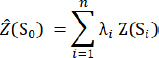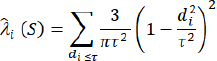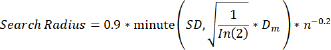| Research Article | ||
Open Vet. J.. 2024; 14(11): 2911-2923 Open Veterinary Journal, (2024), Vol. 14(11): 2911-2923 Research Article Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in EgyptYumna Elsobky1*, Mahmoud Eltholth2,3, Ehsan Abdalla4, Nourhan Eissa1, Ghada Hadad1, Mohamed Nayel5, Akram Salama5, Walid Mousa5, Ahmed Kamal6, Ashraf M. Abu-Seida7, Osama K. Gaidan8 and Mohamed Elkamshishi91Department of Animal Hygiene and Zoonosis, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt 2Department of Health Studies, University of London, Egham, UK 3Department of Animal Hygiene and Preventive Medicine, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt 4Department of Pathobiology/Department of Graduate Public Health, College of Veterinary Medicine, Tuskegee University, Tuskegee, AL 5Department of Animal Medicine and Infectious Diseases, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt 6Department of Birds and Rabbit Medicine, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt 7Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 8Faculty of Veterinary Medicine, Omar Al-mukhtar University, Al Bayda, Libya 9Department of Animal Hygiene and Zoonoses, Faculty of Veterinary Medicine, Matrouh University, Matrouh, Egypt *Corresponding Author: Yumna Elsobky. Department of Animal Hygiene and Zoonosis, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Egypt. Email: yumna.elsobky [at] vet.usc.edu.eg Submitted: 14/08/2024 Accepted: 18/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Highly pathogenic avian influenza (HPAI) (H5N1) has been endemic in Egypt for almost two decades, profoundly impacting both the poultry industry and public health. Egypt stands as a prominent epicenter for HPAI H5N1 outbreaks in Africa, marked by the highest number of positive human cases. Despite continuous governmental efforts, prior research underscored the inadequacy of strategies in controlling the virus spread. Aim: This study identified spatiotemporal patterns and high-risk clusters of HPAI H5N1 outbreaks at the subdistrict level. Methods: This study involved trial tracking of HPAI H5N1 endemicity dynamics, enabling tailored interventions at a regional level based on robust epidemiological investigations to address the persistent challenge of HPAI H5N1 in Egypt. This study illuminated spatiotemporal outbreak dynamics, with specific attention on Menofia governorate. Results: Despite the region’s early poultry impacts, initial outbreaks did not originate from Menofia in studied epidemic waves (EWs). Outbreak risk spatial distribution displayed an escalating pattern at the northern border, followed by risk reduction through the sixth EW. The predominant hot spot region was localized within rural districts, particularly villages, while urbanization coincided with lower outbreak density. Observed smoothed densities revealed epidemic propagation within urban centers, preceding its transition to new areas and establishing direct connections with neighboring cities. Primary cluster prognostication was plausible, occurring in regions previously hosting elevated relative risk clusters during preceding EWs. Identification of enduring pinpoint clusters, persistent for extended durations, indicated close contact dynamics and localized viral circulation within populations. Conclusion: This study highlights the significance of customized regional interventions based on the rigorous epidemiological framework. This approach is pivotal in the profound comprehension of endemicity dynamics, efficiently limits geographical infection spread, and contains outbreaks within delineated areas. Keywords: Endemicity dynamics, Highly pathogenic avian influenza, Risk clusters, Spatiotemporal dynamics, Tailored intervention strategies. IntroductionHighly pathogenic avian influenza (HPAI) subtype H5N1 poses a significant threat to the global poultry industry due to its genetic variability and pandemic potential (Otte et al., 2008). In Egypt, the emergence of HPAI H5N1 in poultry was noted in February, 2006; marking the second African nation to report such an infection (Saad et al., 2007; Aly et al., 2008). Egypt stands out with the highest number of HPAI H5N1 notifications of positive human cases (Harfoot and Webby, 2017; Young et al., 2018) and is considered a significant epicenter for HPAI H5N1 outbreaks in Africa (Ekong et al., 2018). Despite persistent governmental efforts to curtail its dissemination, previous publications have revealed the insufficiency of applied strategies in effectively controlling the HPAI-H5N1 virus (Elsobky et al., 2021). As a result, HPAI H5N1 remains entrenched and exerts severe repercussions on both the poultry sector and public health (Matrosovich et al., 1999; Kalthoff et al., 2010). Egypt’s poultry production predominantly occurs in lower Egypt, with concentrated outbreaks in the Nile Delta region due to dense poultry populations and agricultural activities (Arafa et al., 2012; El-Zoghby et al., 2013; Sheta et al., 2014; Arafa et al., 2016a; Elsobky et al., 2022). The reported outbreak rates, however, may underestimate the virus’s true prevalence and it does not fully reflect the true widespread endemic situation of the virus in the region due to the prevailing policy of under-reporting of the disease in the commercial sector in an attempt to protect their business interests and to prevent the mass culling of poultry stocks (Farnsworth et al., 2010; Kayali et al., 2011). In Egypt, HPAI H5N1Clade 2.2 after its emergence has spread quickly across both backyard and commercial flocks (Saad et al., 2007; Aly et al., 2008). Subsequent epidemic waves (EWs) have been documented since 2006, with Clade 2.2.1 observed during the first wave (Balish et al., 2010; Arafa et al., 2016a). A novel variant, Clade 2.2.1.1, emerged during this time, possibly due to vaccination efforts (Arafa et al., 2016a). Despite interventions, the virus continued to spread among vaccinated backyard and commercial poultry, causing significant social and economic losses (Kayali et al., 2016). Egypt was officially designated as an Avian Influenza endemic nation in 2008, underscoring the virus’s evolving nature (El-Shesheny et al., 2014). Clade 2.2.1.1 further diversified between 2008 and 2011 into two clusters (Arafa et al., 2016b). Notably, these clusters ceased to emerge thereafter (Arafa et al., 2016b), but a new variant (subclade 2.2.1.2) emerged in early 2014, with no significant alterations were found in the circulating viruses until 2016 (Arafa et al., 2015; Abdelwahab et al., 2016). A critical obstacle hindering the successful implementation of control strategies lies in the absence of detailed and meaningful epidemiological investigations (Farnsworth et al., 2010). Country-level aggregated data, at times, obscure smaller, high-risk clusters (Jones and Kulldorff, 2012; Elsobky et al., 2022). Therefore, the detection of the spatial cluster of the outbreaks could have been made accurately when involving finer detailed data in sub-districts within villages, and more reliable surveillance data in addition to up-to-date reported outbreak data (Dhingra et al., 2014). Our primary objective was to develop an innovative epidemiological approach to enhance control policies against HPAI H5N1 in Egypt. This approach centers on identifying spatiotemporal patterns and high-risk clusters at the subdistrict level, with tracking of HPAI H5N1 endemicity dynamics enabling tailored interventions guided by robust epidemiological investigations. This approach has the potential to contribute significantly to reducing virus circulation and mitigating the risk of disease transmission in both poultry and human populations. The success of these strategies hinges on the continuous assessment and evaluation of their implementation, ensuring their adaptability and effectiveness over time. Materials and MethodsDescription of the study areaThe Nile Delta region is about 40,000 km2 which accommodates a substantial portion of Egypt’s population and serves as a habitat for extensive poultry activity, including rearing, trade, and consumption (Abdelwahab and Hafez, 2011). This investigation’s focal domain is the Menofia governorate, a locale recognized for reporting elevated instances of HPAI (Arafa et al., 2016). Menofia governorate is one of the leading poultry-producing governorates in Egypt (ElMasry et al., 2017), where registered and unregistered commercial farms are found, in addition to the backyard which is randomly dispersed small-sized household flocks of mixed birds of different species and different ages with various vaccination protocols (Ali et al., 2013; Fasina et al., 2016). Data collectionIn Menofia governorate, confirmed events of HPAI-H5N1 outbreaks in domestic poultry were officially reported to the Global Animal Disease Information System (EMPRES-I) provided by (FAO, 2017), and the Egyptian Ministry of Agriculture (General Organization for Veterinary Services) official reports for national surveillance from January 2006 to December 2017. All data were integrated into one dataset and the villages were used as an administrative unit. The term outbreak was defined as “the confirmed presence of disease with a clinical expression or not, in at least one individual in a defined location during a specified period” (Toma et al., 1999). The epidemic date and the outbreak centroids were utilized as spatiotemporal characteristics of each outbreak. The governorate map was constructed by (ArcGIS) to facilitate the presentation of data and the interpretation of results. Method of analysisDaily, weekly, and monthly epidemic curves, were constructed to display outbreak magnitude and temporal trends of HPAI-H5N1 outbreaks. An EW is a number of disease outbreaks that rapidly peaked and then gradually decreased till the end of the epidemic (Zhang et al., 2010), and the number of outbreaks was calculated for each EW. The interpolation tools were used to study the HPAIH5N1 outbreak risk. A raster risk map displayed the interpolated surface, providing predictions for each location. The geostatistical analysis surface was derived using ordinary kriging by weighting the surroundings of the reported outbreak events to derive a prediction for the unreported locations. The general formula was formed as a weighted sum of the data values (Oliver and Webster, 1990):
where: Z(si)=the reported outbreaks at the ith location λi=an unknown weight for the number of outbreak events at the ith location s0=the prediction location n=the number of outbreak events. Spatially kernel density analysis (KDE) is a simple non-parametric technique that relies on a few assumptions about the structure of the observed data (Worton, 1989). It is equivalent to a simple diffusion model that is a useful approximation to patterns of distribution frequently found in ecological data (O’Brien et al., 2012). KDE was used to identify high-density areas (Silverman , 1998); and run in Environmental Systems Research Institute ArcMap 10.5 software using reported cases to generate a density surface for each EW. The quartic kernel function (Bailey and Gatrell, 1995; Mastumoto et al., 2021) was given by the following equation:
where: i=1,…,n are the input points. di is the distance between the point s and the observed event in location, si and τ is the radius centered on s. The formula to calculate the bandwidth was as follows (ESRI, 2014):
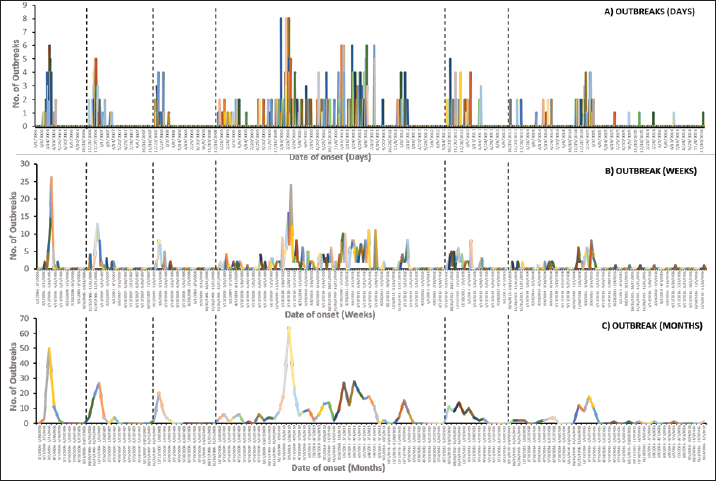
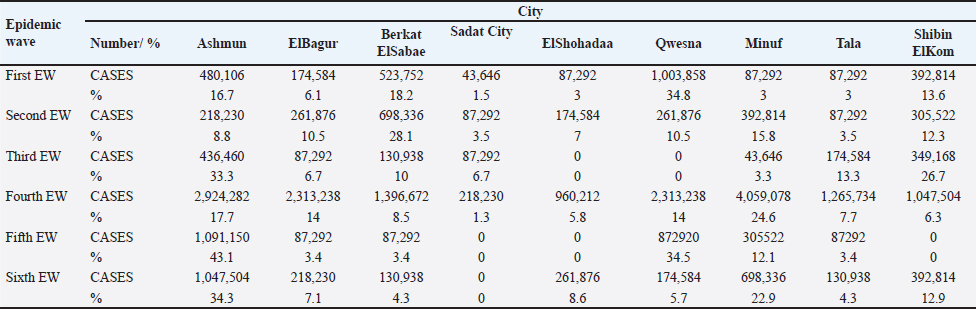
where: SD is the standard distance Dm is the median distance n is the number of points if no population field is used, or if a population field is supplied, n is the sum of the population field values. Kernel density maps for the total number of cases were plotted for each EW to visualize the risk for the disease. Default cells and the output were selected in Square kilometers (Km2). Clustering, defined as an aggregation of diseases within specific spatial and temporal dimensions beyond what could be anticipated, is a fundamental concept in epidemiological analysis (Porta, 2014). Retrospective space-time permutation scan statistics were performed to detect the Spatio-temporal clusters for each EW by investigating whether the outbreaks were interrelated in space and time using (SaTScan 8.2.1 software) (Kulldorff et al. 2005; Onozuka and Hagihara, 2008; Kulldorff, 2010). The scanning window was a cylinder with the spatial and temporal dimensions as circular base and height, respectively. The temporal scanning window was set to <50% of the study period in each EW and a maximum of 50% of outbreaks were allowed in the spatial scanning window (Zhang et al., 2012). The likelihood ratio statistic was used to evaluate the possibility of a true spatiotemporal cluster in a window. The window with the maximum likelihood ratio statistic was considered the primary cluster while the remainder was considered the secondary cluster. Their statistical significance was tested through Monte Carlo simulations of 999 replications (Zhang et al., 2012). The time units of day, week, and month were used, respectively. ArcGIS 10.5 software was used to overlay results from different methods in a map for visual comparisons (ESRI, Redlands). Ethical approvalNot needed for this study. ResultsTemporal distribution of HPAI H5N1 outbreaks in Menofia governorate, EgyptThroughout the study period encompassing 2006 to 2017, six distinct epidemic waves (EW1-6) of H5N1 outbreaks were discerned, as illustrated in Figure 1. The first epidemic wave (first EW) commenced in February 2006, persisted for approximately 3 months, and encompassed 66 reported outbreaks. This initial wave reached its peak in mid-March 2006, subsequently abating in May 2006. The second epidemic wave (second EW) unfolded on November 21, 2006, and exhibited its peak in December of the same year, culminating in mid-April 2007. Notably, this wave spanned 4 months and comprised an estimated 57 outbreaks. Progressing to the third epidemic wave (third EW), the Menofia governorate encountered 30 outbreaks, spanning the duration of three months from December 2, 2007, to mid-March, 2008. The ensuing fourth epidemic wave (fourth EW) was characterized by its prolonged nature, reflecting the endemic status of the disease. This wave persisted from December 16, 2008, through February 11, 2012, resulting in a continuous surge of HPAI H5N1 outbreaks over a span of more than 3 years. The cumulative outbreak count during this extended period amounted to 378. The dynamics of the outbreak presented as four distinct epidemic cycles. The first cycle spanned from January 2009 to August 2009, followed by the second cycle from January 2010 to August 2010. The third cycle persisted from January 2011 to August 2011, with the final cycle peaking in January 2012. The fifth epidemic wave (fifth EW) started in September 2012 and persisted until late May 2013. This wave demonstrated a series of consecutive peaks, notably observed in October 2012, late November 2012, early February 2013, and mid-April 2013, constituting a cumulative total of 58 reported outbreaks. Subsequently, the sixth epidemic wave (sixth EW) emerged in October 2013, culminating on December 10, 2016, and registering 70 continuous outbreaks. A solitary definitive peak was observed during this wave in February 2015, as delineated in Figure 1. Figures 2 and 3 offer a comprehensive depiction of outbreak distribution across localities for each of the six epidemic waves. Over the entire study duration, as depicted in Figure 2A, the localities reporting the highest numbers of outbreaks were Ashmun, Minuf, and Qwesna, in descending order. Following closely were Berkat-ElSabae, ElBagur, and Shibin-ElKom, constituting the second-highest category, succeeded by the third-highest category encompassing Tala, ElShohadaa, and Sadat-City. Table 1 and Figure 2B reveal that during the first epidemic wave (first EW), the predominant outbreak occurrences were observed in Qwesna, Berkat-ElSabae, and Ashmun, representing 34.8%, 18.2%, and 16.7% of the total cases, respectively. Similarly, the second epidemic wave (second EW) witnessed a concentration of outbreaks in Berkat-ElSabae, Minuf, and Shibin-ElKom, accounting for 28.1%, 15.8%, and 12.3% of total cases, respectively, as indicated by Table 1 and Figure 2C. In the context of the third epidemic wave (third EW), Ashmun, Shibin-ElKom, and Tala emerged as prominent outbreak regions, contributing to 33.3%, 26.7%, and 13.3% of total cases, respectively, as denoted in Table 1 and Figure 2D. Transitioning to the fourth epidemic wave (fourth EW), the focal points of outbreak notifications shifted to Minuf, Ashmun, and Qwesna, representing 24.6%, 17.7%, and 14% of total cases, respectively, as highlighted in Table 1 and Figure 2E. The fifth epidemic wave (fifth EW) underscored Ashmun as the locale with the highest outbreak instances within the Menofia governorate, comprising 43.1% of total outbreaks, closely followed by Qwesna at 34.5% of total outbreaks, as depicted in Table 1 and Figure 2F. Finally, during the sixth epidemic wave (sixth EW), the most substantial outbreak notifications in the Menofia governorate emanated from Ashmun, Minuf, and Shibin-ElKom, constituting 34.3%, 22.9%, and 12.9% of total cases, respectively, as illuminated by Table 1 and Figure 2G.
Fig. 1. Epidemic curves of outbreaks of highly pathogenic avian influenza subtype H5N1 in Menofia governorate from January 2006 to December 2016, illustrating A) daily, B) weekly, and C) monthly frequency of outbreaks as a function of time. Vertical lines delineate the six EWs. Table 1. Number of outbreaks during each of the six EWs in each city in Menofia governorate.
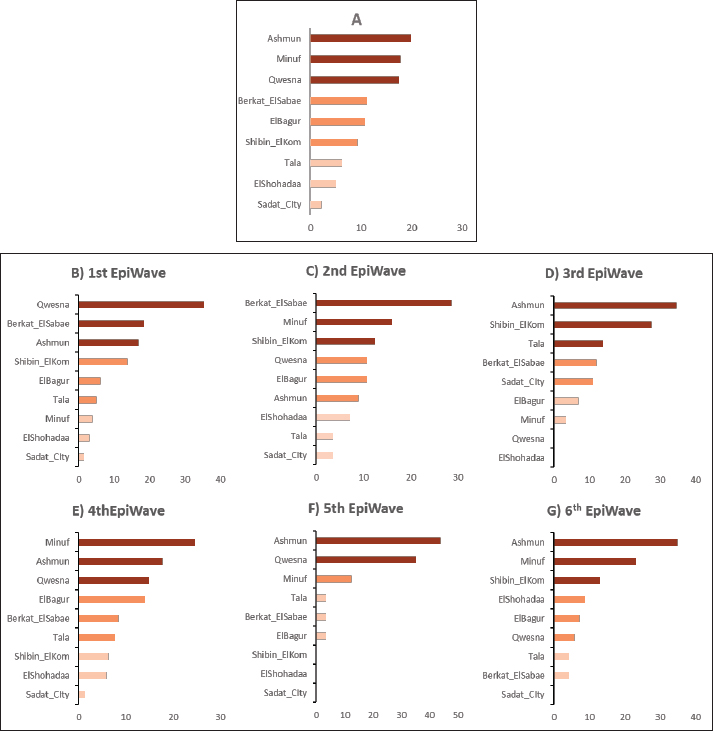
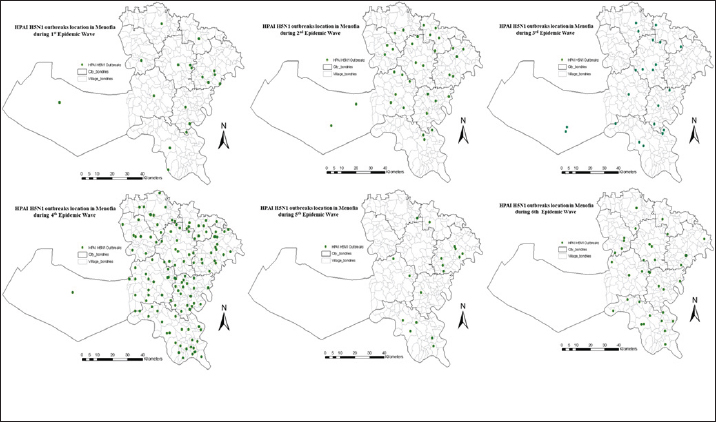
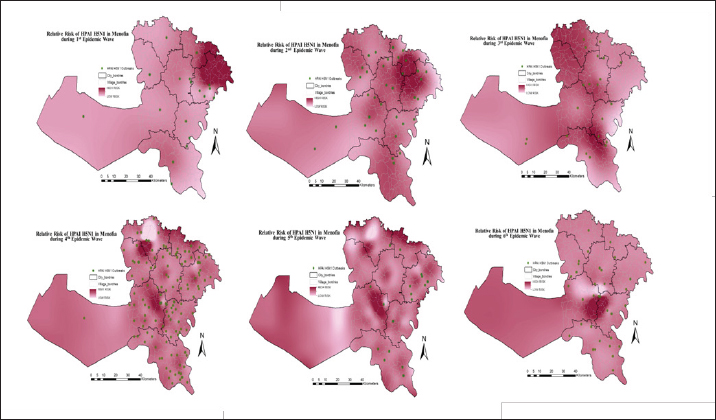
The spatial pattern of HPAI H5N1 outbreak in Menofia governorate, EgyptThe spatial distribution pattern of HPAI H5N1 outbreaks within the Menofia governorate is effectively depicted in Figure 3, which delineates the region into six distinct EWs. To facilitate comprehensive visual comparison, a village-based map was employed through ArcGIS 10.5 software (ESRI, Redlands, CA, USA). Employing an ordinary kriging approach, the raster risk map illustrated in Figure 4 generates an interpolated surface that furnishes predictive insights into the likelihood of outbreak occurrences across various locations within the Menofia governorate during each EW. Notably, during the first EW, Berkat-ElSabae, and Qwesna, situated along the northeast border of the governorate, exhibited the most pronounced predicted risk of HPAI H5N1 spatial occurrences. Subsequently, the subsequent EW witnessed a more widespread dissemination of outbreaks throughout the governorate, characterized by an overall heightened density. The highest predicted risk of outbreak occurrence was particularly concentrated in the northeastern cities (Berkat-ElSabae and Qwesna cities), extending further to encompass the governorate’s central cites (Minuf, ElShohadaa, and Shibin-ElKom cities), ultimately culminating in Ashmun city. In the context of the third EW, a distinct shift in risk distribution was discerned, with the northeast segment of the governorate (encompassing Berkat-ElSabae and Qwesna cities) displaying a notably diminished predicted risk of HPAI H5N1 occurrences. Conversely, the highest predicted risk was prominently identified along the governorate’s northwestern borders (Tala city), extending towards its central region (Shibin-ElKom city), and extending southward to Ashmun city. Transitioning to the fourth EW, outbreak dissemination followed a trend where the northern governorate borders exhibited the highest predicted risk. The fifth EW witnessed a further expansion of outbreaks throughout the entirety of the governorate, with elevated predicted risk observed at both its northern and southern borders. In the sixth EW, a discernible decrease in outbreak density was observed, with the highest predicted risk noted in Minuf and ElBagur. The present investigation delves into a comprehensive depiction of outbreak density and cluster analysis of HPAI H5N1 outbreaks across six distinct EWs within the Menofia governorate. This analytical depiction is notably articulated through the illustrative framework presented in Figure 5. Outbreak density in the Menofia governorate was meticulously delineated via the utilization of adaptive kernel density estimation, wherein varying densities were visually represented by a gradient of monochromatic grey hues, with heightened density translating to a darker coloration. Concurrently, the geographic distribution of outbreaks was symbolized through the use of distinctive green dots. Turning our focus to the spatial dynamics characterizing each of the EWs, it was observed that the initial outbreak wave (first EW) within the Menofia governorate registered its highest outbreak density concentrations within Qwesna and Shibin-ElKom.
Fig. 2. (A) Spatial Distribution of HPAI H5N1 outbreaks in Menofia governorate in the period from 2006 to 2016 (B, C, D, E, F, and G are representing the six epidemic waves). Vertical axis represents the cities, while horizontal axis represents the total no. of outbreaks.
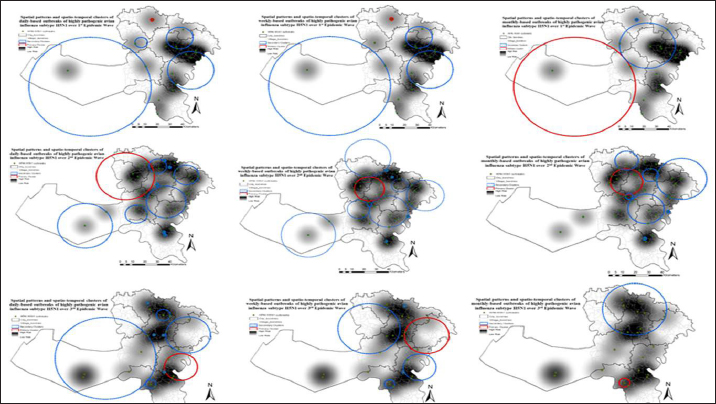
Fig. 3. Spatial distribution of HPAI H5N1 outbreaks in Menofia governorate were divided into corresponding subsets according to the EWs and depicted in the village-based map for visual comparison using ARCGIS 10.5 software (ESRI, Redlands, CA, USA). As the progression of the second EW unfolds; the dispersion of outbreak densities spans across Qwesna, Berkat-ElSabae, ElShohadaa, Minuf, and Ashmun. The third EW showcased a spatial extension of outbreak density that encompassed regions such as Tala, Berkat-ElSabae, Shibin-ElKom, and Ashmun. The fourth EW witnessed a notable proliferation of outbreaks enveloping the entirety of the governorate, with particularly heightened densities concentrated in Qwesna, Berkat-ElSabae, ElBagur, and Ashmun. Shifting our attention to the fifth EW, the distribution of outbreak occurrences became more localized, primarily confined to Qwesna and Ashmun. Finally, within the sixth EW, areas such as Qwesna, Shibin-ElKom, ElShohadaa, Minuf, and Ashmun demonstrated a pronounced surge in outbreak density. The integration of outbreak density and cluster analysis, as substantiated through the comprehensive presentation with the visual representation rendered by Figure 5, enriched our understanding of the intricate spatial dynamics underlying HPAI H5N1 outbreaks across successive EWs within Menofia governorate. Spatiotemporal clustersIn this study, the identification of outbreak clusters across successive EWs in the Menofia governorate was achieved through spatiotemporal cluster analysis. The precise locations and extent of diverse clusters within the Menofia governorate, offering a comprehensive overview can be seen in Figure 5. Over the course of six EWs, the Menofia governorate exhibited discernible spatial patterns and the emergence of spatiotemporal clusters of HPAI subtype H5N1 outbreaks, manifesting at daily, weekly, and monthly intervals. Employing space-time permutation scan statistics, significant spatiotemporal clusters were discerned and characterized by both the most likely cluster (denoted by a red circle) and secondary clusters (indicated by blue dashed circles) within Figure 5. During the initial wave (first EW), the primary and most likely cluster emerged in Tala City towards the conclusion of the wave. Concurrently, secondary clusters encompassed Tala, ElShohadaa, Shibin-ElKom, Minuf, Qwesna, and Berkat-ElSabae, spanning a radius of 26.37 kilometers based on monthly outbreak data. In the second EW, the primary cluster (with a radius of 6.56 km) was manifested in Tala, ElShohadaa, and Shibin-ElKom towards the wave’s end, considering both weekly and monthly outbreak data. Additionally, the primary cluster based on daily outbreak data extended to include Minuf city within a larger radius of 12.60 km. Secondary clusters encompassed the entire governorate during this wave, with notable highest relative risk clusters situated in small villages such as Ashmun, ElBagur, and Shibin-ElKom.
Fig. 4. Raster risk map showing interpolated spatial prediction surface of HPAIH5N1 outbreak risk, based on ordinary kriging with ArcMap version 10.1 (ESRI, Redlands, CA, USA). Daily outbreaks of highly pathogenic avian influenza subtype H5N1 over six EWs were represented by light green dots and outbreak Relative risk from spatial analysis highlighted in monochromatic red (the higher the risk, the darker the color). Transitioning to the third EW, the daily-based analysis revealed a primary cluster at the end of the wave, characterized by a radius of 6.87 km and centered in ElBagur and Ashmun. The monthly-based data, however, showcased a smaller primary cluster (2.14 km radius) exclusively in Ashmun. Furthermore, the weekly analysis detected a primary cluster encompassing ElBagur, Shibin_ElKom, Qwesna, and Berkat-ElSabae, spanning a radius of 9.29 km. Secondary clusters exhibited prevalence across the majority of cities, with prominent most likely clusters identified within the villages of Shibin-ElKom, Ashmun, Berkat-ElSabae, and Tala. The fourth EW featured a primary cluster positioned in ElBagur, Qwesna, and Shibin-ElKom at the wave’s midpoint, encompassing an area with a 10.21 km radius as depicted in Figure 5, based on daily and weekly outbreak data. Contrastingly, the analysis of monthly-based outbreaks revealed a smaller primary cluster (5.17 km radius) in Qwesna and Berkat-ElSabae. Notably, secondary clusters sequentially covered the entire geographic expanse of the governorate during this wave, with the most elevated relative risk clusters predominantly concentrated in Minuf and Tala. By the culmination of the fifth EW, a primary cluster characterized by a 14.01 km radius emerged in Minuf, Ashmun, and ElBagur based on weekly outbreak data. Alternatively, the monthly-based analysis indicated smaller primary clusters (6.46 km radius) in the same cities with the highest relative risk. Within the context of daily outbreak data, the primary cluster encompassed solely Shibin-ElKom and Qwesna, spanning a radius of 4.04 km. Remarkably, the highest relative risk secondary clusters were identified in Tala, Berkat-ElSabae, Ashmun, and Minuf. Transitioning to the sixth EW, primary clusters materialized at various points throughout the wave, spanning daily, weekly, and monthly outbreak data. These primary clusters were consistently accompanied by secondary clusters that pervaded the entirety of the governorate. Within the daily-based analysis, primary clusters (7.97 km radius) were detected in Minuf and Ashmun towards the close of 2014. Likewise, the monthly based analysis unveiled a primary cluster (10.06 km radius) encompassing Minuf, Shibin-ElKom, and ElBagur from January 2015, persisting for 6 months. Finally, the weekly-based examination identified a primary cluster (6.02 km radius) located in Tala, Berkat-ElSabae, and Shibin-ElKom at the end of 2015. Secondary clusters with elevated relative risk were notably situated in Ashmun, Qwesna, ElShohadaa, Shibin-ElKom, ElBagur, and Minuf, encompassing nearly all cities within the Menofia governorate.
Fig. 5. Spatial patterns and spatio-temporal clusters of daily, weekly, and monthly based outbreaks of highly pathogenic avian influenza subtype H5N1 over six EW in Menofia governorate. Outbreaks represented by light green dots and outbreak density from adaptive kernel density estimation highlighted in monochromatic grey (the higher the density, the darker the color). Significant spatio-temporal clusters detected from the space–time permutation scan statistics are illustrated by the most likely cluster (red circle) and by a secondary cluster (blue-dashed circles). DiscussionOur study aimed to investigate the spatiotemporal pattern, identify, and trace the highest risk clusters of HPAI H5N1 outbreaks at the subdistrict/village level within Menofia governorate, from 2006 to 2017 as a trial for tracking the HPAI H5N1 endemicity dynamics for better establishment of effective disease control strategies at that level. Epidemic waves of HPAI H5N1 outbreaks in Menofia governorateThe analysis of HPAI subtype H5N1 outbreaks in Menofia governorate revealed a notable pattern across six distinct EWs, characterized by varying time scales of day, week, and month. These EWs exhibited consistent patterns, underscoring the robust epidemic nature of the disease. The duration of each EW ranged from 3, 5, 3, 38, 7, and 39 months, as depicted in Figure 1. Notably, the epidemic curve within Menofia governorate closely mirrored the temporal patterns observed in Egypt’s broader epidemiological landscape, as elucidated by previous research conducted by (Elsobky et al., 2021; Elsobky et al., 2022), and a global study by (Zhang et al., 2012). A recurring feature of the epidemic curve is the occurrence of disease peaks in December and January, indicative of an apparent non interpreted epidemic cycle. This pattern aligns with the cyclic nature of the disease, highlighting its endemic status. The configuration of the epidemic curve, characterized by a brief vertical span and an extensive horizontal span, underscores the endemicity and suggests an expanding spatial distribution of outbreaks over time within the governorate. Menofia governorate played a pivotal role in the broader Egyptian EW. For instance, the first EW in Menofia governorate commenced merely 1 week following the disease’s initial introduction in Egypt and concluded 2 months prior to the termination of the country’s inaugural EW, according to the EW of HPAI H5N1 outbreaks in Egypt described by Elsobky et al. (2022). Notably, the 2.2.1 Clade emerged during the first EW and persisted throughout the subsequent second and third EWs. Additionally, the 2.2.1.1 Clade, introduced in the second EW, continued to be prevalent during the third EW, ultimately culminating in endemicity by El-Shesheny et al. (2020). Remarkably, Menofia governorate consistently reported higher outbreak numbers during distinct EWs, although it was not the origin of outbreaks. This phenomenon can be attributed to the high density of the poultry population in the governorate, thereby contributing to its prominence in outbreak notifications. Further exploration of the fourth EW in Menofia reveals a distinct epidemiological landscape. This wave was notable for being associated with the coexistence of two clades, namely 2.2.1.1a and 2.2.1.2, coinciding with the emergence of H9N2 (El-Shesheny et al., 2020; Elsobky et al., 2022). It is noteworthy that the fourth EW exhibited a peculiar pattern wherein outbreaks initially occurred during warmer months, suggesting a possible link between clade stability and thermostability (El-Shesheny et al., 2020). This underscores the virus’s capacity for sustained endemicity through adaptive processes (Arafa et al., 2016a; Salaheldin et al., 2018). During the fifth EW, a protracted duration of continuous outbreak notifications was observed, characterized by irregular peaks and altered epidemic cycles. This phenomenon, corroborated by the presence of clades 2.2.1, 2.2.1.1a, and 2.2.1.2, aligns with the concept of viral adaptation and endemicity, a notion that concurs with findings by El-Shesheny et al. (2020). Conversely, the sixth EW culminated without distinct peaks or discernible patterns, potentially attributed to under-reporting and limitations within the surveillance system. This observation resonates with the findings of Elsobky et al. (2022) regarding EWs in Egypt. In essence, the analysis of HPAI H5N1 outbreaks in the Menofia governorate underscores the dynamic interplay between viral evolution, adaptation, and endemicity across distinct EWs. The governorate’s role in shaping the broader epidemiological landscape is pivotal, and the implications of these findings emphasize the importance of vigilance and refined surveillance strategies to address and manage the evolving dynamics of HPAI H5N1 outbreaks to contain outbreaks within delineated areas where the initiation phase starts before the outbreak acceleration phase. The spatial pattern of outbreak density in Menofia governorateThe geographic distribution of HPAI H5N1 outbreaks in Egypt has been extensively studied, consistently revealing a concentration of poultry outbreaks within the densely populated Nile Delta region, while demonstrating comparatively lower densities in upper Egypt (Albayoumi et al., 2013; Kayali et al., 2016; Young et al., 2018; Elsobky et al., 2022). This pattern is attributed to the significant poultry stocks and human population present in the Nile Delta region (Arafa et al., 2012). Notably, more than half of Egypt’s population is in direct proximity to poultry in the Nile Delta region, as reported by Abdelwahab and Hafez (2011). Consequently, the Menofia governorate, situated within lower Egypt, assumes a notable role in the potential zoonotic transmission of H5N1, as outlined in a previous study (Young et al., 2018). As evident from Figure 4, the spatial risk of HPAI H5N1 outbreaks escalates along the governorate’s borders with neighboring provinces, particularly its northern border. Additionally, the elevated risk is observed in locales characterized by numerous small unlicensed farms and backyard poultry. These areas, known for their random distribution across Menofia’s cities, underscore the importance of vigilance and active surveillance. In the context of the sixth EW, a decline in spatial risk is noted, potentially attributed to the under-notification of outbreaks, highlighting the urgency of enhanced surveillance coordination among governorates. Effective control strategies are imperative and should encompass measures such as safeguarding borders, quarantining affected villages, appropriate disposal of deceased birds, restrictions on live bird transportation and markets, and meticulous vaccination coverage coupled with robust biosecurity practices. Notably, the longevity of HPAI H5N1 virus survival under diverse temperatures underscores the significance of these control measures (Stallknecht et al., 1990; Si et al., 2013). Kernel density analyses depicted in Figure 5 elucidate the outbreak dynamics within Menofia governorate, with rural districts, particularly those situated within the delta region, exhibiting prominent hotspots across all six EWs. This phenomenon is linked to the prevalence of household poultry, both registered and unregistered small poultry farms, and informal markets. The pattern also demonstrates a decrease in outbreak density with increased urbanization, elucidating lower densities within more urbanized zones like main city areas and industrial regions such as Sadat City, consistent with findings by Gafi (2018). Observed smoothed densities revealed epidemic propagation within urban centers, preceding its transition to new areas and establishing direct connections with neighboring cities, the same as illustrated by Norström et al. (1999). This perspective indicates that the control measure is not effective in containing outbreaks within delineated areas, which refers to the importance of early actions to control the spread of disease successfully. The identified clusters, both primary and secondary, reinforce the notion of successive outbreak occurrences within Menofia governorate. Primary clusters, consistently identified towards the middle or end of each wave, often align with high relative risk clusters from preceding waves. These clusters are situated within the hot-spot region, albeit with varying locations and sizes. The proactive control of such clusters necessitates intensified interventions at the outset of a wave, thereby curbing spatial disease spread. The sequential emergence of secondary clusters extends to encompass the entirety of the governorate’s geographical expanse, demonstrating enduring persistence over extended periods, frequently spanning months. This phenomenon is concomitantly characterized by the proliferation of discrete pinpoint clusters, indicative of localized dissemination of infection through direct contact within poultry populations, frequently transpiring in the absence of efficacious intervention measures. However, the success of control measures remains limited in effectively compressing spatial disease spread and curtailing epidemic progression. This emphasizes the urgency for tailored disease control strategies operating at subdistrict levels within each governorate, informed by a nuanced understanding of endemicity dynamics. Furthermore, continuous evaluation and refinement of control strategies based on real-world data are essential, given the challenges associated with obtaining accurate and comprehensive outbreak data. However, it is noteworthy to acknowledge certain limitations inherent to this study, including the inability to fully account for various control measures, potential detection bias, and variations in demographic characteristics among at-risk groups. ConclusionThis study highlights the significance of customized regional interventions based on the rigorous epidemiological framework. This approach is pivotal in the profound comprehension of endemicity dynamics, efficiently limits geographical infection spread, and contains outbreaks within delineated areas. AcknowledgmentsNone. Conflict of interestThe authors declare that they have no competing interests. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors’ contributionsYE the first author is the one who did the research and wrote the manuscript’s first draft data collection and processing, and was a major contributor in cleaning, analysis, and interpretation. ME (Eltholth) and EA have given significant intellectual inputs in the final revision and participated in the conceiving and designing of the manuscript. MN, AS, and WM Made significant contributions to the cleaning and interpretation of the data. NE, GH, and AK participated in cleaning, and writing the manuscript. ME (Elkamshishi), AMA, and OG participated in data collection, processing, and writing. All authors critically reviewed the manuscript and approved the final manuscript. Data availabilityAll data generated or analyzed during this study are included in this published article. More tables are available and can be requested from the Corresponding Author. ReferencesAbdelwhab, E.M. and Hafez, H.M. 2011. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol. Infect. 139(5), 647–657. Abdelwhab, E., Hassan, M., Abdel-Moneim, A., Naguib, M., Mostafa, A., Hussein, I., Arafa, A., Erfan, A., Kilany, W. and Agour, M. 2016. Introduction and enzootic of A/H5N1 in Egypt: virus evolution, pathogenicity and vaccine efficacy ten years on. Infect. Genet. Evol. 40, 80–90. Ali, A., Ankers, P., DeHaan, N., Saad, A., Hussein, S., Lubroth, J. and Jobre, Y. 2013. Mapping influenza A (H5N1) virus transmission pathways and critical control points in Egypt. FAO Anim. Prod. Health. Work. Aly, M., Arafa, A. and Hassan, M. 2008. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 52(2), 269–277. Arafa, A., Suarez, D., Kholosy, S., Hassan, M., Nasef, S., Selim, A., Dauphin, G., Kim, M., Yilma, J. and Swayne, D. 2012. Evolution of highly pathogenic avian influenza H5N1 viruses in Egypt indicating progressive adaptation. Arch. Virol. 157(10), 1931–1947. Arafa, A., Naguib, M., Luttermann, C., Selim, A., Kilany, W., Hagag, N., Samy, A., Abdelhalim, A., Hassan, M. and Abdelwhab, E. 2015. Emergence of a novel cluster of influenza A (H5N1) virus clade 2.2. 1.2 with putative human health impact in Egypt, 2014/15. Euro. Surveill. 20(13), 2–8. Arafa, A., El-Masry, I., Khoulosy, S., Hassan, M.K., Soliman, M., Fasanmi, O.G., Fasina, F.O., Dauphin, G., Lubroth, J. and Jobre, Y.M. 2016a. Predominance and geo-mapping of avian influenza H5N1 in poultry sectors in Egypt. Geospat. Health. 11(3), 492. Arafa, A., El-Masry, I., Kholosy, S., Hassan, M.K., Dauphin, G., Lubroth, J. and Makonnen, Y.J. 2016b. Phylodynamics of avian influenza clade 2.2. 1 H5N1 viruses in Egypt. Virol. J. 13(1), 49. Bailey, T.C. and Gatrell, A.C. 1995. Interactive spatial data analysis.Longman Scientific & Technical, vol. 413. Avaialble via http://www.personal.psu.edu/faculty/f/k/fkw/rsoc597/UgandaMaps.pdf (Accessed May 23, 2023) Balish, A.L., Davis, C.T., Saad, M.D., El-Sayed, N., Esmat, H., Tjaden, J.A., Earhart, K.C., Ahmed, L.E., El-Halem, M.A. and Ali, A.H.M. 2010. Antigenic and genetic diversity of highly pathogenic avian influenza A (H5N1) viruses isolated in Egypt. Avian Dis. 54(s1), 329–334. Dhingra, M.S., Dissanayake, R., Negi, A.B., Oberoi, M., Castellan, D., Thrusfield, M., Linard, C. and Gilbert, M. 2014. Spatio-temporal epidemiology of highly pathogenic avian influenza (subtype H5N1) in poultry in eastern India. Spat. Spatiotemporal. Epidemiol.11, 45–57. Ekong, P., Fountain-Jones, N. and Alkhamis M. 2018. Spatiotemporal evolutionary epidemiology of H5N1 highly pathogenic avian influenza in West Africa and Nigeria, 2006–2015. Transbound. Emerg. Dis. 65(1), e70–e82. ELbayoumi, K.M., Mahgoub, K., Mekky, H.M., Hassan, E.R., Amin Girh, Z., Maatouq, A.M., El-Samadony, H.A., Rabie, N.S., Ali, M.A.A. and Kutkat, M.A. 2013. Molecular detection of H5N1, H9N2 and Newcastle disease viruses isolated from chicken in mixed infection in Egypt. World Appl. Sci. J. 27(1), 44–50. ElMasry, I., Elshiekh, H., Abdlenabi, A., Saad, A., Arafa, A., Fasina, F.O., Lubroth, J. and Jobre, Y. 2017. Avian influenza H5N1 surveillance and its dynamics in poultry in live bird markets, Egypt. Transbound. Emerg. Dis. 64(3), 805–814. El-Shesheny, R., Kandeil, A., Bagato, O., Maatouq, A.M., Moatasim, Y., Rubrum, A., Song, M.S., Webby, R.J., Ali, M.A. and Kayali, G. 2014. Molecular characterization of avian influenza H5N1 virus in Egypt and the emergence of a novel endemic subclade. J. Gen. Virol. 95(Pt 7), 1444–1463. El-Shesheny, R., Kandeil, A., Mostafa, A., Ali, M.A. and Webby, R.J. 2021. H5 Influenza viruses in Egypt. Cold Spring Harb. Perspect. Med. 11(6), a038745. Elsobky, Y., Nganwa, D., El Afandi, G., Byomi, A., Reddy, G. and Abdalla, E. 2021. A quantitative risk assessment to evaluate the efficacy of mitigation strategies to reduce highly pathogenic avian influenza virus, subtype H5N1 (HPAI H5N1) in the Menoufia governorate, Egypt. BMC Vet. Res. 17(1), 210. Elsobky, Y., El Afandi, G., Salama, A., Byomi, A., Omar, M. and Eltholth, M. 2022. Spatiotemporal analysis of highly pathogenic avian influenza (H5N1) outbreaks in poultry in Egypt (2006 to 2017). BMC Vet. Res. 18(1), 174. El-Zoghby, E.F., Aly, M.M., Nasef, S.A., Hassan, M.K., Arafa, A.S., Selim, A.A., Kholousy, S.G., Kilany, W.H., Safwat, M. and Abdelwhab, E. 2013. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virol. J. 10(1), 203. Environmental Systems Research Institute (ESRI). 2014. ArcGIS Desktop Help 10.2 Geostatistical analyst. Available via https://resources.arcgis.com/en/help/main/10.2/index.html#//009z00000011000000 FAO. 2017. EMPRES-i-global animal disease information system. Available at: http://empres-i.fao.org/eipws3g/#h=0 Farnsworth, M.L., Hamilton-West, C., Fitchett, S., Newman, S.H., de La Rocque, S., De Simone, L., Lubroth, J. and Pinto, J. 2010. Comparing national and global data collection systems for reporting, outbreaks of H5N1 HPAI. Prev. Vet. Med. 95(3-4), 175–185. Fasina, F.O., Ali, A., Yilma, J., Thieme, O. and Ankers, P. 2016. Production parameters and profitability of the Egyptian household poultry sector: a survey. World’s Poult. Sci. J. 72(1), 17-–188. GAFI, 2018. “Industrial Zones of Governorate”. Ministry of Investment Egypt. Archived from the original on 2018-11-23. Available via: https://www.gafi.gov.eg/English/StartaBusiness/InvestmentZones/Pages/Industrial-Zones.aspx (Accessed November 23, 2023). Cairo, Egypt. Harfoot, R. and Webby, R.J. 2017. H5 influenza, a global update. J. Microbiol. 55(3), 196–203. Jones, S.G. and Kulldorff, M. 2012. Influence of spatial resolution on space-time disease cluster detection. PLoS One. 7(10), e48036. Kalthoff, D., Globig, A. and Beer, M. 2010. (Highly pathogenic) avian influenza as a zoonotic agent. Vet. Microbiol. 140(3-4), 237–245. Kayali, G., Webby, R.J., Ducatez, M.F., El Shesheny, R.A., Kandeil, A.M., Govorkova, E.A., Mostafa, A. and Ali, M.A. 2011. The epidemiological and molecular aspects of influenza H5N1 viruses at the human-animal interface in Egypt. PLoS One. 6(3), e17730. Kayali, G., Kandeil, A., El-Shesheny, R., Kayed, A.S., Maatouq, A.M., Cai, Z., McKenzie, P.P., Webby, R.J., El Refaey, S. and Kandeel, A. 2016. Avian influenza A (H5N1) virus in Egypt. Emerg. Infect. Dis. 22(3), 379–388. Kulldorff, M. 2010. Information Management Services, Inc. (2009) SaTScanTM v8. 0: Software for the spatial and space-time scan statistics. Available via http://www.satscan.org 2010 Kulldorff, M., Heffernan, R., Hartman, J., Assunçao R. and Mostashari, F. 2005. A space–time permutation scan statistic for disease outbreak detection. PLoS Med. 2(3), e59. Matrosovich, M., Zhou, N., Kawaoka, Y. and Webster, R. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73(2), 1146–1155. Matsumoto, P.S.S., Hiramoto, R.M., Pereira, V.B.R., Camprigher, V.M., Taniguchi, H.H., de Raeffray Barbosa, J.E., Cortez, L.R.P., Fonseca, E.d.S., Guimarães, R.B. and Tolezano, J.E. 2021. Impact of the dog population and household environment for the maintenance of natural foci of Leishmania infantum transmission to human and animal hosts in endemic areas for visceral leishmaniasis in Sao Paulo state, Brazil. PLoS One. 16(8), e0256534. Norström, M., Pfeiffer, D.U. and Jarp, J. 1999. A space-time cluster investigation of an outbreak of acute respiratory disease in Norwegian cattle herds. Prev. Vet. Med. 47(1-2), 107–119. O’Brien, S.H., Webb, A., Brewer, M.J. and Reid, J.B. 2012. Use of kernel density estimation and maximum curvature to set marine protected area boundaries: identifying a special protection area for wintering red-throated divers in the UK. Biol. Conser. 156, 15–21. Oliver, M.A. and Webster, R. 1990. Kriging: a method of interpolation for geographical information systems. Int. J. Geograph. Inform. Syst. 4(3), 313–332. Onozuka, D. and Hagihara, A. 2008. Spatial and temporal dynamics of influenza outbreaks. Epidemiology. 19(6), 824–828. Otte, J., Hinrichs, J., Rushton, J., Roland-Holst, D. and Zilberman, D. 2008. Impacts of avian influenza virus on animal production in developing countries. CAB Rev: Persp. Agric. Vet. Sci. Nutr. Natu. Resou. 3(80), 18. Porta, M. 2014. A dictionary of epidemiology. Oxford, UK: Oxford University Press. Saad, M.D., Lu’ay, S.A., Gamal-Eldein, M.A., Fouda, M.K., Khalil, F.M., Yingst, S.L., Parker, M.A. and Montevillel, M.R. 2007. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg. Infec. Dis. 13(7), 1120–1121. Salaheldin, A.H., Kasbohm, E., El-Naggar, H., Ulrich, R., Scheibner, D., Gischke, M., Hassan, M.K., Arafa, A.S.A., Hassan, W.M. and El-Hamid, A. 2018. Potential biological and climatic factors that influence the incidence and persistence of highly pathogenic H5N1 avian influenza virus in Egypt. Front. Microbiol. 9, 528. Sheta, B.M., Fuller, T.L., Larison, B., Njabo, K.Y., Ahmed, A.S., Harrigan, R., Chasar, A., Aziz, S.A., Khidr, A.A.A. and Elbokl, M.M. 2014. Putative human and avian risk factors for avian influenza virus infections in backyard poultry in Egypt. Vet. Microbial. 168(1), 208–213. Si, Y., de Boer, W.F. and Gong, P. 2013. Different environmental drivers of highly pathogenic avian influenza H5N1 outbreaks in poultry and wild birds. PLoS One. 8(1), e53362. Silverman, B.W. 1998. Density estimation for statistics and data analysis, 1st ed. London, UK: Routledge. Doi: 10.1201/9781315140919. Stallknecht, D.E., Shane, S.M., Kearney, M.T. and Zwank, P.J. 1990. Persistence of avian influenza viruses in water. Avian Dis. 34(2), 406–411. Toma, B., Benet, J.J.C., Duford, B.C., Eloit, M.C., Marsh, W.C., Michel, P.C., Moutou, F.C., Sanaa, M.C. and Vaillancourt, J.P. 1999. Dictionary of veterinary epidemiology. Ames, LA: Iowa State University Press. Worton, B.J. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecolog. 70(1), 164–168. Young, S.G., Kitchen, A., Kayali, G. and Carrel, M. 2018. Unlocking pandemic potential: prevalence and spatial patterns of key substitutions in avian influenza H5N1 in Egyptian isolates. BMC Inf. Dis. 18, 314. Zhang, Z., Chen, D., Chen, Y., Liu, W., Wang, L., Zhao, F. and Yao, B. 2010. Spatio-temporal data comparisons for global highly pathogenic avian influenza (HPAI) H5N1 outbreaks. PLoS One. 5(12), e15314. Zhang, Z., Chen, D., Chen, Y., Davies, T.M., Vaillancourt, J.P. and Liu, W. 2012. Risk signals of an influenza pandemic caused by highly pathogenic avian influenza subtype H5N1: spatio-temporal perspectives. Vet. J. 192(3), 417–421. | ||
| How to Cite this Article |
| Pubmed Style Elsobky Y, Eltholth M, Abdalla E, Eissa N, Hadad G, Nayel M, Salama A, Mousa W, Kamal A, Abu-seida AM, Gaidan OK, Elkamshishi M. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Vet. J.. 2024; 14(11): 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 Web Style Elsobky Y, Eltholth M, Abdalla E, Eissa N, Hadad G, Nayel M, Salama A, Mousa W, Kamal A, Abu-seida AM, Gaidan OK, Elkamshishi M. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. https://www.openveterinaryjournal.com/?mno=215642 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.20 AMA (American Medical Association) Style Elsobky Y, Eltholth M, Abdalla E, Eissa N, Hadad G, Nayel M, Salama A, Mousa W, Kamal A, Abu-seida AM, Gaidan OK, Elkamshishi M. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Vet. J.. 2024; 14(11): 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 Vancouver/ICMJE Style Elsobky Y, Eltholth M, Abdalla E, Eissa N, Hadad G, Nayel M, Salama A, Mousa W, Kamal A, Abu-seida AM, Gaidan OK, Elkamshishi M. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 Harvard Style Elsobky, Y., Eltholth, . M., Abdalla, . E., Eissa, . N., Hadad, . G., Nayel, . M., Salama, . A., Mousa, . W., Kamal, . A., Abu-seida, . A. M., Gaidan, . O. K. & Elkamshishi, . M. (2024) Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Vet. J., 14 (11), 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 Turabian Style Elsobky, Yumna, Mahmoud Eltholth, Ehsan Abdalla, Nourhan Eissa, Ghada Hadad, Mohamed Nayel, Akram Salama, Walid Mousa, Ahmed Kamal, Ashraf M. Abu-seida, Osama K. Gaidan, and Mohamed Elkamshishi. 2024. Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Veterinary Journal, 14 (11), 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 Chicago Style Elsobky, Yumna, Mahmoud Eltholth, Ehsan Abdalla, Nourhan Eissa, Ghada Hadad, Mohamed Nayel, Akram Salama, Walid Mousa, Ahmed Kamal, Ashraf M. Abu-seida, Osama K. Gaidan, and Mohamed Elkamshishi. "Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt." Open Veterinary Journal 14 (2024), 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 MLA (The Modern Language Association) Style Elsobky, Yumna, Mahmoud Eltholth, Ehsan Abdalla, Nourhan Eissa, Ghada Hadad, Mohamed Nayel, Akram Salama, Walid Mousa, Ahmed Kamal, Ashraf M. Abu-seida, Osama K. Gaidan, and Mohamed Elkamshishi. "Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt." Open Veterinary Journal 14.11 (2024), 2911-2923. Print. doi:10.5455/OVJ.2024.v14.i11.20 APA (American Psychological Association) Style Elsobky, Y., Eltholth, . M., Abdalla, . E., Eissa, . N., Hadad, . G., Nayel, . M., Salama, . A., Mousa, . W., Kamal, . A., Abu-seida, . A. M., Gaidan, . O. K. & Elkamshishi, . M. (2024) Spatio-temporal dynamics and risk cluster analysis of highly pathogenic avian influenza (H5N1) in poultry: Advancing outbreak management through customized regional strategies in Egypt. Open Veterinary Journal, 14 (11), 2911-2923. doi:10.5455/OVJ.2024.v14.i11.20 |