| Research Article | ||
Open Vet. J.. 2024; 14(11): 2883-2892 Open Veterinary Journal, (2024), Vol. 14(11): 2883-2892 Research Article Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrheaSalma Bessalah1, Asim Faraz2, Ayman Balla Mustafa3*, Syeda Maryam Hussain4, Shamsaldeen Ibrahim Saeed5, Chanda Liaqat6, Waqas Ashraf7, Zeeshan Muhammad Iqbal8, Muhammad Arslan Akbar9 and Mohamed Hammadi11Livestock and Wildlife Laboratory, Arid Lands Institute (I.R.A), University of Gabès, Médenine, Tunisia 2Department of Livestock and Poultry Production, Bahauddin Zakariya University, Multan, Pakistan 3Therapeutic Nutrition Department, Faculty of Health Sciences, Misurata University, Misurata, Libya 4Department of Livestock Production and Management, Pir Mehr Ali Shah-Arid Agriculture University, Rawalpindi, Pakistan 5College of Veterinary Medicine, University of Juba, Juba, South Sudan 6Department of Epidemiology and Public Health, University of Veterinary and Animal Sciences, Lahore, Pakistan 7Department of Microbiology, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan 8Department of Livestock Management, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan 9Department of Breeding and Genetics, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan *Corresponding Author: Ayman Balla Mustafa. Therapeutic Nutrition Department, Faculty of Health Sciences, Misurata University, Misurata, Libya. Email: a.yassien [at] nurs.misuratau.edu.ly Submitted: 11/08/2024 Accepted: 26/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
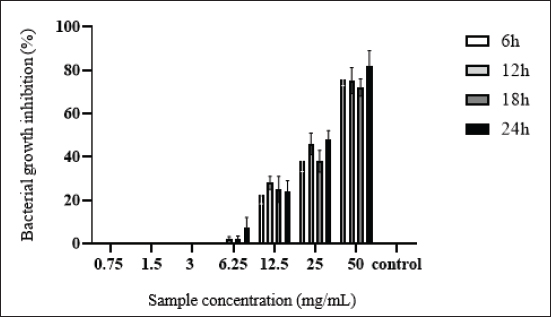
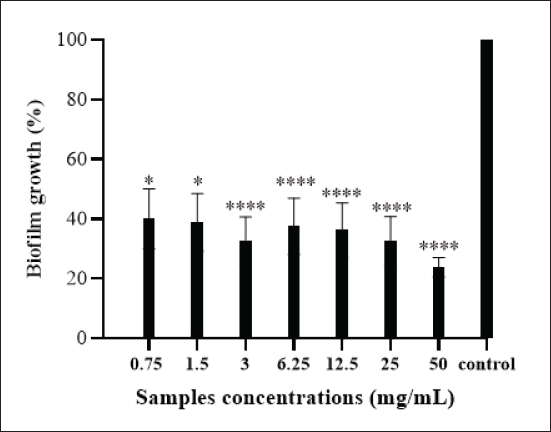
AbstractBackground: Many protective proteins, including lactoferrin and heavy chain antibodies, are present in camel colostrum, giving it a distinctive composition. Beyond a broad spectrum of pathogens, these proteins demonstrate antibacterial properties. Aim: The current research assessed the prophylactic properties of camel colostrum against Escherichia coli F17. Methods: A microbroth dilution method was employed to assess the efficacy of camel colostrum, whereas a crystal violet assay was utilized to determine its antibiofilm potential. Extracellular deoxyribonuclease acid (eDNA) release, swarming, and swimming motilities were also examined. Results: Showed that camel colostrum significantly reduced E. coli-F17 growth by 70% and above at different incubation periods (6–24 hours). The rate of cell attachment gradually decreased from approximately 40% to 24% as the concentration increased from 12.5 to 50 mg/ml. E. coli-F17 developed a biofilm at a rate of 54.8% when exposed to 50 mg/ml of camel colostrum. In contrast, the greatest level of biofilm formation against the tested bacteria (94%) was observed at a concentration of 1.5 mg/ml. A halo zone of camel colostrum ranging from 10 to less than 30 mm at concentrations between 6 and 50 mg/ml also inhibited swimming and swarming capabilities. The treated cells yielded no eDNA. Conclusion: According to these results, camel colostrum inhibits the growth of E. coli-F17 by impeding the swarming and swimming motilities, and biofilm formation. Additionally, camel colostrum incubation with E. coli-F17 diminishes eDNA. To evaluate the potential protective effects of camel colostrum in an animal model, additional research is recommended. Keywords: Biofilm, Camel colostrum, Calf diarrhea, Diarrheagenic bacterial pathogen, eDNA. IntroductionA significant challenge to camel productivity lies in the elevated morbidity and mortality rates of young animals during their initial stages of life, resulting in diminished growth rates and impaired herd performance (Marce et al., 2010). Numerous studies have reported camel calf mortality rates ranging between 30% and 50% (Allan et al., 2023). Infectious diarrhea constitutes a major factor contributing to calf mortality and poor weight gain, thereby impacting herd performance (Lanz Uhde et al., 2008). Despite its significant impact, this disease has received relatively limited attention in comparison to similar conditions in other mammalian species. Effective management of camel-calf diarrhea necessitates a multifaceted approach, encompassing hygiene and sanitation measures, vaccination strategies, nutritional interventions, and targeted antimicrobial therapy when indicated. An evidence-based discussion of these management strategies is vital for reducing the disease burden in camelid populations. Previous research has indicated that diarrhea is frequently linked to the failure of colostrum transfer, resulting in morbidity and mortality (Salhi et al., 2015). Conversely, prior research has identified Escherichia coli strains that express F17 fimbrial adhesins as a pivotal pathogen linked to neonatal diarrhea in various livestock species, including camels (Bessalah et al., 2016; Yeshiwas et al., 2017; Siuce et al., 2020). The efficacy of camel colostrum transfer in preventing and treating neonatal diarrhea has been investigated in a number of studies (Isako et al., 2020). The results indicate that this naturally occurring substance may have a substantial impact on combating this illness. The newborn’s ability to thrive and survive hinges on the amount and quality of colostrum intake, especially its immunoglobulin content. The degree of protection and survival of the newborn is affected by the quantity of colostrum received and the quality of the immunoglobulin content (Tizard., 2001). The antibacterial effect of camel colostrum has been studied in numerous studies (Jrad et al., 2015a,b; Al-Samhary., 2018). However, its antibacterial capacity against E. coli expressing F17 fimbriae, associated with camel calf diarrhea, has not been studied. By addressing the knowledge gaps in camel-calf diarrhea management, this study endeavors to elucidate the mechanisms by which camel colostrum exerts its effects against E. coli expressing F17 fimbrial adhesin in order to enhance the development of effective prevention and control measures. Materials and MethodsCamel colostrumThe camel colostrum utilized in this study was sourced from three healthy camels who were chosen randomly (average age, 6 years) from the LEFS laboratory at the Arid Land Institute in Médenine-Tunisia. The three samples were manually gathered from each of the udders within 24 hours of parturition. Subsequently, they were pooled, freeze-dried, and stored until analysis. Bacterial strains and culture conditionsEscherichia coli-F17 (E. coli-F17) f17/afa/EastI/papC/iroN/iss/iucD, serogroup O64, a field strain initially acquired from camel-calf suffering from diarrhea, was the bacterium utilized in this investigation (Bessalah et al., 2016). Isolate was maintained at −20°C in tryptic soy broth enriched with 0.6% yeast extract and 20% (v/v) glycerol for subsequent procedures. Antibacterial activity of camel colostrum against E. coli-F17 strainThe bacterial inhibition effect of camel colostrum at different concentrations (0.75, 1.5, 3, 6.25, 12.5, 25, and 50 mg/ml) against the E. coli-F17 strain was assessed by the broth microdilution method (Wang et al., 2012). The experimental medium employed was Luria-Bertani (LB) broth, with a bacterial density set at 5 × 105 colony-forming units/ml. 100 μl of bacterial suspension was aseptically transferred into the wells of 96-well assay plates, each containing camel colostrum at varying final concentrations. The wells containing LB medium alone and LB medium with bacterial inoculum served as the negative and positive controls, respectively. After inoculation, the plates were incubated at 37°C for different time intervals (6, 12, 18, and 24 hours). Using a spectrophotometer at 600 nm to measure optical density (O.D) for each well. The bacterial inhibition percentage was calculated using the following formula: Bacterial inhibition (%)=Ic-Is/Ic *100 where IC is the control’s absorbance and IS is the sample’s absorbance (for different concentrations and different time intervals). Antibiofilm activity of camel colostrumTo assess the impact of camel colostrum at different phases of biofilm development, we adapted the methodology outlined by Carrano et al. (2019) with certain adjustments. Primary attachmentTo assess the effect of camel colostrum on the initial adherence of the E. coli-F17 strain, of 1 overnight bacterial growth (with an O.D 1 at 600 nm) was mixed with camel colostrum samples at different concentrations (0.75, 1.5, 3, 6.25, 12.5, 25, and 50 mg/ml). Following incubation at 4°C for 2 hours, the next 200 µl of each mixture was dispensed into the wells of 96-well microtiter plates and incubated for an additional hour at 37°C without agitation. Biofilm formationTo explore the effect of camel colostrum on biofilm formation, after 24 hours, the cell cultures of E. coli-F17 (attenuated to an O.D 0.005 at 600 nm) were combined with varying concentrations of camel colostrum (0.75% to 50% mg/ml). At 4°C, the mixtures were incubated for 2 hours. Then, 200 µl of a mixture containing was dispensed into each well of the polystyrene microtiter plates. The plates were subsequently incubated at 37°C overnight without agitation. Biofilm maturationTo assess the impact of camel colostrum on the maturation of E. coli-F17 biofilms, after 24 hours, bacterial growth (with an O.D 0.01 at 600 nm) were dispensed into sterile 96-well microplates, then incubating for 24 hours. The LB medium was refreshed with a new medium containing camel colostrum in different concentrations; furthermore, the plates were incubated at 37°C for 24 hours without shaking. Biofilm quantificationThe biofilm quantification was performed as described by O’Toole (2011) with minor modifications. The medium of the incubated plates was removed, and the wells were subjected to three times rinses with sterile phosphate-buffered saline to eliminate any suspended bacteria. Afterward, the plates were stained for 5 minutes with 200 μl of 0.1% crystal violet per well. Following PBS washing, the adherent cells were re-solubilized by adding 200 μl of ethanol per well. The released stain’s quantity was quantified by assessing the absorbance at 570 nm. The formula used to calculate the percentage of biofilm formation in E. coli-F17 is as follows: biofilm formation rate (%)=(OD sample / OD control) ×100 Negative control (LB medium alone) and positive control (bacteria suspension alone) were included in the experiment. Swarming and swimming motility assaysThe swimming and swarming motility assays were conducted following established protocols (Lee et al., 2013). In the swimming assay, 5 μl of overnight E. coli-F17 culture with an OD of 0.4 at 600 nm were introduced into the center of the swimming agar medium with varying concentrations of camel colostrum. This medium comprised 1% tryptone, 0.5% NaCl, and 0.3% agar. For swarming assays, 5 μl of E. coli-F17 culture (0.4 OD at 600 nm) were inoculated at the center of the swarming agar medium. The medium comprised 1% peptone, 0.5% NaCl, 0.5% agar, and 0.5% of filter-sterilized d-glucose; with increasing concentrations of camel colostrum. The plates were then incubated at 37°C for 18 hours. After 16 hours of incubation, the decrease in motility was assessed by measuring the swim and swarm zones of the bacteria. Results are expressed using the following formula: Swimming inhibition (%)=(1 - Hs/Hc) ×100, where Hs: the halo obtained for plates containing camel colostrum while the Hc: the halo obtained in plates without camel colostrum. Extracellular DNA (eDNA) detectioneDNA extraction eDNA extraction of E. coli-F17 was performed by salting-out procedure (Miller et al., 1988). Concisely, overnight E. coli-F17 cultures were diluted in fresh LB medium at (1:200), then, co-incubated with camel colostrum at varying concentrations. The samples were incubated at 37°C for 24 hours with agitation. Cell-free supernatants were obtained by passing through 0.22-mm-pore-size filters (Millipore, Bedford, MA, United States) after centrifugation at 12,000 rpm for 10 minutes to remove bacterial cells. The eDNA from the supernatants was isolated using an equal volume of NaCl (4.5 M) and mixed thoroughly by inversion until homogenization. After that, 250 µl of chloroform was added to the mixture, which was then shaken for 10 minutes. Following a 10-minute centrifugation at 12,000 rpm, the aqueous upper phase of the samples was transferred into fresh containers containing an equivalent volume of isopropanol to facilitate the precipitation of the DNA. The solution was mixed by inversion and immediately centrifuged for 10 minutes at 12,000 rpm. The eDNA precipitate underwent a washing step with 70% ethanol, followed by air-drying, before being resuspended in TE buffer (10 mM Tris-Cl, 1 mM EDTA, pH 8.0). Agarose gel electrophoresis Different samples were electrophoresed on a 1% agarose gel by running 20 μl of each sample. The gel was visualized under a gel-doc (Gel DocTM EZ System Bio-Rad). Statistical analysis The experimental data underwent analysis using an ANOVA test, where a p-value of less than 0.05 was deemed statistically significant. The complete array of experiments was conducted in triplicate, and the data were presented as the mean ± SD of triplicate measurements. Ethical approval Not needed for this study. ResultsAntibacterial activitiesThe antibacterial efficacy of camel colostrum against E. coli-F17 is depicted in Figure 1. Various concentrations of camel colostrum (ranging from 0.75 to 50 mg/ml) were employed to elucidate the growth pattern of E. coli-F17 when exposed to camel colostrum. At a higher concentration (50 mg/ml), camel colostrum significantly reduces E. coli-F17 growth (78%), after 6 hours of incubation (p < 0.001). At the same concentration, over 80% of E. coli-F17 growth was inhibited after 24 hours. However, concentrations below 12.5 mg/ml showed lower inhibitory effects (24.1%) at the same time periods, as shown in Figure 1. Camel colostrum exhibits no impact on the bacterial strain’s growth at concentrations of 3mg/ml or lower. Effects of camel colostrum on initial biofilm formationThe activities of camel colostrum to prevent initial E. coli-F17 attachment are presented in Figure 2. Results clearly demonstrated that camel colostrum at 50 mg/ml has relatively better anti-adhesion properties when compared to other concentrations as only 28% of biofilm growth was enregistred ( p < 0.0001). When the concentration of camel colostrum was decreased to 1.5 mg/ml or below, the proportion of biofilm development was increased to approximately 40%. This observation demonstrates statistical significance (p < 0.05) when compared to the control (untreated bacteria).
Fig. 1. Escherichia coli-F17 growth in the presence of various concentrations of camel colostrum at different concentrations using a microbroth dilution assay. Results are presented as the mean of three replicates. Untreated bacteria was used as control.
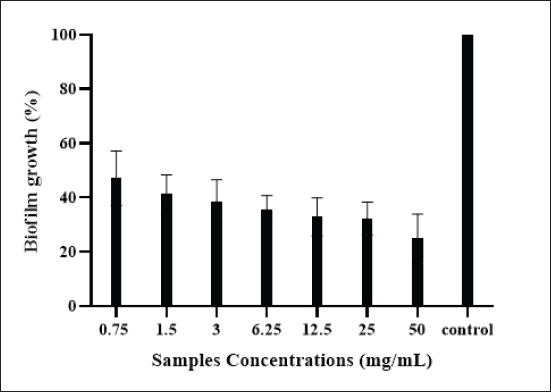
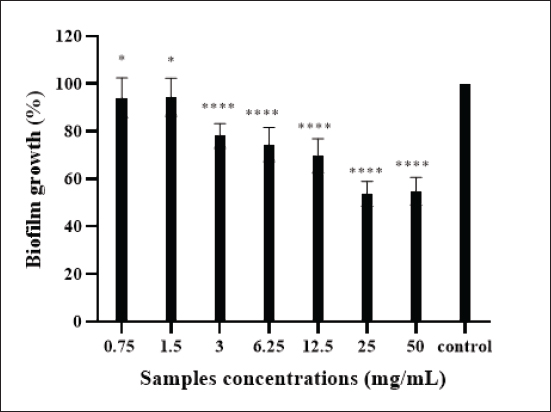
Fig. 2. Effect of camel colostrum at different concentrations on primary attachment of E. coli-F17 to polystyrene surfaces. Untreated E. coli-F17 cells were used as the control. Multiple comparisons were performed using ANOVA (p & 0.05), followed by Tukey’s Test. Significant differences between the tested concentrations and the control are indicated by various asterisks (*), where * denotes p & 0.05 and **** denotes p & 0. 0001. Effects of camel colostrum on biofilm formationBiofilm formation in E. coli-F17 was assessed after exposure to varying concentrations of camel colostrum and is represented in Figure 3. Camel colostrum reduced E. coli-F17 biofilm biomass at 24 hours post-development with the most significant result being obtained at 50 mg/ml. Compared with the control, at 50 mg/ml of camel colostrum, the biofilm reduced significantly to 24.8%. However, in the presence of camel colostrum at 6, 12.5, and 25 mg/ml, the biofilm formation rates in the E. coli-F17 ranged from 32.5% to 35.7%, without a significant difference. Effects of camel colostrum on mature biofilmsThe impact of camel colostrum on mature biofilms of E. coli-F17 was also evaluated and results are depicted in Figure 4. Exposure to camel colostrum at different concentrations ( p < 0.0001), except 0.75 and 1.5 mg/ml, significantly reduced biofilm growth. The biofilm growth rate decreased in a concentration-dependent manner, reaching its lowest level at the highest test concentration. Specifically, at 50 mg/ml of camel colostrum, the biofilm formation level of E. coli-F17 was 54.8%. ( p < 0.0001). Whereas, at a concentration of 1.5 mg/ml, camel colostrum shows the highest biofilm growth in the tested bacteria (94%). Swimming and swarming motility inhibition on E. coli-F17The impacts of camel colostrum on E. coli-F17 swimming and swarming motilities are illustrated in Figure 5. Under controlled conditions (bacteria alone, without camel colostrum), the tested bacteria demonstrated proficient swarming and swimming motilities (>30 mm diameter at 24 hours). The diameter of the halo zone was significantly reduced with camel colostrum concentrations of 6, 12.5, 25, and 50 mg/ml compared to the control ( p < 0.0001). While at 3 mg/ml or less, no inhibition was observed in both models. Effects of camel colostrum on the production of eDNAThe effects of the camel colostrum on eDNA production by E. coli-F17 biofilms were determined, and the result is shown in Figure 6. The untreated bacteria (control) produced significantly higher concentrations of eDNA. However, treating cells with camel colostrum at different concentrations showed no eDNA content of biofilms. DiscussionDiarrhea in young camel calves is a multifaceted condition influenced by a variety of factors such as environmental conditions, management practices, and nutritional elements, along with the involvement of infectious agents (Awoke et al., 2015). Bacteria were cited among the major causes of infectious diarrhea in young calves (less than 3 months), especially E. coli expressing F17 fimbriae (Bessalah et al., 2016). Several studies (Isako et al., 2020; James et al., 2022) have highlighted the heightened risk of diarrhea in calves associated with an insufficient passive transfer of colostrum immunity. Notably, camel calves that do not receive an adequate supply of colostrum are particularly vulnerable to developing diarrhea, as highlighted in the literature (Salhi et al., 2015; Muluneh et al., 2022). Calf diarrhea could be linked to either a qualitative or quantitative deficiency of colostrum and may result in the swift spread of pathogenic E. coli strains, as indicated by Muktar et al. (2015). Therefore, ensuring that the calf suckles promptly and sufficiently is crucial.
Fig. 3. Biofilm formation by E. coli-F17 strain after exposure to increasing concentrations of camel colostrum (from 0.75 to 50 mg/ml). The results are expressed as the mean ± standard deviation (SD) derived from three separate experiments. Untreated bacteria was used as the control.
Fig. 4. The effects of the camel colostrum on E. coli-F17 mature biofilm. Mature biofilms were then exposed to the camel colostrum at various concentrations (from 0.75 to 50 mg/ml) and incubated for an additional 24 hours. The control group consisted of cells that were not treated with camel colostrum. The asterisks (*) and (****) indicate statistical differences between the treated cells and the untreated cells (p & 0.05) and (p & 0.0001), respectively.
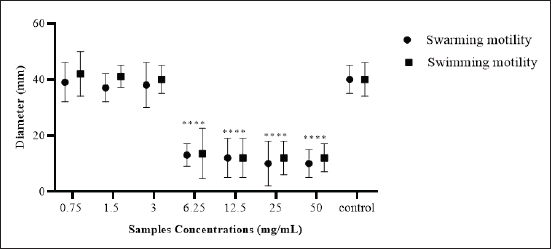
Fig. 5. Quantitative estimation of swimming and swarming motilities of E. coli-F17 after incubation with camel colosturm at different concentrations (from 0.75 to 50 mg/ml). The data from all experiments are reported as the mean of three independant replicates. **** indicates a p-value of less than 0.0001, denoting statistical significance. The current study reveals a pronounced growth inhibitory effect of camel colostrum against E. coli-F17. Here, camel colostrum inhibited up to 82.1% of E. coli-F17 growth at a concentration of 50 mg/ml after 24 hours of culture incubation in a broth microdilution assay. Whereas, a low inhibition activity was recorded at 12.5 mg/ml after 24 hours of incubation (24.1%) and no inhibition at concentrations of 3 mg/ml and below. Jrad et al. (2014) conducted research on the antibacterial activity of digested camel milk, colostrum, and colostral whey proteins and found that colostrum protein has the highest impact on bacteria growth for E. coli XL1 blue and L. innocua LRGIA 01. In another study, Benkerroum et al. (2004) analyzed the effectiveness of camel colostrum as an antibacterial and found a high level of activity against E. coli.
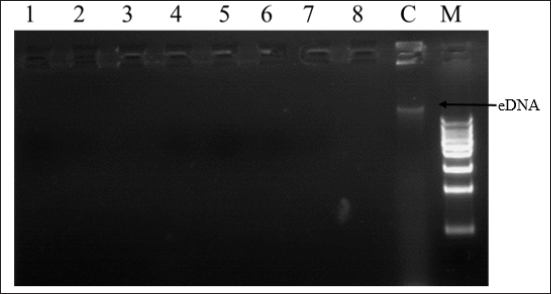
Fig. 6. 1% Agarose gel electrophoresis of eDNA of E. coli-F17 after treatment with camel colostrum; lane 1 to lane 8: bacteria treated with camel colostrum at different concentration (from 0.75 to 50 mg/mLl; lane 8: untreated cell (control); lane 9: 1Kb DNA ladder. While the inhibitory activity of camel colostrum against several food-borne pathogens has been established, no data are currently available regarding its potential to prevent biofilm formation, a key factor in pathogenic E. coli (Öztürk et al., 2023). This report represents the first documentation demonstrating the capacity of camel colostrum to reduce biofilm formation by E. coli-F17 strain. Notably, our findings suggest that camel colostrum inhibits the adhesion of cells to surfaces, thereby impeding the formation of biofilms, especially in the early phases, within the first two hours after E. coli-F17 inoculation. Similarly, camel colostrum exhibited high activity against a pre-established 24-hour-old biofilm. Also, consistent effects on methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa have been documented in other research studies where camel milk whey hydrolysate was utilized to induce changes in cell biofilm formation (Abdel-Hamid et al., 2019). Additionally, in our previous report, we found that natural extracts exhibited higher antibiofilm formation against E. coli-F17 (Bessalah et al., 2023). The capacity of pathogenic strains to establish colonization and subsequently propagate within their preferred habitats is primarily governed by their motility (Weller-Stuart et al., 2017). Swimming and swarming are the most well-known modes of bacterial motility (Josenhans and Suerbaum 2002). The importance of bacterial motility in the virulence of different pathogenic bacteria, such as Escherichia coli, has been well documented (Wood, 2009). Notably, camel colostrum demonstrated a reduction in both swimming and swarming motilities. Our results reveal that in vitro, camel colostrum effectively inhibits bacterial swimming, with inhibition values ranging from 10 to 13.5 mm for camel colostrum higher than 6.25 mg/ml. These findings align with other studies where compounds with antimicrobial activity have shown favorable inhibition of swimming motility in E. coli strains (Hidalgo et al., 2011; Bai et al., 2022). In contrast to swimming motility, which is an individual cellular action, swarming motility is a collective behavior that necessitates communication among bacterial cells to facilitate efficient swarming (Daniels et al., 2004). This communication, referred to as quorum sensing, is an oivotal factor in bacterial virulence. Studies have linked quorum sensing to swarming motility and the expression of virulence factors in enterotoxigenic E. coli (Sturbelle et al., 2015). Hence, it would be fascinating to explore in future research whether camel colostrum possesses anti-quorum sensing properties. Recently, extensive research has centered around eDNA. The role of eDNA in the initial steps of the biofilm formation process, specifically in attachment and aggregation, has been highlighted (Das et al., 2015; Panlilio and Rice, 2021). A prevailing hypothesis suggests that eDNA contributes to the structural stability of the biofilm matrix, promoting hydrophobicity, and cell aggregation, thereby facilitating easier adhesion of cells to surfaces. Current findings revealed that when camel colostrum is applied directly to the E. coli-F17 biofilm, extracellular eDNA degradation and biofilm matrix attenuation occur. Disrupting eDNA interactions similarly poses a threat to the integrity of the matrix. Hence, it is crucial to recognize that even if the biofilm is not entirely disrupted, the creation of access points for additional treatments becomes possible. Thus, the antibiotic susceptibility of the residing pathogenic bacteria can be enhanced. Another interesting point in this study is that eDNA degradation was associated with the weakness of bacteria’s motility. This observation is consistent with the study by Berne et al. (2010), which demonstrated that eDNA facilitates the dispersal of biofilms by inhibiting swarmer motile bacteria from settling in the established biofilm. The interaction of eDNA with the surface-binding regions of the cells facilitates the dispersal of the bacterial population, enabling them to establish new biofilms elsewhere. The potential of camel’s colostrum to disrupt biofilm formation and modulate bacterial motility can be attributed to substances such as lysozymes, lactoferrin, and immunoglobulines (Swelum et al., 2021). Lysosomes break down the cell walls of bacteria, while lactoferrin sequesters iron, depriving bacteria of this essential nutrient and thus inhibiting their growth (El-Fakharany et al., 2017). These natural components make camel colostrum a formidable ally against bacterial infections. ConclusionIn conclusion, our finding revealed that camel colostrum inhibits the growth of E. coli-F17 by different mechanisms, including the modulation of biofilm formation and the impairment of both swarming and swimming motilities. Interestingly, incubation of E. coli-F17 with camel colostrum reduces eDNA release which serves as a protective shield for resistant bacteria against environmental threats. This suggests that camel colostrum may serve as a preventative measure against E. coli-F17 associated with camel diarrhea. Additional research is justified based on the findings reported in this study to assess the potential protective effects of camel colostrum, either in isolation or in combination with antimicrobial compounds, against E. coli-F17 in an animal model. AcknowledgmentsThe authors would like to express their gratitude to Livestock and Wildlife Laboratory, Arid Lands Institute (I.R.A), University of Gabès, 4119 Médenine, Tunisia, Department of Livestock and Poultry Production, Bahauddin Zakariya University, Multan, Pakistan, and Therapeutic Nutrition Department, Faculty of Health Sciences, Misurata University, Misurata, Libya, for assistance in conducting the research. Conflict of interestThe authors have no conflicts of interest to declare that are relevant to the content of this article. FundingNo external funding was available for this study. Author ContributionsInvestigation, S.B., and A.F; resources, S.B., A.F., M.H., A.B.M., and S.I.S.; writing—original draft preparation, S.B., A.F., M.H., A.B.M., C.L, S.I.S.; M.A.A., W.A. and Z.M.I, writing—review and editing, S.B., A.F., S.M.H., A.B.M., M.A.A., W.A. and Z.M.I.. All authors have read and agreed to the published version of the manuscript. Data availabilityThe data presented in this study are available on request from the corresponding author. ReferencesAbdel-Hamid, M., Romeih, E., Saporito, P., Osman, A., Mateiu, R.V., Mojsoska, B. and Jenssen, H. 2020. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 111, 107056. Allan, F.K., Wong, J.T., Lemma, A., Vance, C., Donadeu, M., Abera, S., Admassu, B., Nwankpa, V., Lane, J.K., Smith, W., Kebede, N., Amssalu, K., Fentie, T., Schnier, C. and Peters, A.R. 2023. Interventions to reduce camel and small ruminant young stock morbidity and mortality in Ethiopia. Prevent. Vet. Med. 219, 106005. Al-Samhary, K.I.M. 2018. Immune modulation assessment of antibacterial and antiviral effects of bovine and camel colostrum. Doctoral dissertation, College of Science, Department of Botany and Microbiology, King Saud University, Riyadh, Saudi Arabia. Awoke, K.Z. and Ali, S.M. 2015. Traditional husbandry practices and major challenge of young stock (camel calf) in Fafen Zone, Ethiopian Somali Regional State, Ethiopia Environmental & Analytical. Environ. Anal. Toxicol. 5, 1000321. Bai, Y., Wang, W., Shi, M., Wei, X., Zhou, X., Li, B. and Zhang, J. 2022. Novel antibiofilm inhibitor ginkgetin as an antibacterial synergist against Escherichia coli. Int. J. Mol. Sci. 23, 8809. Benkerroum, N., Mekkaoui, M., Bennani, N. and Hidane, K. 2004. Antimicrobial activity of camel’s milk against pathogenic strains of Escherichia coli and Listeria monocytogenes. Int. J. Dairy Tec. 57, 39–43. Berne, C., Kysela, D.T. and Brun, Y.V. 2010. A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm. Mol. Microbiol. 77, 815–829. Bessalah, S., Fairbrother, J.M. and Salhi, I. 2016. Antimicrobial resistance and molecular characterization of virulence genes, phylogenetic groups of Escherichia coli isolated from diarrheic and healthy camel-calves in Tunisia. Comp. Immunol. Microb. 49, 1–7. Bessalah, S., Khorchani, T., Hammadi, M., Faraz, A. and Mustafa, A.B. 2023. Inhibitory potential of natural plant extracts against Escherichia coli strain isolated from diarrheic camel calves. Open Vet. J. 13, 1082–1090. Carrano, G., Paulone, S., Lainz, L., Sevilla, M.J., Blasi, E. and Moragues, M.D. 2019. Anti-Candida albicans germ tube antibodies reduce in vitro growth and biofilm formation of C. albicans. Rev. Iberoam. Micol. 36, 9–16. Daniels, R., Vanderleyden, J. and Michiels, J. 2004. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 28, 261–289. Das, T., Kutty, S.K., Tavallaie, R., Ibugo, A.I., Panchompoo, J., Sehar, S., Aldous, L., Yeung, A.W., Thomas, S.R., Kumar, N. and Gooding, J.J. 2015. Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci. Rep. 5, 1–9. El-Fakharany, E.M., El-Baky, N.A., Linjawi, M.H., Aljaddawi, A.A., Saleem, T.H., Nassar, A.Y. and Redwan, E.M. 2017. Influence of camel milk on the hepatitis C virus burden of infected patients. Exp. Therap. Med. 13, 1313–1320. Hidalgo, G., Chan, M. and Tufenkji, N. 2011. Inhibition of Escherichia coli CFT073 fliC expression and motility by cranberry materials. Appl. Environ. Microb. 77, 6852–6857. Isako, T., King’ori, A.O.J. and Onjoro, O. 2020. Review of camel calves nutrition and management in Kenya. J. Nat. Sci. Res. 10, 28–36. James, A., Smith, J., Sheldon, J. and Videla, R. 2022. Failure of Passive Transfer in Camel Calves: 4 Cases (2010-2019). Case Rep. Vet. Med. 2022, 8182648. Jrad, Z., El Hatmi, H., Adt, I., Girardet, J.M., Cakir-Kiefer, C., Jardin, J. and Oulahal, N. 2014. Effect of digestive enzymes on antimicrobial, radical scavenging and angiotensin I-converting enzyme inhibitory activities of camel colostrum and milk proteins. Dairy Sci. Tech. 94, 205–224. Jrad, Z., El-Hatmi, H., Adt, I., Khorchani, T., Degraeve, P. and Oulahal, N. 2015a. Antimicrobial activity of camel milk casein and its hydrolysates. Act. Aliment. 44, 609–616. Jrad, Z., Oulahal, N., Adt, I., Khorchani, T., Degraeve, P. and El-Hatmi, H. 2015b. Camel colostrum: nutritional composition and improvement of the antimicrobial activity after enzymatic hydrolysis. E. J. Food Agri. 2015, 384–389. Josenhans, C. and Suerbaum, S. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microb. 291, 605–614. Lanz Uhde, F., Kaufmann, T., Sager, H., Albini, S., Zanoni, R., Schelling, E. and Meylan, M. 2008. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Vet. Rec. 163, 362–366. Lee, J.H., Cho, H.S., Joo, S.W., Chandra Regmi, S., Kim, J.A., Ryu, C.M., Ryu, S.Y., Cho, M.H. and Lee, J. 2013. Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157: H7. Biofouling 29, 1189–1203. Marce, C., Guatteo, R., Bareille, N. and Fourichon, C. 2010. Dairy calf housing systems across Europe and risk for calf infectious disease. Animal 4, 1588–1596. Miller, S.A., Dykes, D.D. and Polesky, H. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. Muktar, Y., Mamo, G., Tesfaye, B. and Belina, D. 2015. A review on major bacterial causes of calf diarrhea and its diagnostic method. J. Vet. Med. Anim. Health. 7, 173–185. Muluneh, B., Shiferaw, D., Teshome, D., Al-Khaza’leh, J. and Megersa, B. 2022. Constraints and incidence of camel calf morbidity and mortality in Borana rangeland, Southern Ethiopia. J. Arid. Environ. 206, 104841. O’Toole, G.A. 2011. Microtiter dish biofilm formation assay. J. Vis. Exp. 47, 2437. Öztürk, F.Y., Darcan, C. and Kariptaş, E. 2023. The determination, monitoring, molecular mechanisms and formation of biofilm in E. coli. Braz. J. Microbiol. 54, 259–277. Panlilio, H. and Rice, C.V. 2021. The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 118, 2129–2141. Salhi, I., Bessalah, S., Mbarek, S.B., Chniter, M., Seddik, M.M., Khorchani, T. and Hammadi, M. 2015. Passive transfer of maternal immunity in the dromedary (Camelus dromedarius), involvement of heavy chain antibodies. Trop. Anim. Health Prod. 47, 613–618. Siuce, J., Maturrano, L., Wheeler, J.C. and Rosadio, R. 2020. Diarrheagenic Escherichia coli isolates from neonatal alpacas mainly display F17 fimbriae adhesion gene. Trop. Anim. Health Prod. 52, 3917–3921. Sturbelle, R.T., de Avila, L.F.D.C., Roos, T.B., Borchardt, J.L., Dellagostin, O.A. and Leite, F.P.L. 2015. The role of quorum sensing in Escherichia coli (ETEC) virulence factors. Vet. Microbiol. 180(3-4), 245–252. Swelum, A.A., El-Saadony, M.T., Abdo, M., Ombarak, R.A., Hussein, E.O.S., Suliman, G., Alhimaidi, A.R., Ammari, A.A., Ba-Awadh, H., Taha, A.E., El-Tarabily, K.A. and Abd El-Hack, M.E. 2021. Nutritional, antimicrobial and medicinal properties of camel’s milk: a review. Saudi J. Biol. Sci. 28, 3126–3136. Tizard, I. 2001. The protective properties of milk and colostrum in non-human species. Adv Nutr Res. 10, 139–-166. Wang, S., Zheng, F., Huang, Y., Fang, Y., Shen, M., Zhu, M. and Shi, X. 2012. Encapsulation of amoxicillin with inlaponite-doped poly (lactic-co-glycolic acid) nano fibers: preparation, characterization, and antibacterial activity. ACS Appl. Mater. Interface. 4, 6393–6401. Weller-Stuart, T., Toth, I., De Maayer, P. and Coutinho, T. 2017. Swimming and twitching motility are essential for attachment and virulence of Pantoea ananatis in onion seedlings. Mol. Plant Pathol. 18, 734–745. Wood, T.K. 2009. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 11, 1–15. Yeshiwas, T. and Fentahun, W.M. 2017. The prevalence of E. coli from diarrheic calves and their antibiotic sensitivity test in selected dairy farms of Debre Zeit, Ethiopia. Adv. Biotec. Microbiol. 6(1), 555–559. | ||
| How to Cite this Article |
| Pubmed Style Bessalah S, Faraz A, Mustafa AB, Hussain SM, Saeed SI, Liaqat C, Ashraf W, Iqbal ZM, Akbar MA, Hammadi M. Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Vet. J.. 2024; 14(11): 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 Web Style Bessalah S, Faraz A, Mustafa AB, Hussain SM, Saeed SI, Liaqat C, Ashraf W, Iqbal ZM, Akbar MA, Hammadi M. Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. https://www.openveterinaryjournal.com/?mno=215166 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.17 AMA (American Medical Association) Style Bessalah S, Faraz A, Mustafa AB, Hussain SM, Saeed SI, Liaqat C, Ashraf W, Iqbal ZM, Akbar MA, Hammadi M. Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Vet. J.. 2024; 14(11): 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 Vancouver/ICMJE Style Bessalah S, Faraz A, Mustafa AB, Hussain SM, Saeed SI, Liaqat C, Ashraf W, Iqbal ZM, Akbar MA, Hammadi M. Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 Harvard Style Bessalah, S., Faraz, . A., Mustafa, . A. B., Hussain, . S. M., Saeed, . S. I., Liaqat, . C., Ashraf, . W., Iqbal, . Z. M., Akbar, . M. A. & Hammadi, . M. (2024) Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Vet. J., 14 (11), 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 Turabian Style Bessalah, Salma, Asim Faraz, Ayman Balla Mustafa, Syeda Maryam Hussain, Shamsaldeen Ibrahim Saeed, Chanda Liaqat, Waqas Ashraf, Zeeshan Muhammad Iqbal, Muhammad Arslan Akbar, and Mohamed Hammadi. 2024. Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Veterinary Journal, 14 (11), 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 Chicago Style Bessalah, Salma, Asim Faraz, Ayman Balla Mustafa, Syeda Maryam Hussain, Shamsaldeen Ibrahim Saeed, Chanda Liaqat, Waqas Ashraf, Zeeshan Muhammad Iqbal, Muhammad Arslan Akbar, and Mohamed Hammadi. "Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea." Open Veterinary Journal 14 (2024), 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 MLA (The Modern Language Association) Style Bessalah, Salma, Asim Faraz, Ayman Balla Mustafa, Syeda Maryam Hussain, Shamsaldeen Ibrahim Saeed, Chanda Liaqat, Waqas Ashraf, Zeeshan Muhammad Iqbal, Muhammad Arslan Akbar, and Mohamed Hammadi. "Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea." Open Veterinary Journal 14.11 (2024), 2883-2892. Print. doi:10.5455/OVJ.2024.v14.i11.17 APA (American Psychological Association) Style Bessalah, S., Faraz, . A., Mustafa, . A. B., Hussain, . S. M., Saeed, . S. I., Liaqat, . C., Ashraf, . W., Iqbal, . Z. M., Akbar, . M. A. & Hammadi, . M. (2024) Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea. Open Veterinary Journal, 14 (11), 2883-2892. doi:10.5455/OVJ.2024.v14.i11.17 |