| Research Article | ||
Open Vet. J.. 2024; 14(11): 2837-2847 Open Veterinary Journal, (2024), Vol. 14(11): 2837-2847 Research Article Hematological and thermographical changes in rat’s model exposed to long-term RF modulated signalsOmar B. Aghaa1* and Bashaer K. Hameed21Department of Clinical Laboratory Science, College of Pharmacy, University of Mosul, Mosul, Iraq 2Department of Biology, College of Education for Women, University of Tikrit, Tikrit, Iraq *Corresponding Author: Omar B. Aghaa. Department of Clinical Laboratory Science, College of Pharmacy, University of Mosul, Mosul, Iraq. Email: patho.omar [at] uomosul.edu.iq Submitted:04/08/2024 Accepted: 28/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
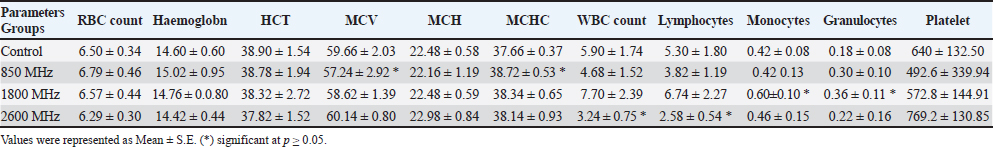
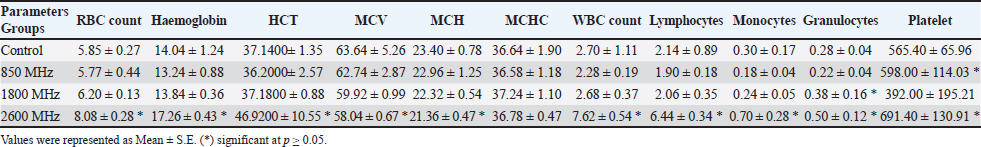
AbstractBackground: Long-term exposure to LTE signals at different frequencies has become a crucial problem in our daily life. Aim: The aim of the study to figure out the thermal influence of LTE signals (850 MHz, 1800 MHz, and 2600 MHz) on hematological values in rat’s model during different periods. Methods: Forty adult male rats were randomly distributed into four equal groups (control, 850 MHz, 1800 MHz, and 2600 MHz exposure groups). The rats were exposed for 2 hours per day over a period of up to 60 days using a radiofrequency generator. Results: The results showed that the different frequencies have different effects on both hematological and thermo-graphical image analysis. Conclusion: The study findings demonstrate that these LTE frequencies have a detrimental effect on the rat model through thermal mechanisms. Keywords: Hematological, Thermographic changes, LTE signals. IntroductionAs the priority of WHO guidelines for standardization of bio-effects studies for radio frequency (RF) exposure on humans and due to the difficulties in conducting such works on human beings, it has been suggested that studies be carried out with another biological system like laboratory animals (Morais et al., 2021). A series of investigations have been conducted since then, with the aim of finding a model system and endpoints that simulate the in vivo situation and have predictive value for potential adverse health effects of chronic or acute RF-EMF exposure in humans (Kim et al., 2020). This includes different ex vivo systems to in vivo, involving diverse animals, cell lines, and plants. The rat is one of the models that has been used extensively for bio-effects and toxicological studies (Quan et al., 2020; Cao et al., 2021). The use of RF-EMF in rats was recommended by the Laboratory Animal Resources Institute and requires ease of access to rats, minimal space requirements for implantation, choice of a wide range of biological end products, and analysis of increased production in-vitro and in-vivo. (Kaur et al., 2023). Although there is a dearth of knowledge about the hematological and thermoregulatory systems of animals exposed to RF-EMW, these systems have a profound influence on the total health and survival of the organism (Abduch et al., 2022). These systems can be taken as a good indicator for judgment of the effect of RF-EMF exposure. Thus, the effect of exposure to RF signals has been studied, with different modulations, simulating the EMW from mobile phones and other wireless communication methods, on the hematological and thermoregulatory systems of rats (Szmigielski et al., 2020). The rise in electronic device usage has raised concerns about the effects of exposure to RF devices on human health. Studies have shown that RF-EMF can impact cognitive functions, cardiac, reproductive, and immune systems (Jagetia, 2022). The human brain reacts to EMW similarly to environmental stress, perceiving it as a threat to survival. Rats exposed to mobile phone radiation experienced increased blood-brain barrier permeability. Different studies have used varying frequencies, modulations, and exposure times of RF-EMW, indicating that it can affect different parts of the body in various ways (Asl et al., 2020). Mobile phones, WLAN, and other radio communication devices are common sources of RF exposure for humans. Investigating the effects of RF-EMF on humans is challenging, but prolonged or acute exposure can be detrimental to health (Negi and Singh, 2021). Electromagnetic radiations (EMRs) from these devices have potentially harmful effects on human health and has become a topic of big debate (Alchalabi et al., 2021). To assess the thermal effects of these radiations, the real-time pattern of RF radiation-induced elevation of skin temperature was assessed using non-invasive thermographycamera. Complete blood count (CBC) provides valuable information in diagnosing and assessing health problems. It is simple and inexpensive, and it provides an indication of the physiological status of an animal (Miglio et al., 2023). Body temperature changes can influence biochemical and physiological processes. In today’s technology-driven population, mobile or cellular phones have become a part of everyday life (Volkoff and Rønnestad, 2020). The aim of the current study was to evaluate the hematological and thermographic changes in rat’s model exposed to long-term RF modulated signals. Material and MethodsAnimals and LTE exposure setupForty adult male rats in good physical condition, averaging between 170 and 190 g, were chosen. The animals were split into three groups: a control group, three exposure groups, and whole-body exposure for 2 hours every day, 7 days a week, for 30 and 60 consecutive days for the period from April to July 2024. The animals were supplied by the University of Mosul’s Faculty of Pharmacy’s laboratory animal research unit. The plastic cages held the animals at room temperature (25°C ± 1°C) and a humidity level (60% ± 10%) with a light/dark cycle that lasted 12 hours. The animals had unlimited access to tap water and standard rat pellets. RF-EMR exposure period was carried out via a specially built acrylic box of 60 cm, 40 cm, and 20 cm. The SAR limit at 0.974 W/kg was chosen for whole-body exposure to mobile phone radiation at 850, 1800, and 2600 MHz LTE frequency for up to 2 hours per day. The SAR is calculated using the formula SAR=(σ/ρ)E², where σ is the conductivity of the tissue (1.34 S/m), ρ is the mass density (1090 kg/m3), and E is the magnitude of the electric field (28.156 V/m). Hematological analysis2 ml of blood was collected from the tail vine after 2,4,6 and 8 weeks, respectively. The collected blood was preserved under −20C using EDTA tubes for later use. A hematological analyzer (Syamex model: K-1000, Japan) was used for CBC estimation according to the manufacturer’s instructions. ThermographyOver the research project, shifts in temperature were recorded and thermographic visualization carried out via an infrared device model FLIR-i5 (FLIR system Inc., USA) (Fig. 1). Each trial came out in a room with adjustable temperatures, offering any shifts in temperature were limited to 0.3°C. In detecting raw temperatures, three key parameters are taken into consideration: the object’s ambient humidity, temperature, and emissions. The infrared technology sensor was set at a 3 m detached from the walls, centered at a 1 m distance, 30°C conveyed Celsius, 28°C ambient temperature, and 60% humidity. FLIR Tools application was applied for interpreting the thermographic snaps, with a reflection of 0.95. Statistical analysisThroughout all 30 days of a trial, thermographs that exist took at multiple times and at every capture location to indicate various intervals. These spans entailed just after the ending of the exposure time, 1 hour, 2 hours, and 4 hours, taken on a weekly basis. The animal’s full body temperatures, or ones of the head, trunk, and tail regions, were measured after the infrared images had been analyzed using FLIR Tools application. Recorded were the mean, highest, and lowest temperatures. A statistical application SPSS V.23 was applied for comparing the data. The data was given as mean ± S.E. To find out the impact between the groups and within each collection, two-way ANOVA was used, with p values of beneath 0.05 being seen as significant. Normality test was tested before starting statistical analysis via kurtosis test for both hematological and thermographical values (−0.24–1.44), (−0.05–0.12), respectively. Ethical approvalAn ethical permit has been issued under No. (UM.VET.2024. 056 in 14/3/2024) by the University of Mosul, College of Veterinary Medicine, Institutional Animal Care and Use Committee. ResultsAfter 2 weeks of exposure, significant changes were noted across all frequency groups compared to the control. The effect revealed an increase in RBC count (6.79 ± 0.46) and hemoglobin (15.02 ± 0.95) levels, while maintaining a similar HCT (38.78 ± 1.94). However, this group experienced a marked decrease in WBC count (4.68 ± 1.52) and a substantial reduction in platelet count (492.6 ± 339.94) compared to the control. The 1800 MHz exposure group showed relatively stable RBC (6.57 ± 0.44) and hemoglobin (14.76 ± 0.80) levels but had an increased WBC count (7.70 ± 2.39) and a slight decrease in platelet count (572.8 ± 144.91). Conversely, the 2600 MHz group displayed a reduction in RBC count (6.29 ± 0.30) and hemoglobin levels (14.42 ± 0.44), with a notable drop in WBC count (3.24 ± 0.75) but an increase in platelet count (769.2 ± 130.85) compared to the control. By the fourth week, distinct trends emerged. The 850 MHz group showed a slight decrease in RBC count (6.01 ± 1.04) and hemoglobin levels (13.76 ± 2.28) compared to the control, with a stable HCT (36.52 ± 6.05) but a continued decrease in WBC count (5.24 ± 1.68) and a significant increase in platelet count (730.0 ± 272.37). The 1800 MHz group maintained RBC (6.22 ± 0.19) and hemoglobin levels (14.36 ± 0.45) similar to the control but exhibited a significant increase in WBC count (4.20 ± 1.12) and a marked rise in platelet count (941.2 ± 110.04). The 2600 MHz group demonstrated decreased RBC count (6.16 ± 0.28) and hemoglobin levels (13.26 ± 0.73), with a further reduction in WBC count (2.58 ± 0.41) and a considerable decrease in platelet count (394.0 ± 113.82).
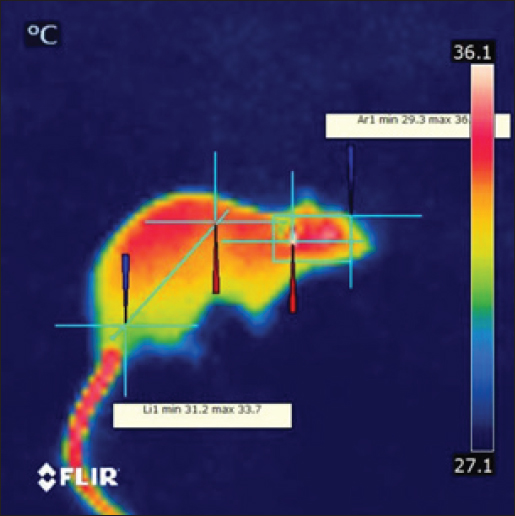
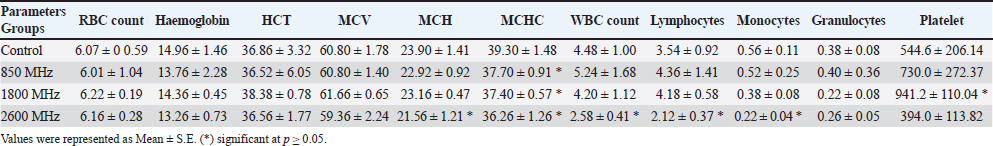
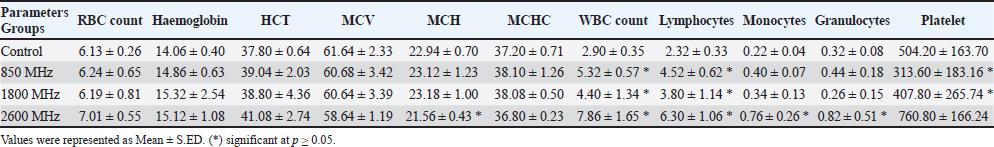
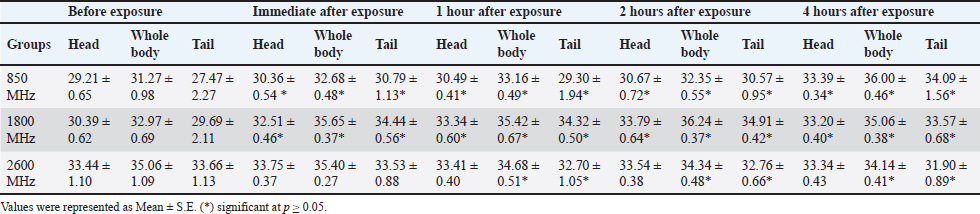
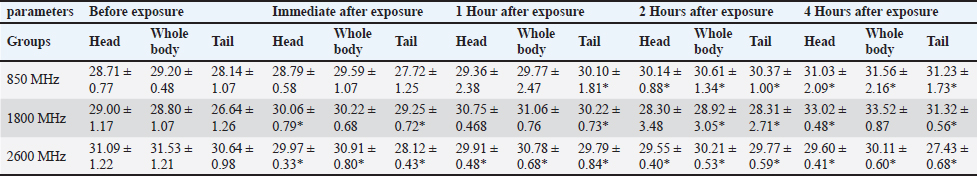
Fig. 1. Prior to the start of the trial, an infrared shot displays a homogeneous appearance of the dermal body temperature in each set of rats. At the six-week mark, the 850 MHz exposure group continued to show increased RBC count (7.18 ± 0.58) and hemoglobin levels (15.72 ± 1.08), with a stable HCT (40.08 ± 2.14). However, the WBC count (5.18 ± 1.70) remained lower, and the platelet count (512.6 ± 339.94) was still significantly reduced compared to the control. The 1800 MHz group exhibited similar RBC (6.77 ± 0.58) and hemoglobin levels (15.22 ± 0.92) to the control but had elevated WBC counts (8.00 ± 2.59) and slightly increased platelet counts (592.8 ± 164.91). The 2600 MHz group showed a continued decrease in RBC count (6.49 ± 0.44) and hemoglobin levels (14.62 ± 0.54), with a significant reduction in WBC count (3.44 ± 0.95) but a notable increase in platelet count (789.2 ± 150.85). After eight weeks, the observed patterns were more pronounced. The 850 MHz group had further increased RBC count (7.28 ± 0.68) and hemoglobin levels (15.92 ± 1.18), with stable HCT (40.18 ± 2.24). The WBC count (5.28 ± 1.80) remained lower, and the platelet count (522.6 ± 349.94) continued to be significantly lower than the control. The 1800 MHz group maintained similar RBC (6.87 ± 0.68) and hemoglobin levels (15.32 ± 1.02) to the control, but WBC counts (8.10 ± 2.69) and platelet counts (602.8 ± 174.91) were elevated. The 2600 MHz group showed decreased RBC count (6.59 ± 0.54) and hemoglobin levels (14.72 ± 0.64), with a further reduction in WBC count (3.54 ± 1.05) but an increased platelet count (799.2 ± 160.85) compared to the control (Tables 1–4). Table 5 presents the thermographic analysis of subjects exposed to LTE frequencies of 850, 1800, and 2600 MHz. Before exposure, the head, whole body, and tail temperatures were 29.21°C ± 0.65°C, 31.27°C ± 0.98°C, and 27.47°C ± 2.27°C, respectively. Immediate post-exposure temperatures increased significantly to 30.36°C ± 0.54°C, 32.68°C ± 0.48°C, and 30.79°C ± 1.13°C (p < 0.05). The trend of significant increases persisted up to 4 hours after exposure. Table 1. Effect of LTE 850, 1800 and 2600 MHz exposure for 2 weeks on haematological values.
Table 2. Effect of LTE 850, 1800 and 2600 MHz exposure for 4 weeks on haematological values.
Table 3. Effect of LTE 850, 1800 and 2600 MHz exposure for 6 weeks on haematological values.
Table 4. Effect of LTE 850, 1800 and 2600 MHz exposure for 8 weeks on haematological values.
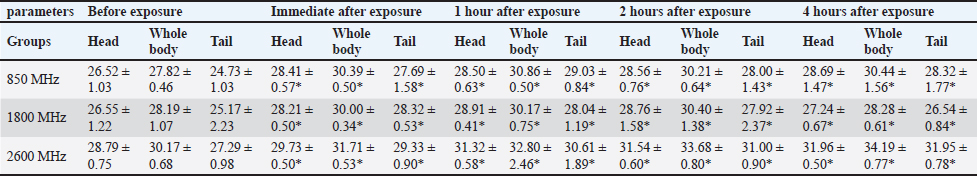
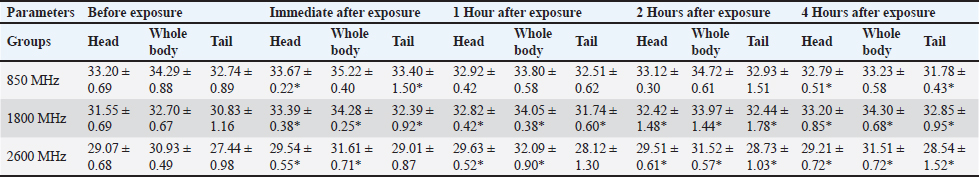
For the 1800 MHz group, Significant temperature increases were observed immediately after exposure (32.51°C ± 0.46°C, 35.65°C ± 0.37°C, 34.44°C ± 0.56°C) and remained elevated up to 4 hours post-exposure (p < 0.05). For the 2600 MHz group, post-exposure temperatures showed a significant rise immediately (33.75°C ± 0.37°C, 35.40°C ± 0.27°C, 33.53°C ± 0.88°C) and continued to show significant increases at 1 and 4 hours (p < 0.05). Table 6 details the thermographic analysis of subjects exposed to LTE frequencies of 850, 1800, and 2600 MHz for 4 weeks, with temperatures measured at the head, whole body, and tail. For post-exposure of the 850 MHz Exposure group, temperatures increased significantly across all time points, with immediate post-exposure readings at 28.41°C ± 0.57°C, 30.39°C ± 0.50°C, and 27.69°C ± 1.58°C (p < 0.05). For 1800 MHz Exposure, significant temperature increases were noted immediately after exposure and persisted up to 4 hours (p < 0.05). For 2600 MHz Exposure, significant rises in temperature were recorded at all time points post-exposure (p < 0.05). Table 7 shows the thermographic analysis for subjects exposed to LTE frequencies of 850, 1800, and 2600 MHz for 6 weeks, with temperatures recorded at multiple time intervals. For 850 MHz Exposure, significant increases were seen immediately post-exposure, with the head temperature at 33.67°C ± 0.22°C (p < 0.05). For 1800 MHz Exposure, post-exposure temperatures showed significant increases at all time points (p < 0.05). For 2600 MHz Exposure, post-exposure measurements showed significant increases immediately and up to 4 hours post-exposure (p < 0.05). Table 8 summarizes the thermographic analysis for subjects exposed to LTE frequencies of 850, 1800, and 2600 MHz for 8 weeks, with temperature data collected before and after exposure. For 850 MHz Exposure, significant temperature increases were recorded, with the immediate post-exposure head temperature at 28.79°C ± 0.58°C (p < 0.05). For 1800 MHz Exposure, significant increases were observed immediately and at subsequent time points (p < 0.05). For 2600 MHz Exposure, significant increases were noted immediately post-exposure and persisted at later time points (p < 0.05) (Fig. 2). DiscussionThe current findings highlight significant hematological changes induced by long-term exposure to LTE frequencies of 850 MHz, 1800 MHz, and 2600 MHz. A previous work stated that RF-EMF affects negatively on hematological values at a frequency of 2.45GHz indicating that this effect is directly related to the altered frequency at a high range (Vijayalaxmi et al., 2001). Other studies found that both 0.9 and 1.8GHz exposure leads to significant changes in Hb, and RBC total count in exposed animals in respect to control animals (Dasdag et al., 2003; Trošić et al., 2012). Table 5. Thermographic image analysis of exposure to LTE 850, 1800 and 2600 MHz for 2 weeks.
Table 6. Thermographic image analysis of exposure to LTE 850, 1800 and 2600 MHz for 4 weeks.
Table 7. Thermographic image analysis of exposure to LTE 850, 1800 and 2600 MHz for 6 weeks.
Table 8. Thermographic image analysis of exposure to LTE 850, 1800 and 2600 MHz for 8 weeks.
This study addressed the thermodynamic impacts on rats of sustained exposure to LTE radiation at 850, 1800, and 2600 MHz. Awareness of the influence of heat of RF waves with bands that go from three KHz to 300 GHz will be easier through thermographic visualization (McNamee and Chauhan, 2009; Nylund, 2011). A disparity in the physique’s thermal regulation system (heat production and heat dissipating), heat generation computed with SAR, and the energy level of an emitted device—which inappropriately exceeds 100 mW per cm2—all trigger a rise in tissue temperature. Three basic pathways govern heat loss: emission to the environs, convective in circulation, and transfer of heat to adjacent tissues (Hamada et al., 2011). Producing heat in a living system takes place as EMR engages with biological materials. This arises when it is absorbed and transmitted to the tissues, and the mechanism of absorption includes heating up, vibrating, rotating, and powered modes (Krewski et al., 2007). The quantity of radiation being absorbed, which is directly dictated by how fast and how long of exposure, impacts the level of excitement induced by biological tissue. Considering it may preheat tissues or hamper cellular activity and expansion, frequency is an essential element in the formation of heat (Güler et al., 2012). There are basically two stages to the thermolytic response of rats to chronic heat stress. The first action is cutaneous vasodilation from exposed vascularized surfaces, typically the hind end, in anticipation of temperature changes with the surrounding environment. Grooming carries out is a subsequent response(Stricker and Hainsworth, 1971). The infrared studies further proved that exposed animals had higher peak and lowest temperatures in various body regions than did unexposed animals. The thermographic spectrum illustrates how the disparity in animals exposed to EMR over an extended period of time (either through high use or chronic exposure) are quite clear, via hot regions scattered over the rats’ bodies. Our results refute a previous paper that Pelletier and associates published. They discovered exposed animals’ tail temperatures were less than those of unexposed animals. They placed the antenna in close vicinity to the exposed rats’ boxes, close to 80 cm distant, and applied low-intensity radiofrequency electromagnetic field continuous waves (900 MHz) (Pelletier et al., 2014). Nevertheless, the study’s findings complied with the data issued by the Usman team. who ultimately came to the view that, in lieu of the control group, animals exposed to 2.46 GHz for 7 hours a day for 4 weeks had higher body temperatures tracked by IRTC appliances, greater excretory rates, and were less active (Usman et al., 2011).
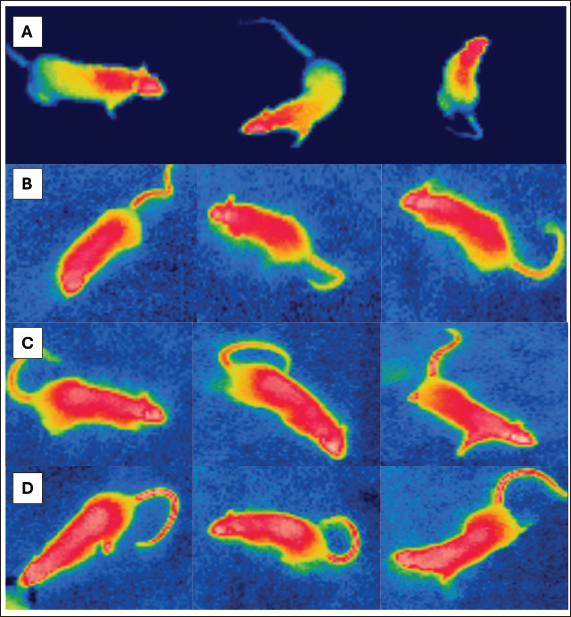
Fig. 2. After the exposure periods ended, IR visuals displayed a notable change in the shade of the hottest areas, (A) Control, (B) 850 MHz, (C) 1800 MHz, and (D) 2600MHz exposure groups. An inclination to associate tail sweating with wasting heat during urination. The tail’s role in thermoregulatory activity and heat stress adaption is backed by an elevation in tail temperature ranging at 33.5°C and 35.5°C. Which enhances tail microvascular vasodilation. Up to 20% of the heat generated by the rat may be lost through vasodilation in the tail. The animal’s tail is a non-evaporative heat loss area, as verified by the use of the surface temperature of the back as a vasodilation factor to see if alterations at 11°C, 20°C, and 30°C having an influence on the critical temperature (Shido and Nagasaka, 1990). Rats in the exposure group adapted to the perpetual heat stress; data implies that after 60 days, there is no disparity between animals that did not get exposed to the heat and those that were. Raised surface temperatures were observed in the head and flank sites on thermogram photographs, emphasizing the value of thermal damage on vital organs in these areas, such as the brain and ovaries, as well as the other vital organs in the body. It was observed that LTE 2600 MHz electromagnetic waves elevated body temperature and widened hot spots in rats relative to the control group. It also validates the outcomes of (Vijayalaxmi and Prihoda, 2008), where noticed that mice exposed to Wi-Fi signals for 7 hours per day for a month saw a rise in surface body temperature attributed to elevated metabolic processes. A recent study demonstrated that the intensity of regional temperature may indicate the amount of energy absorbed by tissues following exposure to radiofrequency EMR from smartphones (Hirata et al., 2021). The investigation by Dasdag et al. (1999) reported that younger rats subjected to moderately intense RF-EMF at 900 MHz for a maximum of 5 weeks adjusted behavioral thermal preference to more intense temperatures in reaction to cold feelings. A research investigation found that 2450 MHz microwaves greatly raised the temperature of rabbit limbs with titanium alloy implants, inducing acute thermal inflammation of nerves and muscles near the implants (Miglio et al., 2023). Although numerous investigations were carried in the 1-3 GHz range (FREI, 1989), overall is no consensus on the non-thermal biological mechanisms underlying RF electromagnetic fields and microwave radiation affecting live humanity. Plenty of studies on microwave radiation’s hematological impacts have yielded inconsistent results. Identifying the cause of inconsistencies can be challenging (Cleary, 1979; Lin et al., 1979), highlighting the need for further research on laboratory animals (Repacholi, 1997) After 24000 MHz irradiation, two strains of irradiated animals displayed considerable leukocytosis, lymphocytosis, and neutrophilia, although one strain possessed inverse response (Smialowicz et al., 1981). In a study conducted by Justesen et al. (1978) rats exposed to intermittent microwaves at 2400 MHz for 90 days (1 hour per day) had an intensity level of 5 mW/cm2 indicating no noteworthy changes in hematological markers (Justesen et al., 1978). Smialowicz et al. (1981) observed no shifts in hematological evidence in rats exposed to 2450 MHz MW fields (Smialowicz et al., 1981). MCREE identified hematological variables influenced by RF/MW exposure in individuals as well as animals. The investigations covered white blood cell count, differential white blood cell count, platelet levels, red blood cell count, mitotic index of hematopoietic stem cells, hematocrit, hemoglobin, and bone marrow megakaryocytes (McRee, 1979). Heat stress and LTE exposure contributed to a considerable drop in total white blood cell count, per a hemato-biochemical study. Data suggest a subtractive connection between RF-EMR and heat stress on WBC levels. Evidence indicates that elevated temperatures may influence immunological responses, specifically T cell populations and lymphocyte proliferation (a kind of white blood cell). Heat exposure (WBGT 29°C ± 1°C) was linked to a notable drop in RBC counts, as well as HB and HCT values. Seley’s (1960) study concluded that heat stress in rodents can reduce adrenocorticotropic hormone levels in the blood, causing decreased RBC counts and HB percentage [20]. Excessive heat exposure (WBGT 33°C ± 1°C) brought about significantly higher blood HB and HCT levels in those exposed compared to the control group. Rats’ elevated HB and HCT levels may be linked to increased nutritional accessibility for HB synthesis or diminished willing appetite at sub-acute heat exposure (Zamanian et al., 2021). The heat-stressed group reported significantly more PCV and RBC values compared to the control group, as could be explained to lower oxygen intake due to high ambient temperature or increased hemoglobin concentration due to dehydration from heat stress. These findings are in line with those of Hussein and Kobeisy (1999). Exposure groups showed a substantial decrease in MCH compared to group 2600 MHz, possibly due to a decrease in HBC in all groups except 2600 MHz, which was not significant. The differential WBC count revealed a substantial decrease in lymphocytes in all groups except the 2600MHZ group compared to the other groups. Prolonged exposure to high temperatures can shrink lymphocytic organs like the spleen and thymus, potentially reducing the number of circulating lymphocytes (Odo et al., 2019). Rats’ hematological parameters were dramatically impacted by radiation exposure, both short and long-term. Hematological examination of rats exposed to EMR revealed considerable increases in white blood cells, lymphocytes, total red blood corpuscles, and packed cell volume. The study found that rats exposed to LTE EMR had considerably lower values for mean corpuscle volume, hemoglobin concentration, and red blood cell distribution width standard deviation. These data were alike Abd Al Abas et al., (2021) who reported that mobile phone radiation had a different impact on hematological values in rats (Al Abas and AL-Hakkak, 2021). ConclusionThe study demonstrates that long-term exposure to LTE frequencies causes significant hemodynamic changes. For instance, the 850 MHz frequency increased RBC and Hb and decreased WBC and platelets count. This frequency rejuvenated WBC, RBC (Red Blood Cell), and hemoglobin levels while a significant increase was observed in platelet count. Lesser RBCs and hemoglobin levels were reported for 2600 MHz frequency while contradictory increased PLT counts (Table 4). These data highlight the differential effects of LTE frequencies on the hematopoietic system, and suggest that additional investigation is needed to understand biological mechanisms and health risks for chronic exposure in view of the increasing spread of wireless technology. AcknowledgmentThe completion of this research paper would not have been possible without the support and guidance of Laboratory staff of University of Ninevah, College of Electronic Engineering, department of Communications for their support by supplying all equipment were needed. The authors are grateful for the invaluable support provided by the University of Mosul, College of Pharmacy throughout the research process. The facilities and resources offered were extremely helpful and have been instrumental in completing this research project successfully. Conflict of interestThe authors had no conflict of interest. FundingThe project has self-funding support. Authors’ contributionsOmar B. Aghaa: was corresponding for animal care and hematological parameters. Bashaer K. Hameed: was corresponding for the thermal treatment and obtaining the blood samples. Article writing was done equally by both authors. Data availabilityAll data are provided in the manuscript. ReferencesAbduch, N.G., Pires, B.V., Souza, L.L., Vicentini, R.R., Zadra, L.E.F., Fragomeni, B.O., Silva, R.M.O., Baldi, F., Paz, C.C.P. and Stafuzza, N.B. 2022. Effect of thermal stress on +thermoregulation, hematological and hormonal characteristics of caracu beef cattle. Animals (Basel). 12(24), 3473. Al Abas, M.A.A. and AL-Hakkak, Z.M.M. 2021. Effect exposure of mobile phone radiation on blood parameters in rats. Iraqi J. Sci. 62(11), 4225–4231. Alchalabi, A.S., Asim, R., Rahim, H. and Abdul Malek, M.F. 2021. Evaluation of the thermal effect of LTE 2600 MHz (4G) electromagnetic field (EMF) exposure: thermographic study on rats. Iraqi J. Vet. Sci. 35(2), 279–285. Asl, J.F., Goudarzi, M. and Shoghi, H. 2020. The radio-protective effect of rosmarinic acid against mobile phone and Wi-Fi radiation-induced oxidative stress in the brains of rats. Pharmacol. Rep. 72, 857–866. Cao, Y., Li, S. and Chen, J. 2021. Modeling better in vitro models for the prediction of nanoparticle toxicity: a review. Toxicol. Mech. Methods. 31(1), 1–17. Cleary, S.F. 1979. Recapitulation: biomedical effects. Bull. N Y Acad. Med. J. Urban Health. 55(11), 1119. Dasdag, S., Ketani, M.A., Akdag, Z., Ersay, A.R., Sari, I., Demirtas, Ö.C. and Celik, M.S. 1999. Whole-body microwave exposure emitted by cellular phones and testicular function of rats. Urol. Res. 55(11), 1119. Dasdag, S., Zulkuf Akdag, M., Aksen, F., Yılmaz, F., Bashan, M., Mutlu Dasdag, M. and Salih Celik, M. 2003. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics, 24(3), 182–188. Güler, A.D., Rainwater, A., Parker, J.G., Jones, G.L., Argilli, E., Arenkiel, B.R., Ehlers, M.D., Bonci, A., Zweifel, L.S. and Palmiter, R.D. 2012. Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nat. Commun. 3(1), 746. Hamada, A.J., Singh, A. and Agarwal, A. 2011. Cell phones and their impact on male fertility: fact or fiction. Open Reprod. Sci. J. 5(4), 125–137. Hirata, A., Kodera, S., Sasaki, K., Gomez-Tames, J., Laakso, I., Wood, A., Watanabe, S. and Foster, K.R. 2021. Human exposure to radiofrequency energy above 6 GHz: review of computational dosimetry studies. Phys. Med. Biol. 66(8), 08TR01. Hussein, S.Y. and Kobeisy, M.A. 1999. Influence of heat stress on growth performance and some blood constituents of Oreochromis niloticus fed ascorbic acid. Assiut Vet. Med. J. 41(81), 17–33. Jagetia, G.C. 2022. Genotoxic effects of wireless communication electromagnetic fields, in: electromagnetic fields of wireless communications: biological and health effects. Boca Raton, FL: CRC Press, 137–188. Justesen, D.R., Ragan, H.A., Rogers, L.E., Guy, A.W., Hjeresen, D.L., Hinds, W.T. and Phillips, R.D. 1978. Compilation and assessment of microwave bioeffects. final report. a selective review of the literature on biological effects of microwaves in relation to the Satellite Power System (SPS). Available via https://www.osti.gov/servlets/purl/6226719 Kaur, P., Rai, U. and Singh, R. 2023. Genotoxic risks to male reproductive health from radiofrequency Radiation. Cells, 12(4), 594. Kim, J., Koo, B.K. and Knoblich, J.A. 2020. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biolm. 21(10), 571–584. Krewski, D., Glickman, B.W., Habash, R.W.Y., Habbick, B., Lotz, W.G., Mandeville, R., Prato, F.S., Salem, T. and Weaver, D.F. 2007. Recent advances in research on radiofrequency fields and health: 2001–2003. J. Toxicol. Environ. Health B. Crit. Rev. 10(4), 287–318. Lin, J.C., Nelson, J.C. and Ekstrom, M.E. 1979. Effects of repeated exposure to 148-MHz radio waves on growth and hematology of mice. Radio. Sci. 14(6S), 173–179. McNamee, J.P. and Chauhan, V. 2009. Radiofrequency radiation and gene/protein expression: a review. Radiat. Res. 172(3), 265–287. McRee, D.I. 1979. Review of Soviet/Eastern European research on health aspects of microwave radiation. Bull. N Y Acad. Med. 55(11), 1133–1151. Miglio, A., Valente, C. and Guglielmini, C. 2023. Red blood cell distribution width as a novel parameter in canine disorders: literature review and future prospective. Animals, 13(6), 985. Morais, L.H., Schreiber, H.L. and Mazmanian, S.K. 2021. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19(4), 241–255. Negi, P. and Singh, R. 2021. Association between reproductive health and nonionizing radiation exposure. Electromagn. Biol. Med. 40(1), 92-102.. Nylund, R. 2011. Proteomics analysis of human endothelial cells after shortterm exposure to mobile phone radiation. Espoo, Finland: Aalto University School of Science, ISBN 978-952-478-657-7. Odo, R.I., Onoja, S.O. and Osuagwu, C.O. 2019. Impact of heat stress on blood and serum biochemistry parameters in rats. Not. Sci. Biol. 11(3), 347–350. Pelletier, A., Delanaud, S., De Seze, R., Bach, V., Libert, J.P. and Loos, N. 2014. Does exposure to a radiofrequency electromagnetic field modify thermal preference in juvenile rats? PLoS One 9(6), e99007. Quan, Y., Sun, M., Tan, Z., Eijkel, J.C.T., Van Den Berg, A., Van Der Meer, A. and Xie, Y. 2020. Organ-on-a-chip: the next generation platform for risk assessment of radiobiology. RSC Adv. 10(65), 39521–39530. Repacholi, M.H. 1997. Radiofrequency field exposure and cancer: what do the laboratory studies suggest? Environ. Health Perspect.105(Suppl. 6), 1565–1568. Selye, H. 1960. The concept of stress in experimental physiology. In: Tanner, J.M., Ed. Stress and Psychiatric disorder. 1st ed. Oxford, Blackwell, pp: 67–75. Shido, O. and Nagasaka, T. 1990. Thermoregulatory responses to acute body heating in rats acclimated to continuous heat exposure. J. Appl. Physiol. 68, 59–65. Smialowicz, R.J., Ali, J.S., Berman, E., Bursian, S.J., Kinn, J.B., Liddle, C.G., Reiter, L.W. and Weil, C.M. 1981. Chronic exposure of rats to 100-MHz (CW) radiofrequency radiation: assessment of biological effects. Radiat. Res. 86(3), 488–505. Stricker, E.M. and Hainsworth, F.R. 1971. Evaporative cooling in the rat: interaction with heat loss from the tail. Quart. J. Exper. Physiol. Cognate. Med. Sci. Transl. Integr. 56(4), 231–241. Szmigielski, S., Bielec, M. and Lipski, S. 2020. Immunologic and cancer-related aspects of exposure to low-level microwave and radiofrequency fields. Modern Bioelectricity, Boca Raton, FL: CRC Press, 861–925. Trošić, I., Pavičić, I., Marjanović, A.M. and Bušljeta, I. 2012. Non-thermal biomarkers of exposure to radiofrequency/microwave radiation. Arh. Hig. Rada. Toksikol. 63(Suppl. 1), 67–72. Usman, A.D., Wan Ahmad, W.F., Ab Kadir, M.Z.A., Mokhtar, M. and Ariffin, R. 2011. Thermographic analysis of Wi-Fi frequencies exposed to unrestrained Swiss albino mice. Int. J. Phys. Sci. 6(36), 8097–8104. Vijayalaxmi, Pickard, W.F., Bisht, K.S., Prihoda, T.J., Meltz, M.L., LaRegina, M.C., Roti Roti, J.L., Straube, W.L. and Moros, E.G. 2001. Micronuclei in the peripheral blood and bone marrow cells of rats exposed to 2450 MHz radiofrequency radiation. Int. J. Radiat. Biol. 77(11), 1109–1115. Vijayalaxmi and Prihoda, T.J. 2008. Genetic damage in mammalian somatic cells exposed to radiofrequency radiation: a meta-analysis of data from 63 publications (1990–2005). Radiat. Res. 169(5), 561–574. Volkoff, H. and Rønnestad, I. 2020. Effects of temperature on feeding and digestive processes in fish. Temperature, 7(4), 307–320. Zamanian, Z., Yousefinejad, S., Dehghan, S.F. and Rahmani, A. 2021. Hemato-biochemical responses of rats co-exposed to heat stress and trichloroethylene vapors. Russian Open Med. J. 10(4), 405. | ||
| How to Cite this Article |
| Pubmed Style Aghaa OB, Hameed BK. Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Vet. J.. 2024; 14(11): 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 Web Style Aghaa OB, Hameed BK. Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. https://www.openveterinaryjournal.com/?mno=213757 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.12 AMA (American Medical Association) Style Aghaa OB, Hameed BK. Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Vet. J.. 2024; 14(11): 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 Vancouver/ICMJE Style Aghaa OB, Hameed BK. Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 Harvard Style Aghaa, O. B. & Hameed, . B. K. (2024) Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Vet. J., 14 (11), 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 Turabian Style Aghaa, Omar B., and Bashaer K. Hameed. 2024. Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Veterinary Journal, 14 (11), 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 Chicago Style Aghaa, Omar B., and Bashaer K. Hameed. "Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals." Open Veterinary Journal 14 (2024), 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 MLA (The Modern Language Association) Style Aghaa, Omar B., and Bashaer K. Hameed. "Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals." Open Veterinary Journal 14.11 (2024), 2837-2847. Print. doi:10.5455/OVJ.2024.v14.i11.12 APA (American Psychological Association) Style Aghaa, O. B. & Hameed, . B. K. (2024) Hematological and thermographical changes in rat’s model exposed to long term RF modulated signals. Open Veterinary Journal, 14 (11), 2837-2847. doi:10.5455/OVJ.2024.v14.i11.12 |