| Research Article | ||
Open Vet. J.. 2024; 14(11): 2817-2826 Open Veterinary Journal, (2024), Vol. 14(11): 2817-2826 Research Article Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of IraqHakeem Jawad Kadhim1*, Abbas Kamil Shlaga1 and Safaa Hussein Ali21Department of Microbiology, College of Veterinary Medicine and Surgery, Shatrah University, Shatrah, Thi-Qar, Iraq 2Department of Physiology, College of Veterinary Medicine and Surgery, Shatrah University, Shatrah, Thi-Qar, Iraq *Corresponding Author: Hakeem Jawad Kadhim. Department of Microbiology, College of Veterinary Medicine and Surgery, Shatrah University, Iraq. Email: hakim.jawad [at] shu.edu.iq Submitted: 02/08/2024 Accepted: 10/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
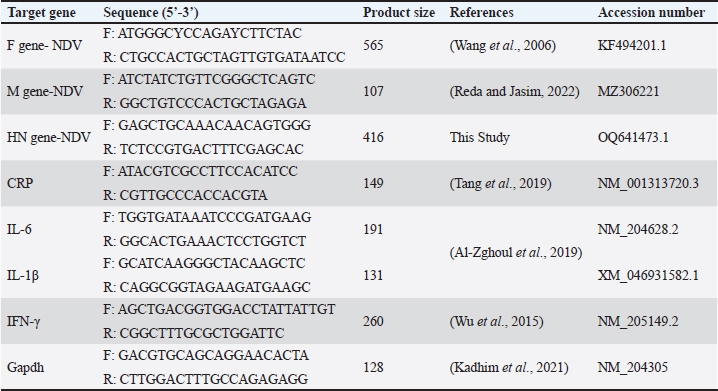
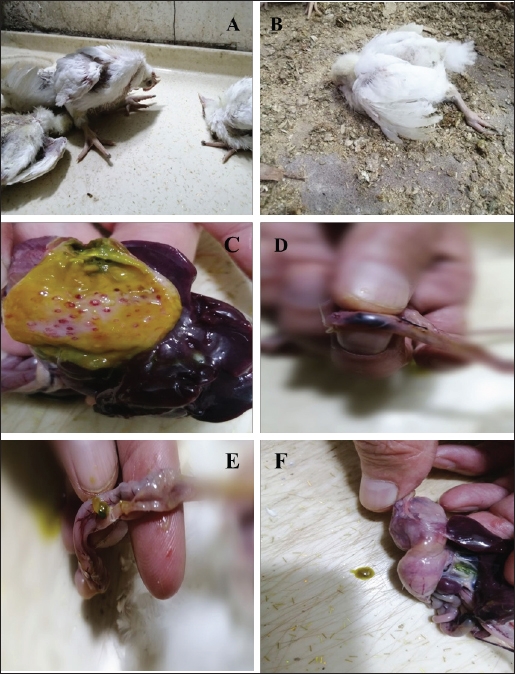
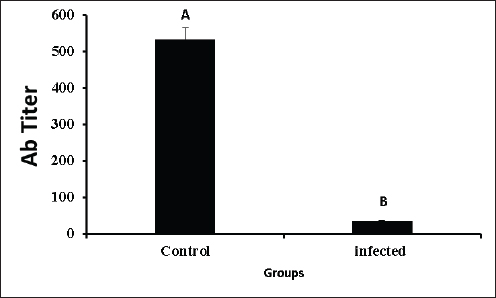
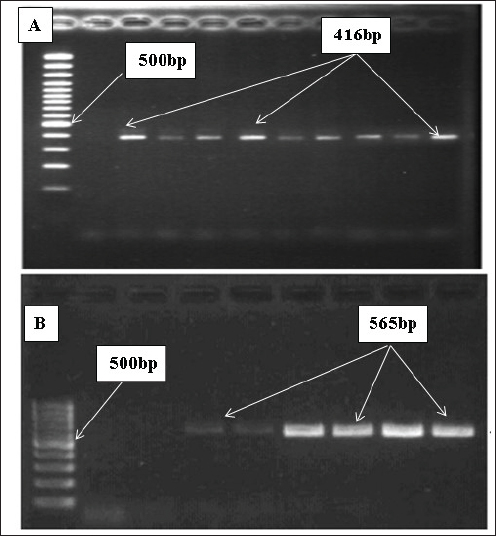
AbstractBackground: In poultry, despite intense vaccination programs for prevention of Newcastle disease (ND), the ND infection still affects, causing high mortality in most vaccinated flocks. Aim: This study aimed to determine whether the genetic material of the ND virus has changed and has become incompatible with the vaccines used in Iraq. Methods: Real-time PCR was used to analyze genetic variation in the fusion (F) and haemaggluatination neuraminidase (HN) genes, as well as mRNA expression changes in inflammatory biomarkers, including C-reactive protein (CRP), interleukin 6, interleukin-1 beta (IL-6, IL-1β), and gamma interferon (IFN-γ). Results: Although the La Sota vaccine was initially used to vaccinate broiler flocks against NDV, we noticed a variety of respiratory, digestive, and nervous signs in many flocks throughout Thi-Qar Province of Iraq. Furthermore, the infected birds showed typical postmortem lesions, such as mottled spleen, proventricular hemorrhages, and cecal tonsil hemorrhages. Blood, liver, and tracheal swabs were collected from infected and healthy broilers aged 3–5 weeks old. NDV infection was initially confirmed using the NDV antigen rapid test, which showed positive results in 52 of the 60 suspected samples (86.66%). However, mean antibody levels in ND-infected birds were significantly lower than those in healthy birds. In contrast, mRNA levels of bio-inflammatory genes were increased, indicating that the birds were infected with the virus and that there was inflammation in the body. To confirm NDV infection, the F and HN genes were sequenced. F gene alignment against NCBI nucleotide sequence data showed that the isolate had 94.68% similarity with the avian orthoavulavirus1 isolate Ph/IR/AMMM116/2018 fusion protein gene. While HN gene alignment showed 95.36% similarity with the NDV strain La Sota hemagglutinin-neuraminidase gene. Conclusion: ND infection resulted in a decrease in antibody titers and an increase in the expression of inflammatory biomarkers genes. The findings suggested that alterations in the nucleic acids of the NDV strains could be the main cause of potential outbreaks in Iraq, and that vaccines appeared to be incompatible with the circulating strain. Thus, it is recommended that, besides the rapid detection test, molecular methods should always be considered in endemic areas or outbreak situations. Keywords: Fusion gene, Haemaggluatination, Neuraminidase, Inflammatory biomarkers, RT-PCR. IntroductionNewcastle disease (ND) is an acute contagious viral disease that affects avian species and causes high rates of morbidity and mortality, resulting in huge economic losses. In addition, Shabbir et al. (2013) found that ND induces various clinical signs in domestic poultry, including respiratory, digestive, and neurological signs. Specifically, the main clinical signs are loss of appetite, depression, thirst, weakness, coughing, sneezing, nasal discharge, greenish diarrhea, head and neck twisting, leg and/or wing paralysis, or paralysis of the entire body. Furthermore, common gross lesions are hemorrhage and ulcers associated with central necrosis in the small intestine, hemorrhage of the proventriculus, pneumonic lungs, hemorrhages in the trachea, air sacs, brain, cecal tonsils, lymphoid tissue lesions, and congested spleen. Clinical signs and gross lesions depend on several factors, including virus strain, host, age, immune state, environmental stress, and co-infection (Waheed et al., 2013). ND is caused by avian paramyxovirus-1 of the genus Avulavirus, family Paramyxoviridae, and order Mononegavirales (Briand et al., 2012). NDV is an enveloped virus with negative polarity and non-segmented single-stranded RNA. The NDV genome contains six proteins, including RNA polymerase (L), haemaggluatination neuraminidase (HN), fusion (F), matrix (M), phosphoprotein (P), and nucleoprotein (NP), in the order of 3’-NP-P-M-F-HN-L-5’ (Kolakofsky et al., 2005). NDV uses HN to interact with the host cells, whereas the F protein is responsible for viral entry. Once fused, viral nucleic acids (nucleocaspids) enter the host cell cytoplasm, where the transcription and translation phases start (Mao et al., 2022). During viral infection, many cells respond by producing cytokines that can enhance or depress cell functions. Cytokines can be categorized based on their function and synthesis location into interleukins, tumor necrosis factors, and chemokines (Mitra and Leonard, 2018). Gamma interferons (IFNγ), interleukin 6 (IL-6), and interleukin 1 beta (IL-1β) are multifunctional cytokines that play essential roles in acute-phase responses, immunological control, and haematopoesis. C-reactive protein (CRP) is an example of an acute-phase protein that is produced as part of an inflammatory response to infection or other stressors in animals (Aliyu et al., 2022). Regardless of sex and age, the ND virus (NDV) can infect approximately 250 avian species and cause severe infection in domestic poultry. However, migratory birds are natural reservoirs for NDV and could be the main factor in transmitting the virus to domestic poultry. Hence, migratory birds may also be responsible for new outbreaks (Bansal et al., 2022). In Iraq, NDV was isolated from infected chickens in Abu Graib, named AG68, and later verified by real-time PCR (Wise et al., 2004). Later, NDV became endemic in Iraq despite the intensive immunization program to control or prevent it, and it is still the main threat to the poultry industry in the country. The possible reason for that might be due to the presence of large marshes in southern Iraq, which are rich in diverse species of migratory birds that are considered reservoirs for the virus. This may shed new strains that are incompatible with vaccines. Consequently, it is crucial to identify endemic serotypes in each province by molecular techniques. The goal of this study was to use real-time PCR to identify genetic variations in NDV within Thi-Qar Province by analyzing the F and HN gene nucleotide sequences. The serum antibody titers for the infected and control birds were measured. Similarly, the mRNA levels of inflammatory biomarkers, including CRP, IL-6, and IL-1β, as well as interferon gamma, were examined to assess the inflammatory response. Materials and MethodsBirds and sample collectionBased on clinical signs and postmortem lesions in broiler flocks at the age of 3–5 weeks. Sixty birds were collected from flocks that showed clinical signs and postmortem lesions similar to those of NDV infection and assigned as the infected group. The initial diagnosis was performed using the NDV antigen rapid kit (Bionote, Korea). Blood, liver, and tracheal swabs were collected and snap-frozen rapidly, except for the blood samples. The blood sample was split into two parts: the first part was used for serological testing, and the second part (200 μl) was mixed with Trizol for total RNA extraction using the Trizol-Chloroform protocol (for gene expression of inflammatory biomarkers). Furthermore, the antibody titer in the serum samples of the infected was calculated using the haemagglutination inhibition test (Reda and Jasim 2022). In addition, in order to find the effects of NDV on the antibody titer, we also need blood samples from healthy birds (control group). The control group referred to the samples that were collected from various flocks of healthy birds that did not show clinical signs of NDV infection and had never been infected with NDV. In addition, control birds were examined using the NDV rapid antigen kit to ensure that the birds are healthy and not infected with NDV. RNA processing, reverse transcription, and real-time PCR as well as conventional PCR for NDV detection and gene sequenceFor the infected birds, we extracted viral RNA from tracheal swabs using a viral Gene-spinTMViral DNA/RNA extraction kit and quantified it. Subsequently, two types of PCR, including RT-PCR and conventional PCR, were performed on the complementary DNA (cDNA), which was generated using Superscript®III (Invitrogen). The matrix (M) gene of NDV was screened in 30 µl for 40 cycles using RT-PCR in the infected samples. Samples were classified as positive for NDV if the cycle threshold (Ct) values less than 32 and as negative if Ct values more than 32. On the other hand, samples having Ct values between 18 and 22 underwent additional testing using conventional PCR, which amplified the F and HN genes in 50 µl for 30 cycles. The amplification of HN and F genes was verified by 1.5% agarose gel electrophoresis. Finally, the PCR products were sequenced using Sanger sequencing by Morgan Company and aligned against data available in the NCBI. RNA processing, reverse transcription, and real-time PCR for expression of inflammatory biomarkersTo address the effects of NDV on mRNA expression of inflammatory biomarkers, we must have healthy birds in order to compare between them. Therefore, samples (liver and blood) were collected from control (healthy) birds (described in the birds and sample collection section). Trizol-Chloroform protocol was used to extract total RNA from blood and liver samples of both control and infected birds, as reported in previous studies (Kadhim et al., 2019; 2020). The extracted RNA was then treated with Dnase1 to remove genomic DNA, and the RNeasy Mini Kit (Qiagen) was employed to purify the extracted RNA. The concentration of purified RNA was measured using nanodrop spectrophotometry. Complementary DNA (cDNA) was synthesized from purified RNA (1µg) using Superscript®III (Invitrogen) as described in our previous publications (Kadhim et al., 2021 2022). We examined the expression of inflammatory biomarkers, including CRP, IL-6, IL-1β, and IFN-γ, as well as the housekeeping gene, glyceraldehyde3-phophate dehydrogenase (GAPDH). Primer details were listed in Table 1. RT-PCR was performed in 30 µl and run in duplicate for 40 cycles. The average Ct and delta Ct values were determined for all genes. The fold changes in mRNA expression of inflammatory biomarkers were calculated after normalization to GAPDH using the 2−ΔΔCt equation (Schmittgen and Livak, 2008). Statistical analysisJohn’s Macintosh Project (JMP®) 18.0 was used to analyze gene expression and antibody titer data. A student t-test and Tukey’s HSD test were calculated to evaluate the significant effects of infection and compared with control. Fold changes in the mRNA levels were calculated and presented as mean ± the standard error of the mean (SEM), with p < 0.05 considered significant statistically. Ethical approvalThe study was performed in accordance with the ethical guidelines for animal research and approved by Research Ethics Committee of College of Veterinary Medicine and Surgery, Shatrah University. ResultsClinical signs and postmortem lesionsThe infected birds showed a variety of clinical signs. The main clinical signs were decreased appetite, coughing, watery eyes, nasal discharge, greenish watery diarrhea, and nervous manifestations such as head and neck twist, legs and/or wings paralysis, or entire body paralysis (Fig. 1A and B). Postmortem examination of infected birds, and spots of necrosis were observed in the gizzard, proventriculus, and intestine (Fig. 1C and D). Furthermore, hemorrhagic spots in the proventriculus were widely found. Regarding PM lesions related to the respiratory system, the air sacs were filled with a white, translucent substance. Also, an enlarged liver and a mottled spleen were observed (Fig. 1E and F). Furthermore, several other lesions were observed, such as dehydrated congested muscles, dehydrated carcass, proventricular gland-tip hemorrhages, congested and hemorrhages in the intestine, and catarrhal tracheitis. NDV antigen rapid testThe test was performed in the fields for infected (with clinical signs) and control (no clinical signs) birds. The results of the infected (suspected) birds showed that 52 (86.65%) of 60 samples were positive, as evidenced by the two lines appearing on the device. In addition, the control birds showed no positive results (0.00%). Hemaggluatination inhibition (HI) testThe HI test was performed to determine antibody titers in infected and control birds. Infected birds had significantly lower antibody titers compared to the control (Fig. 2, p <0.001; T-test=11.32). The mean antibody titer in the control birds was 532.15 ± 32.12, while it was 34.27 ± 2.32 in the infected birds. Molecular diagnosis for NDVUtilizing real-time PCR, the matrix (M) gene, a universal protein for NDV detection, was successfully amplified and detected in 27 samples out of 52; however, the Ct values varied from 18 to 33 cycles. Therefore, samples with Ct values ranging from 18 to 24 were chosen for conventional PCR. The F and HN genes were amplified and identified in the eight infected samples, but the intensity of the bands differed among samples (Fig. 3A and B). Table 1. Primer details used in the current experiment.
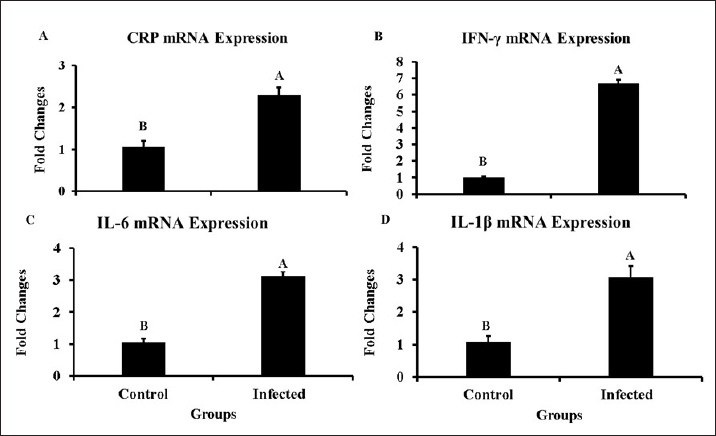
Fig. 1. Clinical signs and postmortem lesions in birds infected with the NDV (A) a neurological sign such as paralysis of legs and wings as well as neck twisting nervous system infection; (B) respiratory signs and paralysis; (C) proventriculus haemorrhage; (D) cecal haemorrhage; (E) haemorrhage and congestion of the intestine; (F) enlargement and congestion of the liver and spleen. Genetic analysis of specific regions within the HN and F genes of NDVThe HN and F genes nucleotide sequences from the infected samples were analyzed by comparing them to the ND virus sequences available in the NCBI database (Fig. 4A and B). The HN gene showed 95.36% sequence homology with the ND virus strain La Sota hemagglutinin-neuraminidase gene (Fig. 4A). Similarly, the sequence of F gene exhibited a 94.68% match with the avian orthoavulavirus 1 isolate Ph/IR/AMMM116/2018 fusion protein gene (Fig. 4B). Gene expression data of inflammatory biomarkersSeveral biomarkers were tested to compare the inflammatory response induced by NDV in infected birds with that in healthy birds (Fig. 5). More specifically, CRP gene expression showed a significant increase in infected birds compared with controls, as documented by approximately two-fold changes (p < 0.01). Furthermore, IL6, Il1β, and IFN-γ mRNA expression increased significantly during infection and showed 3–4-fold changes in mRNA expression compared to their expression in non-infected birds. Remarkably, IFN-γ mRNA expression a greater than a six-fold increase (p < 0.001).
Fig. 2. Mean of antibody titers in the infected and control groups.
Fig. 3. Conventional PCR for products HN (A) and F (B) genes of NDV validated by 1.5% agarose gel electrophoresis.
Fig. 4. An alignment of nucleotide sequences of HN (A) and F (B) genes against available sequences in the NCBI data. DiscussionThis study addressed NDV genetic variations and the mRNA expression of inflammatory biomarkers in infected flocks within Thi-Qar Province of Iraq. The birds exhibited a variety of clinical signs and gross lesions. In this context, Getabalew et al. (2019) reported that respiratory signs may be due to an ND infection in the bronchi or pneumonia. Furthermore, greenish, watery stools can be a sign of digestive complications, such as malabsorption syndrome, dehydration, and malnutrition (Bhutia et al., 2017). During ND infection, when virulent NDV strains infect the central nervous system (CNS), they can replicate in neurons, causing encephalitis and nervous signs, as discussed previously (Cattoli et al., 2011; Ecco et al., 2011). In addition to clinical signs, postmortem examination revealed typical lesions, such as mottled spleen, congested and enlarged liver, periventricular gland-tip hemorrhages, congestion and hemorrhage of the intestine, and catarrhal tracheitis, while other researchers reported the same P.M. lesions (Awad et al., 2020). Notably, the NDV antigen rapid test showed that not all infected birds with clinical signs were positive. The principle of the NDV rapid test kit depends on the antibody-antigen reaction. Therefore, possible explanations for this are test sensitivity, antibody-antigen compatibility, and/or other viral infections. Similarly, the antibody titer decreased significantly in infected birds.
Fig. 5. Changes in the mRNA levels for the inflammatory biomarkers in the infected birds comparing with non-infected birds: A=CRP, B=IFN-γ, C=IL6 and D=IL-1β. To explain the sudden appearance of ND outbreaks in Thi-Qar province of Iraq despite regular and intensive immunization, a molecular approach, PCR, was used to identify genetic variations in the ND virus. RT-PCR is the most specific and efficient approach for detecting NDV (Selim et al., 2022). Three genes, which are the matrix (M) gene, the haemagglutination (HN) gene, and the fusion (f) gene, were amplified successfully by PCR. In this study, the M gene, which is a standard gene used for the detection of NDV infection with high sensitivity, was detected in approximately 27 of 52 positive samples detected by the NDV rapid test. A possible explanation could be the low concentration of viral nucleic acids in the tested sample, which could be supported by the Ct values recorded in the results. In the current study, M gene Ct values ranged from 18 to 33 and appeared to be dependent on the concentration of NDV in the samples, reflecting the intensity of infection. Variations in Ct value suggested that viral concentrations differed between samples. Similarly, previous studies have shown that 26 out of 34 fields were positive when tested by RT-PCR (Hasan et al., 2010). Additionally, Worku et al. (2022) utilized RT-PCR for lung samples and identified NDV in 17 of the 63 samples. Unlike the findings of this study, Ahmed and Odisho (2018) found that 100% of the analyzed samples tested positive using RT-PCR. This could be attributed to the primers used or the local NDV strains. Thereafter, conventional PCR was applied to the positive samples to amplify the F and HN genes. PCR data showed that eight samples displayed strong bands at 400 bp on the gel for the HN gene. An alignment of nucleotide sequences for the HN gene showed 95.36% similarity with the ND virus strain La Sota hemagglutinin-neuraminidase gene. Regarding the F protein, alignment of the nucleotide sequence data showed that the isolate had 94.68% similarity with the avian orthoavulavirus1 isolate Ph/IR/AMMM116/2018 fusion protein gene. Sequence data of the F and HN genes in the infected birds revealed alterations in NDV genetic materials. This could explain the prevalence of ND in broilers. In reviewing the literature, a few papers addressed the mRNA expression of inflammatory biomarkers or cytokines in NDV-infected chickens. Therefore, RT-qPCR was used to observe gene expression changes in several inflammatory biomarkers in the NDV-infected birds. Specifically, the mRNA levels of CRP increased significantly in the liver tissue during infection, resulting in an approximately two-fold change. A high CRP level indicated that there was inflammation somewhere in the bodies of birds, and NDV infection seemed to be responsible for this increase. Further, CRP has been widely used as an indicator for viral infections such as COVID-19, and it has decreased disease mortality (Zheng et al., 2020). The second biomarker measured in this study was interferon gamma (IFN-γ). It is synthesized and secreted by T lymphocytes and natural killer cells and mediates the T-helper type I immune response (Fensterl and Sen, 2009). Furthermore, INF-γ has antiviral roles in birds, including in avian flu, ND, and Marek’s disease (Sawant et al., 2011; Susta et al., 2013). Researchers showed that IFN-γ removes intracellular pathogens, hinders viral replication, activates major histocompatibility complexes I and II, and aids in the processing and presentation of antigens (Schultz and Chisari, 1999; Yeh et al., 1999; Kaiser, 2010). Remarkably, IFN-γ mRNA increased in the infected birds compared with controls; thus, the possible increase in IFN-γ in the current study is conclusive evidence of the immune response to viral infection with NDV. The study found that IL-1β mRNA levels increased significantly in NDV-infected birds compared with those in controls. IL-1β is a key mediator of the inflammatory reaction during viral infections, resulting in the release of IL-6, which is involved in intercellular and vascular cell adhesion and facilitates lymphocyte activation and leukocyte infiltration (Peiró et al., 2017). Furthermore, IL-1β can reduce the proliferation of the virus and repair tissue damage; however, a high amount worsens inflammation and increases lethality rates. Elevated IL-Iβ levels can be observed not only in NDV infections but also in other viral infections, such as avian flu and infectious bronchitis, as previously documented in other studies (Thomas et al., 2009; Wang et al., 2016; Thi and Hong, 2017; Amarasinghe et al., 2018). Notably, IL-Iβ reduction is linked to decreasing severity of pneumonia in H1N1 infection (Kim et al., 2015). The last biomarker tested in infected samples was interlukin-6 (IL-6), which is produced in the body during inflammation. The study found high IL-6 mRNA expression in infected birds compared to the control. IL-6 is an immune protein and pyrogen responsible for fever in both infectious and noninfectious diseases. Furthermore, Queiroz et al. (2022) reported that IL-6 is essential for coronavirus infection in humans, including disease duration and severity. It is synthesized by several types of cells during inflammation, including endothelial (Chi et al., 2001), immune (Tanaka et al., 2014), and fat cells (Fain, 2010). IL-6 is essential for adaptive immunity development because it helps differentiate naïve CD4+ T cells (Tanaka et al., 2014). Therefore, the high temperature resulted from viral infections and severe inflammation that may occur due to the same infection as ND. It is likely that the increase was the result of a strong immune response and the production of more IL-6. The findings of this study were consistent with those of other studies that addressed proinflammatory cytokine profiling and found that NDV predominantly increased the production of interleukin 6 (IL-6) in most cells (Chhabra et al., 2018). ConclusionThe current study evaluated genetic variations in the F and HN genes of the ND virus detected in Thi-Qar province of Iraq. These findings revealed alterations in the nucleotide sequences of the selected genes’ (HN and F), which are known to be responsible for the pathogenicity of the virus. Specifically, modifications in these genes and the incompatibility of the vaccine with endemic strains may be the cause of ND prevalence in Thi-Qar province of Iraq. This could lead to the recurrence of ND infections in poultry, resulting in significant economic losses. In addition to traditional diagnostic techniques, such as HI and rapid tests, this study emphasized the importance of regularly using PCR, a molecular approach, in endemic areas and/or outbreak situations. Furthermore, the infected birds showed a decrease in the antibody titer and an increase in inflammatory biomarkers, highlighting the need to determine the antibody titer as a crucial step in evaluating the immunological condition after immunization to assess the vaccine’s efficacy. AcknowledgmentsThe authors would like to thank College of Veterinary Medicine and Surgery, Shatrah University, for their help with animal husbandry during animal experimentation. This manuscript is a part of Abbas master thesis. Conflict of interestThe authors declare no conflict of interest. FundingThe research received no particular fund. Authors’ contributionsThe authors contributed equally to perform the Manuscript. Data availabilityAll data are included within the manuscript. ReferencesAhmed, A.I. and Odisho, S.M. 2018. Isolation identification and pathotyping of Newcastle disease viruses form naturally infected chickens In Iraqi Kurdistan region. Iraqi J. Agric. Sci. 49(1), 132–141. Aliyu, M., Zohora, F.T., Anka, A.U., Ali, K., Maleknia, S., Saffarioun, M. and Azizi, G. 2022. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 111, 109130. Al-Zghoul, M.B., Saleh, K.M. and Ababneh, M.M.K. 2019. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult. Sci. 98(4), 1805–1819. Amarasinghe, A., Abdul-Cader, M.S., Almatrouk, Z., Van der. Meer, F., Cork, S.C., Gomis, S. and Abdul-Careem, M.F. 2018. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV). Vet. Microbiol. 215, 1–10. Awad, M., Mosad, S. and El-Kenawy, A. 2020. Molecular differentiation between velogenic isolates and lentogenic LaSota strain of Newcastle disease virus. Mansoura Vet. Med. J. 21(4), 193–198. Bansal, N., Singh, R., Chaudhary, D., Mahajan, N.K., Joshi, V.G., Maan, S., Ravishankar, C., Sahoo, N., Mor, S.K., Radzio-Basu, J., Kapur, V., Jindal, N. and Goyal, S.M. 2022. Prevalence of Newcastle disease virus in wild and migratory birds in Haryana, India. Avian Dis. 66(2), 141–147. Bhutia, L.D., Rajkhowa, T.K., Ravindran, R., Arya, R.S., Roychoudhury, P., Mandakini, R.K. and Singh, Y.D. 2017. Occurrence of Newcastle disease in poultry population of Mizoram, India. Indian J. Vet. Pathol. 41(2), 151–154. Briand, F.X., Henry, A., Massin, P. and Jestin, V. 2012. Complete genome sequence of a novel avian paramyxovirus. J. Virol. 86, 7710. Cattoli, G., Susta, L., Terregino, C. and Brown, C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J. Vet. Diagn. Invest. 23(4), 637–656. Chhabra, R., Ball, C., Chantrey, J. and Ganapathy, K. 2018. Differential innate immune responses induced by classical and variant infectious bronchitis viruses in specific pathogen free chicks. Dev. Comp. Immunol. 87, 16–23. Chi, L., Li, Y., Stehno-Bittel, L., Gao, J., Morrison, D.C., Stechschulte, D.J. and Dileepan, K.N. 2001. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J. Interferon Cytokine Res. 21(4), 231–240. Ecco, R., Susta, L., Afonso, C.L., Miller, P.J. and Brown, C. 2011. Neurological lesions in chickens experimentally infected with virulent Newcastle disease virus isolates. Avian Pathol. 40(2), 145–152. Fain, J.N. 2010. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010, 513948. Fensterl, V. and Sen, G.C. 2009. Interferons and viral infections. Biofactors. 35, 14–20. Getabalew, M., Alemneh, T., Akeberegn, D., Getahun, D. and Zewdie, D. 2019. Epidemiology, diagnosis & prevention of Newcastle disease in poultry. Am. J. Biomed. Sci. Res. 16, 50–59. Hasan, A.R., Ali, M.H., Siddique, M.P., Rahman, M.M. and Islam, M.A. 2010. Clinical and laboratory diagnoses of newcastle and infectious bursal diseases of chickens. Bangladesh J. Vet. Med. 8(2), 131–140. Kadhim, H.J., Kang, S.W. and Kuenzel, W.J. 2019. Differential and temporal expression of corticotropin releasing hormone and its receptors in the nucleus of the hippocampal commissure and paraventricular nucleus during the stress response in chickens (Gallus gallus). Brain Res. 1714, 1–7. Kadhim, H.J., Kang, S.W. and Kuenzel, W.J. 2021. Possible roles of brain derived neurotrophic factor and corticotropin releasing hormone neurons in the nucleus of hippocampal commissure functioning within the avian neuroendocrine regulation of stress. Stress. 24(5), 590–601. Kadhim, H.J., Kidd, M Jr, Kang, SW. and Kuenzel, W.J. 2020. Differential delayed responses of arginine vasotocin and its receptors in septo-hypothalamic brain structures and anterior pituitary that sustain hypothalamic-pituitary-adrenal (HPA) axis functions during acute stress. Gen. Comp. Endocrinol. 286, 113302. Kadhim, H.J. and Kuenzel, W.J. 2022. Interaction between the hypothalamo-pituitary-adrenal and thyroid axes during immobilization stress. Front. Physiol. 13, 972171. Kaiser P. 2010. Advances in avian immunology--prospects for disease control: a review. Avian Pathol. 39(5), 309–324. Kim, K.S., Jung, H., Shin, I.K., Choi, B.R. and Kim, D.H. 2015. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 87, 1104–1112. Kolakofsky, D., Roux, L., Garcin, D. and Ruigrok, R.W.H. 2005. Paramyxovirus mRNA editing, the “rule of six” and error catastrophe: a hypothesis. J. Gen. Virol. 86(Pt 7), 1869–1877 Mao, Q., Ma, S., Schrickel, P.L., Zhao, P., Wang, J., Zhang, Y. and Wang, C. 2022. Review detection of Newcastle disease virus. Front. Vet. Sci.9, 936251. Mitra, S. and Leonard, W.J. 2018. Biology of IL-2 and its therapeutic modulation: mechanisms and strategies. J. Leukoc. Biol. 103(4), 643–655. Peiró, C., Lorenzo, Ó., Carraro, R. and Sánchez-Ferrer, C.F. 2017. IL-1β Inhibition in Cardiovascular complications associated to diabetes mellitus. Front. Pharmacol. 8, 363. Queiroz, M.A.F., Neves, P.F.M.D., Lima, S.S., Lopes, J.D.C., Torres, M.K.D., Vallinoto, I.M.V.C., Bichara, C.D.A., Dos Santos, E.F., de Brito, M.T.F.M., da Silva, A.L.S., Leite, M.M., da Costa, F.P., Viana, M.N.D.S.A., Rodrigues, F.B.B., de Sarges, K.M.L., Cantanhede, M.H.D., da Silva, R., Bichara, C.N.C., van den Berg, A.V.S., Veríssimo, A.O.L. and Vallinoto, A.C.R. 2022. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front. Cell. Infect. Microbiol. 12, 922422. Reda, A.M. and Jasim, N.S. 2022. Molecular diagnosis of Newcastle disease virus by RT-RCR and detection fusion gene using conventional PCR. J. Pharm. Negat. 13 (03), 983–994. Sawant, P., Verma, P., Subudhi, P., Chaturvedi, U., Singh, M., Kumar, R. and Tiwari, A. 2011. Immunomodulation of bivalent Newcastle disease DNA vaccine induced immune response by co-delivery of chicken IFN-γ and IL-4 genes. Vet. Immunol. Immunopathol. 144, 36–44. Schmittgen, T.D. and Livak, K.J. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3(6), 1101–1108. Schultz, U. and Chisari, F.V. 1999. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J. Virol. 73(4), 3162–3168. Selim, K., Adel, A., Eid, S. and Shahein, M. 2022. Development of real time reverse transcription loop-mediated isothermal amplification assay for rapid detection of genotype VII of Newcastle disease viruses. Br. Poult. Sci. 63(6), 864–870. Shabbir, M.Z., Zohari, S., Yaqub, T., Nazir, J., Shabbir, M.A., Mukhtar, N., Shafee, M., Sajid, M., Anees, M., Abbas, M., Khan, M.T., Ali, A.A., Ghafoor, A., Ahad, A., Channa, A.A., Anjum, A.A., Hussain, N., Ahmad, A., Goraya, M.U., Iqbal, Z. and Munir, M. 2013. Genetic diversity of Newcastle disease virus in Pakistan: a countrywide perspective. Virol. J. 10, 170. Susta, L., Cornax, I., Diel, D.G., Garcia, S.C., Miller, P.J., Liu, X., Hu, S., Brown, C.C. and Afonso, C.L. 2013. Expression of interferon gamma by a highly virulent strain of Newcastle disease virus decreases its pathogenicity in chickens. Microb. Pathog. 61-62, 73–83. Tanaka, T., Narazaki, M. and Kishimoto, T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6(10), a016295. Tang, H., Finn, R.D. and Thomas, P.D. 2019. TreeGrafter: phylogenetic tree-based annotation of proteins with gene ontology terms and other annotations. Bioinformatics. 35(3), 518–520. Thi, H.T.H. and Hong, S. 2017. Inflammasome as a Therapeutic Target For Cancer Prevention And Treatment. J. Cancer Prev. 22(2), 62–73. Thomas, P.G., Dash, P., Aldridge, J.R.Jr., Ellebedy, A.H., Reynolds, C., Funk, A.J., Martin, W.J., Lamkanfi, M., Webby, R.J., Boyd, K.L., Doherty, P.C. and Kanneganti, T.D. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 30, 566–575. Waheed, U., Siddique, M., Arshad, M., Ali, M. and Saeed, A. 2013. Preparation of Newcastle disease vaccine from VG/GA strain and its evaluation in commercial broiler chicks. Pak. J. Zool. 45(2), 339344. Wang, B., Zhu, J., Li, D., Wang, Y., Zhan, Y., Tan, L., Qiu, X., Sun, Y., Song, C., Meng, C., Ying, L., Xiang, M., Meng, G. and Ding, C. 2016. Newcastle disease virus infection induces activation of the NLRP3 inflammasome. Virology 496, 90–96. Wang, Z., Liu, H., Xu, J., Bao, J., Zheng, D., Sun, C., Wei, R., Song, C. and Chen, J. 2006. Genotyping of Newcastle disease viruses isolated from 2002 to 2004 in China. Ann. N. Y. Acad. Sci. 1081, 228–239. Wise, M.G., Suarez, D.L., Seal, B.S., Pedersen, J.C., Senne, D.A., King, D.J., Kapczynski, D.R. and Spackman, E. 2004. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 42(1), 329–338. Worku, T., Dandecha, M., Shegu, D., Aliy, A. and Negessu, D. 2022. Isolation and molecular detection of Newcastle disease virus from field outbreaks in chickens in Central Ethiopia. Vet. Med. (Auckl.). 13, 65–73. Wu, G., Liu, L., Qi, Y., Sun, Y., Yang, N., Xu, G., Zhou, H. and Li, X. 2015. Splenic gene expression profiling in White Leghorn layer inoculated with the Salmonella enterica serovar Enteritidis. Anim. Genet. 46(6), 617–626. Yeh, H.Y., Winslow, B.J., Junker, D.E. and Sharma, J.M. 1999. In vitro effects of recombinant chicken interferon-gamma on immune cells. J. Interferon Cytokine Res. 19(6), 687–691. Zheng, Z., Peng, F., Xu, B., Zhao, J., Liu, H., Peng, J., Li, Q., Jiang, C., Zhou, Y., Liu, S., Ye, C., Zhang, P., Xing, Y., Guo, H. and Tang, W. 2020. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 81(2), e16–e25. | ||
| How to Cite this Article |
| Pubmed Style Kadhim HJ, Shlaga AK, Ali SH. Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Vet. J.. 2024; 14(11): 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 Web Style Kadhim HJ, Shlaga AK, Ali SH. Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. https://www.openveterinaryjournal.com/?mno=214085 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.10 AMA (American Medical Association) Style Kadhim HJ, Shlaga AK, Ali SH. Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Vet. J.. 2024; 14(11): 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 Vancouver/ICMJE Style Kadhim HJ, Shlaga AK, Ali SH. Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 Harvard Style Kadhim, H. J., Shlaga, . A. K. & Ali, . S. H. (2024) Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Vet. J., 14 (11), 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 Turabian Style Kadhim, Hakeem Jawad, Abbas Kamil Shlaga, and Safaa Hussein Ali. 2024. Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Veterinary Journal, 14 (11), 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 Chicago Style Kadhim, Hakeem Jawad, Abbas Kamil Shlaga, and Safaa Hussein Ali. "Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq." Open Veterinary Journal 14 (2024), 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 MLA (The Modern Language Association) Style Kadhim, Hakeem Jawad, Abbas Kamil Shlaga, and Safaa Hussein Ali. "Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq." Open Veterinary Journal 14.11 (2024), 2817-2826. Print. doi:10.5455/OVJ.2024.v14.i11.10 APA (American Psychological Association) Style Kadhim, H. J., Shlaga, . A. K. & Ali, . S. H. (2024) Clinical and molecular identification of Newcastle disease virus in naturally infected chicks in Thi-Qar province of Iraq. Open Veterinary Journal, 14 (11), 2817-2826. doi:10.5455/OVJ.2024.v14.i11.10 |