| Short Communication | ||
Open Vet. J.. 2024; 14(11): 3100-3107 Open Veterinary Journal, (2024), Vol. 14(11): 3100-3107 Short Communication In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strainsIndah Amalia Amri1*, Purnaning Dhian Isnaeni2 and Jasni Sabri31Laboratory of Microbiology and Immunology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 2Faculty of Agriculture, Universitas Jember, Jember, Indonesia 3Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia *Corresponding Author: Indah Amalia Amri. Laboratory of Microbiology and Immunology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia. Email: indahamaliaamri [at] ub.ac.id Submitted: 20/08/2024 Accepted: 25/10/2024 Published: 30/10/2024 © 2024 Open Veterinary Journal
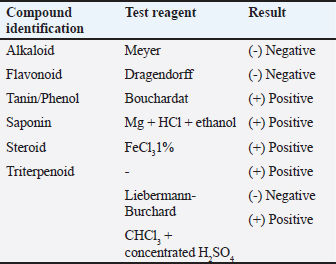
AbstractBackground: Eupatorium odoratum (EO) L (Siam weed) is a highly invasive species that contains various beneficial active compounds. This study was conducted to explore the antibacterial properties of EO ethanol extract against Gram-positive and Gram-negative bacteria. Aim: The aim of this study was to evaluate the antibacterial activity of EO ethanolic extract against various Gram-positive and Gram-negative bacteria to assess its potential as an antimicrobial agent. Methods: The study employed the agar well diffusion method to measure the antimicrobial effectiveness of the EO ethanolic extract. Additionally, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using the broth microdilution assay for different bacterial strains. Results: The results showed that the EO ethanol extract could not inhibit Escherichia coli and Klebsiella pneumoniae. It presented weak to moderate inhibition against Bacillus subtilis, Enterococcus faecalis, and Proteus vulgaris, and exhibited moderate to strong inhibition against Staphylococcus aureus. The MIC and MBC of the EO ethanolic extract against B. subtilis, S. aureus, E. faecalis, E. coli, P. vulgaris, and K. pneumoniae were 12.00 and 12.00 mg/ml; 5.00 and 7.50; 15.46 and 18.98 mg/ml; 75.00 and 87.50 mg/ml; 25.00 and 25.00; and 77.50 and 100.00 mg/ml. Conclusion: It was concluded that while EO ethanolic extract showed moderate to strong effectiveness against S. aureus, it was less effective against other Gram-positive bacteria and showed no activity against the tested Gram-negative bacteria. Therefore, the ethanolic extract of EO was considered to have potential as an antimicrobial agent, particularly against S. aureus. Keywords: Antimicrobial, Ethanolic extract, E. odoratum. IntroductionThe resurgence of infectious diseases since the mid-1970s, including new pathogens and re-emerging older diseases, has challenged the notion that infectious diseases are becoming less of a public health concern. Despite the advancements in healthcare, infectious diseases are still the leading cause of mortality, especially in tropical regions, where they account for nearly half of all deaths. Unfortunately, the lack of specific and effective therapies and the absence of vaccines often complicate the treatment of these diseases. The issue is further compounded by the increasing prevalence of bacterial infections, which substantially impact public health. This also signifies the impending threat to health, which is antimicrobial resistance. Antimicrobial resistance become a crucial health issue that needs to be resolved effectively. The recent rise in antibiotic resistance and the failure of traditional chemotherapy underscores the urgent need to discover new natural antibacterial agents to prevent disease spread and enhance treatment efficacy. The search for alternative antibacterial agents from natural sources has gained popularity. Plants, in their vast biological and chemical diversity, represent a significant resource for medicinal compounds. Traditional plant-based medicines have been instrumental in healthcare. With an estimated 250,000 to 500,000 species, plants have been the cornerstone of traditional medicine throughout human history. Their popularity as a source of medicinal treatments regained momentum in the late 1990s. Even today, in many developing countries, plant-based traditional medicines are vital for healthcare. The antimicrobial properties of plant extracts have attracted much interest due to their potential to treat bacterial infections. Plants are abundant in secondary metabolites such as tannins, alkaloids, terpenoids, and flavonoids which exhibited antimicrobial activities in vitro. These various bioactive compounds found in plants accentuate their potential as a source of innovative antimicrobial agents (Newman and Cragg, 2020). One of the plants widely known for its medicinal purposes is Siam weed [Eupatorium. odoratum (EO) L]. It is considered an invasive weed and is widely spread over almost all regions of Indonesia. In spite of its invasive characteristic, EO has been widely used in Indonesia as a herbal alternative medicine to treat diarrhoea, astringency, spasms, hypertension, inflammations, and topical wounds. Additionally, it has been used as a diuretic, antiprotozoal, hepatotropic agent, and anthelmintic (Andika et al., 2020). These therapeutic properties of EO are most likely due to its bioactive compounds. The chemical composition of Eupatorium species, including diterpenes, has shown a wide range of pharmacological activities, such as antibacterial, antifungal, and anti-inflammatory effects. Despite its widespread use, there is limited research on EO’s microbial activities. Given the impending antibiotic resistance issue, exploring the antibacterial activities of EO against Gram-positive and Gram-negative bacteria becomes crucial. The antimicrobial properties of plants, including EO hold eloquent therapeutic potential in infectious disease treatment. Moreover, the exploration of the antimicrobial potential of EO extract also aligns with sustainable healthcare practices (Ramesh and Subramani, 2018). Evaluating microbial activities of a plant extract can be done in several ways, however, an in vitro study must be the preliminary study to be done. Therefore, this research specifically investigates the potential of EO ethanol extract, a traditionally used medicinal plant, in combating Gram-positive and Gram-negative bacterial infections in vitro. Such studies are essential preliminary investigations to evaluate the efficacy of EO extract against broad-spectrum bacteria. Given the alarming rise in antibiotic resistance, exploring the antibacterial properties of EO is timely and crucial (James et al., 2018). This study aims to contribute to the ongoing search for new and effective antibacterial agents derived from natural sources, aligning with the urgent global need to address the challenges posed by infectious diseases. Further exploration of EO promises to uncover new therapeutic possibilities and contribute to global efforts to improve public health. Materials and MethodsPlant material and ethanol extractSiam weed (EO L) or Komba-komba (local Sulawesi language) leaves were collected between May and July 2023 from Kendari in Southeast Sulawesi. The leaves were sourced from wild plants and were verified by the Herbal Laboratory, Materia Medica Batu. After collection, the plant materials were air-dried and ground into a fine powder using a blender. To prepare the ethanol extracts, 30 g of powdered plant material underwent extraction twice, using 300 ml of 96% ethanol each time. This extraction process was conducted over 48 hours at 25°C–27°C. Next, the filtrates were evaporated using a rotary evaporator. The extracts were then reconstituted in 5% dimethyl sulfoxide (DMSO) to obtain a 100 mg/ml concentration and maintained as stock solutions for antibacterial activity tests, to achieve a concentration of 100 mg/ml, 100 mg of extract was required for each ml of 5% DMSO used (Sangnim et al., 2022). Additionally, the secondary metabolites in the extracts were identified through phytochemical testing and liquid chromatography-mass spectrometry (LC-MS) analysis (Yusuf and Fahriani, 2022). Bacterial strainsThis research used six types of bacteria, consisting of Gram-positive strains (Enterococcus faecalis, Bacillus subtilis, and Staphylococcus aureus) and Gram-negative strains (Klebsiella pneumoniae, Proteus vulgaris, and Escherichia coli). These bacterial isolates were obtained from the Laboratory of Microbiology at the Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia. The selection of these bacterial strains was integral to the study as they facilitated a comprehensive analysis of the antibacterial efficacy of the tested compounds. The bacterial strains were aerobically cultured in Mueller Hinton Broth (MHB) at 37°C for 24 hours for the antibacterial activity assessment. Next, they were suspended in sterile saline to achieve a 0.5 McFarland standard. The resulting bacterial suspensions, with a concentration of 108 CFU/ml, were used for the in vitro antibacterial activity tests, namely the agar well diffusion and microdilution methods (Teh et al., 2017). Determination of antimicrobial activity of EOThe antimicrobial activity of EO was assessed using agar well diffusion and microdilution methods. The agar well diffusion method was used for the initial antibacterial assay using Mueller Hinton Agar (MHA). The 0.5 McFarland standard inoculum of Gram-positive strains (E. faecalis, B. subtilis, and S. aureus) and Gram-negative strains (K. pneumoniae, P. vulgaris, and E. coli) were inoculated on separate MHA plates (Manandhar et al., 2019). The EO extract was then divided into several concentrations (100%, 80%, 60%, 40%, 20%, 10%, and 5%). Seven 5-mm-wide wells that were 3 cm apart were then made in each MHA plate using the yellow micropipette tip. Next, each well was filled with 0.02 ml of the EO extract. The plates were then incubated at 37°C for 24 hours. The inhibition zones were analyzed by measuring the clear area around the wells (Retnaningsih et al., 2019). Determination of minimum inhibitory (MIC) and bactericidal concentrations (MBC) of EOThe microdilution method was employed to determine the EO extract’s MIC and MBC (Kowalska-Krochmal and Dudek-Wicher, 2021; Amri et al., 2023). Two 200 µl dilution series of EO extract in MHB were prepared in 10 Eppendorf tubes. Each tube contained decreasing EO extract concentrations from 10%, 5%, 2.5%, 1.25%, 0.6%, 0.3%, 0.2%, 0.1%, 0.04%, and 0.02%. Each tube was then filled with 100 µl of 0.5 McFarland standard bacterial inoculum and 100 µl triphenyl-tetrazolium chloride (TTC) as the indicator (Rialita et al., 2015). The MIC and MBC of the EO extract were determined by consecutively examining the TTC’s color changes at 24 and 48 hours. Data analysisThis study used a factorial design with EO concentrations as the first variable and bacterial species as the second variable. Each treatment was replicated four times. The inhibition zone data obtained were then analyzed by variance analysis with the EO concentrations as treatments. This analysis was done to determine the smallest EO extract concentration that inhibits bacterial growth. The Tukey HSD test was used to determine the best concentration for inhibiting bacterial growth. The MIC and MBC data were analyzed qualitatively. Results and DiscussionThe LC-MS analysis of EO extractThe LC-MS analysis of the ethanolic extract of EO revealed diverse arrays of bioactive compounds, such as flavonoids, terpenoids, phenolics, and lipids. The most prevalent group was flavonoids, which are well-known for having anti-inflammatory and antioxidant characteristics. Among the notable flavonoids found are 4′,5,6,7-Tetramethoxyflavanone, Ponkanetin, Rubone, Olmelin, Ophiopogonone E, and Tetramethoxyluteolin, each recognized for their potential therapeutic benefits (Tsuchiya et al., 1996). These compounds not only contribute to the extract’s pharmacological activities but also improve its bioactivity via synergistic interactions. Additionally, substances that may enhance the extract’s bioactivity were found, such as Allysine, a derivative of an amino acid, and several other unidentified substances (Table 1). The qualitative analysis of EO extract also revealed the presence of various bioactive constituents including flavonoids, alkaloids, tannins/phenols, terpenoids, and saponins (Table 2). These secondary metabolites found in EO extract are well-documented for their varied medicinal uses such as antibacterial, anti-inflammatory, and wound-healing qualities (Wink, 2015). The presence of such a wide variety of bioactive compounds highlighted EO’s pharmacological significance in conventional medicine and its potential for modern drug discovery. Through the identification and characterization of these substances, researchers can investigate the synergistic interactions that may enhance the therapeutic outcomes and mechanism of action. Moreover, understanding the chemical composition of EO extract is crucial for creating pharmacological preparations and standardized formulations that could be clinically evaluated for treating infectious diseases caused by bacteria. Table 1. LC-MS analysis of an ethanol extract from the leaves of EO.
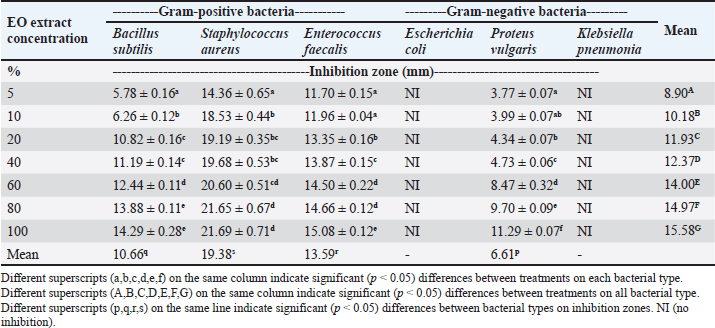
The ethanolic extract of EO had an abundant chemical profile and diverse pharmacological potential, as demonstrated by the LC-MS analysis. Its potential as a source of novel medicinal agents is further confirmed by the presence of flavonoids, terpenoids, phenolics, and other bioactive compounds. The antibacterial activity of EO extractThe results showed that the EO extract inhibited the bacterial growth of all three Gram-positive bacteria but was only effective against P. vulgaris. The diameter of each bacteria’s inhibition zone varied from 3.77 mm at the lowest concentration of 5% of the EO extract against P. vulgaris to 21.69 mm at 100% of the EO extract concentration against S. aureus (Table 3). Based on the inhibition zone categories determined by Lingga et al. (2015), the 5% concentration of EO extract did not inhibit the growth of E. coli and K. pneumonia; has weak inhibition against P. vulgaris; moderate inhibition against B. subtilis; and strong inhibition against S. aureus and E. faecalis. Table 2. Results of qualitative phytochemical analysis of EO extract.
The results revealed that as the concentration of the EO extract increased, the inhibition zone also increased significantly in diameter. Particularly, strong inhibition was observed at the 60% concentration of the EO extract against S. aureus. However, at the 10% concentration, the EO extract demonstrated medium inhibition against two types of Gram-positive bacteria: S. aureus and E. faecalis. The EO extract’s inhibitory activity was significant against all bacteria, although the highest inhibition was only moderate at 15.58 mm wide. Gram-positive bacteria were found to be more susceptible to the EO extract than Gram-negative bacteria. This result might be due to the complex structure of the outer cell membrane of Gram-negative bacteria. Moreover, Gram-negative bacteria possess phospholipid membranes containing lipopolysaccharide components that are impermeable to lipophilic solutions (Hanphakphoom and Krajangsang, 2016). This outer membrane, composed of lipopolysaccharides (LPS) and proteins, acts as a barrier restricting the entry of hydrophobic compounds, including antimicrobial agents, due to the hydrophilic layer of LPS (Nikaido, 2003). EO contains many active compounds that have been proven to have antimicrobial properties. Flavonoids, alkaloids, phenolic, terpenoid, and saponin were the active compounds of the EO extract used in this research. Other researchers have mentioned that EO also contains essential oils (Aziz et al., 2020), acacetin, luteolin, isosakuranetin, percinogenin, and chalcones (Suksamrarn et al., 2004). Flavonoids have been proven to have bactericidal effects and these active compounds are believed to inhibit the growth of B. subtilis, S. aureus, E. faecalis, and P. vulgaris in this study. Flavonoids may disrupt nucleic acid synthesis, cytoplasmic membrane function, and energy metabolism, as well as reduce adhesion, biofilm formation, and membrane permeability (Shamsudin et al., 2022). Moreover, Chromolaena, among other plants like Hypericum and Capella, had been reported to be rich in flavonoids and also exhibited antibacterial properties (Cushnie and Lamb, 2005). Table 3. The inhibition zones of EO extract against various kinds of bacteria.
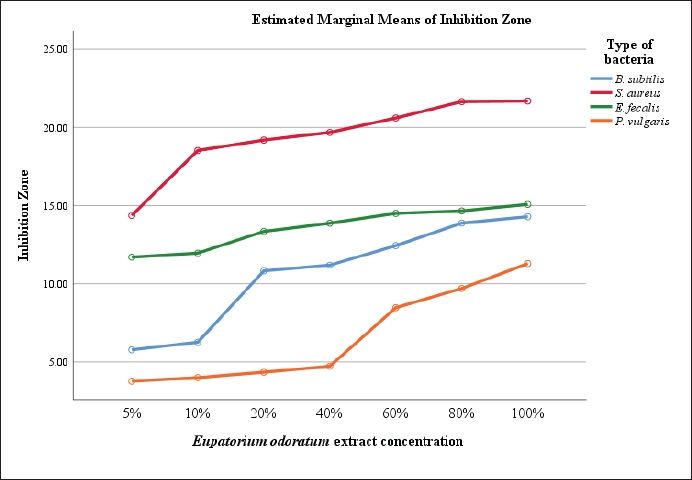
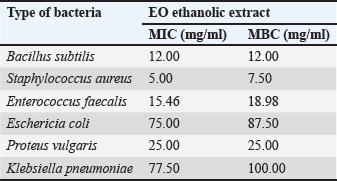
Phytochemical analysis discovered that the EO ethanolic extract also contains alkaloids, which have been linked to antimicrobial activity. Alkaloids’ antibacterial mechanism mainly includes the disruption of cell wall synthesis, cell membrane permeability, bacterial metabolism, nucleic acid synthesis, and protein synthesis in bacteria (Yan et al., 2021). Alkaloids can also inhibit the folic acid enzyme, which is crucial in RNA’s purine and pyrimidine production and DNA biosynthesis (Othman et al., 2019). Alkaloids, widely found in plant extracts, are one of the many secondary metabolites that plants produce to protect themselves from herbivores (by deterring them from eating the plants) and from bacterial and fungal infestation (Adamski et al., 2020). The antimicrobial properties of phenolic acids are dependent on the substitution of the core benzene ring, especially the chain length, number, and position of the substitution (Cueva et al., 2010). Phenolic acids can also acidify the bacterial cytoplasm, which leads to bacterial cell death (Kumar and Goel, 2019). Moreover, as phenolics are partly lipophilic, their ability to acidify the cytoplasm by passing through the bacterial cell membrane differs depending on the number of hydroxyl, methoxy, and carboxyl functional groups, and the saturation of the alkyl side chain (Eceveit et al., 2022). Additionally, because different molecules had diverse structures and chemical compositions, they exhibited a range of antimicrobial effects. These effects included permeabilizing and destabilizing the bacterial plasma membrane or inhibiting the extracellular enzyme activity of bacteria (Takó et al., 2020). Terpenes and their derivatives are secondary metabolites commonly found in essential oils and have been proven to possess antimicrobial activities against both susceptible and resistant pathogens (Mahizan et al., 2019). Essential oils, containing various terpenes, have been reported to exhibit significant antimicrobial properties by interfering with multiple microbial survival processes. Previous research also reported that terpenoids from 70% EO ethanolic extract concentrations developed inhibition zones between 14 and 27 mm against S. aureus (Rofida and Nurwahdaniati, 2015). Terpenoids can inhibit bacteria due to their ability to interfere with crucial microbial survival processes, including oxygen uptake, oxidative phosphorylation (Mahizan et al., 2019), cell wall synthesis, energy metabolism, and cytoplasmic membrane function (Barbieri et al., 2017). Saponins were able to impair the permeability of the bacterial outer membrane. Around 90% of the surface of the cell walls of Gram-negative bacteria, which do not contain natural cholesterol, were covered with LPS. Saponins interacted with the lipid part of LPS and formed complex compounds, thereby increasing the permeability of the bacterial cell wall and leading to the destruction of the bacterial cell (Cankaya and Somuncuoglu, 2021). The S. aureus was observed as the most susceptible bacteria to the EO extract based on the moderate inhibition zone of 14.36 mm at the 5% concentration. This inhibition gradually increased to a strong inhibition zone of 21.69 mm at the 100% concentration of the EO extract (Fig. 1). The least susceptible bacterium to EO extract was P. vulgaris, a Gram-negative bacterium which only showed a weak to moderate inhibition zone at an average of 6.61 mm. Although, the EO extract showed only weak inhibitory ability at 5% to 40% concentration against P. vulgaris, but at the 60% concentration, the inhibition zone significantly increased and continued until the 100% concentration. Although the EO extract concentration required to inhibit bacterial growth differed for each bacterium, the results showed that the inhibitory zones grew as the concentration increased. This finding implies that the EO ethanolic extract possesses antimicrobial activities at relatively higher concentrations. Additionally, the variability of the results obtained among the different bacterium types showed that each bacteria has different defense mechanisms against the antimicrobial compounds of EO. MIC and MBC of EO extractThe microdilution assay is essential for determining a compound’s MIC and MBC. The MIC is described as the lowest concentration (mg per ml solution) of an antimicrobial agent required to inhibit visible bacterial growth in vitro, while the MBC represents the lowest concentration that results in bacterial cell death. Meanwhile, the microdilution broth assay allows for the quantitative investigation of the bacteriostatic and bactericidal activity of the EO extract against specific bacteria (Hossain et al., 2022). The results of the MIC and MBC The MIC values in Table 4 indicate that at those concentrations, the extract can inhibit bacterial growth, but it did not inhibit the growth of all tested bacterial cells. The different MIC values signify the different susceptibilities of bacterial types against the EO extract. The lowest MIC value shown by EO ethanolic extract was 5 mg/ml against S. aureus, and the highest MIC value was 77.5 mg/ml against K. pneumoniae. These results suggest that the EO extract can start to inhibit bacterial growth at a 5 mg/ml concentration, depending on the type of bacteria. S. aureus is the most susceptible bacterium, followed by B. subtilis, E. faecalis, P. vulgaris, and E. coli. The least susceptible bacterium to the EO extract is K. pneumoniae.
Fig. 1. The inhibition zone of various concentrations of EO extract on different bacteria. Table 4. The MIC and MBC of EO extract against various Gram-positive and Gram-negative bacteria.
Typically, the MBC value is higher than the MIC. However, if the MIC and MBC values are identical, the antimicrobial effect of a compound is high against that specific bacterium. The EO ethanolic extract demonstrated varying MIC and MBC values for each type of bacteria. The lowest MBC value of the EO extract in this research is 7.5 mg/ml against S. aureus. Meanwhile, the highest MBC value is 100 mg/ml against K. pneumoniae. Specific bacteria have different susceptibilities against certain compounds due to their different defense mechanisms. Thus, an antimicrobial agent might be effective against certain bacteria while not affecting other types of bacteria. Some bacterial defense mechanisms include biofilm production, cell wall, cell membrane, encased efflux pumps, and the alteration of intracellular materials and gene regulation (Zhou et al., 2015). Overall, the MIC and MBC values obtained in this research imply that at 100 mg/ml concentration, the EO ethanolic extract can kill Gram-positive and Gram-negative bacteria. The importance of determining MIC and MBC values has been emphasized in the development of novel microbial agents, particularly due to the rising of antibiotic resistance. Microdilution assays remained a cornerstone in antimicrobial research, offering reliable data that is important for assessing the efficacy of new bioactive compounds (Wiegand et al., 2008). The resurgence of natural products in drug discovery, particularly new antimicrobial agents, was compromised largely by plant extracts (Newman and Cragg, 2020). The potential of EO extract as an antimicrobial agent, as demonstrated by the MIC and MBC values, aligns with these findings. ConclusionThe ethanolic extract of EO demonstrated significant antibacterial activity, particularly against Gram-positive bacteria. Its pharmacological potential is supported by the presence of varied bioactive compounds, including alkaloids, phenolics, terpenoids, and flavonoids. The extract’s effectiveness in inhibiting bacterial growth is confirmed by microdilution assay findings, which show strong antibacterial qualities with an MBC value of 7.5 mg/ml and the lowest MIC value of 5 mg/ml against S. aureus. This study validates EO as a viable option for developing new antimicrobial agents. AcknowledgmentsThe authors would like to express their gratitude to Brawijaya University for funding this research through the DPP SPP 2023 Faculty of Veterinary Medicine and to the research team for their invaluable contributions. Conflict of interestThe authors declare no conflict of interest. Authors’ contributionsConceptualization, I.A.A.; methodology, I.A.A.; formal analysis, I.A.A. and P.D.I.; writing—original draft preparation, I.A.A.; writing—review and editing, I.A.A. and P.D.I. All authors have read and agreed to the published version of the manuscript. ReferencesAdamski, Z., Blythe, L.L., Milella, L. and Bufo, S.A. 2020. Biological activities of alkaloids: from toxicology to pharmacology. Toxins 12(4), 210. Amri, I.A., Ramadani, N.F., Hamidah, F., Dameanti, F.N.A.E.P. and Adrenalin, S.L. 2023. Potential antibacterial effects of ethanol extract and essential oil of Origanum vulgare on Klebsiella pneumonia and Staphylococcus aureus. World Vet. J. 13(4), 486–491. Andika, B., Halimatussakdiah, H. and Amna, U. 2020. Qualitative analysis of secondary metabolite compounds in siam weed (Chromolaena odorata L.) leaf extracts from Langsa City, Aceh. Quimica: Jurnal Ilmiah Sains dan Terapan. 2(2), 1–6. Aziz, N.A., Mohamad, M., Mohsin, H.F., Hazalin, N.A.M.N. and Hamid, K.A. 2020. The pharmacological properties and medicinal potential of Chromolaena odorata: a review. Int. J. Pharm. Nutraceut. Cosmet. Sci. 2, 30–41. Barbieri, R., Coppo, E., Marchese, A., Daglia, M., Sobarzo-Sánchez, E., Nabavi, S.F. and Nabavi, S.M. 2017. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol. Res. 196, 44–68. Cankaya, I.I. and Somuncuoglu, E.I. 2021. Potential and prophylactic use of plants containing saponin-type compounds as antibiofilm agents against respiratory tract infections. Evid. Based Complement. Alternat. Med. 2021, 6814215. Cueva, C., Moreno-Arribas, M.V., Martín-Álvarez, P.J., Bills, G., Vicente, M.F., Basilio, A., Rivas, C.L., Requena, T., Rodríguez, J.M. and Bartolomé, B. 2010. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 161(5), 372–382. Cushnie, T.P. and Lamb, A.J. 2005. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 26(5), 343–356. Ecevit, K., Barros, A.A., Silva, J.M. and Reis, R.L. 2022. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2(4), 460–498. Hanphakphoom, S. and Krajangsang, S. 2016. Antimicrobial activity of Chromolaena odorata extracts against bacterial human skin infections. Modern Appl. Sci. 10(2), 159–171. Hossain, M.L., Lim, L.Y., Hammer, K., Hettiarachchi, D. and Locher, C. 2022. A review of commonly used methodologies for assessing the antibacterial activity of honey and honey products. Antibiotics 11, 975. James, J.M., Geethumol, C.U., Anilkumar, A.S. and Selvaraj, G. 2018. Phytochemical screening, antibacterial and allelopathic effects of few invasive plants of kerala. Plant Sci. Today. 5(4), 175–181. Kowalska-Krochmal, B. and Dudek-Wicher, R. 2021. The minimum inhibitory concentration of antibiotics: methods, interpretation, clinical relevance. Pathogens 10(2), 165. Kumar, N. and Goel, N. 2019. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst). 20, 24. Lingga, A.R., Usman, P. and Evy, E.R. 2015. Antibacterial test of Torch ginger (Nicolaia speciosa Horan) stem extract against Staphylococcus aureus and Escherichia coli. JOM Faperta. 2(2), 1–15. Mahizan, N.A., Yang, S.K., Moo, C.L., Song, A.A., Chong, C.M., Chong, C.W., Abushelaibi, A., Lim, S.E. and Lai, K.S. 2019. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 24(14), 2631. Manandhar, S., Luitel, S. and Dahal, R.K. 2019. In vitro antimicrobial activity of some medicinal plants against human pathogenic Bacteria. J. Trop. Med. 2019, 1895340. Newman, D.J. and Cragg, G.M. 2020. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. 2020. J. Nat. Prod. 83(3), 770–803. Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67(4), 593–656. Othman, L., Sleiman, A. and Abdel-Massih, R.M. 2019. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. (10), 911. Ramesh, P. and Subramani, A. 2018. Effect of antimicrobial activity of Eupatorium odoratum against clinical microbes. Int. J. Sci. Res. Biol. Sci. 5(5), 30–35. Retnaningsih, A., Primadiamanti, A. and Marisa, I. 2019. Inhibitory effect of ethanol extract of papaya seeds against Escherichia coli and Shigella dysenteriae using well diffusion method. J. Analis. Farmasi. 4(2), 122–129. Rialita, T., Rahayu, W.P., Nuraida, L. and Nurtama, B. 2015. Antimicrobial activity of Red ginger (Zingiber officinale Var. Rubrum) and Red galangal (Alpinia purpurata K. Schum) essential oils against pathogenic and food-spoiling bacteria. Agritech 35(1), 43–52. Rofida, S. and Nurwahdaniati, N. 2015. Antibacterial activity of Chromolaena odorata (L) King leaves with bioautography. Pharmacy 12(1), 29–36. Sangnim, T., Meeboon, P., Phongsewalak, P., Prasongdee, P., Sriamornsak, P., Singh, I. and Huanbutta, K. 2022. Development and evaluation of liquid plaster loaded with Chromolaena odorata leaf extract endowed with several beneficial properties to wound healing. Gels 8(2), 72. Shamsudin, N.F., Ahmed, Q.U., Mahmood, S., Ali Shah, S.A., Khatib, A., Mukhtar, S., Alsharif, M.A., Parveen, H. and Zakaria, Z.A. 2022. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules 27(4), 1149. Suksamrarn, A., Chotipong, A., Suavansri, T., Boongird, S., Timsuksai, P., Vimuttipong, S. and Chuaynugul, A. 2004. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res. 27(5), 507–511. Takó, M., Kerekes, E.B., Zambrano, C., Kotogán, A., Papp, T., Krisch, J. and Vágvölgyi, C. 2020. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants (Basel) 9(2), 165. Teh, C.H., Nazni, W.A., Nurulhusna, A.H., Lee, H.L. and Nishibuchi, M. 2017. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 17, 36. Tsuchiya, H., Sato, M., Miyazaki, T., Fujiwara, S., Tanigaki, S., Ohyama, M., Tanaka, T. and Iinuma, M. 1996. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 50(1), 27–34. Wiegand, I., Hilpert, K. and Hancock, R.E. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3(2), 163–175. Wink, M. 2015. Modes of action of herbal medicines and plant secondary metabolites. Medicines (Basel) 2(3), 251–286. Yan, Y., Li, X., Zhang, C., Lv, L., Gao, B. and Li, M. 2021. Research progress on antibacterial activities and mechanisms of natural alkaloids: a review. Antibiotics 10, 318. Yusuf, H. and Fahriani, M. 2022. Anticancer activity and apoptotic induction of Chromolaena odorata Linn leaves extract and fractions on hepatocellular carcinoma cell lines (hepg2). J. Natural. 22(1), 57–67. Zhou, G., Shi, Q.S., Huang, X.M. and Xie, X.B. 2015. The three bacterial lines of defense against antimicrobial agents. Int. J. Mol. Sci. 16(9), 21711–21733. | ||
| How to Cite this Article |
| Pubmed Style Amri IA, Isnaeni PD, Sabri J. In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Vet. J.. 2024; 14(11): 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 Web Style Amri IA, Isnaeni PD, Sabri J. In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . https://www.openveterinaryjournal.com/?mno=215037 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.39 AMA (American Medical Association) Style Amri IA, Isnaeni PD, Sabri J. In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Vet. J.. 2024; 14(11): 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 Vancouver/ICMJE Style Amri IA, Isnaeni PD, Sabri J. In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 Harvard Style Amri, I. A., Isnaeni, . P. D. & Sabri, . J. (2024) In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Vet. J., 14 (11), 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 Turabian Style Amri, Indah Amalia, Purnaning Dhian Isnaeni, and Jasni Sabri. 2024. In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Veterinary Journal, 14 (11), 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 Chicago Style Amri, Indah Amalia, Purnaning Dhian Isnaeni, and Jasni Sabri. "In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains ." Open Veterinary Journal 14 (2024), 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 MLA (The Modern Language Association) Style Amri, Indah Amalia, Purnaning Dhian Isnaeni, and Jasni Sabri. "In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains ." Open Veterinary Journal 14.11 (2024), 3100-3107. Print. doi:10.5455/OVJ.2024.v14.i11.39 APA (American Psychological Association) Style Amri, I. A., Isnaeni, . P. D. & Sabri, . J. (2024) In vitro evaluation of the antimicrobial efficacy of Eupatorium odoratum ethanol extract against Gram-positive and Gram-negative bacterial strains . Open Veterinary Journal, 14 (11), 3100-3107. doi:10.5455/OVJ.2024.v14.i11.39 |