| Research Article | ||
Open Vet. J.. 2024; 14(11): 2901-2910 Open Veterinary Journal, (2024), Vol. 14(11): 2901-2910 Research Article A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, BrazilReiner Silveira de Moraes1, Diego Ribeiro1, Alessandra Melchert1, Henry David Mogollón García2, Doughlas Regalin3, Raphael Lúcio Andreatti Filho1, Regina Kiomi Takahira1, Rogério Giuffrida4, Adriano Sakai Okamoto1 and Priscylla Tatiana Chalfun Guimarães-Okamoto1*1Department of Veterinary Clinics, School of Veterinary Medicine and Animal Science, São Paulo State University (Unesp), Botucatu, Brazil 2Institute of Biology, Campinas State University (Unicamp), São Paulo, Brazil 3School of Veterinary Medicine and Animal Bioscience, Federal University of Jataí (UFJ), Brazil 4Department of Veterinary Clinics and One Health, School of Veterinary Medicine, University of Western São Paulo (Unoeste), São Paulo, Brazil *Corresponding Author: Priscylla Tatiana C. G. Okamoto. Department of Veterinary Clinics, School of Veterinary Medicine and Animal Science, São Paulo State University (Unesp), Botucatu, Brazil. Email: tatiana.okamoto [at] unesp.br Submitted: 13/08/2024 Accepted: 24/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
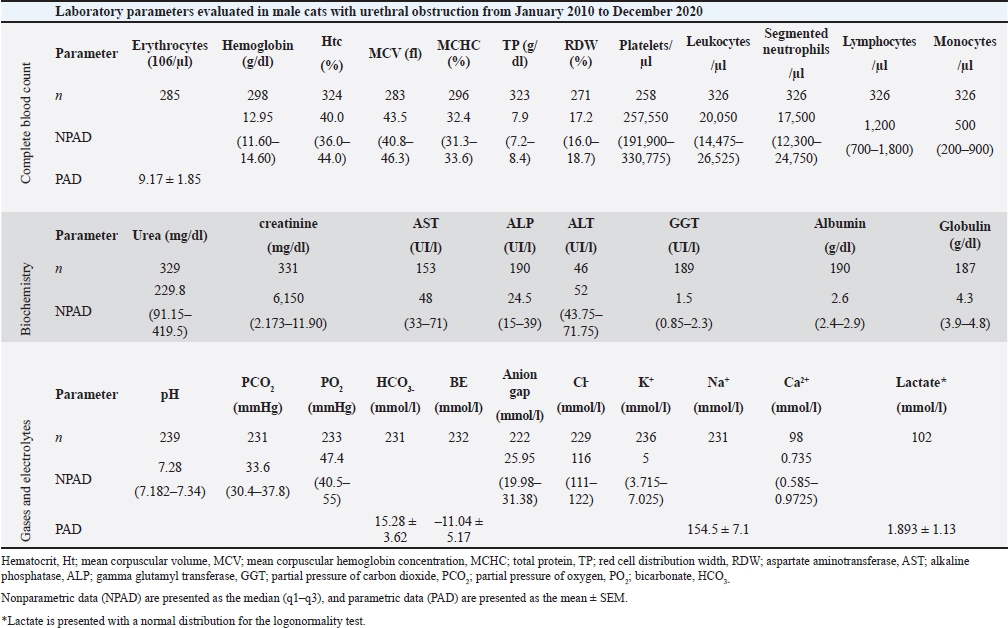
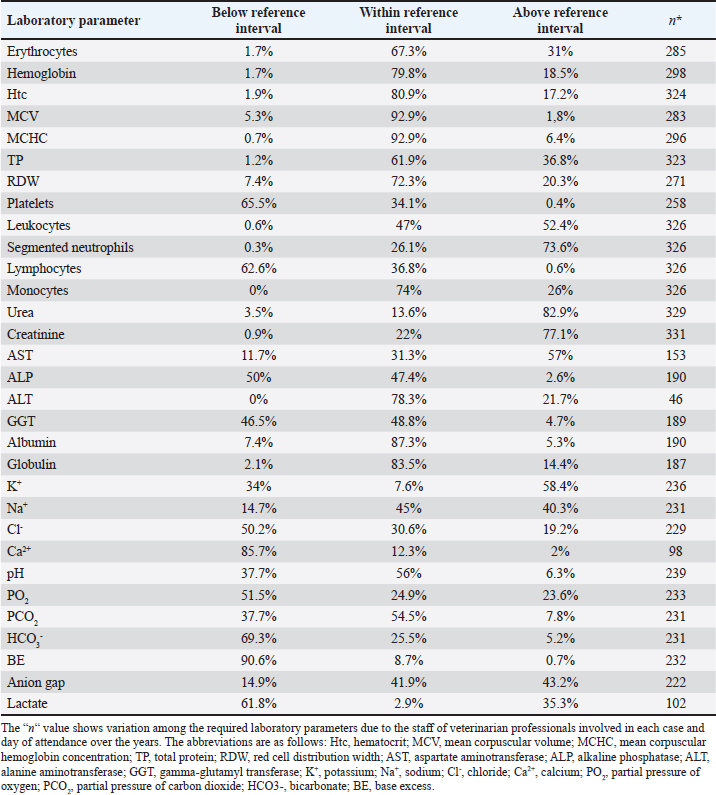
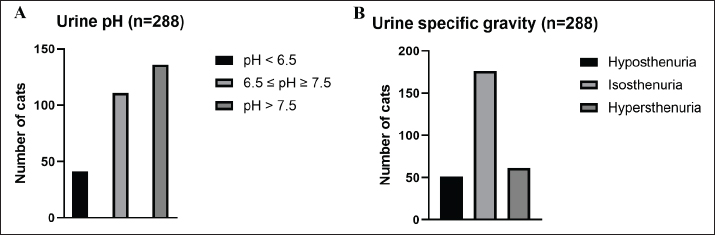
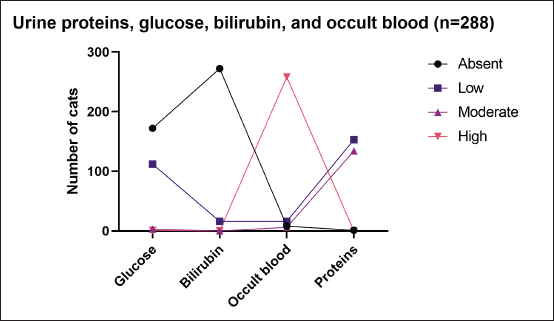
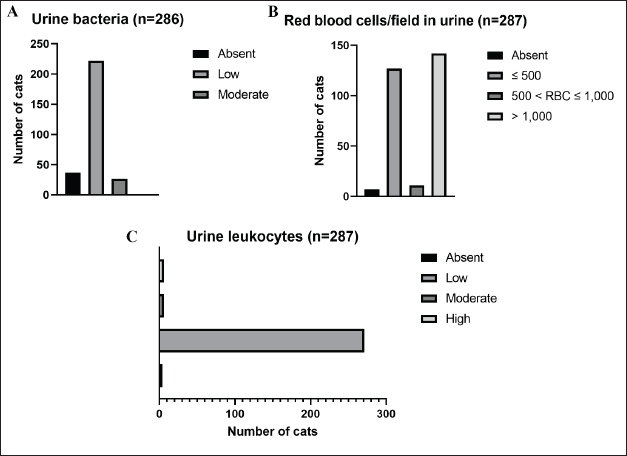
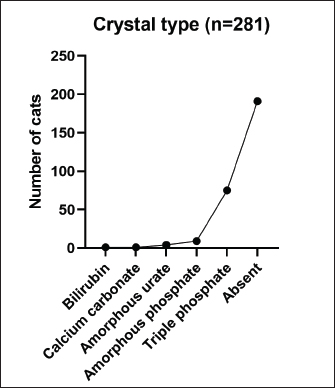
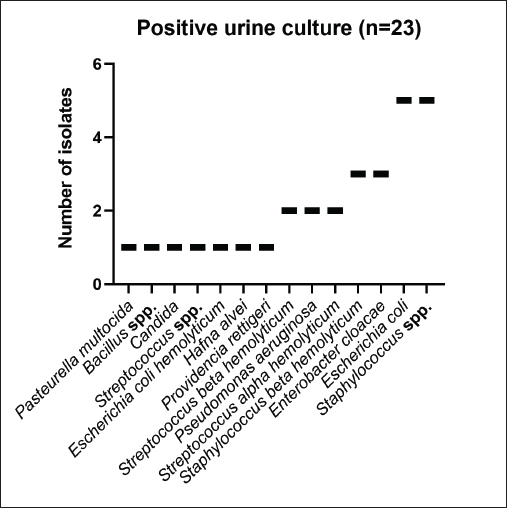
AbstractBackground: Urinary retention in obstructed male cats leads to changes in blood and urine compounds, which, combined with the time of obstruction, are linked to the worsening of the animal’s clinical status. Aim: This study aimed to describe the primary laboratory changes in male cats with urethral obstruction (UO). Methods: Medical records of 386 male cats diagnosed with UO and treated at the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine and Zootechnics (FMVZ), UNESP—Botucatu, between 2010 and 2020 were reviewed. Data on sex, age, breed, body weight, and reproductive status were evaluated. Results: Over the years, complete blood count, renal and hepatic biochemistry, blood gas analysis, electrolytes, urinalysis, and urine culture were performed upon request. Anaemia was not representative in obstructed cats. Thrombocytopenia was identified in 65.5% (169/258) of patients, and neutrophilic leukocytosis was identified in 52.4% (170/326) of patients. High urea and creatinine values were detected in 82.9% (273/329) and 77.1% (256/331) of the patients, respectively. Acidemia, increased PO2, metabolic acidosis, hypochloremia, hyperkalemia, hypernatremia, and hypocalcemia were observed in 37.6% (90/239), 51.5% (120/233), 69.3% (160/231), 50.2% (115/229), 58.5% (138/239), 40.2% (93/231), and 85.7% (84/98) of the cats, respectively. Urinalysis revealed an acidic pH, isosthenuria, a low proportion of proteins, and a high presence of occult blood and erythrocytes per field (> 1,000). Finally, 19.49% had positive cultures. Escherichia coli and Staphylococcus spp. are commonly isolated. Conclusion: This study provides a description of laboratory changes and those most prevalent in the population under study. UO may result mainly in isolated or combined laboratory changes such as azotemia, acidemia, metabolic acidosis, hyperkalemia, hypocalcemia, acidic pH, and occult blood in the urine. Additional laboratory changes may be present; however, they must be deeply investigated as comorbidities might be associated with them. Therefore, the description of laboratory changes in large populations, such as in male cats with UO, provides a reference for veterinarians regarding the hematological and urinary changes expected in obstructed male cats and instigates the search for further studies in the field. Keywords: Blood abnormality, Felis catus, FLUTD, Urethra, Urine blockage. IntroductionUrethral obstruction (UO) in male cats is a feline lower urinary tract (FLUTD) complication commonly associated with causes such as feline idiopathic cystitis (FIC), urolithiasis, urethral plugs, neoplasms, or anatomical defects (Piyarungsri et al., 2020). Urinary retention affects the glomerular filtration rate (Muller et al., 2022), leading to the disruption of blood and urine compounds (Dinallo et al., 2022). These conditions, combined with the time of obstruction, are often linked to deterioration of the animal’s clinical condition, resulting in a poorer prognosis and a greater risk of mortality (Segev et al., 2011). The inability to excrete nitrogenous compounds such as urea and the inability to tubularly reabsorb creatinine may lead to an increase in both compounds in the blood, which may exhibit varying proportions (Abdel-Saeed et al., 2020). Among the changes in blood gas analysis and electrolytes, a significant increase in phosphorus, magnesium, lactate, potassium, and sodium levels, along with a significant reduction in blood pH and PO2, can be identified in male cats obstructed for a period of ≥ 36 hours (Neri et al., 2016). Nevertheless, a significant increase in ionized calcium levels can be expected (Segev et al., 2011). In the complete blood count (CBC), an increase in hematocrit (Htc), total leukocyte count, and absolute neutrophil count, along with a significant decrease in total lymphocyte and monocyte counts, can be expected (Abdel-Saeed et al., 2020). Notably, hypoproteinaemia can also be identified (Lamb et al., 2018). Urine retention in cats with UO can lead to hematuria, proteinuria, and crystalluria (Seo et al., 2021). Additionally, microhematuria (> 10 red blood cells (RBCs) per field) is frequently observed in cats with lower urinary tract disease due to various causes. Moreover, pyuria may be more common in cats with urinary tract infections (UTIs) than in those with FICs (Dorsch et al., 2014). Furthermore, pyuria, occult blood, and bacteriuria can also be detected (Neri et al., 2016). When bacteria and leukocytes are present in urinalysis, urine culture becomes an important diagnostic tool for urogenital infections, preferably collected through cystocentesis to reduce the risk of contamination by microbial agents from the lower genitourinary tract (Sævik et al., 2011). Australian, European, and Norwegian studies have reported Escherichia coli as the most commonly isolated microbial agent in the urine of cats with lower urinary tract disease, specifically bacterial cystitis, with the majority having an estimated abundance of > 100,000 CFU/ml of urine (Litster et al., 2007; Passmore et al., 2008; Sævik et al., 2011). Hematological evaluation in obstructed cats supports diagnosis and enables monitoring of the animal’s clinical progression (Balakrishnan and Drobatz, 2013). Unlike previous studies with both nonobstructive and obstructive FLUTD, this study provides a descriptive presentation of the hematological and urinary changes in 386 male cats with UO treated at the Veterinary Hospital of São Paulo State University in Botucatu, Brazil, from 2010 to 2020. Materials and MethodsData collectionMedical records of male and female cats attending the Veterinary Teaching Hospital of the FMVZ, UNESP—Botucatu, were accessed and carefully reviewed from January 2010 to December 2020 for the selection of male cats diagnosed with UO presenting signs of the lower urinary tract (urine retention, hematuria, stranguria, pollakiuria, periuria, and dysuria). Data on CBC, renal and hepatic biochemistry, blood gas analysis, electrolytes, urinalysis, and urine culture were assessed upon availability in the records. In the selection, female cats with UO, male cats with nonobstructive FLUTD, and duplicate records were excluded from the study. Also, comorbidities, whether of infectious, parasitic, fungal, or traumatic causes (car accident, fight with other animals) were excluded. In the selection of animals, there was no predilection for age, reproductive status, episode of obstruction, breed, and body weight. For the laboratory evaluation, only examinations carried out exclusively by the Veterinary Clinical Laboratory Service of the FMVZ at Unesp, Botucatu/SP were included to avoid discrepancies due to different analysis methods, other laboratories, and staff. The CBC was used to analyze the erythrocyte count, hemoglobin, Htc, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), total protein (TP), platelets, total leukocytes, segmented neutrophils, lymphocytes, and monocytes. Urea and creatinine were analyzed to assess renal function. To assess hepatic function, aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), and albumin were analyzed. In addition, globulin was evaluated. For blood gas and electrolyte analysis, venous blood samples were evaluated for pH, partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), bicarbonate (HCO3-), base excess (BE), anion gap, chloride (Cl-), potassium (K+), sodium (Na+), calcium (Ca2+), and lactate. For urine analysis, only sample results obtained by cystocentesis were considered. The urine specific gravity (USG), pH, presence or absence of occult blood, crystals, leukocytes, and bacteria in the urine sediment were noted. Additionally, the main isolated microbial agents were registered. Statistical analysisDescriptive analyses were performed on the parameters of CBC, renal and hepatic biochemistry, blood gas and electrolyte analysis, urinalysis, and urine culture. A transformation log was applied to the lactate variable. The data are presented as the means ± standard error of the mean (SEM) for parameters with a normal distribution and medians (first and third quartiles) for parameters without a normal distribution. When applicable, the data were presented as a frequency distribution. Statistical analyses were performed via the Epi Info version 7.2.3.1 package. Ethical approvalThis study was approved by the Animal Ethics Committee (CEUA) of the Faculty of Veterinary Medicine and Zootechnics of São Paulo State University (FMVZ, UNESP—Botucatu) under protocol number 0235/2021. ResultsIn total, 386 male cats were diagnosed with UO. The mean body weight was 4.24 ± 1.11 kg, and the mean age was 8.2 ± 4.5 years. The data concerning reproductive status revealed that 60.9% of the male cats were neutered and 39.1% were intact. Among the cat breeds, 91.8% were domestic cats, 5.7% were Siamese, 2.1% were Persian, and 0.3% were Turkish Angora. The number of laboratory parameters required for each animal varied substantially over the years. Thus, the difference in the number of male cats for each laboratory parameter was influenced by the staff of veterinarians in different clinical scenarios. The results of the data collection for CBC, biochemistry, blood gas analysis, and electrolytes are presented as the means ± SEMs and medians (first–third quartiles) in Table 1. The percentages of male cats below, within, and above the reference interval for each parameter were also evaluated (Table 2). The frequency distributions for urine pH (Fig. 1A), USG (Fig. 1B), and proteins (Fig. 2) revealed an acidic pH, isosthenuria, and a low proportion of proteins. The absence of glucose and bilirubin (Fig. 2), in addition to the high prevalence of occult blood (Fig. 2), was observed. Acetone, urobilinogen, bilirubin, and bile salts were not identified in any of the cats for which urinalysis was performed. In the examination of the urine sediments, bacteria were not present in a representative proportion of obstructed male cats according to the categorical scale (Fig. 3A). The number of red blood cells per ˣ 400 field did not show a pattern in the frequency distribution analysis (Fig. 3B). The same scenario was observed for leukocytes (Fig. 3C). In the crystal analysis, the absence of these compounds stood out (Fig. 4). Among the male cats with UO, 118 (30.56%) had urine cultures performed. Of these, 23% (19.49%; CI 95%: 12.34%–26.64%) showed positive microbial growth. Among these, Escherichia coli (E. coli) and Staphylococcus spp. were the most commonly isolated microorganisms, with five cats having more than one microorganism isolated in the urine culture (Fig. 5). However, in the urine sediments of these culture-positive male cats, 12 (57.1%; CI 95%: 34.02%–78.18%) presented leukocyturia, and 19 (95%; CI 95%: 72.1%–99.8%) presented bacteriuria. Moreover, among the culture-negative male cats, 89 (94.68%; CI 95%: 90.14%–99.22%) presented with leukocyturia, and 77 (85.6%; CI 95%: 76.6%–92.1%) presented with bacteriuria. DiscussionThis retrospective study revealed that significant changes in CBC parameters are not commonly expected in obstructed male cats without comorbidities. As noted by Tion et al. (2015), both dogs and cats typically present CBC values within the normal range unless there is an underlying condition contributing to the obstruction, such as mycoplasma infection (Hagiwara, 2009). Thrombocytopenia, a condition characterized by low platelet counts, is considered rare in cats, with an estimated prevalence of 1.2% (Ellis et al., 2018). This study suggested that thrombocytopenia in cats may be associated with inflammation in FLUTD patients. This inflammation can lead to platelet activation and aggregation, as well as their removal by the mononuclear phagocytic system (Ellis et al., 2018). Consequently, the observed neutrophilia and lymphopenia are likely indicative of a stress-induced leukogram secondary to FLUTD, as described by Tariq et al. (2014). This aligns with the findings of this study, in which more than 50% of the cats exhibited leukocytosis due to neutrophilia. Lymphopenia has also been observed in more than 60% of cats. With respect to renal function, azotemia was detected in more than 75% of the animals in this study, which is in line with previous research by Neri et al. (2016). This result is consistent with expectations for cats with obstructive FLUTD, as reported in previous studies (Abdel-Saeed et al., 2020; Seo et al., 2021). Additionally, an increase in serum AST levels was observed in more than 55% of the cats, which is believed to be associated with factors such as liver fat accumulation and anorexia/hyporexia, as suggested by Fascetti et al. (1997), an inflammatory condition, and a higher body condition score, as described by Okada et al. (2017) and Kobayashi et al. (2020). In this study, venous blood samples were collected to assess the acid‒base status and ventilation of patients. As expected, metabolic acidosis, a common metabolic disorder in cats with UO, was confirmed in more than 50% of the cats. In metabolic acidosis, the concentration of bicarbonate ions decreases, resulting in a negative BE (Rieser, 2013). This study revealed reductions in both of these variables, which is consistent with findings in obstructive FLUTD in previous research (Fröhlich et al., 2016; Neri et al., 2016). Notably, PCO2 levels can range from normal to reduced, with the majority of the cats in this study exhibiting values within this range. Additionally, half of the animals in the study had PO2 levels below the minimum reference value (27.6 mmHg), which is in line with findings by Gonzalez and Waddell (2016). Importantly, while venous blood is useful for assessing metabolic and electrolyte disturbances and providing information about ventilation through PCO2 assessment, it is not reliable for assessing oxygenation (Gonzalez and Waddell, 2016). Compared with those of Neri et al. (2016), the prevalence of cats with hyperkalaemia in this study was 26.7% greater, a common occurrence in obstructed cats due to the inability of the kidneys to excrete potassium, resulting in the antiport of hydrogen and potassium (Canei et al., 2021). The percentages of cats with low ionized calcium concentrations were greater than those previously reported (Drobatz and Hughes, 1997). These authors demonstrated a positive correlation between ionized calcium and pH and chloride levels, along with a negative correlation with potassium, urea, and creatinine, findings that align with the data from obstructed cats in our study. Table 1. Median (first–third quartiles) and mean ± SEM for CBC, biochemical and blood gas and electrolyte analysis variables in male cats with UO.
Table 2. Percentual evaluation of laboratory parameter values below, within, and above the reference interval in male cats with UO admitted to the Veterinary Teaching Hospital of the FMVZ, Unesp, Botucatu, from January 2010 to December 2020.
The urinary profile of obstructed cats revealed isosthenuria in most cases, which was lower than that previously reported by Gerber et al. (2005) but similar to that reported by Abdel-Saeed et al. (2020). The majority of the cats presented high levels of red blood cells per field and microscopic hematuria. This prevalence is consistent with the findings of Neri et al. (2016). The high incidence of hematuria or occult blood in the urine presented in our study is consistent with the findings of Dinler et al. (2021) and can result from various pathological processes that damage the mucosa and vasculature of the urogenital tract. These include infections, inflammation, and neoplasia, as described by Forrester (2004). Consequently, microscopic hematuria can be expected in both obstructed and unobstructed FLUTDs. Regarding crystalluria in obstructed cats, the majority noted the absence of crystals in the urine. This finding is in line with that of Gerber et al. (2005) and contrasts with that of Abdel-Saeed et al. (2020), who reported struvite crystals in all patients with obstructive FLUTD analyzed in their study.
Fig. 1. Frequency distribution of (A) urine pH and (B) USG in 288 male cats diagnosed and treated for UO at the Veterinary Teaching Hospital, FMVZ, UNESP, Botucatu, São Paulo, from 2010 to 2020.
Fig. 2. Frequency distribution of glucose, bilirubin, occult blood, and protein in the urine of 288 male cats diagnosed and treated for UO at the Veterinary Teaching Hospital, FMVZ, UNESP, Botucatu, São Paulo, from 2010 to 2020. Regarding the low adherence of urine samples sent for analysis, it is mainly related to the non-collection of urine samples on the first day of presentation to the emergency room. In order to maintain some consistency in the findings of this study, only urinalysis performed from samples collected on the first day were considered. Therefore, samples collected after any medical intervention were not included, explaining the 30.56% rate. Similarly, the low rate of positive urine culture may have been influenced by the exclusion of urine culture performed on any other day rather than the first day of presentation. Besides the small number of animals with urine culture performed on the first day of presentation, the most commonly isolated microorganisms, E. coli and Staphylococcus spp. are somehow, in agreement with previous studies (Dorsch et al., 2014; Teichmann-Knorm et al., 2018). In this study, although a similar proportion of isolates were found for both microorganisms, different results could have been found if a higher number of animals had urine culture performed in the first day of presentation. Considering that E. coli and Staphylococcus spp. were the bacteria most commonly isolated in equal proportions in this study, it contrasts with previous studies where E. coli was the predominant pathogen (Gerber et al., 2005; Litster et al., 2007; Dorsch et al., 2014). In culture-positive cats, the prevalence of E. coli isolation ranged from 39% to 59% (Dorsch et al., 2019), which was higher than our findings. In both human and animal UTIs, E. coli is the most frequently identified microorganism (Chapman et al., 2006). Escherichia coli strains from phylogenetic group B2 are known for their extraintestinal virulence, including UTIs (Clermont et al., 2000), which may explain the presence of E. coli in obstructed cats with UTIs (Dorsch et al., 2014), as identified in this study. In contrast, Enterococcus spp., a commonly cited UTI isolate, was not found in this study. When positive and negative urine cultures from cats were compared with bacteria in the urinary sediment, no correlation was detected between the two tests. These findings underscore the importance of urine culture as the gold standard for detecting subclinical bacteriuria and UTIs, especially when clinical signs are absent. Subclinical bacterial infections are a common concern and are attributed to the indiscriminate use of antimicrobials in both humans (Cortes-Penfield et al., 2017) and animals (Weese et al., 2019).
Fig. 3. Frequency distribution of (A) bacteria, (B) red blood cells per field, and (C) leukocytes in the urine sediments in male cats with UO at the Veterinary Teaching Hospital, FMVZ, UNESP, Botucatu, São Paulo, from 2010 to 2020.
Fig. 4. Frequency distribution of crystal types in 281 male cats diagnosed and treated with UO at the Veterinary Teaching Hospital, FMVZ, UNESP, Botucatu, São Paulo, from 2010 to 2020. The limitations of this study include the variation in the number of tests requested for each animal, which resulted in discrepancies in the number of animals included in the evaluation of each laboratory variable. Similarly, low adherence to urine culture resulted in a low number of animals in which urine cultures were performed. Although the investigation of laboratory changes based on comorbidities in male cats is believed to provide a deeper understanding of the disease in the species, the variation in the number of animals per group would not provide sufficient statistical power in this study, explaining the exclusion of comorbidities in the methodology, and therefore becoming a limitation of the study.
Fig. 5. Main urine isolates in 23 obstructed male cats with bacterial growth among all the male cats with UO that had urine culture performed at the Veterinary Teaching Hospital, FMVZ, UNESP, Botucatu, São Paulo, from 2010 to 2020. The laboratory evaluation of male cats with UO is essential to exclude the underlying causes of obstruction and identify comorbidities and dysfunctions resulting from the obstructive condition. Data collection in this study allowed the description of possible laboratory changes and those most prevalent in the population under study. The description resulting from data collection with a significant number of animals makes this document reference material for veterinarians regarding the changes expected in an obstructed male cat. Therefore, it is important to note that while certain laboratory abnormalities such as azotemia, acidemia, metabolic acidosis, hyperkalemia, hypocalcemia, acidic pH, and occult blood in the urine are commonly expected in UO, each patient must be thoroughly evaluated based on their clinical condition. This is because UO may result from an underlying illness not directly related to the urinary tract. AcknowledgmentsThe authors are immensely grateful to the archives sector of the Veterinary Hospital of the Faculty of Veterinary Medicine and Zootechnics of Unesp, Botucatu, São Paulo, for making the data for this investigation available. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Author contributionConceptualization: Reiner Silveira de Moraes, Priscylla Tatiana Chalfun Guimarães-Okamoto; Methodology: Reiner Silveira de Moraes, Henry David Mogollón García; Data Collection: Reiner Silveira de Moraes; Data Processing: Reiner Silveira de Moraes, Henry David Mogollón García, Diego Ribeiro; Data Analysis & Interpretation: Reiner Silveira de Moraes, Henry David Mogollón García, Diego Ribeiro; Writing-Original Draft: Reiner Silveira de Moraes, Diego Ribeiro; Writing-Review & Editing: Alessandra Melchert, Doughlas Regalin, Raphael Lúcio Andreatti Filho, Rogério Giufrida, Regina Kiomi Takahira, Adriano Sakai Okamoto, Priscylla Tatiana Chalfun Guimarães-Okamoto; Supervision: Priscylla Tatiana Chalfun GuimarãesOkamoto Critical Review: Henry David Mogollón García, Alessandra Melchert, Rogério Giufrida, Regina Kiomi Takahira, Priscylla Tatiana Chalfun Guimarães-Okamoto. Data availabilityAll the data is presented in this manuscript. Any additional data are available from the authors upon reasonable request and with permission from the corresponding author. ReferencesAbdel-Saeed, H., Tahon, R.R. and Farag, H.S. 2020. Diagnostic and epidemiological studies on obstructive feline lower urinary tract disease (FLUTD) with special reference to anatomical findings in Egyptian Tomcats. Bulg. J. Vet. Med. 24, 383–394. Balakrishnan, A. and Drobatz, K.J. 2013. Management of urinary tract emergencies in small animals. Vet Clin. North Am. Small Anim. Pract. 43, 843–867. Canei, D.H., Pereira, M.E., Freitas, M.N., Trevisan, Y.P.A., Zorzo, C., Bortolini, J., Mendonça, A.J., Sousa, V.R.F. and Almeida, A.B.P.F. 2021. Biochemical, electrolytic, and cardiovascular evaluations in cats with urethral obstruction. Vet. World. 14, 2002–2008. Chapman, T.A., Wu, X.Y., Barchia, I., Bettelheim, K.A., Driesen, S., Trott, D., Wilson, M. and Chin, J.J.C. 2006. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 72, 4782–4795. Clermont, O., Bonacorsi, S. and Bingen, E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66, 4555–4558. Cortes-Penfield, N.W., Trautner, B.W. and Jump, R. 2017. Urinary tract infection and asymptomatic bacteriuria in older adults. Infect. Dis. Clin. North Am. 31, 673–688. Dinallo, H.R., Giuffrida, R., Azevedo, M.G.P., Santarém, C.L., Andrade, S.M.C.F., Brinholi, R.B., Motta, Y.P., Schmidt, S.E.M., Ferreira, J.C.P., García, H.D.M., Brito, A.F., Melchert, A. and Okamoto, P.T.C.G. 2022. Acute-phase proteins in cats with obstructive feline lower urinary tract disease. Vet. Clin. Pathol. 51, 77–83. Dinler Ay, C., Tuna, G.E., Ulutaş, B. and Voyvoda, H. 2021. Clinicopathological characteristics of cats with obstructive lower urinary tract disease in the Aydın province (Turkey). Kocatepe Vet. J. 14, 474–481. Dorsch, R., Remer, C., Sauter-Louis, C. and Hartmann, K. 2014. Feline lower urinary tract disease in a German cat population: a retrospective analysis of demographic data, causes and clinical signs. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 42, 231–329. Dorsch, R., Teichmann-Knorrn, S. and Lund, H.S. 2019. Urinary tract infection and subclinical bacteriuria in cats—a clinical update. J. Feline Med. Surg. 21, 1023–1038. Drobatz, K.J. and Hughes, D. 1997. Concentration of ionized calcium in plasma from cats with urethral obstruction. J. Am. Vet. Med. Assoc. 211, 1392–1395. Ellis, J., Bell, R., Barnes, D.C. and Miller, R. 2018. Prevalence and disease associations in feline thrombocytopenia: a retrospective study of 194 cases. J. Small Anim. Pract. 59, 531–538. Fascetti, A.J., Mauldin, G.E. and Mauldin, G.N. 1997. Correlation between serum creatine kinase activities and anorexia in cats. J. Vet. Intern. Med. 11, 9–13. Forrester, S.D. 2004. Diagnostic approach to hematuria in dogs and cats. Clin. North Am. Small Anim. Pract. 34, 849–66. Fröhlich, L., Hartmann, K., Sautter-Louis, C. and Dorsch, R. 2016. Postobstructive diuresis in cats with naturally occurring lower urinary tract obstruction: incidence, severity and association with laboratory parameters on admission. J. Feline Med. Surg. 18, 809–817. Gerber, B., Boretti, F.S., Kley, S., Laluha, P., Müller, C., Sieber, N., Unterer, S., Wenger, M., Flückiger, M., Glaus, T. and Reusch, C.E. 2005. Evaluation of clinical signs and causes of lower urinary tract disease in European cats. J. Small Anim. Pract. 46, 571–577. Gonzalez, A.L. and Waddell, L.S. 2016. Blood gas analysers. Top. Companion Anim. Med. 31, 27–34. Hagiwara, M.K. 2009. Anemia in cats: Is it Mycoplasma? In the Proceedings of the World Small Animal Veterinary Association Congress. São Paulo, Brazil. Kobayashi, M., Okada, Y., Ueno, H., Mizorogi, T., Ohara, K., Kawasumi, K., Suruga, K., Kadokura, K., Ohnishi, Y. and Arai, T. 2020. Effects of supplementation with anti-inflammatory compound extracted from herbs in healthy and obese cats. Vet. Med. (Auckland, N.Z.) 11, 39–44. Lamb, C.R., Cortellini, S. and Halfacree, Z. 2018. Ultrasonography in the 2602 diagnosis and management of cats with ureteral obstruction. J. Feline Med. Surg. 20, 15–22. Litster, A.L., Moss, S.M., Honnery, M., Rees, B. and Trott, D.J. 2007. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: recognition of Staphylococcus felis as a possible urinary tract pathogen. Vet. Microbiol. 121, 182–188. Litster, A., Moss, S., Platel, J. and Trott, D.J. 2009. Occult bacterial lower urinary tract infections in cats-urinalysis and culture findings. Vet. Microbiol. 136, 130–134. Muller, K.M., Burkitt-Creedon, J.M. and Epstein, S.E. 2022. Presentation variables associated with the development of severe postobstructive diuresis in male cats following relief of urethral obstruction. Front. Vet. Sci. 9, 783874. Neri, A.M., Machado, L.H.A., Okamoto, P.T.C.G., Filippi, M.G., Takahira, R.K., Melchert, A. and Lourenço, M.L.G. 2016. Routine screening examinations in attendance of cats with obstructive lower urinary tract disease. Top. Companion Anim. Med. 31, 140–145. Okada, Y., Kobayashi, M., Sawamura, M. and Arai, T. 2017. Comparison of visceral fat accumulation and metabolome markers among cats of varying BCS and novel classification of feline obesity and metabolic syndrome. Front. Vet. Sci. 14, 4–17. Passmore, C.A., Sherignton, J. and Stegemann, M.R. 2008. Efficacy and safety of cefovecin for the treatment of urinary tract infections in cats. J. Small Anim. Pract. 49, 295–301. Piyarungsri, K., Tangtrongsup, S., Thitaram, N., Lekklar, P. and Kittinuntasilp, A. 2020. Prevalence and risk factors for feline lower urinary tract disease in Chiang Mai, Thailand. Sci. Rep. 10, 196. Rieser, T.M. 2013. Arterial and venous blood gas analyses. Top. Companion Anim. Med. 28, 86–90. Sævik, B.K., Trangerud, C., Ottesen, N., Sørum, H. and Eggertsdóttir, A.V. 2011. Causes of lower urinary tract disease in Norwegian cats. J. Feline Med. Surg. 13, 410–417. Segev, G., Livne, H., Ranen, E. and Lavy, E. 2011. Urethral obstruction in cats: predisposing factors, clinical, clinicopathological characteristics and prognosis. J. Feline Med. Surg. 13, 101–108. Seo, S., Na, H., Choi, S., Choi, H., Lee, Y. and Lee, K. 2021. Ultrasonographic and clinical findings in cats with feline lower urinary tract disease. J. Vet. Clin. 38, 63–68. Tariq, A., Rafique, R., Abbas, S.Y., Khan, M.N., Huma, I., Perveen, S. and Kamran, M. 2014. Feline lower urinary tract (FLUTD) – an emerging problem of recent era. J. Vet. Sci. Anim. Husb. 2, 1–4. Teichmann-Knorrn, S., Reese, S., Wolf, G., Hartmann, K. and Dorsch, R. 2018. Prevalence of feline urinary tract pathogens and antimicrobial resistance over five years. Vet. Rec. 183, 21. Tion, M.T., Dvorska, J. and Saganuwan, S.A. 2015. A review on urolithiasis in dogs and cats. Bulg. J. Vet. Med. 18, 1–18. Weese, J.S., Blondeau, J., Boothe, D., Guardabassi, L.G., Gumley, N., Papich, M., Jessen, L.R., Lappin, M., Rankin, S., Westropp, J.L. and Sykes, J. 2019. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 247, 8–25. | ||
| How to Cite this Article |
| Pubmed Style Moraes RSD, Ribeiro D, Melchert A, García HDM, Regalin D, Filho RLA, Giuffrida R, Takahira RK, Okamoto AS, Guimarães-okamoto PTC. A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Vet. J.. 2024; 14(11): 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 Web Style Moraes RSD, Ribeiro D, Melchert A, García HDM, Regalin D, Filho RLA, Giuffrida R, Takahira RK, Okamoto AS, Guimarães-okamoto PTC. A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. https://www.openveterinaryjournal.com/?mno=214235 [Access: January 28, 2026]. doi:10.5455/OVJ.2024.v14.i11.19 AMA (American Medical Association) Style Moraes RSD, Ribeiro D, Melchert A, García HDM, Regalin D, Filho RLA, Giuffrida R, Takahira RK, Okamoto AS, Guimarães-okamoto PTC. A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Vet. J.. 2024; 14(11): 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 Vancouver/ICMJE Style Moraes RSD, Ribeiro D, Melchert A, García HDM, Regalin D, Filho RLA, Giuffrida R, Takahira RK, Okamoto AS, Guimarães-okamoto PTC. A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Vet. J.. (2024), [cited January 28, 2026]; 14(11): 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 Harvard Style Moraes, R. S. D., Ribeiro, . D., Melchert, . A., García, . H. D. M., Regalin, . D., Filho, . R. L. A., Giuffrida, . R., Takahira, . R. K., Okamoto, . A. S. & Guimarães-okamoto, . P. T. C. (2024) A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Vet. J., 14 (11), 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 Turabian Style Moraes, Reiner Silveira De, Diego Ribeiro, Alessandra Melchert, Henry David Mogollón García, Doughlas Regalin, Raphael Lucio Andreatti Filho, Rogério Giuffrida, Regina Kiomi Takahira, Adriano Sakai Okamoto, and Priscylla Tatiana Chalfun Guimarães-okamoto. 2024. A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Veterinary Journal, 14 (11), 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 Chicago Style Moraes, Reiner Silveira De, Diego Ribeiro, Alessandra Melchert, Henry David Mogollón García, Doughlas Regalin, Raphael Lucio Andreatti Filho, Rogério Giuffrida, Regina Kiomi Takahira, Adriano Sakai Okamoto, and Priscylla Tatiana Chalfun Guimarães-okamoto. "A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil." Open Veterinary Journal 14 (2024), 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 MLA (The Modern Language Association) Style Moraes, Reiner Silveira De, Diego Ribeiro, Alessandra Melchert, Henry David Mogollón García, Doughlas Regalin, Raphael Lucio Andreatti Filho, Rogério Giuffrida, Regina Kiomi Takahira, Adriano Sakai Okamoto, and Priscylla Tatiana Chalfun Guimarães-okamoto. "A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil." Open Veterinary Journal 14.11 (2024), 2901-2910. Print. doi:10.5455/OVJ.2024.v14.i11.19 APA (American Psychological Association) Style Moraes, R. S. D., Ribeiro, . D., Melchert, . A., García, . H. D. M., Regalin, . D., Filho, . R. L. A., Giuffrida, . R., Takahira, . R. K., Okamoto, . A. S. & Guimarães-okamoto, . P. T. C. (2024) A retrospective description of blood and urine alterations in 386 male cats with urethral obstruction in Botucatu, São Paulo, Brazil. Open Veterinary Journal, 14 (11), 2901-2910. doi:10.5455/OVJ.2024.v14.i11.19 |