| Research Article | ||
Open Vet. J.. 2024; 14(11): 2827-2836 Open Veterinary Journal, (2024), Vol. 14(11): 2827-2836 Research Article Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on hematological indices, lipid profile parameters, and blood glucose level of Sprague Dawley rats during lactation and offspring growthHasan Basri1,2, Slamet Widiyanto3*, Hendry T. S. S. G. Saragih4 and Zuprizal Zuprizal51Biology Doctoral Study Program, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Biology Study Program, Faculty of Mathematics and Natural Sciences, Universitas Islam Al-Azhar, Mataram, Indonesia 3Department of Animal Physiology, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Laboratory of Animal Development Structure, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia 5Department of Animal Nutrition and Feed Science, Faculty of Animal Science, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Slamet Widiyanto. Department of Animal Physiology, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: slametbio [at] ugm.ac.id Submitted: 03/08/2024 Accepted: 17/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
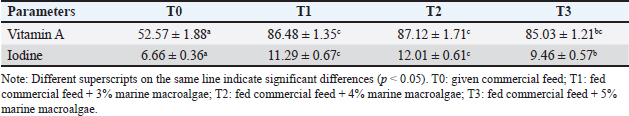
AbstractBackground: The lactation period is a crucial period where the nutritional status and the mother’s environment influence milk production, impacting organ differentiation, function, and structure in the baby’s body. Aim: The study aimed to determine the impact of providing lactating rats with quail egg supplements enriched with marine macroalgae Eucheuma spinosum on their physiological condition (blood cells, lipids, blood glucose, antioxidant activity, and prolactin hormone levels) and the growth of their offspring. Methods: The study involved 25 lactating Sprague Dawley white rats aged 3 months old and weighing approximately 200 g divided into five treatment groups thus; T0 as the control, T1 with quail eggs enriched with commercial feed, T2 with quail eggs enriched with 3% of marine macroalgae, T3 with quail eggs enriched with 4% of marine macroalgae, and T4 with quail eggs enriched with 5% of marine macroalgae, which received one quail egg for 21 days. At the end of the study period, the following parameters were assayed: vitamin A, iodine, weight and body length of rat pups, red blood cell count, hemoglobin levels, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, white blood cell count, lymphocytes, neutrophils, red blood cell distribution width, platelet distribution width, and mean platelet volume. Also, cholesterol levels, high-density lipoprotein, low-density lipoprotein, blood glucose levels, prolactin hormone, antioxidant activity with SOD and MDA. Results: The study result found that adding marine macroalgae to the quail feed significantly increased the vitamin A and iodine content in the quail egg yolks. Furthermore, the weight and body length of the rat pups in the supplemented groups significantly increased (p < 0.05) compared to the control group. However, it did not have a significant impact (p > 0.05) on the rats’ blood parameters, cholesterol, blood glucose, antioxidant activity, and prolactin hormone levels. Conclusion: In conclusion, providing lactating rats with quail eggs enriched with marine macroalgae E. spinosum support the growth and development of the rat offspring without negatively impacting the rats’ overall health parameters. Keywords: Marine macroalgae, Quail eggs, Lactation, Haematological, Antioxidant activity. IntroductionLactation is a crucial period where the nutritional status and the mother's environment influence milk production, impacting organ differentiation, function, and structure in the baby's body (Marshall et al., 2022; Carretero-Krug et al., 2024). The lactation period begins with milk secretion and includes all changes in the mammary glands from the beginning of pregnancy to the breastfeeding period (Neville et al., 2023). During lactation, nutritional needs increase compared to normal conditions. These nutrients are utilized to increase blood volume, as well as the growth of mammary glands in preparation for breastfeeding and to increase body metabolism. Inadequate nutrition during lactation can lead to decreased milk production, complications for the mother and baby, an increased risk of cardiovascular disease (Crozier et al., 2009; Dos et al., 2014; Biberoglu et al., 2016), and causes oxidative stress due to excessive metabolic load during lactation (Toy et al., 2009; Berchieri-Ronchi et al., 2011). These nutritional needs must be maintained from pregnancy to lactation. Complete nutrition is essential for recovery after childbirth and preparation for milk production. The nutritional requirements for milk formation are particularly high because breast milk is the sole source of nutrition for children's growth and development (Prawitasari et al., 2019). Under normal conditions, breast milk provides all the nutritional needs for growth, development, and the child's immunological response (Lokossou et al., 2022). The calories expended during lactation by the mother must be balanced with nutritional intake for the energy obtained from food to be used for milk production (Kim and Yi, 2020). Balanced energy and protein supplementation can increase fetal growth and reduce the risk of fetal mortality at birth, low birth weight, and delivery at early gestational age, particularly in pregnant and lactating mothers who are malnourished (Imdad and Bhutta, 2011; Salmuth et al., 2021). Furthermore, various important micronutrients are necessary for achieving nutritional needs during breastfeeding, including folic acid, calcium, iron, zinc, iodine, and vitamins (Delange, 2007; Mousa et al., 2019). Most nutritional needs are provided through the food consumed by the parent. Quail eggs known for their high nutritional content, are a readily available functional food source that can be further enriched through the feed provided, offering essential nutrients for the growth and development of the fetal (Miranda et al., 2015; Jeke et al., 2018). The nutritional content of quail eggs includes fat, protein, carbohydrates, fiber, essential fatty acids, vitamins, minerals, lipids, and bioactive compounds as antioxidants (Basri et al., 2018; Liu et al., 2024). The nutritional value of quail eggs can be enhanced by using appropriate feed additives, such as macroalgae. Macroalgae can be used as a valuable source of feed additives due to their abundant availability and easy access of the resource. Furthermore, the nutritional composition of macroalgae comprises carbohydrates, protein, lipids, minerals, dietary fiber, amino acids, and bioactive compounds, all of which are potentially suitable for inclusion in quail feed (Øverland et al., 2019; Kulshreshtha et al., 2020). Macroalgae can be used as feed additives to improve health, productivity, and the overall quality of meat and eggs (Hajati and Zaghari, 2019). Previous studies on the addition of macroalgae, specifically Macrocystis pyrifera flour, has been found to increase the nutritional content of chicken eggs (Rendón et al., 2003). Limited research has been conducted on the impact of quail eggs enriched with marine macroalgae as a nutritional supplement during lactation. Previous studies have found that consuming chicken eggs and milk during lactation can significantly increase breastmilk production (Achalapong, 2016). Similarly, studies on administering egg protein have indicated a significant increase in the weight and body length of rat pups (Naeem et al., 2023). Furthermore, a study involving the supplementation of one organic quail egg per day to hematology showed no significant differences in results; however, it was observed that organic quail egg supplementation could maintain the hematological status of erythrocytes, hemoglobin (Hb), and blood pH in lactating white rats (Basri et al., 2018). Based on these findings, the objective of this study is to investigate the effects of quail eggs enriched with Eucheuma spinosum on the physiological condition of lactating rats including hematological indicies, lipid profile parameters, blood glucose, antioxidant activity, and the hormone prolactin, the weight and body length of rat pups. Materials and MethodsStudy areaThe research was conducted at the Integrated Research and Testing Laboratory, Tropical Livestock Research Center Laboratory, Faculty of Animal Husbandry, Gadjah Mada University. ProceduresFemale Sprague Dawley rats were used in the study. The rats were housed at 26°C with 70% humidity under artificial lighting (12 hours light and 12 hours dark) (Gopalakrishnan et al., 2018). They were fed for 15 hours (17:00–07:00), a 9-hour fasting period (08:00–16:00), and ad libitum access to feed and drinking water. The rats were given standard feed (Rat Bio) produced by PT. Citra Ina Feed Mill contains 60% carbohydrates, 20% protein, 4% fat, 4% crude fiber, 12% calcium, and 0.7% phosphorus. The study treatment involved quail eggs enriched with marine macroalgae of the E. spinosum (Fig. 1). Each rat was given one quail egg per day for 21 days, with the eggs boiled for 2 minutes and then cut into small pieces before being given to the rats every day at 16:00 (Basri et al., 2018). Research designThis is an experimental study with a completely randomized design. The study used 25 lactating white Sprague-Dawley rats aged 3 months old and weighing around 200 g. The rats were divided into 5 treatments: T0: control treatment T1: treatment with quail eggs enriched with commercial feed T2: treatment with quail eggs enriched with commercial feed + 3% marine macroalgae T3: treatment with quail eggs enriched with commercial feed + 4% marine macroalgae T4: treatment with quail eggs enriched with commercial feed + 5% marine macroalgae. Each treatment group consisted of 5 replications, with each replication including 1 lactating white rat. At the end of the lactation period, blood sampling was performed. Blood samples were collected from the orbital sinus of the eye after prior anesthesia using ketamine and xylazine. A 1 ml blood sample was collected using microcapillary hematocrit and placed in an ethylen diamine tetra acetic acid vacutainer tube. The blood samples were then centrifuged at 3,000 rpm speed for 5–15 minutes.
Fig. 1. Marine macroalgae E. spinosum. The mating process of the ratsThe mating process of white rats commences by placing male and female rats in separate cages with transparent bases for 1 day. The initial step involves placing three female white rats, which have undergone the estrus phase, along with one male white rat in a cage measuring 37 × 30 × 20 cm. The mating process typically takes place during nighttime and extends over a period of 5 days. Sample analysis methodThe UV-Vis Spectrophotometer method was used to analyze the Vitamin A content (Nielsen, 2010; Nisa et al., 2017). Additionally, the iodine concentration in egg yolk was determined using the spectrophotometer method at 440 nm (Sumaiya et al., 2016). A complete blood analysis, including RBC, HGB, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, white blood cell, lymphocytes, neutrophils, red blood cell distribution width, platelet distribution width, and mean platelet volume, was conducted using the hematology analyzer method (Binev, 2023). Cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels were analyzed using the CHOD-PAP method. Blood glucose was measured using the GOD-PAP method, using UV-Vis spectrophotometry (Apriyanto et al., 2021). SOD, MDA, and prolactin hormone levels were measured using an ELISA kit from the Bioassay Technology Laboratory (Shanghai, China). The CAT numbers of each kit were: SOD CAT No. E0168Ra, MDA CAT No. E0156Ra, Prolactine CAT No. E0190Ra. The development of rat pups was observed, including body weight gain measured using a digital scale with an accuracy of 0.01 g and body length observed using a caliper (Mahmood et al., 2012; Iwansyah et al., 2017). Data analysisThe data obtained were analyzed using variance, and if a significant difference was found, the Duncan test was conducted at a 95% significance level. The analysis was performed using SPSS 22 for Windows software. Ethical approvalThe study was carried out with approval from the Ethical Clearance Commission of the Integrated Research and Testing Laboratory (LPPT) at Gadjah Mada University, with the number 00026/04/LPPT/VIII/2023. ResultsThe vitamin A and iodine content in quail eggs significantly increased (p < 0.05) in the T1, T2, and T3 groups compared to the control group (Table 1). The highest increase in vitamin A content was observed in the T2 group. This increase in vitamin A levels was attributed to the addition of E. spinosum to the quail feed. The vitamin A content in E. spinosum was 31.56 µg/100 g, and it increased the highest vitamin A content to 87.12 ± 1.71 µg/100 g in quail-fed 4% marine macroalgae. The marine macroalgae species E. spinosum contains 57.77 ppm of iodine. Quail eggs supplemented with 4% marine macroalgae feed have the highest iodine content at 12.01 ± 0.1 ppm (Table 1). The body weight and body length of the rat pups were measured every week to determine the effects of the treatments (Table 2). Results of the body weight and body length of the rat pups on days 7, 14, and 21 showed a significant increase (p < 0.05) compared to the control group (Table 2). The administration of quail eggs to groups T1, T2, T3, and T4 significantly increased the body weight and body length of the rat pups compared to treatment T0. The highest increase in body weight and body length of the rat pups was found in group T4, while the lowest values were found in the control group. Providing lactating rats with quail egg supplements enriched with marine macroalgae did not result in any significant changes in the levels of various hematological parameters compared to the control group (Table 3). The lipid components examined in this study were cholesterol, HDL, LDL, blood glucose, SOD, MDA and prolactin hormone. According to the results, cholesterol, HDL, LDL, blood glucose, SOD, MDA, and prolactin hormone levels did not show significant changes compared to the control group (Table 4). DiscussionVitamin A and iodine contentThe concentration of vitamin A in egg yolk is closely linked to the concentration of vitamin A in the feed consumed by poultry (Lima and Souza, 2018; Meléndez-Martínez, 2019). Previous studies have shown that providing macroalgal extract can increase the vitamin A content in broilers egg (Ivarsson et al., 2023). It is important to note that vitamins are required in small amounts but play a critical role in primary biological functions such as growth, embryonic development, epithelial cell differentiation, and immune function. Vitamin A deficiency can lead to various disorders, including lack of appetite, decreased development and immunity, and increased disease susceptibility (Khan et al., 2023). The process of transferring vitamin A from the feed to quail eggs involves the absorption of vitamin A from the feed consumed by the quail, which is then stored in the egg yolk. Since vitamin A is fat-soluble, it is absorbed along with the fat in the feed and carried to the liver. During egg formation, vitamin A is transferred through the bloodstream to the ovaries for the synthesis of egg yolk precursors (Khan et al., 2023). The high level of iodine in the quail eggs is due to the high transfer of iodine from the feed to the eggs. Previous research has shown that feed containing 13 ppm iodine can increase the iodine content in chicken egg yolks (Sumaiya et al., 2016). Similarly, other studies have indicated a linear correlation between the iodine content in feed mixtures of up to 5 mg/kg and the iodine content in egg yolks (Słupczynska et al., 2014). The iodine in feed is absorbed through the digestive tract and transported via the bloodstream to various tissues and organs, including the ovaries (Andersson and Braegger, 2022). The developing egg follicle (ovum) accumulates iodine in the ovary. As the mature egg follicle moves to the oviduct, where the shell and egg white are formed, iodine accumulates in the egg components. Iodine is a micronutrient necessary to produce thyroid hormones, which are essential for regulating metabolism, growth, and development (Andersson and Braegger, 2022). Sufficient iodine concentration in breast milk is crucial for providing optimal neonatal thyroid hormones and preventing impaired neurological development in breastfed babies (Azizi and Smyth, 2009). Table 1. Vitamin A (µg/100 g) and iodine (ppm) content in quail eggs enriched with macroalgae E. spinosum.
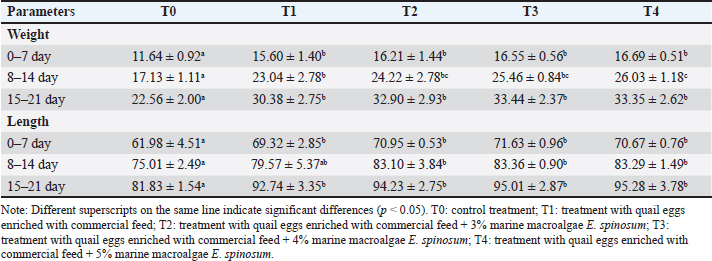
Table 2. Body weight (g) and length (mm) of rat pups supplemented with quail eggs enriched with E. spinosum.
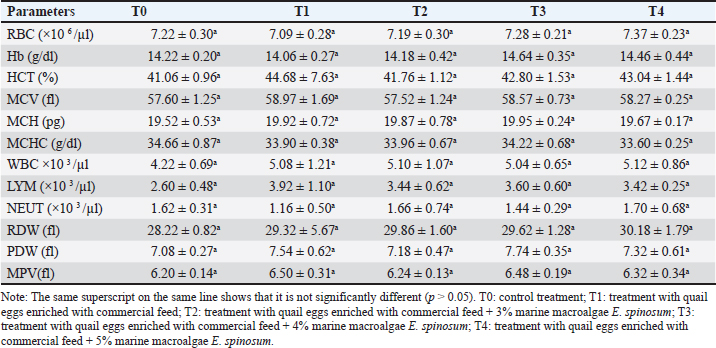
Table 3. Hematology profile of lactating rats given quail eggs supplementation enriched with macroalgae E. spinosum.
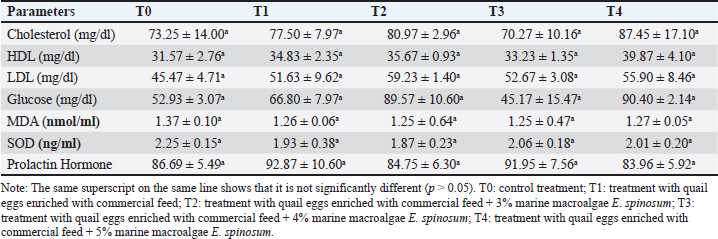
Table 4. Serum biochemistry, oxidative stress status and prolactin hormone in lactating rats supplemented with quail egg enriched with macroalgae E. spinosum
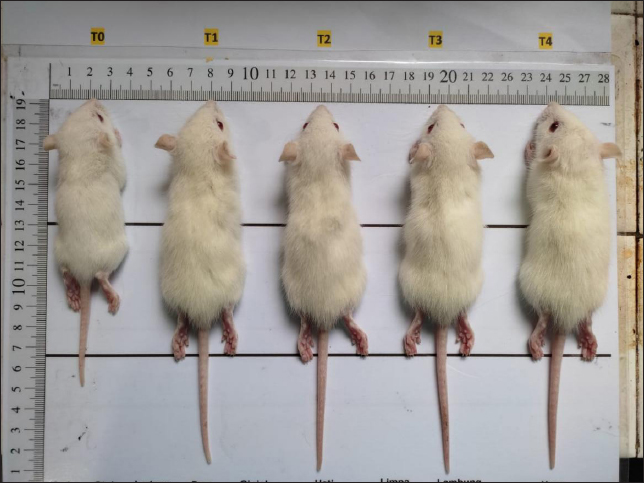
Body weight and length of rat pupsThere was a linear increase in the body weight and body length of the rat pups starting on days 7, 14, and 21 in the groups given quail egg supplementation. The highest increase in body weight and body length of the rat pups was found in group T4, while the lowest values were found in the control group. Based on these results, it was found that supplementation of quail eggs enriched with E. spinosum was able to increase the body weight and body length of the rat pups (Fig. 2). Quail eggs enriched with E. spinosum contain high levels of nutrients, particularly protein. Body length and weight are crucial parameters that need to be measured to understand the impact of high protein intake on the growth of the offspring (Pastuszewska et al., 2008). Previous research has also reported that providing egg protein to mothers significantly increased the body weight and length of rat pups compared to the control group (Naeem et al., 2023). Consistent with the previous statement, a high correlation was found between body length and weight with high protein consumption (Pastuszewska et al., 2008). Another study reported that providing one egg per day for 6 months to children in Ecuador resulted in a 47% reduction in stunting prevalence and a 74% reduction in underweight, thus helping the offspring achieve normal growth (Iannotti et al., 2017).
Fig. 2. Rat pups aged 21 days after being given treatment, including: T0: control treatment; T1: treatment with quail eggs enriched with commercial feed; T2: treatment with quail eggs enriched with commercial feed + 3% marine macroalgae E. spinosum; T3: treatment with quail eggs enriched with commercial feed + 4% marine macroalgae E. spinosum; T4: treatment with quail eggs enriched with commercial feed + 5% marine macroalgae E. Spinosum. Hematological indiciesHematological examination is valuable for diagnosing and monitoring metabolic diseases (Dunlea et al., 2023). Hematological status is a useful indicator for assessing health conditions during lactation (Wrzecińska et al., 2023). Concerning the levels of various hematological parameters, the study results showed that there are no significant changes in the treatment groups compared to the control. Basri et al., (2018) found that there is no impact on red blood cell and Hb levels in lactating white rats when given organic quail eggs and suggested that supplementing lactating rats with quail eggs enriched with marine macroalgae can help maintain their hematological condition and prevent physiological disorders during the lactation period. Normal hematological levels indicate the absence of metabolic disorders, diseases, organ damage, drug influence, or stress in rats (Lager and Jordan, 2012; Kuhn et al., 2017; Gwozdzinski et al., 2021). Serum biochemistry, oxidative stress status, and prolactin hormoneThis study showed a similar result to the previous research that providing organic quail egg supplements during the lactation period had no impact on the lipid profile parameters such as total cholesterol, LDL, HDL, and triglycerides (Purba et al., 2018). The lack of effect on increasing plasma lipid levels during lactation is attributed to increased distribution of lipids to the placenta and mammary glands to meet the lipid needs of rat offspring (Zhang et al., 2017). Additionally, the similar feed consumed may have caused the lack of effect of quail egg supplementation on cholesterol, LDL, and HDL levels in lactating rats. The cholesterol content influences cholesterol levels synthesized in the liver and small intestine in the diet (Caponio et al., 2020). The body has a specific mechanism for managing excess cholesterol from food intake, involving reducing endogenous absorption and synthesis, and increasing cholesterol excretion through bile (Fernandez and Murillo, 2022). Excess cholesterol in cells inhibits HMG-CoA activity (Altmann et al., 2004), thus causing the body to maintain a balance in plasma cholesterol levels (Fernandez and Murillo, 2022). Cholesterol is crucial for the development of brain tissue, nerve myelination, and serves as a fundamental ingredient for many enzymes (Lawrence and Lawrence, 2011). The research on the effect of quail eggs on blood glucose levels revealed no significant difference compared to the control group. However, in group T3, glucose levels decreased, possibly due to metabolic changes (Hussein, 2013). Metabolic changes indicate an increase in the rat's nutritional requirements for the growth of her offspring (Zhu et al., 2015). Giving quail eggs may not significantly impact blood glucose levels. A homeostasis process occurred in groups T1, T2, and T4, maintaining normal blood glucose levels. Homeostasis is achieved when glucose enters the liver. The small intestine and liver absorb glucose, and excess glucose is converted into glycogen and stored in the liver and muscles, with a small amount stored in the brain (Kanungo et al., 2018; Zhang et al., 2019). Findings from this investigation of MDA's lipid peroxidation indicate no significant increase compared to the control, and there was no decrease in SOD levels across all treatments. Throughout the study, SOD and MDA levels remained constant, indicating the absence of significant oxidative stress. MDA and SOD levels fluctuate in response to physiological imbalances during the lactation period. Previous research also supports the observation that MDA levels in lactating rats do not significantly increase compared to controls (Zheng et al., 2015). This absence of oxidative stress in lactating rats may be attributed to the direct energy metabolism to the offspring via milk (Garratt et al., 2011). The physiological regulation of antioxidant systems plays a crucial role in maintaining the oxidant-antioxidant balance, thus preventing oxidative damage to macromolecules (Garratt et al., 2011). The data suggests that antioxidants are physiologically regulated in response to reactive oxygen species (ROS) in various tissues of lactating rats, contributing to oxidant-antioxidant balance during peak lactation (Zheng et al., 2015). The increase in metabolic rate during lactation leads to a rise in the production of ROS, potentially disrupting macromolecules, which can be mitigated by antioxidants (Selman et al., 2000; Salmon et al., 2001; Dowling and Simmons, 2009; Garratt et al., 2011; Xu et al., 2014). According to the research findings, the treatment group given quail eggs did not show a significant increase in prolactin hormone levels compared to the control. This contrasts with previous research, suggesting that high-protein foods can significantly stimulate prolactin secretion (Velázquez-Villegas et al., 2015). In rats, the lactation period lasts for 21 days after birth (Grases-Pintó et al., 2021). The levels of secreted prolactin are influenced by various factors, including the frequency and duration of offspring breastfeeding (Cregan et al., 2002; Wati et al., 2023), maternal psychology (Nagel et al., 2022), and the length of the lactation period. Prolactin hormone levels naturally decrease over time (Powe et al., 2010). Given that blood sampling in this study was conducted at the end of the third week, which also marks the end of the lactation period, no increase in prolactin hormone levels would likely be observed in the blood samples. ConclusionThe research results indicate that the addition of 4% of marine macroalgae E. spinosum to quail feed can effectively increase vitamin A and iodine levels in quail egg yolks. Furthermore, the T4 treatment, which involves enriching quail eggs with 5% marine macroalgae, is shown to increase the weight and body length of rat pups. However, this treatment did not have a significant impact on hematological levels, lipids, antioxidant activity, and the hormone prolactin compared to the control treatment. Overall, the study suggests that incorporation of quail eggs enriched with marine macroalgae E. spinosum support the growth and development of the rats offspring without negatively impact the health of the rats. Further research is needed to explore the potential beneficial effects of quail eggs enriched with marine macroalgae on other health parameters in lactating rats. AknowledgmentsThe authors acknowledge the scholarship provided by Indonesia Endowment Funds for Education (LPDP) and Center for Higher Education Funding (BPPT) in 2022. Conflict of interestThe authors have declared no conflicts of interest. FundingThe study was funded by the Centre for Higher Education Funding (BPPT) and the Indonesia Endowment Fund for Education (LPDP). Authors’ contributionsHB and SM concept and design, HB analisis/interpretasi data and drafting manuscript, SM HG ZZ critical revision of manuscript, HB HG statistical analysis, HB admin, technical or material support, SH HG ZZ Supervision and final approval. Data availabilityAll data from this study are provided in the manuscript. ReferencesAchalapong, J. 2016. Effect of egg and milk supplement on breast milk volume at 48 and 72 hours postpartum: a randomized-controlled trial. Thai. J. Obstet. Gynaecol. 24(1), 20–25. Altmann, S.W., Davis, H.R., Zhu, L.J., Yao, X., Hoos, L.M., Tetzloff, G., Iyer, S.P., Maguire, M., Golovko, A., Zeng M., Wang, L., Murgolo, N. and Graziano, M.P. 2004. Niemann-pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303(5661), 1201–1204. Andersson, M. and Braegger, C.P. 2022. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 43(3), 469–506. Apriyanto, Y.S., Iriyanti, N. and Tugiyanti, E. 2021. The effect of supplementation of avocado seed flour (Persea americana Mill.) in feed on blood lipids profile and egg yolk cholesterol of japanese Quail (Corturnix-corturnix japonica). Anim. Prod. 23(1), 10–17. Azizi, F and Smyth, P. 2009. Breastfeeding and maternal and infant iodine nutrition. Clin. Endocrinol. (Oxf). 70(5), 803–809. Basri, H., Saraswati, T.R. and Isdadiyanto, S. 2018. Hematological status of rats (Ratt2us norvegicus L.) in the lactation period after giving supplements organic quail eggs. IJBR. 6(1), 1–4. Berchieri-Ronchi, C.B., Kim, S.W., Zhao, Y., Correa, C.R., Yeum, K.L. and Ferreira, A.L. 2011. Oxidative stress status of highly prolific sows during gestation and lactation. Animal. 5(11), 1774–1779. Biberoglu, E., Biberoglu, K., Kirbas, A., Daglar, K., Genc, M. and Avci, A. 2016. Circulating and myometrial markers of oxidative stress in pregnant women with fetal growth restriction. J. Obstet. Gynaecol. Res. 42(1), 29–35 Binev, R. 2023. Investigations on some indicators in the blood of cattle with orosthenic activity tongue rolling. J. Adv. Vet. Anim. Res. 10(2), 336–341. Caponio, G.R., Wang, D.Q., Ciaula, A.D., Angelis, M.D. and Portincasa, P. 2020. Regulation of cholesterol metabolism by bioactive components of soy proteins: novel translational evidence. Int. J. Mol. Sci. 22(1), 227. Carretero-Krug. A., Montero-Bravo, A., Morais-Moreno, C., Puga, A.M., Samaniego-Vaesken, M.L., Partearroyo, TG. and Varela-Moreiras, L. 2024. Nutritional status of breastfeeding mothers and impact of diet and dietary supplementation: a narrative review. Nutrients. 16(2), 301. Cregan, M.D., Mitoulas, L.R. and Hartmann, P.E. 2002. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp. Physiol. 87(2), 207–214. Crozier, S.R., Robinson, S.M., Godfrey, K.M., Cooper, C. and Inskip, H.M. 2009. Women's dietary patterns change little from before to during pregnancy. J. Nutr. 139(10), 1956–1963. Delange, F. 2007. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 10(12a), 1571–1580. Dos, Q. Santos, R., Sichieri, Marchioni, D.M. and Verly Junior E. 2014. Brazilian pregnant and lactating women do not change their food intake to meet nutritional goals. BMC Pregnancy Childbirth. 14(186), 1–7. Dowling, D.K and Simmons, L.W. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proceedings of the Royal Society B: Biological Sciences, 276(1663), 1737–1745. Dunlea, E., Crushell, E., Cotter, M., Blau, N. and Ferreira, C.R. 2023. Clinical and biochemical footprints of inherited metabolic disease. XVI. Hematological abnormalities, Mol. Genet. Metab. 140(4), 107735 Fernandez, M.L and Murillo, A.G. 2022. Is there a correlation between dietary and blood cholesterol? evidence from epidemiological data and clinical interventions. Nutrients. 14(10), 2168. Garratt, M., Vasilaki, A., Stockley, P., McArdle, F., Jackson, M. and Hurst, J.L. 2011. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. Biol. Sci. 278(1708), 1098–1106. Gopalakrishnan, K., Teitelbaum, S.L., Wetmur, J., Manservisi, F., Falcioni, L., Panzacchi, S., Gnudi, F., Belpoggi, F. and Chen, J. 2018. Histology and transcriptome profiles of the mammary gland across critical windows of development in sprague dawley rats. J. Mammary Gland Biol. Neoplasia. 23(3), 149–163. Grases-Pintó, B., Abril-Gil, M., Torres-Castro, P., Castell, M., Rodríguez-Lagunas, M.J., Pérez-Cano, F.J. and Franch, A. 2021. Rat milk and plasma immunological profile throughout lactation. Nutrients. 13(4), 1257. Gwozdzinski, K., Pieniazek, A. and Gwozdzinski, L. 2021. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxid. Med. Cell Longev. 2021, 1–19. Hajati, H. and Zaghari, M. 2019. Spirulina in Poultry Nutrition. Cambridge Scholars Publishing, Cambridge, UK. Hussein, E. 2013. Biochemical and histopathologichal studies on the liver of rats administrated with different concentrations of aqueous extrack of Glycyrrhiza glaborus. Glob. Vet. 10(5), 491–495. Iannotti, L.L., Lutter, C.K., Stewart, C.P., Gallegos Riofrío, C.A., Malo, C., Reinhart, G ., Palacios, A., Karp, C., Chapnick, M., Cox, K. and Waters, W.F. 2017. Eggs in early complementary feeding and child growth: a randomized controlled trial. Pediatrics.140(1), 2016–3459. Imdad, A and Bhutta, Z.A. 2011. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health. 11(Suppl. 3), S17. Ivarsson, E., Wall, H., Boyner, M., Cervin, G., Pavia, H. and Wattrang, E. 2023. Effects of algal supplementation in feed to broiler breeders on transfer of nutrients and antibodies to chicks and quality of hatchlings. Animal. 17(12), 101020. Iwansyah, A.C., Damanik, M.R.M., Kustiyah, L. and Hanafi, M. 2017. Potensi fraksi etil asetat daun Coleus amboinicus L. dalam meningkatkan produksi susu, berat badan tikus dan anaknya. J. Gizi. Pangan. 2(1), 61–68. Jeke, A., Phiri, C., Chitindingu, K. and Taru, P. 2018. Ethnomedicinal use and pharmacological potential of Japanese quail (Coturnix coturnix japonica) birds meat and eggs, and its potential implications on wild quail conservation in Zimbabwe: A review. Cogent. Food Agric. 4(1), 1507305 Kanungo, S., Wells, K., Tribett, T. and El-Gharbawy, A. 2018. Glycogen metabolism and glycogen storage disorders. Ann. Transl. Med. 6(24), 474. Khan, R.U., Khan, A., Naz, S., Ullah, Q., Puvaˇca N.,, Laudadio, V., Mazzei, D., Seidavi, A., Ayasan, T. and Tufarelli, V. 2023. Pros and cons of dietary vitamin a and its precursors in poultry health and production: a comprehensive review. Antioxidants, 12(5), 1131. Kuhn, V., Diederich, L., Keller, T.C.S., Kramer, C.M., Lückstädt, W. and Panknin, C. 2017. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox. Signal. 26(13), 718–742. Kulshreshtha, G., Hincke, M.T., Prithiviraj, B. and Critchley, A. 2020. A review of the varied uses of macroalgae as dietary supplements in selected poultry with special reference to laying hen and broiler chickens. J. Mar. Sci. Eng. 8(7), 536. Kim, S.Y. and Yi, D.Y. 2020. Components of human breast milk: from macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 63(8), 301–309. Lager, K and Jordan, E. The metabolic profile for the modern transition dairy cow. The Mid-South Ruminant Nutrition Conference, 2012, pp 9–16. Lawrence, R.A and Lawrence, R.M. 2011. Breastfeeding: a guide for the medical profession, 7th Ed. Philadelphia, PA: Saunders Elsevier. Lima, H. J.D. and Souza, L.A.Z. 2018. Vitamin A in the diet of laying hens: enrichment of table eggs to prevent nutritional deficiencies in humans. World Poult. Sci. J. 74(4), 619–626. Liu, Y., Song, M., Bai, H., Wang, C., Wang, F. and Yuan, Q.. 2024. Curcumin improves the egg quality, antioxidant activity, and intestinal microbiota of quails during the late laying period. Poult. Sci. 103(1), 103233. Lokossou, G. A. Kouakanou, G,L., Schumacher, A. and Zenclussen, A.C. 2022. Human breast milk: from food to active immune response with disease protection in infants and mothers. Front. Immunol. 13, 849012. Mahmood, A., Omar, M.N. and Ngah, N. 2012. Galactagogue effects of Musa x paradisiaca flower extract on lactating rats. Asian Pac. J. Trop. Med. 5(11), 882–886. Meléndez-Martínez, A.J. 2019. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 63(15), e1801045. Miranda, J., Anton, X., Redondo-Valbuena, C., Roca-Saavedra, P., Rodriguez, J. and Lamas, A.. 2015. Egg and egg-derived foods: effects on human health and use as functional foods. Nutrients. 7(1), 706–729. Marshall, N.E., Abrams, B., Barbour, L.A., Catalano, P., Christian, P., Friedman, J.E., Hay, W.W., Jr, Hernandez, T.L., Krebs, N.F., Oken, E., Purnell, J.Q., Roberts, J.M., Soltani, H., Wallace, J. and Thornburg, K.L. 2022. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am. J. Obstet. Gynecol. 226(5), 607–632. Mousa, A., Naqash, A. and Lim, S. 2019. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. 11(2), 443. Nagel, E.M., Howland, M.A., Pando, C., Stang, J., Mason, S.M., Fields, D.A. and Demerath, E.W. 2022. Maternal Psychological Distress And Lactation And Breastfeeding Outcomes: A Narrative Review. Clin. Ther. 44(2), 215–227. Naeem, M., Rahim, M.A., Nisa, M.U., Khalid, K., Ahmad, N. and Khalid, N. and Awuchi, C.G. 2023. Digestibility, intake, and growth performance of egg protein replaced with vegetable protein in weaning food. Cogent Food and Agriculture. 9(1), 2258800. Neville, M.C., Demerath, E.W., Hahn-Holbrook, J., Hovey, R.C., Martin-Carli, J., McGuire, M.A., Newton, E.R., Rasmussen, K.M., Rudolph, M.C. and Raiten, D.J. 2023. Parental factors that impact the ecology of human mammary development, milk secretion, and milk composition-a report from "breastmilk ecology: genesis of infant nutrition (BEGIN)" Working Group 1. Am. J. Clin. Nutr. 117 (Suppl. 1), S11–S27. Nielsen, S.S. 2010. Food Analysis. 4th Edition, Food Science Text Series, Newyork, NY: Springer, pp: 602. Nisa, R.K., Saraswati dan, T.R., Yuniwarti, E.Y.W. 2017. Kadar kolesterol dan vitamin A pada telur itik pengging, itik tegal dan itik magelang. Buletin Anatomi dan Fisiologi, 2(2), 114–119. Øverland, M., Mydland, L.T. and Skrede, A. 2019. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food. Agric. 99(1), 13–24. Pastuszewska, B., Taciak, M., Ochtabińska, A., Tuśnio, A., Misztal, T., Romanowicz, K. and Morawski, A. 2008. Nutritional value and physiological effects of soya-free diets fed to rats during growth and reproduction. J. Anim. Physiol. Anim. Nutr. (Berl). 92(1), 63–74. Powe, C. E., Allen, M., Puopolo, K.M., Merewood, A., Worden, S., Johnson, J.C., Fleischman, A. and Welt, C.K. 2010. Recombinant human prolactin for the treatment of lactation insufficiency. Clin. Endocrinol. (Oxf). 73(5), 645–653. Prawitasari, S., Saraswati, T.R. and Tana, S. 2019. Liver histological structure of rats (Rattus norvegicus) in the lactation period after supplemented with organic quail eggs. J. Phys.: Conf. Ser., pp: 1217. Purba, S.L., Saraswati, R.T. and Isdadiyanto, S.. 2018. The effect of organic quail egg supplementation on the blood lipid profile of white mice (Rattus Norvegicus L.) during the lactation period. J. Phys.: Conf. Ser., pp: 1025. Rendón, U., Carrillo, S., Arellano, L.G., Casas, M.M., Pérez, F. and Avila, E. 2003. Chemical composition of the residue of alginates (Macrocystis pyrifera) extraction. Its utilization in laying hens feeding. Cuban J. Agricult. Sci. 37, 287–293. Salmon, A.B., Marx, D.B. and Harshman, L.G. 2001. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution, 55(8), 1600–1608. Salmuth, V.V., Brennan, E., Kerac, M. and McGrath, M. 2021. Frison S, Lelijveld N. Maternal-focused interventions to improve infant growth and nutritional status in low-middle income countries: a systematic review of reviews. PLoS One. 16(8), e0256188. Selman, C., McLaren, J.S., Himanka, M.J. and Speakman, J.R. 2000. Effect of longterm cold exposure on antioxidant enzyme activities in a small mammal. Free Radic. Biol. Med. 28(8), 1279–1285. Słupczynska, M., Jamroz, D., Orda, J. and Wiliczkiewicz, A. 2014. Effect of various sources and levels of iodine, as well as the kind of diet, on the performance of young laying hens, iodine accumulation in eggs, egg characteristics, and morphotic and biochemical indices in blood. Poult. Sci. 93(10), 2536–2547. Sumaiya, S., ,Nayak, S., Baghel, R.P., Nayak, A., Malapure, C.D. and Kumar, R. 2016. Effect of dietary iodine on production of iodine enriched eggs. Vet. World. 9(6), 55–558. Toy, H., Camuzcuoglu, H., Arioz, D.T., Kurt, S., Celik, H. and Aksoy, N. 2009. Serum prolidase activity and oxidative stress markers in pregnancies with intrauterine growth restricted infants. J. Obstet. Gynaecol. Res. 35(6), 1047–1053. Velázquez-Villegas, L.A., López-Barradas, A.M., Torres, N., Hernández-Pando, R., León-Contreras, J.C. and Granados, O. 2015. Prolactin and the dietary protein/carbohydrate ratio regulate the expression of SNAT2 amino acid transporter in the mammary gland during lactation. Biochim. Biophys. Acta. 1848(5), 1157–1164. Wati, L.R., Sargowo, D., Nurseta, T. and Zuhriyah, L. 2023. The role of protein intake on the total milk protein in lead-exposed lactating mothers. Nutrients, 15(11), 2584. Wrzecińska, M., Kowalczyk, A., Czerniawska-Piątkowska, E., Kordan, W. and Araujo, J.P. Examination of the haematological profile of pregnant Polish Holstein-Friesian black-and-white cattle in the early stage. J. Vet. Res. 67(3), 415–425. Xu, Y.C., Yang, D.B., Speakman, J.R. and Wang, D.H. 2014. Oxidative stress in response to natural and experimentally elevated reproductive effort is tissue dependent. Funct. Ecol. 28(2), 402–410. Zheng, G.X., Lin, J.T., Zheng, W.H., Cao, J. and Zhao, Z.J. 2015. Energy intake, oxidative stress and antioxidant in mice during lactation. Dongwuxue Yanjiu. 36(2), 95–102. Zhang, Y., Kallenberg, C., Hyatt, H.W., Kavazis, A.N. and Hood, W.R. 2017. Change in the lipid transport capacity of the liver and blood during reproduction in rats. Front. Physiol. 8, 517. Zhang, X., Yang, S. Chen, J. and Su, Z. 2019. Unraveling the regulation of hepatic gluconeogenesis. Front. Endocrinol. (Lausanne). 9, 802. Zhu, W., Mu, Y., Liu, J. and Wang, Z.. 2015. Energy requirements during lactation in female Apodemus chevrieri (Mammalia: Rodentia: Muridae) in the Hengduan Mountain Region. Italian J. Zool. 82(2), 165–171. | ||
| How to Cite this Article |
| Pubmed Style Basri H, Widiyanto S, Saragih HT, Zuprizal Z. Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Vet. J.. 2024; 14(11): 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 Web Style Basri H, Widiyanto S, Saragih HT, Zuprizal Z. Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. https://www.openveterinaryjournal.com/?mno=214105 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.11 AMA (American Medical Association) Style Basri H, Widiyanto S, Saragih HT, Zuprizal Z. Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Vet. J.. 2024; 14(11): 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 Vancouver/ICMJE Style Basri H, Widiyanto S, Saragih HT, Zuprizal Z. Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 Harvard Style Basri, H., Widiyanto, . S., Saragih, . H. T. & Zuprizal, . Z. (2024) Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Vet. J., 14 (11), 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 Turabian Style Basri, Hasan, Slamet Widiyanto, Hendry T.s.s.g. Saragih, and Zuprizal Zuprizal. 2024. Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Veterinary Journal, 14 (11), 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 Chicago Style Basri, Hasan, Slamet Widiyanto, Hendry T.s.s.g. Saragih, and Zuprizal Zuprizal. "Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth." Open Veterinary Journal 14 (2024), 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 MLA (The Modern Language Association) Style Basri, Hasan, Slamet Widiyanto, Hendry T.s.s.g. Saragih, and Zuprizal Zuprizal. "Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth." Open Veterinary Journal 14.11 (2024), 2827-2836. Print. doi:10.5455/OVJ.2024.v14.i11.11 APA (American Psychological Association) Style Basri, H., Widiyanto, . S., Saragih, . H. T. & Zuprizal, . Z. (2024) Investigating the effect of quail egg supplementation enriched with marine macroalgae Eucheuma spinosum on haematological indices, lipid profile parameters and blood glucose level of Sprague Dawley rats during lactation and offspring growth. Open Veterinary Journal, 14 (11), 2827-2836. doi:10.5455/OVJ.2024.v14.i11.11 |