| Research Article | ||
Open Vet. J.. 2024; 14(12): 3232-3240 Open Veterinary Journal, (2024), Vol. 14(12): 3232-3240 Research Article Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast GabonPatrice Makouloutou-Nzassi1,2*, Neil Michel Longo-Pendy2, Lucien Keurtis Ayefegue Nguema3, Silas Sevidzem Lendzele4, Felicien Bangueboussa2, Bernie Bouchedi1, Gael Darren Maganga3,5 and Larson Boundenga2,61Département de Biologie et Ecologie Animale, Institut de Recherche en Ecologie Tropicale (IRET/ CENAREST), Libreville, Gabon 2Unité de Recherche en Ecologie de la Santé, Centre Interdisciplinaire de Recherches Médicales de Franceville, Franceville, Gabon 3Department of Zootechnology, Institut National Supérieur d’ Agronomie et de Biotechnologies, Université des Sciences et Techniques de Masuku, Franceville, Gabon 4Department of Health and Environment, Laboratoire d’écologie des maladies transmissibles (LEMAT), Université Libreville Nord, Libreville, Gabon 5Unité Emergence des Maladies Virales, Centre Interdisciplinaire de Recherches Médicales de Franceville, Franceville, Gabon 6Department of Anthropology, Durham University, Durham, UK *Corresponding Author: Patrice Makouloutou-Nzassi. Département de Biologie et Ecologie Animale, Institut de Recherche en Ecologie Tropicale (IRET/ CENAREST), Libreville, Gabon. Email: patmak741 [at] gmail.com Submitted: 01/08/2024 Accepted: 21/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Gastrointestinal parasites (GIPs) pose a significant global challenge to the poultry industry, affecting health, welfare, and production performance. Few studies have been conducted in Gabon on the prevalence of these infections in chickens. Aim: This cross-sectional survey aims to assess the presence and diversity of GIP among chickens in the M’passa department. Methods: Between April and October 2022, we randomly collected 402 fecal samples from local and exotic chicken breeds from four semi-intensive poultry farms and 11 free-range chicken sites in the M’passa department, southeast Gabon. These samples were analyzed for GIP using flotation and sedimentation methods. Results: This study found 14 GIP eggs and oocytes in 72.9% (293/402) of examined chickens. Capillaria spp. (39.5%) and Ascaridia (31.1%) species were the most frequently identified parasites. Other identified parasites included Eimeria spp. (20.1%), Strongyloides avium (16.9%), Choanotaenia infundibulum (13.4%), Hymenolepis spp. (10.4%), Chilomastix gallinarum (7.7%), and Entamoaba. (1.7%). Single infections occurred in 39.3% (115/293, 95% IC: 33.7–44.9) of cases, while mixed infections were recorded in 60.7% (178/293, 95% IC: 55.1–66.3). The study also identified significant differences in prevalence among local and exotic breeds, genders, and age groups. Conclusion: This study revealed a high prevalence of GIP in Gabon chickens, potentially harming their health and productivity. We recommend implementing effective control measures against these infections to enhance the health and productivity of chickens in the region. Keywords: Chickens, Gastrointestinal parasites, Gabon, Prevalence, Risk factors. IntroductionDomesticated birds, known as poultry, are reared by humans for meat, eggs, and occasionally feathers to support livelihoods. This group includes chickens, ducks, geese, and turkeys. Poultry is a vital source of protein and farm fertilizer (Jegede et al., 2015). The most commonly raised poultry is domestic chicken (Gallus gallus domesticus) (Asumang et al., 2019; Lawal et al., 2020). Over the past few decades, poultry production has steadily risen globally (Mottet and Tempio, 2017). According to the Food and Agriculture Organization, developing countries account for approximately 75% of the existing 15 billion chickens (Ferdushy et al., 2016). This sector is recognized as an important mechanism for alleviating problems associated with poverty in developing countries in terms of food security and malnutrition (Ola-Fadunsin, 2017; Ola-Fadunsin et al., 2019b). Gastrointestinal parasites (GIPs) significantly hinder poultry production, leading to economic losses from reduced productivity, poorer feed conversion rates, and inadequate weight gain. They cause issues such as decreased egg production, catarrh, anorexia, diarrhea, intestinal obstruction, emaciation, anemia, weakness, paralysis, poor feathering, and even death (Jegede et al., 2015; Shifaw et al., 2021). Additionally, GIP can increase disease susceptibility and weaken the immune response to vaccinations (Pleidrup et al., 2014; Saadoon Ibadi Al-Alwani and Hamle Abid, 2023). Gastrointestinal helminths can also transmit pathogens like Histomonas meleagridis, resulting in high morbidity and up to 20% mortality in chicken flocks (McDougald, 2005; Clark and Kimminau, 2017). GIP that infects poultry include; cestodes, nematodes, trematodes, and protozoans (Nnadi and George, 2010; Shifaw et al., 2021). Nematodes are the most significant intestinal worms in the poultry industry due to their species diversity and the diseases they cause (Hafiz et al., 2015; Ola-Fadunsin et al., 2019a,b). In Gabon, animal husbandry plays a marginal role in the economy, representing less than 1% of the GDP (Maganga et al., 2019). Poultry and pig farming constitutes 80% of peri-urban rearing activities in Libreville, Gabon’s capital, with laying hens being the most commonly raised (Maganga et al., 2019). Reports from Libongui indicate the presence of farms with over 11,000 chickens (Libongui, 2022). Gabon’s national production shows an estimated 3 million eggs in 2019, and 3,920 tons of chicken meat in 2013 (Lacroix, 2014; Direct, 2020). Semi-intensive and backyard poultry farming is expanding in Haut-Ogooué Province, Gabon; however, there are no reports on the GIP of chickens in this region. This study aims to determine the prevalence and species diversity of GIP in chickens in southeastern Gabon, filling a critical knowledge gap in the region. Materials and MethodsStudy areaThe study took place in the M’passa departments of Haut-Ogooué province in southeastern Gabon (Coordinates 13°8′S and 13°35′E) (Fig. 1). This region features an equatorial climate with four alternating seasons of rainy and dry weather. Average temperatures range from 24.4°C to 26.8°C, and annual rainfall typically falls between 2000 and 2250 mm. There is currently no information available on chicken husbandry in this province. Sample size determinationThe study’s sample size was calculated using Thrusfield’s formula (Thrusfield, 2018) with 5% precision and a predicted prevalence of 25.0%, as reported by Makouloutou-Nzassi et al. (2024).
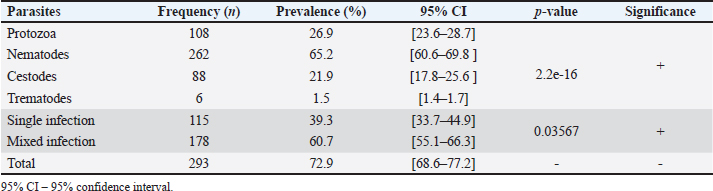
Fig. 1. Location of the sampling zone. N=1.962*Pexp(1-exp) d2 where: N=required sample Pexp=Expected prevalence D=desired absolute precision N=1.962*0.25 (1–0.25) 0.052 N=3.8416*0.25 (0.75)=288 0.0025 The study’s sample size was set at 288, but to minimize sampling errors, it was increased to 402 chickens. SamplingThis study utilized a simple random sampling technique from April to October 2022, investigating four semi-intensive poultry farms and 11 free-range chicken sites. The selection of the farms and sites was based on an agreement with the managers and owners after discussing the study’s objectives. Domestic poultry were chosen for their availability, regardless of age and sex. A total of 402 fecal samples were collected from individual domestic poultry (201 from local and 201 from exotic breeds) using swabs from the rectum or directly from the ground after defecation. The samples were placed in sterile, labeled Falcon tubes with physiological serum and kept on ice for transport to the Ecological Health Research Unit at the Interdisciplinary Center for Medical Research of Franceville (CIRMF) for processing and testing. Data on breeding system, breed, sex, and age were recorded via a survey questionnaire. Sample examinationParasite eggs, cysts, oocysts, and larvae in fecal samples were identified using two qualitative techniques: flotation with a saturated salt solution and sedimentation with physiological saline (Chauhan, 2007). These parasitic forms were examined at X40 and X100 magnification using an optical microscope with a camera (Realux France, ToupcamTM). Identification was based on the morphology of the shell, egg nucleus, oocysts, cysts, and the head and tail of the larva (Ashford and Crewe, 2003; Boundenga et al., 2018; Maganga et al., 2019). Gastrointestinal parasitic species were identified using keys by Soulsby (1968). Statistical analysisThe data obtained from parasitological analyses and questionnaires were entered into Microsoft Office Excel 2016 (Microsoft Corp., Redmond, WA). The statistical tests were conducted using R Studio 4.3.2 software (“Posit,” 2024). Host-related risk factors, such as breeding system, breed, sex, and age, were considered explanatory variables for GIP infection using chi-square and Fisher tests. The test results were considered significant at a 5% significance level. Ethical approvalThis study used non-experimental animals and conducted non-invasive sampling. The fecal samples collected were obtained through non-invasive and non-painful procedures following the protocols at Institut National Supérieur d’Agronomie et Biotechnologies. All poultry owners were adults and were informed about the nature of this study. ResultsPrevalence and diversity of GIPThis study aimed to determine the prevalence and diversity of GIP in domestic poultry in M’passa department of Gabon (Fig. 2). As shown in Table 1, the overall prevalence of infection with GIP in domestic chickens (Gallus gallus domesticus) was 72.9% (293/402; 95% CI:68.6–77.2). Nematodes had the highest prevalence at 65.2% (or 262/402), followed by protozoans at 26.9% (or 108/402), cestodes at 21.9% (or 88/402), and trematodes had the lowest prevalence of 1.5% (6 cases) (p < 0.05).
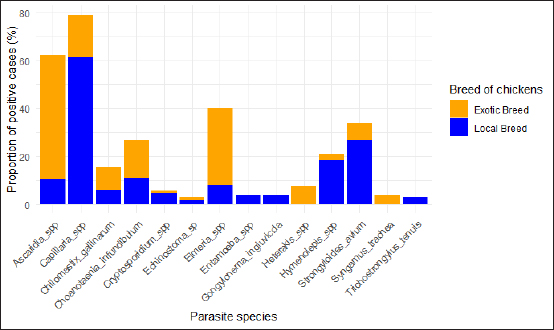
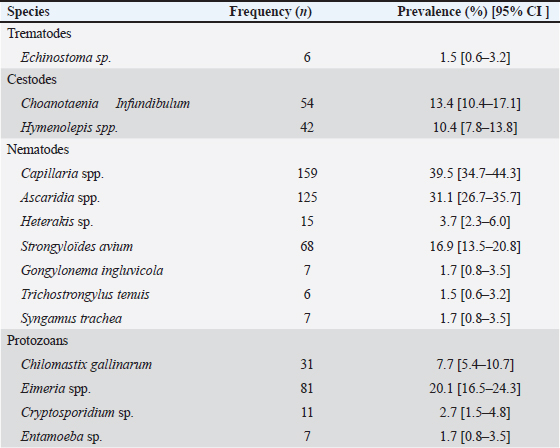
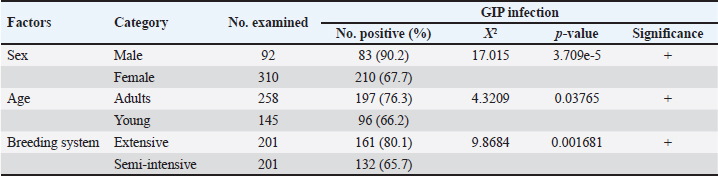
Fig. 2. Diversity of GIP with breed of chicken. Parasitic diversity included 14 genera: seven nematodes, two cestodes, one trematode, and four protozoa (Table 2). Capillaria spp. (39.5%) and Ascaridia spp. (31.1%) were the most prevalent nematodes, followed by Strongyloides avium (16.9%). Trichostrongylus tenuis (1.5%) was the least encountered. Among cestodes, Choanotaenia infundibulum had the highest prevalence at 13.4% (or 54/402), followed by Hymenolepis spp. (10.4% or 42/402). For protozoa, 20.1% (or 81/402) of chickens were infected with Eimeria spp., and 7.7% (or 31/402) with Chilomastix gallinarum; other protozoan prevalence ranged from 1% to 2.7% (Table 2). Helminths had a prevalence of 70.4% (283/402), while protozoa accounted for 26.9% (108/402). Single infection was recorded in 39.3% (115/293) of cases, while mixed infections included two species in 21.6% (or 87/293), three species in 14.7% (or 59/293), and four or more species in 10.9% (or 32/293). Prevalence of GIP and host-related risk factorsThe gastrointestinal parasitic infections showed a significant association (p < 0.05) with the sex, age, and breeding system of the chickens. Males had a higher prevalence (90.2%) than females (67.7%), adults (76.6%) were more affected than young chickens (66.2%), and extensive breeding systems exhibited a greater prevalence (80.1%) compared to semi-intensive systems (65.7%) (Table 3). The extensive breeding system (p-value <2.2e-16) also strongly correlated with Capillaria spp. occurrence, while the semi-intensive system (p=4.654e-5) was strongly linked to Heterakis spp. presence. For Hymenolepis spp., extensive management (p=1.106e-7) and adults ( p=0.01687) were strongly associated with their frequency. Extensive system (p=1.038e-7) and females (p=5.791e-5) were risk factors for S. avium occurrence. The extensive system was the sole risk factor for Gongylonema ingluvicola ( p=0.01482) and Entamoeba spp. ( p=0.01482). For T. tenuis, the extensive system ( p=0.01482) and males ( p=0.02653) were associated risk factors. In this study, we found that adult individuals ( p=0.02615) showed a significant association with Ascaridia sp., while young individuals ( p=0.0007075) were strongly linked to C. infundibulum. Additionally, we observed a strong association of females (p=6.338e-15) with Capillaria spp., and the semi-intensive farming system was significantly linked ( p=0.01482) to the occurrence of Syngamus trachea. DiscussionEpidemiological studies are important for preventing and controlling parasitic poultry diseases (Györke et al., 2013; Shifaw et al., 2021). This study is the first of its kind to be conducted on poultry endoparasites in the country. We found that 72.9% of the sampled population was infected with one or more GIP species. In other studies, Asumang et al. (2019) reported a prevalence rate of 65.5% in Pankrono-Kumasi, Ghana, Sarba et al. (2019) reported 92.1% in West Shoa zone central, Ethiopia, and Idika et al. (2016) reported 96.8% in Nsukka region, Nigeria. The differences in prevalence rates among these studies may be due to factors such as sampling periods, sample size, study design, geographical area, and climatic conditions. The prevalence of GIP observed in our study may be attributed to agro-ecological conditions, exposure to specific intermediate hosts, and the poultry management system. This study revealed that approximately 60.7% of the chickens in the study area were found to harbor multiple species of GIP, supporting the hypothesis that parasitic infestations tend to co-circulate in chickens (Ben Slimane, 2016; Coroian et al., 2024). The combined detrimental effects of these infestations on the host’s metabolism significantly contribute to early chick mortality and other production losses among adult chickens (Nnadi and George, 2010). Moreover, this finding aligned with the results reported in the Giwa Local Government Areas of Nigeria (Junaidu et al., 2014), and in Mekelle town, Ethiopia (Berhe et al., 2019), which recorded a polyspecific parasitism rate of 60.5% and 87.7%, respectively. Table 1. Overall prevalence of GIP of domestic poultry (Gallus gallus domesticus) (N=402).
Table 2. Species-specific prevalence of GIP of domestic chicken (Gallus gallus domesticus) (N=402).
Table 3. Association of potential risk factors for GIP infection in chickens in the study area.
The results of this study indicated that 14 species of GIP affect poultry in the study area. Like our findings, Poulsen et al. (2000) reported eighteen GIP species among chickens in the upper eastern region of Ghana, and sixteen species in the KwaZulu-Natal province of South Africa (Mukaratirwa and Khumalo, 2010). In contrast, Permin et al. (1997) reported 29 GIP species in rural scavenging poultry in Tanzania, while Magwisha et al. (2002) found 26 GIP species in free-range chickens in Morogoro, Tanzania. Outside of Africa, nine GIP species were reported in India (Kumar et al., 2015), seven species in Trinidad (Baboolal et al., 2012), six species in Bangladesh (Alam et al., 2014), six species in Iran (Badparva et al., 2015), and four species in Poland (Tomza-Marciniak et al., 2014). The differences in the number of GIP species detected in this study compared to other studies may be attributed to environmental and climatic variations, mainly the temperature and moisture which support the development of these helminths. Our findings demonstrate the presence of diverse species of GIP affecting poultry in the M’passa Department. This study found Capillaria spp. and Ascaridia sp. to be the most prevalent parasite species in the examined chickens, with a prevalence rate of 39.5% and 31.1%, respectively. This is consistent with Shifaw’s systematic review of the prevalence of gastrointestinal nematodes in chickens which found that A. galli, H. gallinarum, and Capillaria spp. were the most common identified parasites (Shifaw et al., 2021). Ascaridia galli’s direct transmission and resilient eggs allow them to survive long outside the host. The eggs, when excreted in feces, develop into an infective stage, contaminating feed and water sources. They can remain infective for years in deep litter systems, depending on environmental factors such as temperature, humidity, pH, and ammonium concentration (Thapa et al., 2015; Shohana et al., 2023; Singh et al., 2023). Consequently, without proper management practices, feed and water can quickly become contaminated, especially as farm handlers may inadvertently transport these eggs from other locations (Sharma et al., 2019). A statistically significant difference was found in the prevalence of GIP among chickens in extensive versus semi-intensive management systems. This suggests that as the production system becomes more modernized and hygienic, the prevalence of the GIP decreases, a finding corroborated by studies from Mekelle Town, Ethiopia (Berhe et al., 2019), and Akure, Nigeria (Afolabi et al., 2016). The lower prevalence in semi-intensive farms may be attributed to better hygiene and feeding practices that inhibit helminth growth and transmission (Chauhan, 2007; Asumang et al., 2019). Conversely, in the extensive system, chickens often ingest intermediate hosts while foraging, leading to higher parasite exposure. The differences observed in Eimeria spp., Heterakis spp., Hymenolepis spp., S. avium, Gongylonema ingluvicola, Entamoeba spp., T. tenuis, and Syngamus trachea are likely due to their widespread presence in nature and their status as common digestive endoparasites in gastrointestinal parasitism (Kaingu et al., 2010; Mwale and Masika, 2011; Lozano et al., 2019; Coroian et al., 2024). Considering the sex parameter, a statistically significant difference in infection rate between male and female chickens is evident, with males showing higher susceptibility to parasites. This finding aligns with a study in Nigeria (Jegede et al., 2015), that also reported higher infection levels in male chickens of both breeds. This difference may result from chance or dietary habits, as males typically have a more active diet, increasing their exposure to helminth infections (Ozougwu et al., 2021). Additionally, their selective feeding may lead them to consume intermediate hosts like earthworms, which create a better environment for parasite development (Sonaiya, 1990; Ola-Fadunsin, et al., 2019a,b). This study found a significant difference (p < 0.05) among the age groups, with adult chickens showing the highest infection rate at 76.6%. This suggests that age influences GIP infection in chickens. Possible reasons include the presence of maternal immunity in chicks and older birds and increased exposure to helminth ova and coccidian oocysts in their environment (Jegede et al., 2015). Additionally, adult poultry tend to be more social, resulting in a greater exposure rate, while younger individuals are less familiar with their surroundings and venture out less frequently (Nnadi and George, 2010). ConclusionThis study recorded a high prevalence of GIP in chickens in southeastern Gabon, with nematodes and cestodes being the most common, including Capillaria spp, Ascaridia spp, C. infundibulum, and Hymenolepis spp. The occurrence of these parasites was influenced by the chickens’ management system, sex, and age. We recommend enhancing producer awareness of GIP prevention and treatment, engaging veterinary extension agents in farm management, and offering support to safeguard poultry health, welfare, and public health. However, this study did not investigate the impact of gastrointestinal parasitic infections on chicken production parameters. Further research is needed to evaluate how these infections affect mortality, weight gain, and egg-laying. AcknowledgmentsThe authors extend their gratitude to the poultry owners in the M’Passa department who participated in the study, and to the staff members of the Unité de Recherches en Écologie de la Santé, CIRMF, for their assistance during the laboratory work. Conflict of interestThe authors declared that they have no conflict of interest. FundingThis study receives no specific grant funding. Authors’ contributionPMN: Conception, Data curation, Formal analysis, Writing original draft, Supervision, NMLP: Data acquisition, Data curation. LKAN: Data acquisition, Data curation SSL: Writing, review the manuscript. BB: Data acquisition. FB: Data acquisition, Data curation. GDM: Supervision, Conception, Validation. LB: Supervision, Conception, Validation. Data availabilityThe data supporting this article’s findings are available in the manuscript and from PMN upon reasonable request. ReferencesAfolabi, O.J., Simon-Oke, I.A. and Olasunkanmi, A.O. 2016. Intestinal parasites of domestic chicken (Gallus gallus domesticus) in Akure, Nigeria. J. Biomed [Internet]. 1(4). (Accessed 22 May 2024). Alam, M., Mostofa, M., Khan, M., Alim, M., Rahman, A. and Trisha, A. 2014. Prevalence of gastrointestinal helminth infections in indigenous chickens of selected areas of Barisal District, Bangladesh. Bangladesh J. Vet. Med. 12(2), 135–139. Ashford, R. and Crewe, W. 2003. Parasites of homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Boca Raton, FL: CRC Press. Asumang, P., Akoto Delali, J., Wiafe, F., Kamil, Z., Iddrisu Balali, G., Afua Dela Gobe, V., Nketiah Siaw, W. and Pinamang, G. 2019. Prevalence of gastrointestinal parasites in local and exotic breeds of chickens in Pankrono–Kumasi, Ghana. J. Parasitol. Res. 2019, e5746515. Baboolal, V., Suratsingh, V., Gyan, L., Brown, G., Offiah, N.V., Adesiyun, A.A. and Basu, A.K. 2012. The prevalence of intestinal helminths in broiler chickens in Trinidad. Vet. Arh. 82(6), 591–597. Badparva, E., Ezatpour, B., Azami, M. and Badparva, M. 2015. First report of birds infection by intestinal parasites in Khorramabad, west Iran. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 39(4), 720–724. Ben Slimane, B. 2016. Prevalence of the gastro-intestinal parasites of domestic chicken Gallus domesticus Linnaeus, 1758 in Tunisia according to the agro-ecological zones. J. Parasit. Dis. 40(3), 774–778. Berhe, M., Mekibib, B., Bsrat, A. and Atsbaha, G. 2019. Gastrointestinal helminth parasites of chicken under different management system in Mekelle Town, Tigray Region, Ethiopia. J. Vet. Med. 2019, e1307582. Boundenga, L., Moussadji, C., Mombo, I.M., Ngoubangoye, B., Lekana-Douki, J.B. and Hugot, J.P. 2018. Diversity and prevalence of gastrointestinal parasites in two wild Galago species in Gabon. Infect. Genet. Evol. 63, 249–256. Chauhan, H.V.S. 2007. Poultry diseases, diagnosis and treatment. New Delhi, India: New Age International. Clark, S. and Kimminau, E. 2017. Critical review: future control of blackhead disease (Histomoniasis) in poultry. Avian Dis. 61(3), 281–288. Coroian, M., Fábián-Ravasz, T.Z., Dobrin, P.R. and Györke, A. 2024. Occurrence of Eimeria spp. and intestinal helminths in free-range chickens from Northwest and Central Romania. Anim. Open Access J. MDPI 14(4), 563. Direct, I.G. 2020. Elevage : Le Gabon a produit 3 millions d’œufs en 2019. Direct Infos Gabon. Available via https://directinfosgabon.com/elevage-le-gabon-a-produit-3-millions-doeufs-en-2019/ (Accessed 02 June 2024). Ferdushy, T., Hasan, M.T. and Golam Kadir, A.K.M. 2016. Cross sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free range chickens in Narsingdi district, Bangladesh. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 40(3), 818–822. Györke, A., Pop, L. and Cozma, V. 2013. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite 20, 50. Hafiz, A.B., Muhammad, A.R., Muhammad, A.A., Imran, A.K., Abdul, A., Zahid, M. and Shaukat, H.M. 2015. Prevalence of Ascaridia galli in white leghorn layers and Fayoumi-Rhode Island red crossbred flock at government poultry farm Dina, Punjab, Pakistan. Trop. Biomed. 32(1), 11–16. Idika, I.K., Obi, C.F., Ezeh, I.O., Iheagwam, C.N., Njoku, I.N. and Nwosu, C.O. 2016. Gastrointestinal helminth parasites of local chickens from selected communities in Nsukka region of southeastern Nigeria. J. Parasit. Dis. 40(4), 1376–1380. Jegede, O.C., Asadu, I.A., Opara, M., Obeta, S.S. and Olayemi, D.O. 2015. Gastrointestinal parasitism in local and exotic breeds of chickens reared in Gwagwalada Guinea Savannah zone of Nigeria. Sokoto J. Vet. Sci. 13(3), 25–30. Junaidu, H.I., Luka, S.A. and Mijinyawa, A. 2014. Prevalence of gastrointestinal helminth parasites of the domestic fowl (Gallus-gallus domesticus) slaughtered in Giwa Market, Giwa Local Government, Area, Kaduna State, Nigeria. J. Nat. Sci. Res. 4(19), 120–125. Kaingu, F.B., Kibor, A.C., Shivairo, R., Kutima, H., Okeno, T.O., Waihenya, R. and Kahi, A.K. 2010. Prevalence of gastro-intestinal helminthes and coccidia in indigenous chicken from different agro- climatic zones in Kenya. AJAR 5(6), 458–462. Kumar, S., Garg, R., Ram, H., Maurya, P.S. and Banerjee, P.S. 2015. Gastrointestinal parasitic infections in chickens of upper gangetic plains of India with special reference to poultry coccidiosis. J. Parasit. Dis. 39(1), 22–26. Lacroix, I. 2014. Gabon—production alimentaire: viande (poulet) (tonnes) | Statistiques Available via https://perspective.usherbrooke.ca/bilan/servlet/BMTendanceStatPays?codePays=GAB&codeTheme=5&codeStat=RSA.FAO.MeatChicken (Accessed 02 June 2024). Lawal, R.A., Martin, S.H., Vanmechelen, K., Vereijken, A., Silva, P., Al-Atiyat, R.M., Aljumaah, R.S., Mwacharo, J.M., Wu, D.D., Zhang, Y.P., Hocking, P.M., Smith, J., Wragg, D. and Hanotte, O. 2020. The wild species genome ancestry of domestic chickens. BMC Biol. 18(1), 13. Libongui, G.E. 2022. Agriculture en zone urbaine et périurbaine de Libreville: dynamiques spatiales, acteurs et enjeux environnementaux (phdthesis). Le Mans, France: Le Mans Université (Accessed 07 December 2023). Lozano, J., Anaya, A., Palomero Salinero, A., Lux Hoppe, E.G., Gomes, L., Paz-Silva, A., Rebelo, M.T. and Madeira de Carvalho, L. 2019. Gastrointestinal parasites of free-range chickens: a worldwide issue. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 76(2), 110–117. Maganga, G.D., Kombila, L.B., Boundenga, L., Kinga, I.C.M., Obame-Nkoghe, J., Tchoffo, H., Gbati, O.B. and Awah-Ndukum, J. 2019. Diversity and prevalence of gastrointestinal parasites in farmed pigs in Southeast Gabon, Central Africa. Vet. World 12(12), 1888–1896. Magwisha, H.B., Kassuku, A.A., Kyvsgaard, N.C. and Permin, A. 2002. A comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickens. Trop. Anim. Health Prod. 34(3), 205–214. Makouloutou-Nzassi, P., Bouchedi, B., Mamgombi-Pambou, J.B., Longo-Pendy, N.M., N’dilimabaka, N., Bangueboussa, F., Koumba, S., Matoumba, A.M., Boundenga, L., Maganga, G.D. and Mintsa-Nguema, R. 2024. Prevalence of Cryptosporidium spp. infection in rodents and chickens in Franceville, Gabon. Vet. World 17(7), 1523–1529. McDougald, L.R. 2005. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Dis. 49(4), 462–476. Mottet, A. and Tempio, G. 2017. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. J. 73(2), 245–256. Mukaratirwa, S. and Khumalo, M.P. 2010. Prevalence of helminth parasites in free-range chickens from selected rural communities in KwaZulu-Natal province of South Africa. J. S. Afr. Vet. Assoc. 81(2), 97–101. Mwale, M. and Masika, P.J. 2011. Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. AJAR 6(9), 2033–2038. Nnadi, P.A. and George, S.O. 2010. A cross-sectional survey on parasites of chickens in selected villages in the Subhumid zones of South-Eastern Nigeria. J. Parasitol. Res. 2010, e141824. Ola-Fadunsin, S.D. 2017. Investigations on the occurrence and associated risk factors of avian coccidiosis in Osun State, Southwestern Nigeria. J. Parasitol. Res. 2017, e9264191. Ola-Fadunsin, S.D., Ganiyu, I.A., Rabiu, M., Hussain, K., Sanda, I.M., Musa, S.A., Uwabujo, P.I. and Furo, N.A. 2019a. Gastrointestinal parasites of different avian species in Ilorin, North Central Nigeria. J. Adv. Vet. Anim. Res. 6(1), 108–116. Ola-Fadunsin, S.D., Uwabujo, P.I., Sanda, I.M., Ganiyu, I.A., Hussain, K., Rabiu, M., Elelu, N. and Alayande, M.O. 2019b. Gastrointestinal helminths of intensively managed poultry in Kwara Central, Kwara State, Nigeria: its diversity, prevalence, intensity, and risk factors. Vet. World 12(3), 389–396. Ozougwu , J.C., Imakwu, C.A., Eziuzor, S.C., Ekeleme, J.E., Okeke, O.P., Amana, G.U. and Ogbodo, J.C. 2021. Prevalence of intestinal helminthes with respect to age, sex and breeds of chicken slaughtered at Eke Awka Market, Awka, Anambra State, Nigeria. Asian J. Biol. 1–7. Permin, A., Magwisha, H., Kassuku, A.A., Nansen, P., Bisgaard, M., Frandsen, F. and Gibbons, L. 1997. A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. J. Helminthol. 71(3), 233–240. Pleidrup, J., Dalgaard, T.S., Norup, L.R., Permin, A., Schou, T.W., Skovgaard, K., Vadekær, D.F., Jungersen, G., Sørensen, P. and Juul-Madsen, H.R. 2014. Ascaridia galli infection influences the development of both humoral and cell-mediated immunity after Newcastle Disease vaccination in chickens. Vaccine 32(3), 383–392. Posit. 2024. Posit. Available via https://www.posit.co/ (Accessed 29 September 2024). Poulsen, J., Permin, A., Hindsbo, O., Yelifari, L., Nansen, P. and Bloch, P., 2000. Prevalence and distribution of gastro-intestinal helminths and haemoparasites in young scavenging chickens in upper eastern region of Ghana, West Africa. Prev. Vet. Med. 45(3-4), 237–245. Saadoon Ibadi Al-Alwani, W. and Hamle Abid, H. 2023. Comparative effect of Ascaridia galli infection on the two laying hen lines on the liver function and immune response. Arch. Razi Inst. 78(3), 1065–1070. Sarba, E.J., Bayu, M.D., Gebremedhin, E.Z., Motuma, K., Leta, S., Abdisa, K., Kebebew, G. and Borena, B.M. 2019. Gastrointestinal helminths of backyard chickens in selected areas of West Shoa Zone Central, Ethiopia. Vet. Parasitol. Reg. Stud. Rep. 15, 100265. Sharma, N., Hunt, P.W., Hine, B.C. and Ruhnke, I. 2019. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poult. Sci. 98(12), 6517–6526. Shifaw, A., Feyera, T., Walkden-Brown, S.W., Sharpe, B., Elliott, T. and Ruhnke, I. 2021. Global and regional prevalence of helminth infection in chickens over time: a systematic review and meta-analysis. Poult. Sci. 100(5), 101082. Shohana, N.N., Rony, S.A., Ali, M.H., Hossain, M.S., Labony, S.S., Dey, A.R., Farjana, T., Alam, M.Z., Alim, M.A. and Anisuzzaman, 2023. Ascaridia galli infection in chicken: Pathobiology and immunological orchestra. Immun. Inflamm. Dis. 11(9), e1001. Singh, R., Gupta, I. and Patil, R.D. 2023. Ascariasis in poultry: a comprehensive review. Pharma Innov. 12(11S), 699–704. Sonaiya, B. 1990. Strategies for sustainable animal agriculture in developing countries. Available via https://www.fao.org/4/t0582e/T0582E26.htm (Accessed 31 May 2024). Soulsby, E.J.L. 1968. Helminths, arthropods and protozoa of domesticated animals. (Accessed 06 November 2022). Thapa, S., Hinrichsen, L.K., Brenninkmeyer, C., Gunnarsson, S., Heerkens, J.L.T., Verwer, C., Niebuhr, K., Willett, A., Grilli, G., Thamsborg, S.M., Sørensen, J.T. and Mejer, H. 2015. Prevalence and magnitude of helminth infections in organic laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 214(1-2), 118–124. Thrusfield, M. 2018. Veterinary epidemiology. Hoboken, NJ: John Wiley & Sons. Tomza-Marciniak, A., Pilarczyk, B., Tobiańska, B. and Tarasewicz, N. 2014. Gastrointestinal parasites of free-range chickens. Ann. Parasitol. 60(4), 305–308. | ||
| How to Cite this Article |
| Pubmed Style Makouloutou-nzassi P, Longo-pendy NM, Nguema LKA, Lendzele SS, Bangueboussa F, Bouchedi B, Maganga GD, Boundenga L. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Vet. J.. 2024; 14(12): 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 Web Style Makouloutou-nzassi P, Longo-pendy NM, Nguema LKA, Lendzele SS, Bangueboussa F, Bouchedi B, Maganga GD, Boundenga L. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. https://www.openveterinaryjournal.com/?mno=212105 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.8 AMA (American Medical Association) Style Makouloutou-nzassi P, Longo-pendy NM, Nguema LKA, Lendzele SS, Bangueboussa F, Bouchedi B, Maganga GD, Boundenga L. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Vet. J.. 2024; 14(12): 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 Vancouver/ICMJE Style Makouloutou-nzassi P, Longo-pendy NM, Nguema LKA, Lendzele SS, Bangueboussa F, Bouchedi B, Maganga GD, Boundenga L. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 Harvard Style Makouloutou-nzassi, P., Longo-pendy, . N. M., Nguema, . L. K. A., Lendzele, . S. S., Bangueboussa, . F., Bouchedi, . B., Maganga, . G. D. & Boundenga, . L. (2024) Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Vet. J., 14 (12), 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 Turabian Style Makouloutou-nzassi, Patrice, Neil Michel Longo-pendy, Lucien Keurtis Ayefegue Nguema, Silas Sevidzem Lendzele, Felicien Bangueboussa, Bernie Bouchedi, Gael Darren Maganga, and Larson Boundenga. 2024. Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Veterinary Journal, 14 (12), 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 Chicago Style Makouloutou-nzassi, Patrice, Neil Michel Longo-pendy, Lucien Keurtis Ayefegue Nguema, Silas Sevidzem Lendzele, Felicien Bangueboussa, Bernie Bouchedi, Gael Darren Maganga, and Larson Boundenga. "Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon." Open Veterinary Journal 14 (2024), 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 MLA (The Modern Language Association) Style Makouloutou-nzassi, Patrice, Neil Michel Longo-pendy, Lucien Keurtis Ayefegue Nguema, Silas Sevidzem Lendzele, Felicien Bangueboussa, Bernie Bouchedi, Gael Darren Maganga, and Larson Boundenga. "Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon." Open Veterinary Journal 14.12 (2024), 3232-3240. Print. doi:10.5455/OVJ.2024.v14.i12.8 APA (American Psychological Association) Style Makouloutou-nzassi, P., Longo-pendy, . N. M., Nguema, . L. K. A., Lendzele, . S. S., Bangueboussa, . F., Bouchedi, . B., Maganga, . G. D. & Boundenga, . L. (2024) Prevalence of gastrointestinal parasites in chickens (Gallus gallus domesticus) and associated risk factors in M’passa department, Southeast Gabon. Open Veterinary Journal, 14 (12), 3232-3240. doi:10.5455/OVJ.2024.v14.i12.8 |