| Research Article | ||
Open Vet. J.. 2024; 14(12): 3296-3308 Open Veterinary Journal, (2024), Vol. 14(12): 3296-3308 Research Article The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus)Aceng Ruyani1,2*, Emi Suswati1, Dhea Prita Pratiwi3, Rina Elvia1, Oktoviani Oktoviani3 and Deni Parlindungan2,41Graduate School of Science Education, Bengkulu University, Bengkulu, Indonesia 2Conservation Education for Sustainability of Bio-Resources (CESB-R), Bengkulu University, Bengkulu, Indonesia 3Department of Medicine, Bengkulu University, Bengkulu, Indonesia 4Department of Science Education, Bengkulu University, Bengkulu, Indonesia *Corresponding Author: Aceng Ruyani. Graduate School of Science Education, Bengkulu University, Bengkulu, Indonesia. Email: ruyani [at] unib.ac.id Submitted: 22/08/2024 Accepted: 05/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
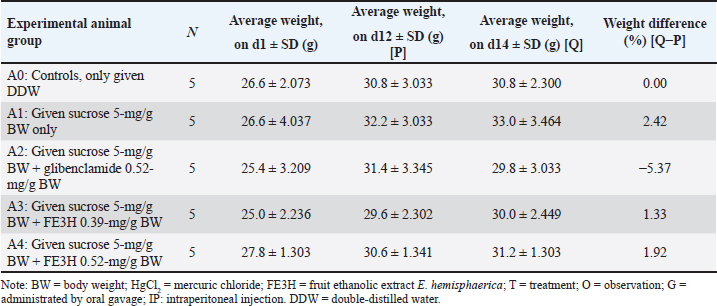
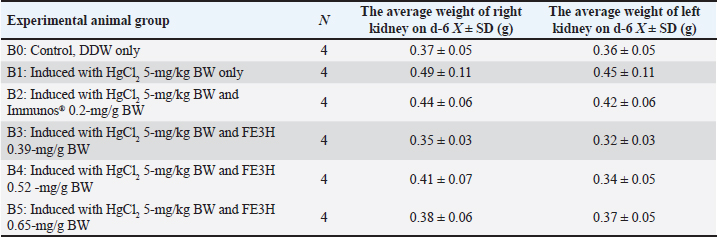
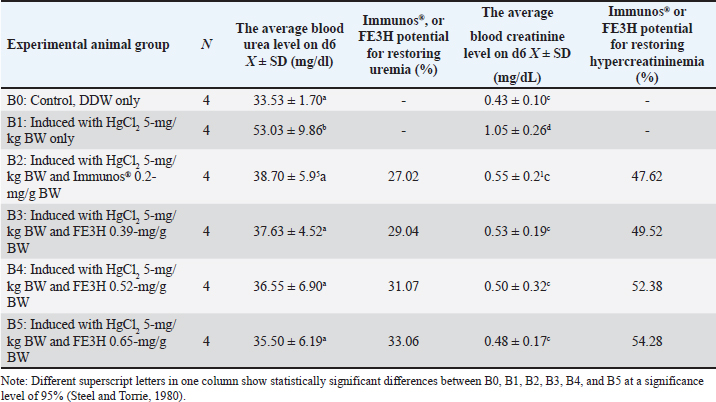
AbstractBackground: Leaf ethanolic extract of Etlingera hemisphaerica (LE3H) has the potential to restore glucose, triglyceride, and uric acid disorders and reduce mercury toxicity. High levels of glucose (hyperglycemia), urine (uremia), and creatinine (hypercreatininemia) in the blood cause real health problems. Meanwhile, the phytochemical content in fruit ethanolic extract of E. hemisphaerica (FE3H) is higher than that of LE3H. Aim: This study evaluated the potential of FE3H as a solution for hyperglycemia, uremia, and hypercreatininemia in mice. Methods: Quercetin levels in FE3H were determined by high-performance liquid chromatography. The first (A) stage used 25 male mice divided into five groups. On day (d)1, the body weight (BW) of the mice was weighed. On d2–11, 5-mg/gBW sucrose was given by gavage, and then, on d12, BW and blood glucose level of mice were determined. On d13, 0.52-mg/gBW glibenclamide was given in A2, 0.39-mg/gBW FE3H was given in A3, and 0.52-mg/g BW FE3H was given in A4 by gavage. Control, A0, was only given double-distilled water (DDW). On d14, BW and blood glucose levels of the mice were determined. The second (B) stage used 24 male mice divided into six groups. On d1, B1–B5 were injected intraperitoneally 5-mg/kg BW HgCl2; then, on d3–5, they were given by gavage 0.2-mg/g BW Immunos® in B2, 0.39-mg/gBW FE3H in B3, 0.52-mg/gBW FE3H in B4, and 0.65-mg/gBW FE3H in B5. Controls, B0 and B1, were only given DDW. On d6, the mice were killed by cervical dislocation. The weight of the kidney was determined, and then, the urea and creatinine levels were measured in blood samples from the heart. Results: FE3H contains 98.92 ± 12.88 µg/g quercetin. Sucrose tends to increase BW, and then, 0.39-mg/g BW FE3H treatment tends to restore BW close to control. Sucrose significantly increased blood glucose, and then, 0.39- and 0.52-mg/gBW FE3H treatment restored significantly blood glucose similar to control. HgCl2 increases kidney weight, and then, 0.65-mg/gBW FE3H treatment tends to restore kidney weight close to control. HgCl2 significantly increased urea and creatinine, and then, 0.52- and 0.65-mg/gBW FE3H treatment significantly restored urea and creatinine similar to the control. Conclusion: FE3H, which is high in flavonoid quercetin, has the potential to restore hyperglycemia by 47.38%–48.18%, uremia by 29.04%–33.06%, and hypercreatininemia by 49.52%–54.28% in mice. Keywords: Etlingera hemisphaerica, Sucrose, Mercury chloride, Hyperglycemia, Uremia, Hypercreatininemia. IntroductionForest honje [Etlingera hemisphaerica (Blume) R.M.Sm] was employed as traditional herbs and spices (http://www.theplantlist.org/tpl1.1/record/kew-243067) by several ethnic groups in Bengkulu, Indonesia. Leaves of E. hemisphaerica have high polyphenol content, which is good for curing dysentery. Ascorbic acid, minerals, and antioxidants are also present in E. hemisphaerica. Several research reports on E. hemisphaerica have been published in the form of five papers in reputable international journals and can be accessed online via the following link [https://pubmed.ncbi.nlm.nih.gov/?term=Etlingera+hemisphaerica]. In Mus musculus with hyperglycemia and hypertriglyceridemia, 0.39-mg/g body weight (BW) leaf ethanolic extract of E. hemisphaerica (LE3H) may potentially lower blood glucose (36.2%) and triglyceride (21.19%) levels (Ruyani et al., 2014). Researchers looking for herbal medicine found that giving 5-mg/kg BW mercuric chloride (HgCl2) significantly increased the number of white blood cells. However, giving HgCl2 followed by 0.39-mg/g BW LE3H may have lowered the number of white blood cells. Because HgCl2 treatment therapy lowered erythrocyte counts while raising leukocyte counts, LE3H therapy was safe for erythrocyte and leukocyte counts. The liver weight was increased by 5-mg/kg BW HgCl2, and the control decreased when LE3H treatment was used at a dose of 0.39 mg/kg BW. Researchers using histology found that HgCl2 treatment made tissues swell and less extracellular space than the control group. However, giving 0.39-mg/g BW LE3H made the symptoms better. The results showed that LE3H protects the livers of mice from the harm induced by HgCl2 (Ruyani et al., 2017). On the other hand, giving HgCl2 and then 0.39-mg/g BW LE3H treatment kept the number of blood cells close to the control. Leukocytes and erythrocytes decreased when 5-mg/kg BW HgCl2 was given. LE3H treatment could protect this protein profile since the control condition after HgCl2 delivery showed a new 125-kDa protein and caused a 48-kDa protein to be overexpressed. When found in the blood of M. musculus, LE3H may help protect against HgCl2 overdose. Because of this, LE3H pills might help protect people who are exposed to HgCl2 (Ruyani et al., 2019). In an earlier work, LE3H was checked to see if it could protect M. musculus from HgCl2’s teratogenic effects. The eight things measured were resorbed egg, dead fetus, living fetus, morphologically normal living fetus, deformed living fetus, the number of Malformed Living Fetus (MLFs), the length of Morphologically Normal Living Fetus (MNLF), and the weight of MNLF. Four of the values for LE3H were significantly different from the controls (50.00%), but seven of the values for HgCl2 were significantly different (87.50%), showing that HgCl2 was much more likely to cause congenital disabilities than LE3H. LE3H changed the teratogenicity of HgCl2 in two ways: it went up by 22.50% and down by 67.50%. Therefore, LE3H lessened the effects of HgCl2 on M. musculus, which caused birth defects (Ruyani et al., 2020). Later, studies show that 0.39-mg/g BW LE3H might be a natural substance that can greatly reduce the problems with fetal anatomy and endochondral ossification that are caused by 5-mg/kg BW HgCl2 on M. musculus during the time after conception (Ruyani et al., 2021). Leaves of E. hemisphaerica are always easy to find and make great research tools. The community often collects the blooming stage of the plant for different uses, which makes it hard to get to the fruit of E. hemisphaerica. We must examine how the phytochemicals in leaves and fruit are similar and different. Many papers (Ruyani et al., 2014; 2017; 2019; 2020; and 2021) have looked into and written about the possibilities of the LE3H. New research suggests that LE3H and FE3H, an ethanolic fruit extract from E. hemisphaerica, can help mice with hyperuricemia. For mice, 0.01-mg/g BW allopurinol is the same as 0.13-mg/g BW LE3H when used to treat high blood pressure. The FE3H lowers uric acid better than the LE3H when mice have hyperuricemia (Karyadi et al., 2023). Therefore, we need to study how FE3H can regulate the amounts of glucose, urea, and creatinine in mice. Materials and MethodsExtract preparationIn the Lebong Regency of Bengkulu Province, Indonesia, fruits of E. hemisphaerica were found. The plant’s identity was confirmed by the Indonesian Institute of Sciences’ Research Center for Plant Conservation and Botanical Gardens in Bogor, Indonesia (http://lipi.go.id/; Number B-1750/IPH.3./3./KS/V/2019). Fresh E. hemisphaerica fruit weighing 6.26 kg was collected after being cleaned and cut into small pieces. The fruit was dried in the air for 10 days and then ground up to 1.36 kg of dried fruit. The powder was mixed with 96% ethanol for 7 days, concentrated with a rotating evaporator at 50 °C, and the filtrate was cooled down (Sopi et al., 2013). This made an ethanolic extract of E. hemisphaerica (FE3H) fruit that was dark brown. Once the ethanol in the concentrated extract has evaporated, the FE3H crude extract can be used as a test sample for this study (Ruyani et al., 2017; 2019; 2020). Phytochemical screening of FE3HHigh-performance liquid chromatography (HPLC; Knauer Smartline) (Macêdo et al., 2021) was used to measure the amount of quercetin in FE3H at Gadjah Mada University in Yogyakarta, Indonesia (https://lppt.ugm.ac.id). DosageThe dosage of FE3H (0.39-, 0.52-, and 0.65-mg/g BW) was according to Ruyani et al. (2014, 2017, 2019, 2020). A 5-mg/g BW sucrose was given to mice for days (d)2–11 to make them develop hyperglycemia. It was used 0.52-mg/g BW glibenclamide, a sulfonylurea diabetes drug, as a positive control (POM: GKL9520905004A2) was given by gavage. There was one intraperitoneal (IP) injection of 5-mg/kg BW HgCl2 (Merck, Germany; Product No. 104 417) (Chowdhury and Arora, 1982; Saxena and Kumar, 2004; El-Desoky et al., 2013). These are the positive controls for LE3H therapy, and 0.2-mg/g BW Immunos® (POM SD 121542741) was given by gavage from PT Lapi Laboratories (Serang, Indonesia). The Immunos® has 500 mg of echinacea, 50 mg of ascorbic acid, 15 mcg of selenium, and 10 mg of zinc picolinate (Ruyani et al., 2014). This dietary supplement strengthens the immune system against both short- and long-term diseases. In Bengkulu, Indonesia, it was bought at a drugstore. Group of experimental animalsAs an experimental animal, 49 male Swiss Webster mice (M. musculus) from the Animal Test Center, School of Life Sciences and Technology (SITH), Bandung Institute of Technology (ITB), Bandung, Indonesia, were employed. The first (A) stage used 25 male mice aged 6–8 weeks with 25–35-g BW, which was divided into five (A0–A4) groups. On day (d)1, the BW of the mice was weighed. On d2–11, 5-mg/g BW sucrose was given by gavage, and then, on d12, BW and blood glucose level of mice were determined. On d13, 0.52-mg/g BW glibenclamide was given by gavage in A2, 0.39-mg/g BW FE3H was given by gavage in A3, and 0.52-mg/g BW FE3H was given by gavage in A4. Control, A0, was only given double-distilled water (DDW). On d14, BW and blood glucose levels of the mice were determined (Table 1). In the second (B) stage, 24 male mice aged 6–8 weeks with 25–35-g BW, which was divided into six groups (B0–B5) were used. On d1, B1–B5 were injected IP 5-mg/kg BW HgCl2; then, on d3–5, they were given by gavage 0.2-mg/g BW Immunos® in B2, 0.39-mg/g BW FE3H in B3, 0.52-mg/g BW FE3H in B4, and 0.65-mg/g BW FE3H in B5. Controls, B0 and B1, were only given DDW. On d6, the mice were killed by cervical dislocation (CD), and then, the abdominal and thoracic cavities were dissected. The weight of the kidney was determined, and then, the urea and creatinine levels were measured in blood samples from the heart (Table 1). Blood glucose level determinationTo check the blood glucose level in M. musculus, a small amount of blood from the tip of the tail was dripped onto a glucose strip that was then put into a glucometer brand Accu-Chek® (https://www.accu-chek.com/meters). The glucometer was previously set to the code on the glucose strip box. After the blood is on the strip, wait 5 seconds before using the glucometer to check the blood sugar level. The number shown on the glucometer is the blood glucose level of the mice in mg/dl (Andrikopoulos et al., 2008). Blood urea and creatinine level determinationThe mice were killed by CD, the abdomen and chest were dissected, and 0.5 ml of blood was taken with a needle from the heart to determine urea and creatinine levels. The blood samples that had been taken were then put into non-ethylenediamine tetra acid tubes (Microtainer®) To obtain serum, the samples were centrifuged for 15 minutes at a speed of 3,000 rotations per minute (rpm). Serum was immediately initialized from red blood cells for 1 hour after sampling. The ultraviolet (UV) method with a frequency of 340 nm was used to measure the amounts of blood urea in plasma. Here is how to find out how much urea is in your blood. Before using the BT35i, prepare 0.5 ml of blood, put it into a cloth activator, and spin it at 3,000 rpm for 15 minutes. You will then need to take serum and make blood urea reagent. The sample must then be put into the machine (Syahputra et al., 2021). Colorimetric testing with a 490-nm frequency was used to measure the amounts of creatinine in blood plasma. If the environment is alkaline, creatinine and picric acid should join to form a yellow–orange complex molecule. To measure blood urea amounts, create a cloth activator and put 0.5 ml of blood in it. Centrifugation at 3,000 rpm for 15 minutes was performed to obtain blood serum. Then, a blood urea reagent was made, and the sample was put into the BT35i device (Gul et al., 2021). Statistical analysisMultiple tests (analysis variance, the one-way classification) and the least significant difference (LSD; Waller–Duncan’s test) were used to make the data from this study more general (Steel and Torrie, 1980). Ethical approvalEthical approval was granted through the local committee of animal care and use at the Committee on the Ethics of Animal Experiments, Department of Medicine within the University of Bengkulu, Indonesia (No. 107/UN30.14.9/ LT/2020, March 31, 2020) before starting this study. ResultsQuercetin is a flavonoid that is found in many fruits and veggies. It is known to be anti-inflammatory, antiviral, and antimicrobial. HPLC was used to measure the amount of quercetin in FE3H and was found to be 98.92 ± 12.88 µg/g (Fig. 1 and Table 2). Subjects A1–A4 usually gain between 10.07% and 23.62% of their BW after 9 days (d2–11) of 5-mg/g BW sucrose treatment. In addition, testing with 0.39-mg/g BW FE3H and 0.52-mg/g BW FE3H showed that 0.39-mg/g BW FE3H was most effective (1.33%) at restoring BW to a level similar to the control condition (Table 3). Animals A1–A4 had much higher blood sugar levels than controls after being given 5-mg/g BW sucrose for 11 days (d2–11). According to the results, giving two doses of FE3H was enough to bring blood sugar levels back to the level of the control group (A0). The same effect was observed on diabetes with both FE3H and glibenclamide. FE3H has the potential to restore hyperglycemia by 47.38%–48.18% (Table 4). The right kidney (B1: 0.49 ± 0.11) and the left kidney (B1: 0.45 ± 0.11) weights on day 6 tended to rise more than those of the control (B0: 0.37 ± 0.05 and 0.36 ± 0.05) after receiving HgCl2 5-mg/kg BW on day 1. The right and left kidney weights (B2–B5) tended to return to the weight of the control kidney (B0) after the administration of HgCl2 on day 1 and three doses of FE3H on days 3–5. In the instance of HgCl2 poisoning, FE3H and Immunos® displayed a related phenomenon (Table 5 and Fig. 1). On day 6, levels of urea (B1: 53.03 ± 9.86b) and creatinine (B1: 1.05 ± 0.26b) following administration of 5-mg/kg BW HgCl2 were substantially greater than those of the control (B0: 33.53 ± 1.70a and 0.43 ± 0.10a). Although three doses of FE3H were administered after the administration of HgCl2 on day 1, the blood levels of urea (B2: 38.70 ± 5.95a, B3: 37.63 ± 4.52a, B4: 36.55 ± 6.90a, and B5: 35.50 ± 6.19a) and creatinine (B2: 0.55 ± 0.21c, B3: 0.53 ± 0.19a, B4: 0.50 ± 0.32a, and B5: 0.48 ± 0.17a) were comparable to the control (B0) on day 6. When HgCl2 caused elevated blood urea and creatinine levels, FE3H and Immunos® demonstrated a related occurrence. FE3H has the potential for restoring uremia 29.04%–33.06% and hypercreatininemia 49.52%–54.28% (Table 6). Table 1. Research design to determine the potential of fruit ethanolic extract E. hemisphaerica (FE3H) to restore hyperglycemia, uremia, and hypercreatininemia in M. musculus. Standard food and drink were given ad libitum by experimental animals.
Fig. 1. Amount of quercetin in FE3H was detected by HPLC. Table 2. Results of quantitative analysis of quercetin levels in the FE3H using HPLC [https://lppt.ugm.ac.id].
Table 3. Average BW of M. musculus on day 1 (d1), day 12 (d12), and day 14 (d14). On day (d)1, BW of the mice was weighed. On d2–11, 5-mg/g BW sucrose was given by gavage, and then, on d12, BW and blood glucose level of mice were determined. On d13, 0.52-mg/g BW glibenclamide was given by gavage in A2, 0.39-mg/g BW FE3H was given by gavage in A3, and 0.52-mg/g BW FE3H was given by gavage in A4. Control, A0, was only given DDW. On d14, BW and blood glucose levels of the mice were determined.
Table 4. Average glucose level of M. musculus was on day 12 (d12) and day 14 (d14). On day (d)1, BW of the mice was weighed. On d2–11, 5-mg/g BW sucrose was given by gavage, and then, on d12, BW and blood glucose level of mice were determined. On d13, 0.52-mg/g BW glibenclamide was given by gavage in A2, 0.39-mg/g BW FE3H was given by gavage in A3, and 0.52-mg/g BW FE3H was given by gavage in A4. Control, A0, was only given DDW. On d14, BW and blood glucose levels of the mice were determined.
Table 5. Average weight right and left kidneys on day 6 (d6). On d1, B1–B5 were injected IP 5-mg/kg BW HgCl2; then, on d3–5, they were given by gavage 0.2-mg/g BW Immunos® in B2, 0.39-mg/g BW FE3H in B3, 0.52-mg/g BW FE3H in B4, and 0.65-mg/g BW FE3H in B5. Controls, B0 and B1, were only given DDW. On d6, the mice were killed by CD, and then, the abdominal and thoracic cavities were dissected. The weight of the kidney was determined, and then, the urea and creatinine levels were measured in blood samples from the heart.
DiscussionA phytochemical study of the fruit ethanolic extract of E. hemisphaerica (FE3H) was done using HPLC (Knauer Smartline) and UV-vis spectrophotometry (1800 Shimadzu). The following six test parameters are given in order of their amounts: flavonoids (32.91% w/w), tannins (20.89% w/w), phenol (19.88% w/w), sucrose (2.96% w/w), alkaloids (2.05% w/w), and saponins (1.64% w/w). The phytochemical content of leaf ethanolic extract of E. hemisphaerica (LE3H) and FE3H was compared. FE3H had higher amounts of five test parameters: flavonoids, alkaloids, tannins, sucrose, and phenol. In the meantime, LE3H had more saponins than FE3H (2.32% w/w vs. 1.64% w/w). The most important phytochemicals in LE3H and FE3H are flavonoids (Karyadi et al., 2023; Table 7). Flavonoid quercetin is found in many fruits and veggies and is known to have anti-inflammatory, antiviral, and antimicrobial properties (Petrillo et al., 2022). This study used HPLC to measure the amount of quercetin in FE3H and found it to be 98.92 ± 12.88 µg/g (Table 2 and Fig. 1). Also, it was said that quercetin is a strong antioxidant that can grab reactive oxygen species, reactive nitrogen species, and reactive chlorine species. This quercetin can bind to transition metal ions and work as a reducing agent (Carrillo-Martinez et al., 2024). The literature shows that blood glucose (Yarahmadi et al., 2021), urea (Balkrishna et al., 2023), and creatinine (Vrbjar et al., 2023) levels are related to the presence of quercetin. Technically speaking, hyperglycemia means high blood glucose. Diabetes happens when the body does not have enough insulin or cannot use it correctly. The 9-day (d2–11) 5-mg/g BW sucrose treatment changed the blood glucose levels of M. musculus (Tables 3 and 4). Mus musculus had high blood sugar levels after this treatment, which is called hyperglycemia. High-sucrose diets can cause hyperglycemia in mice (Burchfield et al., 2018). Extremely high blood glucose levels, above 180–200 mg per deciliter (mg/dl), usually cause diabetes symptoms. Clodi et al. (2023) stated that in critical illness, hyperglycemia is associated with increased mortality. Based on the currently available evidence, intravenous insulin therapy should be initiated when blood glucose is above 180 mg/dl. After initiation of insulin therapy, blood glucose should be maintained between 140 and 180 mg/dl. Sucrose-induced hyperglycemia can cause diabetes and obesity, but a plant product or the drug glibenclamide can stop these problems (Ngueguim et al., 2016). In this study, glibenclamide (0.52-mg/g BW) treatment led to similar blood glucose levels in the treated group compared to the control group (Table 4), and BW tended to drop (Table 3). FE3H (0.39 or 0.52-mg/g BW) also showed that glibenclamide can stop sucrose from raising blood sugar levels (Table 4). There is an old glibenclamide drug that includes the sulfonylurea molecule and is very important for treating type 2 diabetes (T2D) mellitus. By stopping ATP-sensitive K+ channels, the drug works to depolarize cells and cause insulin to be released (Luzi and Pozza, 1997). Evidence shows that FE3H can reverse hyperglycemia. Glibenclamide and FE3H have similar effects on hyperglycemia, and glibenclamide has the potential to restore hyperglycemia by 60.98% (Table 4). Table 6. Average blood of urea and creatinine level on day 6 (d-6). On d1, B1–B5 were injected IP 5-mg/kg BW HgCl2; then, on d3–5, they were given by gavage 0.2-mg/g BW Immunos® in B2, 0.39-mg/g BW FE3H in B3, 0.52-mg/g BW FE3H in B4, and 0.65-mg/g BW FE3H in B5. Controls, B0 and B1, were only given DDW. On d6, the mice were killed by CD, and then, the abdominal and thoracic cavities were dissected. The weight of the kidney was determined, and then, the urea and creatinine levels were measured in blood samples from the heart.
Table 7. Comparison of phytochemical content of leaf ethanolic extracts E. hemisphaerica (LE3H) and fruit ethanolic extract E. hemisphaerica (FE3H) (Karyadi et al., 2023).
The three main plant chemicals that make up FE3H are flavonoids, tannins, and phenols (Karyadi et al., 2023). Table 2 shows that quercetin is a powerful flavonoid with many positive effects. It lowers blood pressure, fights hyperlipidemia and hyperglycemia, and stops cancer and other diseases by being antiviral, anticancer, anti-inflammatory, and antimicrobial (Hosseini et al., 2021). There are also claims that flavonoids can help people with diabetes, but more studies are needed to understand how they can treat diabetes (Al-Ishaq et al., 2019) fully. Tea from the red honeybush plant (Cyclopia genistoides) has no caffeine or much tannin. The different honeybush tea extracts, especially the aqueous and ethyl acetate extracts, slowed down the breakdown of lipids and sugars linked to T2D, lowered blood sugar, and controlled oxidative damage to the pancreas (Xiao et al., 2020). Researchers found that the water-based solution of Raphia hookeri leaves can act as an antioxidant and stop enzymes from breaking down carbohydrates because it contains phenolic compounds. Free radicals can cause oxidative stress in pancreatic cells, which may be helped by the extract. It may also lower blood sugar levels, which are important for treating T2D. However, it is strongly suggested that more clinical trials and in vivo studies can be done (Dada et al., 2017). The phytochemical parts of FE3H—flavonoids, tannins, and phenol—have been found to help lower blood sugar. FE3H has the potential to restore hyperglycemia by 47.38%–48.18% (Table 4). “Urine in the blood,” or uremia, is most common in people with end-stage renal disease and chronic kidney disease. On the other hand, if kidney function is lost quickly, it could also be due to severe renal damage. Urea hurts many types of cells in direct and indirect ways. Polyneuropathy is a typical sign of uremia, especially when renal replacement therapy is started too late (Zemaitis et al., 2023). For example, uremia can also lead to neurological problems. The kidney is the organ that mercury ions are most likely to damage. Blocking the urinary tract can also stop mercury ions from building up in the kidney tissue. In the same tests, this decrease is less strong than with inulin, however. Based on these results, gamma-glutamyl transpeptidase (gam-GT), an enzyme found in the kidneys, may help remove mercury from the tubule lumen. The death caused by mercury chloride and the effects on the buildup of organic ions in kidney slices were worse when nonprotein sulfhydryl was low because glutathione levels dropped quickly and production was stopped (Berndt et al.,1985). Male rats of the Long Evans breed were kept alive for a long time after mercuric chloride (HgCl2) caused uremia. Over time, the intramolecular structure of muscle glycogen changed, but the amount of glycogen in the muscles did not change significantly (Mannan and Rahman, 1977). In this research, kidney weights tended to rise more after being given 5-mg/kg BW of HgCl2 on day 1 (B1) compared to the control group (B0). In addition, giving HgCl2 and then treating the kidney weight with Immunos® (B2) and FE3H (B3–B5) tends to get closer to the state of control kidney weights (B0). The left and right kidneys behaved similarly, though the right kidney usually weighed more than the left kidney (Table 4 and Fig. 2). Compared to controls, giving HgCl2 raised blood urea levels by a large amount. After treatment with HgCl2 and FE3H, blood urea levels returned to where they were in the control group (Table 5). This shows that HgCl2 leads to uremia (Levine et al., 2003; Levine and Saltzman, 2003). In the meantime, both Immunos® and FE3H work to stop uremia. Immunos® has the potential to restore uremia by 27.02% (Table 6). Reports say that flavonoids change some parts of nitrogen metabolism in animals with experimental uremia. According to research, flavonoids robinine and hyperine lowered the amount of free ammonia in the rat brain and raised the amount of glutamine amide nitrogen and protein amide nitrogen. Robinine also slightly reduced urea synthesis and arginase activity in liver slices from rats that had their kidneys removed (Sokolova and Liubartseva, 1979). In treating uremia and hypercreatinemia (Hsieh et al., 2013), quercetin, a natural antioxidant, works much better. Another finding said that puerarin, an isoflavone, can stop vascular hardening in uremic rats by reducing inflammation (Liu et al., 2019). A substance called RG-tannin can lower the amounts of urea and creatinine in mice (Yokozawa et al., 1991). Zingerone, a phenolic alkenone found in ginger, was used to treat kidney damage caused by cecal closure and puncture surgery in mice, and it was tested by measuring blood urea nitrogen and serum creatinine (Lee et al., 2019). Scientists say that cyclophosphamide lowers the functions of the kidneys, liver, and antioxidant enzymes. This makes blood urea nitrogen and creatinine levels rise greatly. Meanwhile, saponins might protect the liver and kidneys from the harmful effects of cyclophosphamide (Golmohammadi et al., 2023). Phytochemicals in FE3H, such as flavonoids, tannins, phenol, and saponins, have been shown to help lower uric acid levels. Flavonoids exhibit the most promising potential (Karyadi et al., 2023) (Table 7). FE3H has the potential to restore uremia by 29.04%–33.06% (Table 6).
Fig. 2. Average weight right and left kidneys on day 6 (d-6). On d1, B1–B5 were injected IP 5-mg/kg BW HgCl2; then, on d3–5, they were given by gavage 0.2-mg/g BW Immunos® in B2, 0.39-mg/g BW FE3H in B3, 0.52-mg/g BW FE3H in B4, and 0.65-mg/g BW FE3H in B5. Controls, B0 and B1, were only given DDW. On d6, the mice were killed by CD, and then, the abdominal and thoracic cavities were dissected. The weight of the kidney was determined, and then, the urea and creatinine levels were measured in blood samples from the heart. Being busy or moving makes muscles make creatinine, a waste product. The kidneys will control how much creatinine is in the blood. Depending on a person’s age, gender, daily actions, and BW, their normal creatinine levels may be different. Normal amounts of creatinine in the body for adult men are between 0.6 and 1.2 mg/dl, and for adult women, they are between 0.5 and 1.1 mg/dl. Creatinine levels should be normal, but if they are too high or too low, it could mean that the kidneys are not working properly. In people with hypercreatininemia (>1.3 mg/dl), levels of creatinine are higher than usual (Akrom et al., 2017; Manna et al., 2005). Treatment with HgCl2 raised creatinine levels much more than the control group (Table 6), and kidney weights were also generally higher (Table 5 and Fig. 2). Many studies have shown that HgCl2 can cause hypercreatininemia (Moreira-Rodrigues et al., 2010; Chan et al., 2020; Ijaz et al., 2021; Goel et al., 2023). This fact fits with those results. According to this study, hypercreatininemia can be improved by 47.62% when Immunos® is given as a supplement. Immunos® is a supplement that boosts the immune system against short- and long-term infections. It is well-known outside of the supplement industry. The potential and process for the immune system are thought to be similar to those of FE3H (Table 6). FE3H may help lower hypercreatininemia, and flavonoids play the biggest part (Karyadi et al., 2023), especially when quercetin is present (Table 2). Multiple study reports must be used to back up this claim. Quantitative measures of biochemical, such as blood urea nitrogen and serum creatinine, show that quercetin completely stops HgCl2-induced acute kidney damage. In particular, quercetin greatly lowered the buildup of Hg in the kidneys (Shin et al., 2015). Quercetin stopped the mercurial-induced oxidation of glutathione. Findings show that quercetin’s ability to protect mitochondria from mercurial-induced dysfunction is linked to removing oxidant species made when methylmercury (MeHg) or HgCl2 is present. There was the first demonstration of quercetin’s ability to protect against mercurial-induced toxicity, pointing to its ability to block mercurial-dependent hydrogen peroxide production as a possible molecular method of protection (Franco et al., 2007). Human-derived liver cells are damaged by DNA damage and oxidative stress caused by HgCl2 and MeHg. The flavonoid lessens these effects. Although the amounts of metals and flavonoids used in this study were based on human exposure, results suggest that quercetin may protect people from the harmful effects of the metal (Barcelos et al., 2011). Scientists also found that quercetin lowered the levels of several substances in the blood, including malondialdehyde, serum/plasma creatinine, blood urea nitrogen, urine protein, urine albumin, and superoxide dismutase (Hu et al., 2022). Quercetin greatly lowered the BW, blood glucose, creatinine, and blood urea nitrogen levels of diabetes mice (Zhu et al., 2024). As a potential drug for treating diabetic nephropathy, quercetin makes clinical prediction and therapy easier (Feng et al., 2022). Results from earlier research agree with this, showing that FE3H has the potential for restoring hypercreatininemia 49.52%–54.28% (Table 6). More research should be done on the useful natural substances in the ethanol extract of E. hemisphaerica using the right proteome methods (Ruyani et al., 2023). ConclusionThe ethanolic extract of fruit E. hemisphaerica (FE3H) that was high in flavonoid quercetin has the potential for restoring hyperglycemia by 47.38%–48.18%, uremia by 29.04%–33.06%, and hypercreatininemia by 49.52%–54.28% in mice. AcknowledgmentsWe express our gratitude to Shyfa Fatihatunnisa Ruyani, M.Si., for her grammatical suggestions in preparing this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research was funded by the Hibah Penelitian Dasar 2019 (Nomor 779/U30.15/LT/2019) from the Directorate General of Higher Education, Republic Indonesia government. Authors’ contributionsAR: initiator of research idea, recipient of research funding, finalization of publication paper, and corresponding author; ES and RE: E. hemisphaerica fruit extract preparation, hyperglycemia research activity, and data collection; DPP and O: uremia and hypercreatininemia research activity, and data collection; RE and O: generalization through statistical tests; DP: research project administrator, and drafter of report. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityThe data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. ReferencesAkrom, E., Darmawan, N. and Maulida, A. 2017. Faktor-faktor berhubungan dengan kejadian hiperkreatininemia pada pasien berisiko sindrom metabolik di puskesmas Jetis I. Pharmaciana 7(2), 205–216. Al-Ishaq, R.K., Abotaleb, M., Kubatka, P., Kajo, K. and Büsselberg, D. 2019. Flavonoids and their anti-biabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules 9(9), 430. Andrikopoulos, S., Blair, A.R., Deluca, N., Fam, B.C. and Proietto, J. 2008. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 295, E1323–32. Balkrishna, A., Sinha, S., Kumar, A., Arya, V., Gautam, A.K., Valis, M., Kuca, K., Kumar, G. and Amarowicz, R. 2023. Sepsis-mediated renal dysfunction: pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed. Pharmacother. 165, 115183. Barcelos, G.R.M., Angeli, J.P.F., Serpeloni, J.M., Grotto, D., Rocha, B.A., Bastos, J.K., Knasmüller, S. and Júnior, F.B. 2011. Quercetin protects human-derived liver cells against mercury-induced DNA-damage and alterations of the redox status. Mutat. Res. 726(2), 109–115. Berndt, W.O., Baggett, J.M., Blacker, A. and Houser, M. 1985. Renal glutathione and mercury uptake by kidney. Fundam. Appl. Toxicol. 5, 832–839. Burchfield, J.G., Kebede, M.A., Meoli, C.C., Stöckli, J., Whitworth, P.T., Wright, A.L., Hoffman, N.J., Minard, A.Y., Ma, X., Krycer, J.R., Nelson, M.E., Tan, S.-X., Yau, B., Thomas, K.C., Wee, N.K.Y., Khor, E.C., Enriquez, R.F., Vissel, B., Biden, T.J., Baldock, P.A., Hoehn, K.L., Cantley, J., Cooney, G.J., James, D.E. and Fazakerley, D.J. 2018. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 293, 5731–5745. Carrillo-Martinez, E.J., Flores-Hernández, F.Y., Salazar-Montes, A.M., Nario-Chaidez, H.F. and Hernández-Ortega, L.D. 2024. Quercetin, a flavonoid with great pharmacological capacity. Molecules 29(5), 1000. Chan, T.Y.K., Chan, A.P.L. and Tang, H.L. 2020. Nephrotic syndrome caused by exposures to skin-lightening cosmetic products containing inorganic mercury. Clin. Toxicol. (Phila). 58(1), 9–15. Chowdhury, A.R. and Arora, U. 1982. Toxic effect of mercury on testes in different animal species. Indian J. Physiol. Pharmacol. 26, 246–249. Clodi, M., Resl, M., Abrahamian, H., Föger, B. and Weitgasser, R. 2023. Therapie der Hyperglykämie bei erwachsenen, kritisch kranken PatientInnen (Update 2023). Wien. Klin. Wochenschr. 135, 272–274. Dada, F.A., Oyeleye, S.I., Ogunsuyi, O.B., Olasehinde, T.A., Adefegha, S.A., Oboh, G. and Boligon, A.A. 2017. Phenolic constituents and modulatory effects of Raffia palm leaf (Raphia hookeri) extract on carbohydrate hydrolyzing enzymes linked to type-2 diabetes. J. Tradit. Complement. Med. 7, 494–500. El-Desoky, G.E., Bashandy, S.A., Alhazza, I.M., Al-Othman, Z.A., Aboul-Soud, M.A.M. and Yusuf, K. 2013. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS One 8, e59177. Feng, X., Bu, F., Huang, L., Xu, W., Wang, W. and Wu, Q. 2022. Preclinical evidence of the effect of quercetin on diabetic nephropathy: a meta-analysis of animal studies. Eur. J. Pharmacol. 921, 174868. Franco, J.L., Braga, H.C., Stringari, J., Missau, F.C., Posser, T., Mendes, B.G., Leal, R.B., Santos, A.R.S., Dafre, A.L., Pizzolatti, M.G. and Farina, M. 2007. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem. Res. Toxicol. 20(12), 1919–1926. Goel, H., Printz, R., Shiota, C., Estes, S., Pannala, V., Hameed, M.D.M.A., Shiota, M. and Wallqvist, A. 2023. Assessing kidney injury induced by mercuric chloride in Guinea Pigs with in vivo and in vitro experiments. Int. J. Mol. Sci. 24(8), 7434. Golmohammadi, M.G., Banaei, S., Timar, M. and Abedi, A. 2023. Saponin protects against cyclophosphamide-induced kidney and liver damage via antioxidant and anti-inflammatory actions. Physiol. Int. 110, 108–120. Gul, H.F., Dolanbay, T., Simsek, A.T. and Aras, M. 2021. Evaluation of blood urea, creatinine, and glucose levels as biochemical indicators of the type and severity of traumatic brain injury. Turk. Neurosurg. 31, 333–338. Hosseini, A., Razavi, B.M., Banach, M. and Hosseinzadeh, H. 2021. Quercetin and metabolic syndrome: a review. Phytother. Res. 35, 5352–5364. Hsieh, C.L., Peng, C.C., Chen, K.C. and Peng, R.Y. 2013. Rutin (quercetin rutinoside) induced protein-energy malnutrition in chronic kidney disease, but quercetin acted beneficially. J. Agric. Food. Chem. 61, 7258–7267. Hu, T., Yue, J., Tang, Q., Cheng, K.W., Chen, F., Peng, M., Zhou, Q. and Wang, M. 2022. The effect of quercetin on diabetic nephropathy (DN): a systematic review and meta-analysis of animal studies. Food Funct. 13(9), 4789–4803. Ijaz, M., Arshad, A., Awan, M., Tariq, M., Ali, S., Ali, S., Shafiq, M., Ahmed, S., Sheas, M., Iftikhar, M., Ahmed, S., Nasir, M., Kausar, G., Javed, A. and Safdar, W. 2021. Exploring the potential of curry leaves on mercury-induced hepatorenal toxicity in an animal model. Food Sci. Nutr. 10(2), 499–506. Karyadi, B., Adika, A., Melani, N., Abas, D., Parlindungan, E., Nursaadah, A. and Ruyani, A. 2023. Potential of leave and fruit ethanolic extract of Etlingera hemisphaerica as antihyperuricemic in mice (Mus musculus). Pak. J. Biol. Sci. 26, 63–71. Lee, B.S., Lee, C., Yang, S., Ku, S.K. and Bae, J.S. 2019. Renal protective effects of zingerone in a mouse model of sepsis. BMB Rep. 52, 271–276. Levine, S. and Saltzman, A. 2003. Acute uremia produced in rats by nephrotoxic chemicals is alleviated by protein deficient diet. Ren. Fail. 25, 517–523. Levine, S., Saltzman, A. and Branch, C. 2003. A model for perinephric fluid accumulation in uremic rats with toxic nephrosis. Toxicol. Lett. 146, 9–15. Liu, H., Zhang, X., Zhong, X., Li, Z., Cai, S., Yang, P., Ou, C. and Chen, M. 2019. Puerarin inhibits vascular calcification of uremic rats. Eur. J. Pharmacol. 855, 235–243. Luzi, L. and Pozza, G. 1997. Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol. 34, 239–244. Macêdo, S.K.S., Almeida, T.S., Filho, J.M.T.A., Lima, K.S.B., Libório, R.C., Costa, M.M., Neto, P.J.R., Rolim, L.A. and Nunes, X.P. 2021. Phytochemical identification and quantification of quercetin in Triplaris gardneriana wedd. leaves by HPLC-DAD with evaluation of antibacterial activity. Nat. Prod. Res. 35(18), 3083–3088. Manna, R., Mirk, P., Sallustio, G., Brisinda, G., Izzi, D., La Regina, M., Nucera, G., Maria, G., Montalto, M. and Ghirlanda, G. 2005. Hypercreatininemia and hyperglycemia: diabetic nephropathy or “inverted peritoneal auto-dialysis”? Clin. Nephrol. 63(2), 167–169. Mannan, A. and Rahman, M.S. 1977. Influence of mercuric chloride induced uremia on intramolecular structure of muscle glycogen in rats. Bangladesh Med. Res. Counc. Bull. 3(2), 87–93. Moreira-Rodrigues, M., Henriques-Coelho, T., Moura, C., Vasques-Nóvoa, F., Sampaio-Maia, B., Pestana, M. and Leite-Moreira, A.F. 2010. Cardiac dysfunction in HgCl2-induced nephrotic syndrome. Exp. Biol. Med. (Maywood) 235(3), 392–400. Ngueguim, F.T., Esse, E.C., Dzeufiet, P.D.D., Gounoue, R.K., Bilanda, D.C., Kamtchouing, P. and Dimo, T. 2016. Oxidised palm oil and sucrose induced hyperglycemia in normal rats: effects of Sclerocarya birrea stem barks aqueous extract. BMC Complement Altern. Med. 16, 47. Petrillo, A.D., Orrù, G., Fais, A. and Fantini, M.C. 2022. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother. Res. 36(1), 266–278. Ruyani, A., Kartika, E., Parlindungan, D., Putra, R.J., Sundaryono, A. and Susanta, A. 2021. Leaf ethanolic extract of Etlingera hemesphaerica Blume mitigates defects in fetal anatomy and endochondral ossification due to mercuric chloride during the post-implantation period in Mus musculus. PLoS One 16(3), e0247467. Ruyani, A., Muthmainnah, D., Putri, R.Z.E., Yulisa, T. and Sundaryono, A. 2017. Hepatoprotective effect of leaf ethanolic extract Etlingera hemisphaerica blume to recovery mercuric chloride toxicity on mice. Prosiding Seminar Nasional Pascasarjana (SNP) Unsyiah, Universitas Syiah Kuala (Unsyiah), Banda Aceh, Indonesia. pp: 136–147. Ruyani, A., Parlindungan, D., Kartika, E., Putra, R.J., Sundaryono, A. and Susanta, A. 2020. Leaf ethanolic extract of Etlingera hemesphaerica Blume alters mercuric chloride teratogenicity during the post-implantation period in Mus musculus. Toxicol. Res. 36, 131–138. Ruyani, A., Parlindungan, D., Samitra, D., Rozi, R.Z.E., Fauziah, U.M., Umar, L.A. and Sari, K. 2023. Etlingera hemisphaerica alters one-dimensional profile of serum proteins due to mercury chloride in rats (Rattus norvegicus). Pak. J. Biol. Sci. 26(9), 482–492. Ruyani, A., Putri, R.Z.E., Jundara, P., Gresinta, E., Ansori, I. and Sundaryono, A. 2019. Protective effect of leaf ethanolic extract Etlingera hemisphaerica Blume against mercuric chloride toxicity in blood of mice. J. Diet. Suppl. 16, 51–65. Ruyani, A., Sundaryono, A., Rozi, Z.F., Samitra, D. and Gresinta, E. 2014. Potential assessment of leaf ethanolic extract Honje (Etlingera hemisphaerica) in regulating glucose and triglycerides on mice (Mus musculus). Int. J. Sci. 3, 70–76. Saxena, P.S. and Kumar, M. 2004. Modulatory potential of Spirulina fusiformis on testicular phosphatases in Swiss albino mice against mercury intoxication. Indian J. Exp. Biol. 42, 998–1002. Shin, Y.J., Kim, J.J., Kim, Y.J., Kim, W.H., Park, E.Y., Kim, I.Y., Shin, H.S., Kim, K.S., Lee, E.K., Chung, K.H., Lee, B. and Kim, H.S. 2015. Protective effects of quercetin against HgCl2-induced nephrotoxicity in Sprague-Dawley rats. J. Med. Food. 18(5), 524–534. Sokolova, V.E. and Liubartseva, L.A. 1979. Effect of flavonoids on aspects of nitrogen metabolism in experimental uremia. Vopr. Med. Khim. 25, 379–382. Sopi, R., Hayat, B. and Hayat Khan, M.F. 2013. Bronchodilatory effect of ethanolic extract of the leaves of Nyctanthes arbortristis. Pharmacognosy Res. 5, 169–172. Steel, R.G. and Torrie, J. 1980. Principles and procedures of statistics: a biometrical approach. New York, NY: McGraw-Hill International Book Company. Syahputra, R.A., Harahap, U., Dalimunthe, A., Pandapotan, M. and Satria, D. 2021. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon 7, e07434. Vrbjar, N., Vlkovicova, J., Snurikova, D., Kalocayova, B., Zorad, S., Culafic, T., Tepavcevic, S., Tothova, L., Radosinska, D., Kollarova, M. and Radosinska, J. 2023. Alterations in oxidative stress markers and Na,K-ATPase enzyme properties in kidney after fructose intake and quercetin intervention in rats. Life (Basel) 13(4), 931. Xiao, X., Erukainure, O.L., Beseni, B., Koorbanally, N.A. and Islam, M.S. 2020. Sequential extracts of red honeybush (Cyclopia genistoides) tea: chemical characterization, antioxidant potentials, and anti-hyperglycemic activities. J. Food Biochem. 44, e13478. Yarahmadi, A., Sarabi, M.M., Sayahi, A. and Zal, F. 2021. Protective effects of quercetin against hyperglycemia-induced oxidative stress in hepatic HepG2 cell line. Avicenna. J. Phytomed. 11(3), 269–280. Yokozawa, T., Fujioka, K., Oura, H., Nonaka, G. and Nishioka, I. 1991. Effects of rhubarb tannins on uremic toxins. Nephron 58, 155–160. Zemaitis, M.R., Foris, L.A., Katta, S. and Bashir, K. 2024. Uremia, 2023. Treasure Island, FL: StatPearls Publishing. Available via https://www.ncbi.nlm.nih.gov/books/NBK441859/ Zhu, X., Zhang, C., Liu, L., Xu, L. and Yao, L. 2024. Senolytic combination of dasatinib and quercetin protects against diabetic kidney disease by activating autophagy to alleviate podocyte dedifferentiation via the Notch pathway. Int. J. Mol. Med. 53(3), 26. | ||
| How to Cite this Article |
| Pubmed Style Ruyani A, Suswati E, Pratiwi DP, Elvia R, Oktoviani O, Parlindungan D. The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Vet. J.. 2024; 14(12): 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 Web Style Ruyani A, Suswati E, Pratiwi DP, Elvia R, Oktoviani O, Parlindungan D. The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). https://www.openveterinaryjournal.com/?mno=215503 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.14 AMA (American Medical Association) Style Ruyani A, Suswati E, Pratiwi DP, Elvia R, Oktoviani O, Parlindungan D. The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Vet. J.. 2024; 14(12): 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 Vancouver/ICMJE Style Ruyani A, Suswati E, Pratiwi DP, Elvia R, Oktoviani O, Parlindungan D. The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 Harvard Style Ruyani, A., Suswati, . E., Pratiwi, . D. P., Elvia, . R., Oktoviani, . O. & Parlindungan, . D. (2024) The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Vet. J., 14 (12), 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 Turabian Style Ruyani, Aceng, Emi Suswati, Dhea Prita Pratiwi, Rina Elvia, Oktoviani Oktoviani, and Deni Parlindungan. 2024. The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Veterinary Journal, 14 (12), 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 Chicago Style Ruyani, Aceng, Emi Suswati, Dhea Prita Pratiwi, Rina Elvia, Oktoviani Oktoviani, and Deni Parlindungan. "The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus)." Open Veterinary Journal 14 (2024), 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 MLA (The Modern Language Association) Style Ruyani, Aceng, Emi Suswati, Dhea Prita Pratiwi, Rina Elvia, Oktoviani Oktoviani, and Deni Parlindungan. "The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus)." Open Veterinary Journal 14.12 (2024), 3296-3308. Print. doi:10.5455/OVJ.2024.v14.i12.14 APA (American Psychological Association) Style Ruyani, A., Suswati, . E., Pratiwi, . D. P., Elvia, . R., Oktoviani, . O. & Parlindungan, . D. (2024) The potential of fruit ethanolic extract Etlingera hemisphaerica as a solution for hyperglycemia, uremia, and hypercreatininemia in mice (Mus musculus). Open Veterinary Journal, 14 (12), 3296-3308. doi:10.5455/OVJ.2024.v14.i12.14 |