they are live attenuated vaccines (Liu et al., 2021). Several scientists already demonstrated different mathematical model, focusing on equilibrium stability and control strategies to stabilize LSD-free states. Through mathematical model and simulation, the effectiveness of the propose control schemes are demonstrated for stabilizing the disease-free equilibrium point (Alfwzan et al., 2023; El-Mesady et al., 2024). Different plant extracts were already reported for potential antiviral properties against poxviruses, including variola virus (smallpox) and vaccinia virus. Several researches indicate compounds like flavonoids and phenolic acids may combat poxviruses (Kotwal et al., 2005). Our study focuses on evaluating the antiviral mechanism, and biosafety of these selected active compounds against LSDV. To, reduce clinical signs in infected animals, the screened molecules can be used as a supportive treatment. In our study, active antimicrobial compounds from different plant extracts were taken against LSDV, conducted in silico experiments was performed to explore antiviral efficacy to identify active potent compounds against LSDV. ADMET was analyzed to assess the biosafety and efficacy of the selected active compounds (Fig. 1). Molecular docking and simulations were studied to reveal the insight of interaction and stability of the inhibitors with LSDV ankyrin proteins, DNA and RNA polymerases, glycoproteins, and membrane proteins. Materials and MethodsSequence data retrievalThe FASTA format files of the LSDV ankyrin proteins (NP_150581.1, NP_150582.1, NP_150586.1), DNA (NP_150473.1) and RNA (NP_150489.1, NP_150503.1, NP_150505.1, NP_150519.1, NP_150530.1) polymerases, glycoproteins (NP_150556.1, NP_150557.1, NP_150560.1), and membrane proteins (NP_150534.1, NP_150538.1, NP_150540.1, NP_150546.1) were retrieved from NCBI database (https://www.ncbi.nlm.nih.gov/). Sequences were taken to model structures, docking, and simulations accordingly.
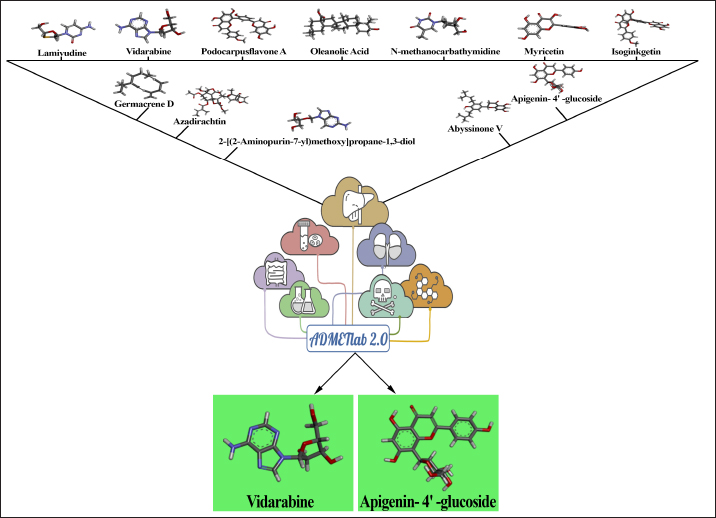
Fig. 1. Selection of potential bioactive compounds and ADMET pharmacokinetic profiling of apigenin-4’–glucoside and vidarabine. Homology modeling and structural refinementHomology modeling method was used to build the three-dimensional structures of LSDV proteins, was modeled via SWISS-MODEL server (Guex et al., 2009; Waterhouse et al., 2018). Template structures were taken with high sequence similarity to the target LSDV proteins, specifically ankyrin proteins (5y4f, 5le7.2.A), DNA (5n2g), and RNA (6ric, 7amv, 6rfl, 6rid) polymerases, glycoproteins (3k7b, 2ox9, 7ul6), and membrane proteins (6v36, 8c8g, 8c03, 8ss6, 6c6l, 6v36) to build model structures. The selected templates, derived from structurally resolved proteins with known homologous sequences, served as the basis for generating the structural models. The SWISS-MODEL server employs advanced algorithms to predict the atomic coordinates of the target proteins, incorporating template-based modeling methodologies. Subsequently, the generated models underwent meticulous validation using state-of-the-art tools such as PROCHECK (Laskowski et al., 1993). The 3D models were then improved using 3Drefine (http://sysbio.rnet.missouri.edu/3Drefine/) online protein structure refinement servers in a two-step procedure (Bhattacharya et al., 2013). Pharmacokinetic profiling and phytochemicals selectionThirteen active phytochemical compounds (Podocarpusflavone A, Isoginkgetin, Vidarabine, Lamivudine, Mycophenolic acid, Azadirachtin, N-methanocarbathymidine, 2-[(2-Aminopurin-7-yl)methoxy]propane-1,3-diol, Germacrene D, Oleanolic Acid, myricetin, apigenin- 4’ –glucoside, Abyssinone V) were taken from the previously reported literature which were implicated in potential therapeutic interventions against capripoxvirus (Bagla et al., 2012; Sadgrove et al., 2020; Kim et al., 2022; Mustafa et al., 2023; Chiem et al., 2023;). Subsequently, the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was utilized to download the structure data file format for each of these compounds. To ascertain their drug-likeness-based absorption rates, distribution within the body, metabolism, excretion, and potential toxicity properties, an analysis was conducted using the ADMETlab 2.0 web server (Lei et al., 2016). Molecular dockingMolecular docking analyses were conducted using AUTO DOCK v4.2.6 to explore the potential binding interactions between the identified active phytochemical compounds and key LSDV proteins, including ankyrin proteins, DNA and RNA polymerases, glycoproteins, and membrane proteins (Forli et al., 2016). The docking process aimed to predict the binding modes and affinities of the phytochemicals within the active sites of the target proteins. In order to run the Autodock genetic algorithm, the ligands were ready for docking, and the grid size (X=60; Y=60; Z=55) and center were specified. The binding energy scores were computed, providing quantitative insights into the strength of the ligand-protein interactions. Post-docking, the results were further scrutinized and visualized using Discovery Studio Visualizer v20.1.0.19295 to gain a comprehensive understanding of the molecular interactions, including hydrogen bonds, hydrophobic contacts, and other key binding features (Riyaphan et al., 2021). MD simulationUsing the GROMACS software, 5 nanosecond simulation was executed with the integration of both NPT (constant Number of particles, Pressure, and Temperature) and NVT (constant Number of particles, Volume, and Temperature) ensembles (Bekker et al., 1993). The simulation maintained a constant temperature of 310 K, mimicking physiological conditions. The CHARMM27 force field was employed to model the intermolecular interactions and energetics. Throughout the simulation, the trajectories of the phytochemicals within the LSDV protein binding sites were closely monitored. Post-simulation, the root mean square deviation (RMSD) was calculated to quantify the overall stability and deviation of the ligand-protein complexes over time. Additionally, the root mean square fluctuation (RMSF) was assessed to elucidate the flexibility and dynamic fluctuations of specific residues within the LSDV protein complexes. Ethical approvalThis article does not contain studies with animal or human subjects performed by any of the authors. So, the requirement of ethical approval is inapplicable for this article. ResultsSelection of active compounds through ADMET studyNumerous phytochemicals were already reported as potential candidates for combating capripoxvirus, including Podocarpusflavone A, Isoginkgetin, Vidarabine, Lamivudine, Mycophenolic acid, Azadirachtin, N-methanocarbathymidine, 2-[(2-Aminopurin-7-yl)methoxy]propane-1,3-diol, Germacrene D, Oleanolic Acid, myricetin, apigenin-4’–glucoside, and Abyssinone V. While these compounds have shown promise in various studies, the selection of the most suitable candidate for further investigation necessitates a meticulous evaluation of drug-likeness properties, encompassing absorption rates, distribution within the body, metabolism, excretion, and potential toxicity. To address this crucial aspect, we conducted a comprehensive analysis using the ADMETlab 2.0 web server. This analytical approach facilitates a systematic assessment of the pharmacokinetic properties of each candidate, aiding in the identification of the most promising compound for subsequent in-depth studies. Following this rigorous analysis, apigenin-4’-glucoside and Vidarabine emerged as the most favorable candidate; demonstrating superior drug-likeness attributes (Supplementary Table 1: can be requested from the corresponding author). Molecular dockingTo identify potential antiviral compounds against LSDV, a comprehensive molecular docking study was conducted. Following an initial screening based on ADMET parameters, a total of sixteen protein structures were taken for docking analysis to reveal interactomics. Interactome profile of apigenin-4’-glucosideApigenin-4’-glucoside emerged as one of the most promising candidates, exhibiting favorable pharmacokinetic properties. The subsequent molecular docking analyses involved a total of sixteen docking complex generation (Fig. 2), exploring the interactions between apigenin-4’-glucoside and various LSDV proteins, including ankyrin proteins, DNA polymerase, RNA polymerase, membrane proteins, and glycoproteins. Specifically, three docking interactions were investigated using apigenin-4’-glucoside, the ankyrin protein (NP_150581.1) exhibited a total of 17 interactions, the majority of which were van der Waals contacts, two covalent hydrogen bond interactions, and two pi-cation interactions with the corresponding biding free energy of −7.6 kcal/mol; the ankyrin protein (NP_150582.1) showed 13 interactions in all, four of which were covalent hydrogen bond interactions, one pi-sigma interaction, one carbon-hydrogen interaction, and most of which were van der Waals interactions with corresponding biding free energy of −6.5 kcal/mol; a total of 15 interactions were detected in the ankyrin protein (NP_150586.1), including five van der Waals contacts, four conventional hydrogen bonds, and one pi-sigma interaction with corresponding biding free energy of −7.1 kcal/mol. In the case of DNA polymerase (NP_150473.1) and apigenin-4’-glucoside complex revealed a total of 15 interactions with a binding energy of −7.5 kcal/mol. Three docking studies were carried out for glycoproteins, unraveling intricate ligand-protein interactions; glycoprotein (NP_150556.1) showed total 12 interactions with corresponding binding free energy of −6.8 kcal/mol; with glycoprotein (NP_150557.1) revealed total 16 interactions with corresponding binding free energy of −7 kcal/mol; and finally, glycoprotein (NP_150560.1) showed total 7 interactions with corresponding binding energy of −5.4 kcal/mol. Membrane proteins were the focus of four docking interactions; first membrane protein (NP_150534.1) showed a total of 11 interactions with corresponding binding free energy of −6 kcal/mol; with corresponding binding free energy −5.7 kcal/mol membrane protein (NP_150538.1) showed total 11 interactions; membrane protein (NP_150539) shoed total 13 interactions with binding free energy of −5.3 kcal/mol; membrane protein (NP_150546.1) showed total 6 interactions with binding free energy of −5.7 kcal/mol.
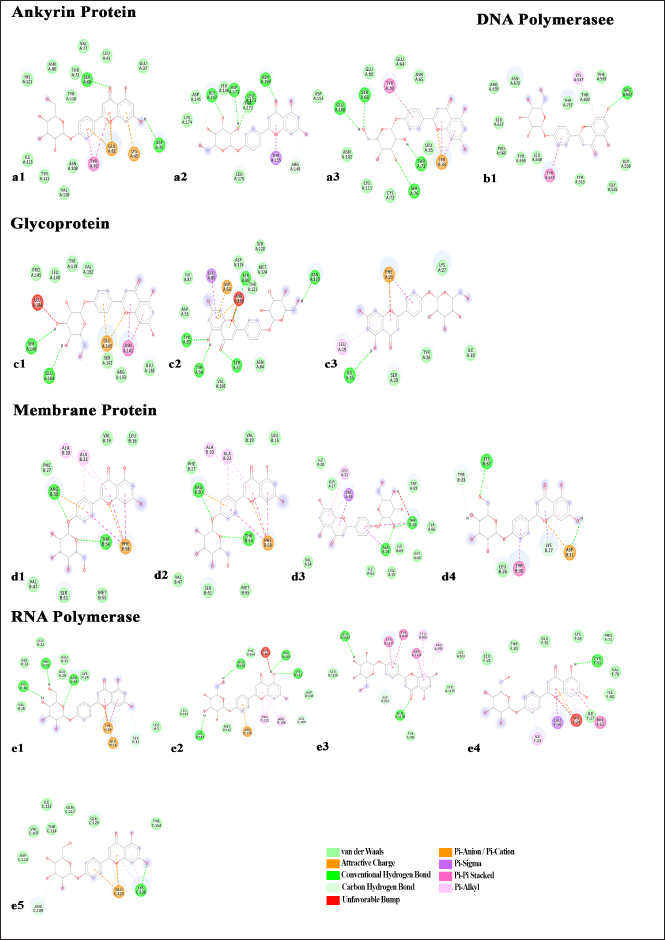
Fig. 2. Interactome profile of Ankyrin protein (a1 – a3), DNA polymerase (b1), Glycoprotein (c1 – c3), membrane protein (d1 – d4) and RNA polymerase (e1 – e5); with Apigenin-4’-glucoside.
Fig. 3. Interactome profile of Ankyrin protein (a1 – a3), DNA polymerase (b1), Glycoprotein (c1 – c3), membrane protein (d1 – d4) and RNA polymerase (e1 – e5); with Vidarabine. Additionally, RNA polymerase interactions (NP_150489.1) showed 13 in all, 3 of which were covalent hydrogen bond, 2 Pi-Cationa, and 1 Cation Hydrogen bond with corresponding binding free energy of −6.7 kcal/mol; A total of 13 interactions were detected in the RNA polymerase (NP_150503.1) with corresponding binding free energy of −6.9 kcal/mol; on the other hand RNA polymerase (NP_150505.1) showed total 12 interactions with corresponding binding free energy of −8.9 kcal/mol; RNA polymerase (NP_150519.1) showed total 13 interactions with corresponding binding free energy of −6.1 kcal/mol; Lastly total 10 interactions were detected with RNA Polymerase (NP_150530.1) with corresponding binding free energy of −5.9 kcal/mol. A total number of interactions of apigenin-4’-glucoside with Ankyrin protein, DNA polymerase, Glycoprotein, membrane protein and RNA polymerase were represented in Figure 4. The highest number of interactions were documented in case of Apigenin-4’-glucoside when compared with Vidarabine. Interactome profile of VidarabineVidarabine also emerged as one of the most promising drug candidates, exhibiting favorable pharmacokinetic properties. The subsequent molecular docking analyses involved a total of sixteen docking complex generation (Fig. 3), exploring the interactions between Vidarabine and various LSDV proteins, including ankyrin proteins, DNA polymerase, RNA polymerase, membrane proteins, and glycoproteins. Three docking interactions were investigated using Vidarabine, the ankyrin protein (NP_150581.1) exhibited a total of 12 interactions, with corresponding biding free energy of -6 kcal/mol; the ankyrin protein (NP_150582.1) showed 12 interactions in all, with corresponding biding free energy of −4.9 kcal/mol; A total of 13 interactions were detected in the ankyrin protein (NP_150586.1), with corresponding biding free energy of −5.8 kcal/mol. In the case of DNA polymerase, a single docking interaction was studied (NP_150473.1) and revealed a total of 12 interactions with providing insights into the binding energy of −5.8 kcal/mol with apigenin-4’-glucoside. Three docking study were carried out for glycoprotein proteins, unraveling intricate ligand-protein interactions; glycoprotein (NP_150556.1) showed total 10 interactions with corresponding binding free energy of −5.2 kcal/mol; with glycoprotein (NP_150557.1) revealed total 11 interactions with corresponding binding free energy of −5.7 kcal/mol; and lastly glycoprotein (NP_150560.1) showed total 5 interactions with corresponding binding energy of −3.4 kcal/mol. Membrane proteins were the focus of four docking interactions; first membrane protein (NP_150534.1) showed total 9 interactions with corresponding binding free energy of −4.7 kcal/mol; with corresponding binding free energy −3.5 kcal/mol membrane protein (NP_150538.1) showed total 5 interactions; membrane protein (NP_150539) showed total 7 interactions with binding free energy of −4.1 kcal/mol; membrane protein (NP_150546.1) showed total 5 interactions with binding free energy of −3.9 kcal/mol. Additionally, RNA polymerase interactions (NP_150489.1) showed 9 in all with corresponding binding free energy of −4.7 kcal/mol; A total of 10 interactions were detected in the RNA polymerase (NP_150503.1) with corresponding binding free energy of −5.6 kcal/mol; on the other hand RNA polymerase (NP_150505.1) showed total 4 interactions with corresponding binding free energy of −4.2 kcal/mol; RNA polymerase (NP_150519.1) showed total 9 interactions with corresponding binding free energy of −4.6 kcal/mol; Lastly, total 10 interactions were detected with RNA Polymerase (NP_150530.1) with corresponding binding free energy of −5.3 kcal/mol. Total number of interactions Vidarabine with Ankyrin protein, DNA polymerase, Glycoprotein, membrane protein, and RNA polymerase were represented in Figure 4. Molecular dynamics (MD) simulationThe MD simulation (Five nanoseconds) for the docking complexes resulted RMSD values which provided key insights into the stability and dynamic behavior of the apigenin-4’-glucoside with different LSDV protein interactions (Fig. 5). The RMSD analysis revealed the robustness of docking complexes maintained a relatively stable trajectory throughout the simulation time. The deviations from the initial structures remained within acceptable ranges, indicating a consistent and equilibrium state of the ligand-protein complexes. This observation suggested that the binding interactions between apigenin-4’-glucoside and LSDV proteins were robust and persist minimal structural fluctuations. Complementary to RMSD, the RMSF analysis offered a detailed perspective on the flexibility and fluctuation patterns of specific residues within the LSDV proteins during the simulation. Significantly, the conserved binding sites displayed minimal fluctuation, confirming the reliability and sustained binding of apigenin-4’-glucoside with the target proteins. DiscussionLumpy skin disease is a serious globally distributed infection that is classified as threatening for all ages and breeds of cattle. The integration of molecular docking and MD simulations has unveiled compelling insights into the promising antiviral properties of apigenin-4’-glucoside against LSDV. The initial screening based on ADMET parameters identified apigenin-4’-glucoside as a stand-out candidate, showcasing favorable pharmacokinetic attributes. Subsequent molecular docking analyses, involving total 15 simulations, interactions between apigenin-4’-glucoside and key LSDV proteins like Ankyrin proteins, DNA polymerase, RNA polymerase, Membrane proteins, and Glycoproteins. Apigenin- 4’ –glucoside, Myricetin, and Abyssinone-V were already reported as antiviral compounds. These antiviral compounds are found to possess significant pharmacodynamics properties with negligibly toxic after studying their toxicity indexing. These molecules were already reported to be effective against herpes virus and found to inhibit the action of polymerase, ATPase, and membrane integrity which are critical for virus survival (Kwofie et al., 2022). The detailed examination of docking complexes revealed diverse binding affinities and interactome profiles across different proteins of LSDV. Notably, the specific docking interactions demonstrated distinct binding sites and associated binding energies. Docking studies revealed interesting binding modes of apigenin-4’-glucoside and Vidarabine with Ankyrin, DNA polymerase, RNA polymerase, membrane protein, and glycoprotein interactions crucial for inhibiting viral life cycle. The MD simulation over a 5 nanoseconds period, with analyses of RMSD and RMSF indicating the stability and robustness of the apigenin-4’-glucoside with different LSDV protein complexes. The low RMSD deviation values indicated a conserved and stable trajectory, while the RMSF analysis indicated minimal deviations into the binding pockets, showing stable binding interactions. Apigenin is already reported that it can inhibit viral DNA, mRNA, and proteins synthesis, without affecting other steps of viral life cycle in an in vitro and in vivo antiviral testing against buffalopox virus (Khandelwal et al., 2020). Several studies already documented that, apigenin is a non-toxic, non-allergic, anti-cancerous molecule. Apigenin is effective of restricting for the proliferation of cancer cell, also it can inhibit metastasis by via regulating different pathway like Akt signaling pathway (Ashrafizadeh et al., 2020; Javed et al., 2021; Naponelli et al., 2024). Several in silico study was already reported that the polyphenolic apigenin having promising antiviral properties against HSV-1, HIV, SARS-CoV, and Influenza Virus (Montenegro-Landívar et al., 2021; Lee et al., 2023). Similar to our findings, apigenin was found to represent as an anti-viral activity against Ankyrin, DNA polymerase, RNA polymerase, membrane protein, and glycoprotein of LSDV. Through this vetinformatics approach apigenin-4’-glucoside emerges as a potent antiviral compound which exhibiting promising interactions with key viral proteins of LSDV. Apigenin analysis represented as a remarkable potent antiviral agent. Consequently, it shows the inhibitory effect against major five protein of LSDV, which shows interesting prospect in the field of LSDV therapy.
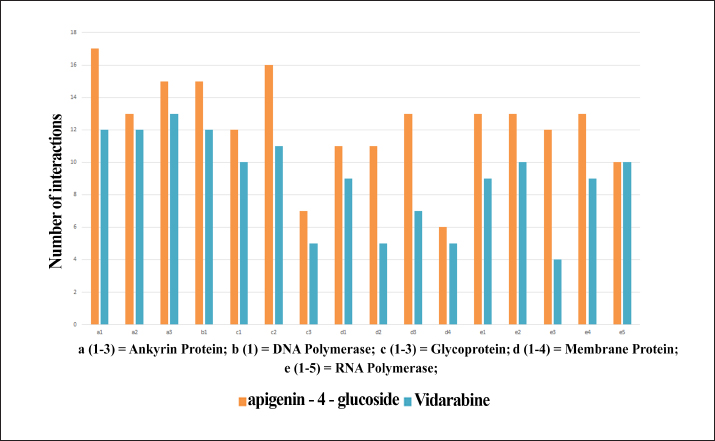
Fig. 4. Total number of protein ligand interactions (y axis) of between selected bioactive compounds with (y axis) ankyrin protein (a1 – a3), DNA polymerase (b1), Glycoprotein (c1 – c3), membrane protein (d1 – d4) and RNA polymerase (e1 – e5); orange color bar represent docking interaction with apigenin-4’-glucoside and cyan color bar represent docking interaction with vidarabine.
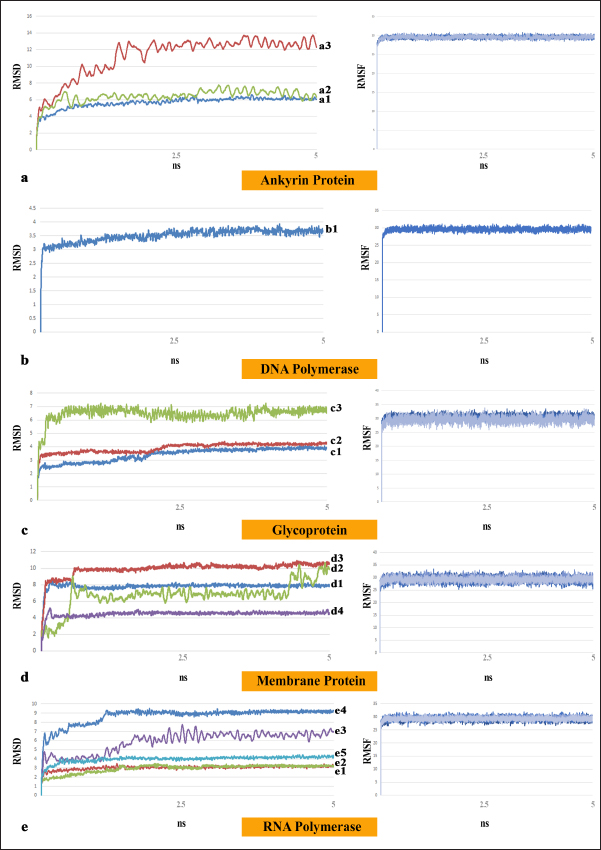
Fig. 5. MD simulation result of five docked complexes including their number of interactions with apigenin-4’-glucoside; a. represents 5ns RMSD and RMSF of ankyrin protein complexes with total number of interactions; b. represents 5ns RMSD and RMSF of DNA polymerase complex with total number of interactions; c. represent 5ns RMSD and RMSF of glycoprotein complexes with total number of interactions; d. represent 5ns RMSD and RMSFof membrane protein complexes with total number of interactions; e. represent 5ns RMSD and RMSF of RNA polymerase complexes with total number of interactions. ConclusionLumpy skin disease is a major transboundary illness that has a huge global economic impact on the dairy industry. Cattle of all ages and breeds are afflicted by LSDV, a member of the Capripoxvirus genus, which has already reported in different parts of the world and most recently, India. This underscores the necessity of attempts to manage and eradicate the virus. The strong antiviral activity of apigenin-4’-glucoside against LSDV is revealed by the integration of molecular docking and dynamics, and is evidenced by its positive ADMET values and varied binding affinities across important viral proteins. Specific binding mechanisms with ankyrin, membrane, glycoproteins, DNA polymerase, and RNA polymerase are revealed by detailed investigations. MD simulations highlight extended binding interactions by highlighting the durability and stability of apigenin-4’-glucoside complexes with LSDV proteins. Its ability to inhibit LSD, however, needs to be investigated through appropriate in vitro and in vivo testing. AcknowledgmentsThis work was supported by College of Veterinary Science, Mamnoor, Warangal, PVNRTVU, Telangana, and Oriental Institute of Science and Technology, Midnapore, West Bengal, in the frame of this research Programme. Conflict of interestAll the authors declared that this article content has no conflict of interest. FundingNo funding to declare. Authors’ ContributionsThe Study and Method development by A.S.P, material preparation, analyzing data by V.K, experiments performed A.S and S.B, interpreted the results V.K and A.S.P, and manuscript is written by A.S.P. Data availabilityThe data that supports the findings of this study are available in the supplementary material of this article, including Supplementary Table 1, that can be requested from the corresponding author. ReferencesAkther, M., Akter, S.H., Sarker, S., Aleri, J.W., Annandale, H., Abraham, S. and Uddin, J.M. 2023. Global burden of lumpy skin disease, outbreaks, and future challenges. Viruses, 15, 1861. Alfwzan, W.F., DarAssi, M.H., Allehiany, F.M., Khan, M.A., Alshahrani, M.Y. and Tag-eldin, E.M. 2023. A novel mathematical study to understand the lumpy skin disease (LSD) using modified parameterized approach. Results Phys. 51, 106626. Allam, A.M., Elbayoumy, M.K., Abdel-Rahman, E.H., Hegazi, A.G. and Farag, T.K. 2020. Molecular characterization of the 2018 outbreak of lumpy skin disease in cattle in Upper Egypt. Vet World. 13, 1262–1268. Ashrafizadeh, M., Bakhoda, M.R., Bahmanpour, Z., Ilkhani, K., Zarrabi, A., Makvandi, P., Khan, H., Mazaheri, S., Darvish, M. and Mirzaei, H. 2020. Apigenin as tumor suppressor in cancers: biotherapeutic activity, nanodelivery, and mechanisms with emphasis on pancreatic cancer. Front. Chem. 8, 829. Bagla, V.P., McGaw, L.J. and Eloff, J.N. 2012. The antiviral activity of six South African plants traditionally used against infections in ethnoveterinary medicine. Vet Microbiology. 155, 198–206. Batra, K., Kumar, A., Kumar, V., Nanda, T., Maan, N.S. and Maan, S. 2015. Development and evaluation of loop-mediated isothermal amplification assay for rapid detection of Capripoxvirus. Vet World. 8, 1286–1292. Bekker, H., Berendsen, H.J.C., Dijkstra, E.J. and Achterop, S. 1993. Gromacs: a parallel computer for molecular dynamics simulations, Physics computing 92. Edited by R.A. de Groot and J. Nadrchal. Singapore, World Scientific, pp: 252–256 Bhanuprakash, V., Indrani, B.K., Hosamani, M. and Singh, R.K. 2006. The current status of sheep pox disease. Comp Immunol Microbiol Infect Dis. 29, 27–60. Bhattacharya, D. and Cheng, J. 2013. 3Drefine: consistent protein structure refinement by optimizing hydrogen bonding network and atomic-level energy minimization. Proteins. 81, 119–131. Brenner, J., Haimovitz, M., Oron, E., Stram, Y., Fridgut, O., Bumbarov, V., Kuznetzova, L., Oved, Z., Waserman, A., Garazzi, S., Perl, S., Lahav, D., Edery, N. and Yadin, H. 2006. Lumpy skin disease LSD. in a large dairy herd in Israel. Isr. J. Vet. Med. 61, 73–77 Buller, R.M., Arif, B.M., Black, D.N., Dumbell, K.R., Esposito, J.J., Lefkowitz, E.J., McFadden, G., Moss, B., Mercer, A.A., Moyer, R.W., Skinner, M.A., Tripathy, D.N. Family Poxviridae. In M, Fauquet A, Mayo J, Maniloff U Eds. 2005. Virus taxonomy: classification and nomenclature of viruses. Eighth Report of the International Committee on Taxonomy of Viruses, 117–133. Chiem, K., Nogales, A., Lorenzo, M., Vasquez, D.M., Xiang, Y., Gupta, Y.K., Blasco, R., de la Torre, J.C. and Mart Nez-Sobrido, L. 2023. Antivirals against monkeypox infections. bioRxiv. 2023. 04.19.537483. El-Mesady, A., Elsadany, A.A., Mahdy, A.M.S. and Sonbaty, A. 2024. Nonlinear dynamics and optimal control strategies of a novel fractional-order lumpy skin disease model. J. Comput. Sci. 79, 102286. Forli, S., Huey, R., Pique, M.E., Sanner, M.F., Goodsell, D.S. and Olson, A.J. 2016. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protocols. 11, 905–919. Gari, G., Abie, G., Gizaw, D., Wubete, A., Kidane, M., Asgedom, H., Bayissa, B., Ayelet, G., Oura, C.A. and Roger, F., Tuppurainen, E.S. 2015. Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine. 33, 3256–3261. Givens M.D. 2018. Review: risks of disease transmission through semen in cattle. Animal. 12, 165–171. Guex, N., Peitsch, M.C. and Schwede, T. 2009. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 30, 162–173. Javed, Z., Sadia, H., Iqbal, M.J., Shamas, S., Malik, K., Ahmed, R., Raza, S., Butnariu, M., Cruz-Martins, N. and Sharifi-Rad, J. 2021. Apigenin role as cell-signaling pathways modulator: implications in cancer prevention and treatment. Cancer Cell Int. 21, 189. Khandelwal, N., Chander, Y., Kumar, R., Riyesh, T., Dedar, R.K., Kumar, M., Gulati, B.R., Sharma, S., Tripathi, B.N., Barua, S. and Kumar, N. 2020. Antiviral activity of Apigenin against buffalopox: novel mechanistic insights and drug-resistance considerations. Antiviral Res. 181, 104870. Kim, Y., Kim, J., Son, S.R., Kim, J.Y., Choi, J.H. and Jang, D.S. 2022. Chemical constituents of the flowers of Pueraria lobata and their cytotoxic properties. Plants (Basel). 11, 1651. Klement, E., Broglia, A., Antoniou, S.E., Tsiamadis, V., Plevraki, E., Petrović, T., Polaček, V., Debeljak, Z., Miteva, A., Alexandrov, T., Marojevic, D., Pite, L., Kondratenko, V., Atanasov, Z., Gubbins, S., Stegeman, A. and Abrahantes, J.C. 2020. Neethling vaccine proved highly effective in controlling lumpy skin disease epidemics in the Balkans. Prev Vet Med. 181, 104595. Kotwal, G.J., Kaczmarek, J.N., Leivers, S., Ghebremariam, Y.T., Kulkarni, A.P., Bauer, G., De Beer, C., Preiser, W. and Mohamed, A.R. 2005. Anti-HIV, anti-poxvirus, and anti-SARS activity of a nontoxic, acidic plant extract from the Trifollium species Secomet-V/anti-vac suggests that it contains a novel broad-spectrum antiviral. Ann N Y Acad Sci. 1056, 293–302. Kwofie, S.K., Annan, D.G., Adinortey, C.A., Boison, D., Kwarko, G.B., Abban, R.A. and Adinortey, M.B. 2022. Identification of novel potential inhibitors of varicella-zoster virus thymidine kinase from ethnopharmacologic relevant plants through an in-silico approach. J Biomol Struct Dyn. 40, 12932–12947. Laskowski, R.A., MacArthur, M.W., Moss, D.S. and Thornton, J.M. 1993. PROCHECK - a program to check the stereochemical quality of protein structures. J. App. Cryst. 26, 283–291. Lee, I. G., Lee, J., Hong, S.H. and Seo, Y.J. 2023. Apigenin’s therapeutic potential against viral infection. Front Biosci (Landmark Ed). 28, 237. Lei, T., Li, Y., Song, Y., Li, D., Sun, H. and Hou, T. 2016. ADMET evaluation in drug discovery: 15. Accurate prediction of rat oral acute toxicity using relevance vector machine and consensus modeling. J Cheminform. 8, 6. Liu, L., Wang, X., Mao, R., Zhou, Y., Yin, J., Sun, Y. and Yin, X. 2021. Research progress on live attenuated vaccine against African swine fever virus. Microb Pathog. 158, 105024. Montenegro-Landívar, M.F., Tapia-Quirós, P., Vecino, X., Reig, M., Valderrama, C., Granados, M., Cortina, J.L. and Saurina, J 2021. Polyphenols and their potential role to fight viral diseases: an overview. Sci Total Environ. 801, 149719. Mustafa, G., Mahrosh, H.S., Salman, M., Ali, M., Arif, R., Ahmed, S. and Ebaid, H. 2023. In Silico analysis of honey bee peptides as potential inhibitors of Capripoxvirus DNA-directed RNA polymerase. Animals (Basel). 13, 2281. Naponelli, V., Rocchetti, M.T. and Mangieri, D. 2024. Apigenin: molecular mechanisms and therapeutic potential against cancer spreading. Int. J. Mol. Sci. 25, 5569. Riyaphan, J., Pham, D.C., Leong, M.K. and Weng, C.F. 2021. In Silico approaches to identify polyphenol compounds as α-Glucosidase and α-Amylase inhibitors against type-II diabetes. Biomolecules. 11, 1877. Sadgrove, N.J., Oliveira, T.B., Khumalo, G.P., Vuuren, S.F.V. and van Wyk, B.E. 2020. Antimicrobial isoflavones and derivatives from erythrina fabaceae.: structure activity perspective sar and qsar. on experimental and mined values against Staphylococcus Aureus. Antibiotics (Basel). 9, 223. Sprygin, A., Pestova, Y., Wallace, D.B., Tuppurainen, E. and Kononov, A.V. 2019. Transmission of lumpy skin disease virus: a short review. Virus res. 269, 197637. Tuppurainen, E.S. and Oura, C.A. 2012. Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transbound Emerg Dis. 59, 40–48. Tuppurainen, E.S., Stoltsz, W.H., Troskie, M., Wallace, D.B., Oura, C.A., Mellor, P.S., Coetzer, J.A. and Venter, E.H. 2011. A potential role for ixodid hard. tick vectors in the transmission of lumpy skin disease virus in cattle. Transbound Emerg Dis. 58, 93–104. Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., Heer, F.T., de Beer, T.A.P., Rempfer, C., Bordoli, L., Lepore, R. and Schwede, T. 2018. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, 296–303. Wolff, J., Moritz, T., Schlottau, K., Hoffmann, D., Beer, M. and Hoffmann, B. 2020. Development of a safe and highly efficient inactivated vaccine candidate against lumpy skin disease Virus. Vaccines. 9, 4. | ||
| How to Cite this Article |
| Pubmed Style Krishna SV, Sarkar A, Banerjee S, Panja AS. Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Vet. J.. 2024; 14(11): 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 Web Style Krishna SV, Sarkar A, Banerjee S, Panja AS. Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. https://www.openveterinaryjournal.com/?mno=211312 [Access: January 22, 2026]. doi:10.5455/OVJ.2024.v14.i11.9 AMA (American Medical Association) Style Krishna SV, Sarkar A, Banerjee S, Panja AS. Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Vet. J.. 2024; 14(11): 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 Vancouver/ICMJE Style Krishna SV, Sarkar A, Banerjee S, Panja AS. Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Vet. J.. (2024), [cited January 22, 2026]; 14(11): 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 Harvard Style Krishna, S. V., Sarkar, . A., Banerjee, . S. & Panja, . A. S. (2024) Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Vet. J., 14 (11), 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 Turabian Style Krishna, S. Vamshi, Aniket Sarkar, Suchandan Banerjee, and Anindya Sundar Panja. 2024. Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Veterinary Journal, 14 (11), 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 Chicago Style Krishna, S. Vamshi, Aniket Sarkar, Suchandan Banerjee, and Anindya Sundar Panja. "Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach." Open Veterinary Journal 14 (2024), 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 MLA (The Modern Language Association) Style Krishna, S. Vamshi, Aniket Sarkar, Suchandan Banerjee, and Anindya Sundar Panja. "Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach." Open Veterinary Journal 14.11 (2024), 2806-2816. Print. doi:10.5455/OVJ.2024.v14.i11.9 APA (American Psychological Association) Style Krishna, S. V., Sarkar, . A., Banerjee, . S. & Panja, . A. S. (2024) Promising antiviral inhibitors against lumpy skin disease: A vetinformatics approach. Open Veterinary Journal, 14 (11), 2806-2816. doi:10.5455/OVJ.2024.v14.i11.9 |