| Research Article | ||
Open Vet. J.. 2024; 14(11): 2924-2935 Open Veterinary Journal, (2024), Vol. 14(11): 2924-2935 Research Article Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar ratZahraa A. Greash1, Layla Omran Elmajdoub2*, Eman Fayad3 and Ali H. Abu Almaaty11Zoology Department, Faculty of Science, Port Said University, Port Said, Egypt 2Zoology Department, Faculty of Science, Misurata University, Misurata, Libya 3Department of Biotechnology, College of Sciences, Taif University, Taif, Saudi Arabia *Corresponding Author: Layla Omran Elmajdoub. Zoology Department, Faculty of Science, Misurata University, Misurata, Libya. Email: elmajdoublayla [at] sci.misuratau.edu.ly Submitted: 15/08/2024 Accepted: 05/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
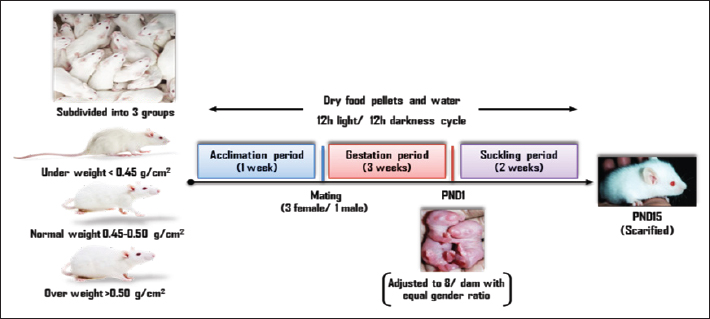
AbstractBackground: Cognitive impairment and attention deficit disorder have been on the rise among generations in recent times. A significant portion of the brain involved in learning and cognition is the hippocampus. Its development begins in utero till weaning. The mother’s body mass index (BMI) before pregnancy indicates her health; however, little data links maternal BMI before pregnancy to fetal hippocampal health outcomes. Aim: The study aimed to estimate the extent to which pre-pregnancy maternal BMI relates to their offspring brain status, and thus to what extent to this stage of life may be an opportunity for mental and cognitive development. Methods: Thirty-six naive female albino rats (Rattus norvegicus) at 8 weeks of age with an average weight of 190–220 g body weight were obtained and assigned to three experimental groups according to their body mass index into; under-, over-, and normal weight. Following one week of habituation, all females were allowed to mate (3 female/ 1 male). On postnatal day 1 (PND1), pups were randomly adjusted to 8/dam with an equal gender ratio. On 15 days postpartum, all pups were sacrificed. Hippocampi were removed and processed for histological investigations, Glial fibrillary acidic protein immunohistochemically, and flow cytometric assessments of apoptosis. Measurements of the cognitive brain were carried out. Results: The present findings manifested elevation in the inflammatory and apoptotic markers in the hippocampus of underweight mothers-offspring yielding a lower cognitive ability than overweight mothers-offspring compared to those whose mothers with normal weight before conception. The male offspring were more affected than female offspring especially those born to pre-pregnancy underweight mothers. Conclusion: The study concluded that there may be a connection between a mother’s pre-pregnancy BMI and her offspring’s cognitive capacities, which calls for more study to gain a deeper knowledge and to create interventions that target the physical health of the mother prior to pregnancy in order to enhance their offspring’s health and cognitive outcomes. Keywords: BMI, Cognition, Mother, Offspring, Pre-pregnancy. IntroductionPhysical, mental, and social well-being are all aspects of health that are directly influenced by lifestyle. The body mass index (BMI), is an anthropometric weight-for-height metric that is logically connected to the percentage of body fat (WHO, 1995), so the World Health Organization has proposed using BMI to monitor the measurement of obesity and underweight in the population level (WHO, 2000). Type 2 diabetes, ischemic stroke, hypertension, ischemic heart disease, osteoarthritis, colon-, breast-, and endometrial cancer are among the diseases that account for the worldwide burden of illness caused by high BMI (Ezzati et al., 2002; James et al., 2004; Finucane et al., 2011). The prevalence of obesity has increased in developing nations over the last 20 years as a result of the population’s adoption of modern lifestyles that include less physical exercise and excessive eating of inexpensive, high-energy foods (Hossain et al., 2007). At least 1.1 billion overweight adults, including 312 million obese according to estimates by the International Obesity Task Force (Haslam and James, 2005). The World Health Organization states that Egypt has the 18th highest rate of obesity prevalence globally (ProCon.org, 2020), where 49.5% of Egyptian females are suffering from obesity according to the 100 Million Health Initiative. Between the ages of 20 and 39 of non-pregnant women, 26% are overweight, and 29% are obese. Contrarily, maternal underweight is also frequent despite the current obesity pandemic. Low BMI has been characterized as a persistent energy deficit in underweight adults for their height as a result of insufficient food intake (Finucane et al., 2011). In low- and middle-income countries, underweight women at 15–44 years of age are still high, and are used to compute the global burden of disease related to malnutrition (Ezzati et al., 2002; Fishman et al., 2004; Black et al., 2008). Some underweight females of reproductive age have short stature, causing a higher risk of intrauterine growth restriction and higher neonatal outcomes which can result in more cesarean delivery (Fishman et al., 2004; Ronsmans et al., 2006). Prenatal life is a delicate time for brain development. Over 99% of the human neocortex is formed during intrauterine life, resulting in an astonishing diversity of fetal functions and activities (Salihagic Kadic and Predojevic, 2012). Many of the cognitive deficits and impairments that appear in childhood and adulthood may be due to defects in the brain during prenatal life (Salihagic Kadic et al., 2009). The hippocampus is a complex structure deeply ingrained in the temporal lobe of the brain. It plays a significant part in memory and learning. Whether a high mother’s BMI impairs fetal growth and brain development is less certain, it is linked to unfavorable long-term consequences for the offspring, such as obesity, worse social and cognitive functioning, and a higher chance of psychiatric disorders (Spann et al., 2020). However, the obesity pandemic is spreading throughout the Middle East and Egypt among, and the issue gets more serious when developing countries take into account the tendency towards a more “Western” lifestyle, where the Western diet style spread, is an obesogenic diet known as fast-, junk- and cafeteria food where high-fat dairy products, processed meats, fried foods, prepackaged foods, traditional animal products, refined grains, corn, eggs, red meat, high-sugar drinks, candy, and sweets (Steyn et al., 2004; Wright et al., 2011). The populations of such countries may be more susceptible to obesity and metabolic syndrome as a result of this “Westernization” (Bahrami et al., 2006). The media and society as a whole exert significant pressure on people to be thin (Wan et al., 2003), and eating and weight reduction habits are found to be significantly influenced by views of ideal weight (Gross et al., 2005). This has led to an obvious low BMI growing for the only purpose of gaining the pleasure of body satisfaction, disregarding the risks. Understanding how a mother’s BMI affects the health of her offspring is crucial for appropriately risk-stratifying a pregnancy at the outset, as is customarily required, so this study aims to estimate the extent to which pre-pregnancy maternal BMI relates to their offspring brain status, and thus to what extent to this stage of life may be an opportunity for mental and cognitive development. Material and MethodsExperimental animalsThirty-six naive female albino rats (Rattus norvegicus) at 8 weeks of age with average body weight 190–220 g were acquired from Suez Canal University, Faculty of Veterinary Medicine’s animal house, and were assigned to three experimental groups based on their body mass index as explained in Engelbregt et al. (2001) into; Group 1: Underweight with body mass index < 0.45 g/cm2 Group 2: Normal weight with body mass index 0.45–0.50 g/cm2 Group 3: Overweight with body mass index >0.50 g/cm2. All animals were housed in suitable plastic cages with sawdust bedding at Port Said University, Faculty of Science’s animal house, each group containing 4 cages of 3 animals (n=12). Dry food pellets and water were provided ad libitum with animal house conditions maintained on a 12 hours light: 12 hours darkness cycle. Experimental designFollowing 1 week of habituation to the housing conditions, all females were allowed to mate with normal fertile males at a ratio of 3 females/ 1 male overnight. In the next morning, males were removed from the cage, and vaginal smears were conducted for all females. Successful mating was confirmed by the presence of sperm in the smear, which was designated as zero-day gestation. Body mass was not measured during pregnancy and lactation to avoid maternal stress factors on offspring development. Before parturition, each dam was housed separately in a cage to deliver spontaneously and left undisturbed with their litters for 24 hours. PND1, pups were randomly adjusted to 8 per dam with a gender ratio of 1 male to 1 female as possible to ensure adequate and standardized nutrition during the suckling period. The pup’s weight was not recorded till the sacrificed day to avoid maternal and pup stress. On 15 days postpartum, diethyl ether was used to quickly dislocate the cervical vertebrae after anesthetizing each pup. Following confirmation of death, decapitation is performed then removing of the brain (Fig. 1). Histopathological investigationsHaematoxylin and eosin stain After the separation of hippocampi, they were fixed immediately in 10% phosphate-buffered formalin (pH 7) and processed into paraffin blocks. Histological sections of 5 µm thicknesses were prepared and stained with hematoxylin and eosin (Weesner, 1968), and then investigated under a bright field light microscope (OPTIKA, Italy). Glial fibrillary acidic protein (GFAP) immunohistochemical stainOn glass slides coated with polylysine, 5 μm thickness histological sections of paraffin-embedded, formalin-fixed hippocampal tissues were positioned. Tissue sections were packed at 65°C for the entire night. Then, they were deparaffinized in xylene and rehydrated in decreasing alcohol grades. For GFAP, the modified Avidin-Biotin immunoperoxidase method was utilized. The GFAP anti-rat primary antibody, manufactured by DAKO, was utilized at a working dilution of 1:50. Under a bright field light microscope (OPTIKA, Italy), the sections were inspected, photographed, and subjected to analysis using ImageJ software, version 1.48v (developed by Wayne Rasband, National Institutes of Health, USA) and Java engine (64-bit).
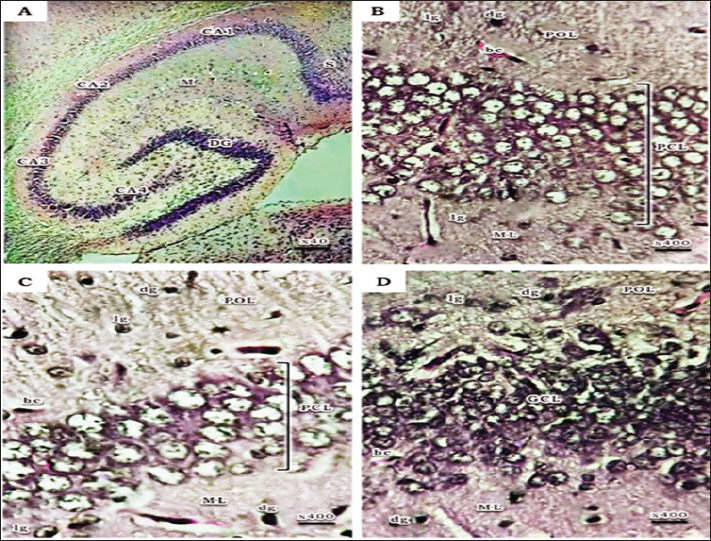
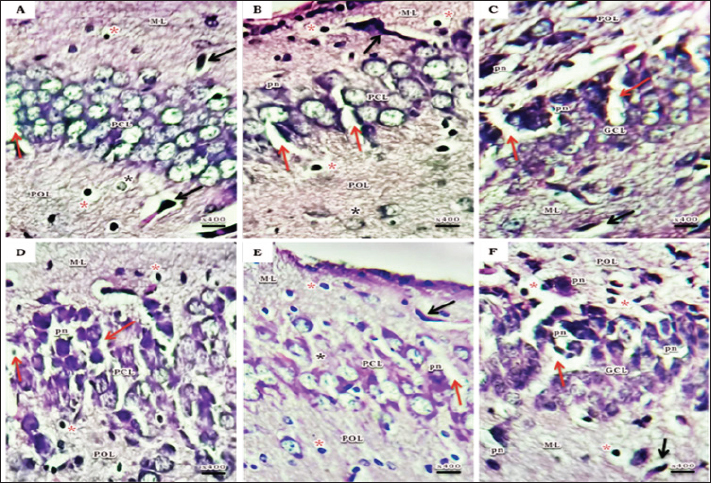
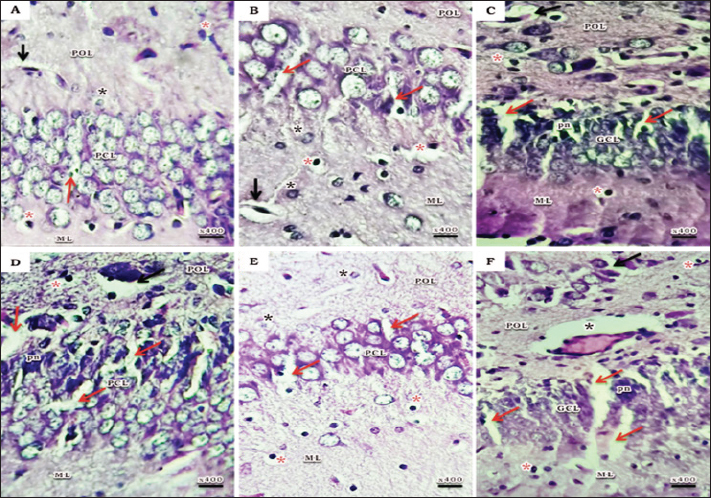
Fig. 1. Schematic timeline of experimental design. Flow cytometric assessments of apoptosisThe Becton Dickinson FACScan Fluorescence Activated Cell Analyzer (Becton Dickinson, Sunnyvale, CA, USA) was used for flow cytometric analysis. After lysing and suspending the hippocampal tissues of 15d postnatal pups at a concentration of 0.1–0.3 × 106/ml in Tris-EDTA buffer (pH 74) (Sigma-Aldrich Co.), the tissues were fixed in 80% ethanol, stained with annexin V (annexin V-FITC) conjugated with fluorescein isothiocyanate, incubated for 15 minutes at room temperature, and then examined for apoptosis. Cognitive brain measurementThe cognitive brains of 15d postnatal pups were estimated based on the cognitive equivalence equation (Bauchot and Stephan, 1966); E—[0.065 × P0.27] (P estimated as body mass in g and E estimated as brain mass in g). Statistical analysisThe SPSS (version 15) software package for Windows (SPSS Inc., Chicago, IL, USA) was used to conduct statistical analysis, which compared the variations between studied groups with one-way post-hoc analysis of variance, and significance was considered at (p < 0.05). Ethical approvalThis study was conducted according to the Animal Care guidelines and bioethics of the Egyptian Committee and all procedures have been approved by the Scientific Research Ethics Committee No. 5 (25 October 2023) at Faculty of Science, Port Said University (ERN: PSU.Sci.18). ResultsHistopathological investigationsHaematoxylin and eosin stain The hippocampus proper comprised of two interlocking structures: c-shaped cornu ammonis (CA) partitioned into four regions (CA1-CA4) and v-shaped dentate gyrus (DG) enfolded around CA4 (Fig. 2A), to unify the comparison between groups; higher magnifications of CA1, CA3, and DG were clarified in all groups. Both CA1 and CA3 regions of a 15-day postnatal offspring of a pre-pregnancy normal-weight mother consisted of well-defined three layers; polymorphic layer and molecular layer displayed lightly and deeply stained glial cells, and the pyramidal cell layer constituted the main pyramidal cells with large rounded vesicular nuclei (Fig. 2B and C). DG region also consists of three layers; a compact granular layer with dark nuclei cells, a molecular layer containing deeply stained glial cells, and a polymorphic layer with both lightly and deeply stained glial cells (Fig. 2D). The present study manifested abnormal structural organization of the hippocampus of a 15-day postnatal offspring of both a pre-pregnancy underweight and overweight mother (Figs. 3 and 4), concerning offspring of a pre-pregnancy underweight mother; gender showed varying degrees of histopathological appearance resembling in CA1 region exhibits disorganized pyramidal neurons with dark, shrunken, pyknotic nuclei, and degenerated neurons in pyramidal cell layer, many lightly and deeply stained glial cells nuclei with pericellular haloes and dilated blood capillaries noticed in polymorphic and molecular layers, also CA3 region reveals lightly stained pyramidal cells cytoplasm, few with pyknotic nuclei and some degenerated with vacuoles space in pyramidal cell layer; many deeply stained glial cells nuclei with pericellular haloes and dilated blood capillaries in polymorphic and molecular layers, and DG region displays disorganized dark shrunken granule cell bodies having pyknotic nuclei with pericellular vaculation in granular layer, many deeply stained glial cells nuclei with pericellular haloes and dilated blood capillaries in polymorphic and molecular layers were noticed (Fig. 3). On the other hand, hippocampus of a 15-day postnatal offspring of a pre-pregnancy overweight mother; CA1 region exhibits somewhat regularly arranged pyramidal cell bodies in pyramidal cell layer in female and loosely packed in male offspring, few of them are shrunken with pyknotic nuclei with pericellular vaculation and the others are apparently normal; few lightly and deeply stained glial cells nuclei with pericellular haloes and blood vessel with perivascular halo in polymorphic and molecular layers were noticed, also CA3 region showed few large lightly stained nuclei pyramidal cells, few normal pyramidal cells and some degenerated neurons in pyramidal cell layer, few lightly and many deeply stained nuclei of glial cells with wide pericellular space and dilated blood vessel with wide perivascular halo observed in polymorphic and molecular layers, and DG region displays few dark granule cell with pyknotic nuclei and some degenerated neurons with vacuoles space in GCL, polymorphic and molecular layers exhibit some deeply stained glial cells nuclei with pericellular haloes, dilated and congested blood vessel with wide perivascular halo as well (Fig. 4).
Fig. 2. A photomicrograph of a histological section of the hippocampus of a 15-day postnatal offspring of a pre-pregnancy normalweight mother. (A) The hippocampus proper comprised of two interlocking structures: C-shaped Cornu Ammonis (CA1-CA4 regions) continued as subiculum (S) and V-shaped Dentate Gyrus (DG), the molecular layer (M) inside the concavity of both CA and DG can be noticed. (B) CA1 region showing its three layers; Polymorphic layer (POL) containing lightly stained (lg) and deeply stained (dg) glial cells, Pyramidal cell layer (PCL) containing 5-6 compact layers of pyramidal cells with large rounded vesicular nuclei, and Molecular layer (ML) containing lightly stained glial cells. (C) CA3 region with few layers of large pyramidal cells (PCL), Polymorphic layer (POL) and Molecular layer (ML) show many lightly stained (lg) and deeply stained (dg) glial cells. (D) DG region shows its three layers; compact granular layer (GCL) with dark nuclei cells, Molecular layer (ML) contains deeply stained glial cells (dg), and Polymorphic layer (POL) with both lightly stained (lg) and deeply stained (dg) glial cells. H&E. Abbreviations; bc, blood capillary
Fig. 3. A photomicrograph of a histological section of the hippocampus of a 15-day postnatal offspring of a pre-pregnancy underweight mother. (A-C) Female offspring and (D-F) Male offspring showing abnormal structure, (A&D) CA1 region exhibits few layers of pyramidal neurons in (A), disorganized with dark, shrunken, pyknotic nuclei (pn) in (D) and degenerated neurons (red arrow) in PCL; many lightly (black star) and deeply (red star) stained glial cells nuclei with pericellular haloes and dilated blood capillaries (black arrow) can be noticed in POL and ML. (B& E) CA3 region reveals lightly stained pyramidal cells cytoplasm (black star), few with pyknotic nuclei (pn) and some degenerated with vacuoles space (red arrow) in PCL; many deeply stained glial cells nuclei with pericellular haloes (red star) and dilated blood capillaries (black arrow) in POL and ML. (C& F) DG region displays disorganized dark shrunken granule cell bodies having pyknotic nuclei (pn) with pericellular vaculation (red arrow) in GCL; many deeply stained glial cells nuclei with pericellular haloes (red star) and dilated blood capillaries (black arrow) can be seen in POL and ML. H&E. Abbreviations; CA1, Cornu Ammonis 1; CA3, Cornu Ammonis 3; DG, Dentate Gyrus; GCL, granular layer; ML, Molecular layer; PCL, Pyramidal cell layer; POL, Polymorphic layer. GFAP immunohistochemical stainIn the hippocampus regions of male offspring born to underweight mothers, there were more GFAP-positive astrocytes than those born to overweight mothers, and this was also true for female offspring but in a lower percentage than male offspring (Fig. 5). Flow cytometric assessments of apoptosisA higher incidence of apoptosis in male offspring’s hippocampus than in females; especially in the pre-pregnancy underweight mother group was observed (Fig. 6). Cognitive brain measurementThe underweight mother-offspring had lower cognitive ability than the overweight mother-offspring, when compared to the normal-weight pre-pregnancy mother-offspring (Fig. 7). DiscussionSeveral features included in this present study enhance understanding of pre-pregnancy maternal BMI associations with their offspring’s hippocampus development that may affect their cognition ability; healthy rats without concomitant medical disorders were used. Moreover, all rats were fed the same standard diet all over the study period which offers a strong model for isolating the relationship between diet type and offspring outcomes. This is important because most studies present their findings in the context of high-fat maternal diets (Niculescu and Lupu, 2009; Vucetic et al., 2010; Naef et al., 2011; Sullivan et al., 2014; Cunha et al., 2015; Glendining et al., 2018). The entire range of maternal BMI levels was also taken into account. Numerous earlier research has compared different weight groups as either obese or not (Tozuka et al., 2010; Robinson et al., 2013; Grissom et al., 2014; Li et al., 2016; Salzwedel et al., 2019), this can make it challenging to determine if their results apply to BMI sequence or just to obesity specifically. Finally, the cognitive abilities of the offspring were assessed using body and brain weights, which is a more comprehensive approach than previous studies that only considered variations in brain weight and physical activity (Tozuka et al., 2010; Cunha et al., 2015).
Fig. 4. A photomicrograph of a histological section of the hippocampus of a 15-day postnatal offspring of a pre-pregnancy overweight mother. (A-C) Female offspring and (D-F) Male offspring showing abnormal structure, (A&D) CA1 region exhibits somewhat regularly arranged pyramidal cell bodies in PCL in (A) and loosely packed in (D); few of them are shrunken with pyknotic nuclei (pn) with pericellular vaculation (red arrow) and the others are apparently normal; few lightly (black star) and deeply (red star) stained glial cells nuclei with pericellular haloes and blood vessel with perivascular halo can be seen (black arrow) in POL and ML. (B& E) CA3 region showed few large lightly stained nuclei pyramidal cells, few normal pyramidal cells and some degenerated neurons (red arrow) can be seen in PCL; few lightly (black star) and many deeply stained nuclei of glial cells with wide pericellular space and dilated blood vessel with wide perivascular halo can be observed in POL and ML. (C& F) DG region displays few dark granule cell with pyknotic nuclei (pn) and some degenerated neurons with vacuoles space (red arrow) in GCL; POL and ML exhibit some deeply stained glial cells nuclei with pericellular haloes (red star), both dilated (black arrow) and congested (black star) blood vessel with wide perivascular halo can be noticed. H&E. Abbreviations; CA1, Cornu Ammonis 1; CA3, Cornu Ammonis 3; DG, Dentate Gyrus; GCL, granular layer; ML, Molecular layer; PCL, Pyramidal cell layer; POL, Polymorphic layer. The present study manifested abnormal structural organization of the hippocampus of a 15-day postnatal offspring of both a pre-pregnancy underweight and overweight mother, which came with a line of evidence that both conditions were linked to chronic, low-grade inflammation in the mother, which may be detrimental to placental development by increasing its inflammation and so reducing the fetus’s access to nutrients (Gaccioli et al., 2013), yielding adverse pregnancy outcomes and poor fetal development (Basatemur et al., 2013; Yu et al., 2013).
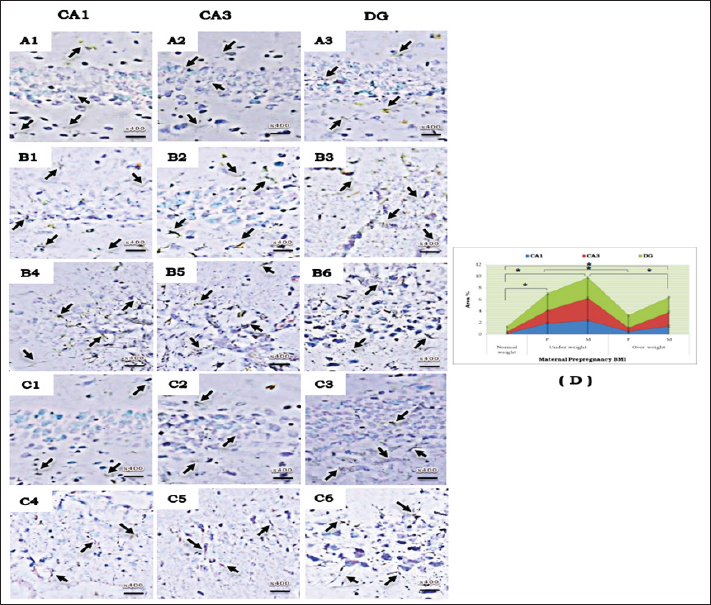
Fig. 5. A photomicrograph of GFAP immunohistochemical staining in the different areas of hippocampus (CA1, CA3, and DG) of a 15-day postnatal offspring in different experimental groups. (A1-A3) a pre-pregnancy normal-weight mother; (B1-B6) a pre-pregnancy underweight mother; (C1-C6) a pre-pregnancy overweight mother; (B1-B3& C1-C3) female offspring; (B4-B6& C4-C6) male offspring. The arrows indicate the astrocyte positive immunoreactions intensity in the different areas. (D) Chart illustrating the Area % of GFAP positive astrocyte in CA1, CA3 and DG regions of the different experimental groups, star means significant at p < 0.05 comparing between groups. Abbreviations; CA1, Cornu Ammonis 1; CA3, Cornu Ammonis 3; DG, Dentate Gyrus. Regarding the varying degrees of hippocampal defects among offspring sexes, it is agreed upon by other studies that females may be more shielded from adverse events in utero because estrogen has neuroprotective and anti-inflammatory properties (Gillies et al., 2004; Shivers et al., 2015). This explains why the development of the male brain is more vulnerable than that of the female offspring to the environmental characteristic alteration that faces fetal development in utero (Dearden and Balthasar, 2014), as well as raising the neurodevelopmental disorders in males than in females, such as in autism spectrum (Zhang et al., 2020) and attention deficit hyperactivity disorders (Valera et al., 2010). Interestingly, in this present study, the hippocampal neurons of offspring born to underweight and overweight mothers before pregnancy were damaged, as evidenced not only by histopathological changes but also by increased GFAP immunostaining. GFAP is a protein specific to astrocytes that enables them to interact with and affect the surrounding neural vasculature (Vasile et al., 2017). Astrocytes undergo a unique response called “reactive astrocytosis” which is a general inflammatory reaction when nearby neurons are affected; the astrocytes become active, start to multiply, and release pro-inflammatory cytokines and neurotoxins detrimentally (Li et al., 2019). The presence of an increase in glial cell nuclei numbers in the histological examination confirmed the reactive astrocytosis. Additionally, the observed dilated congested blood capillaries in hippocampal sections of offspring mostly corresponded to the inflammatory process elicited by the astrocytes. If a mother had a normal weight before becoming pregnant, the hippocampal regions of her offspring showed a decrease in astrocytes and inflammation caused by astrocytosis. This observation was indirectly confirmed by a relative decrease in GFAP immunoreactivity, which reflected a decline in astrocytes. The normal histological examination of her offspring’s hippocampal regions further confirmed this observation.
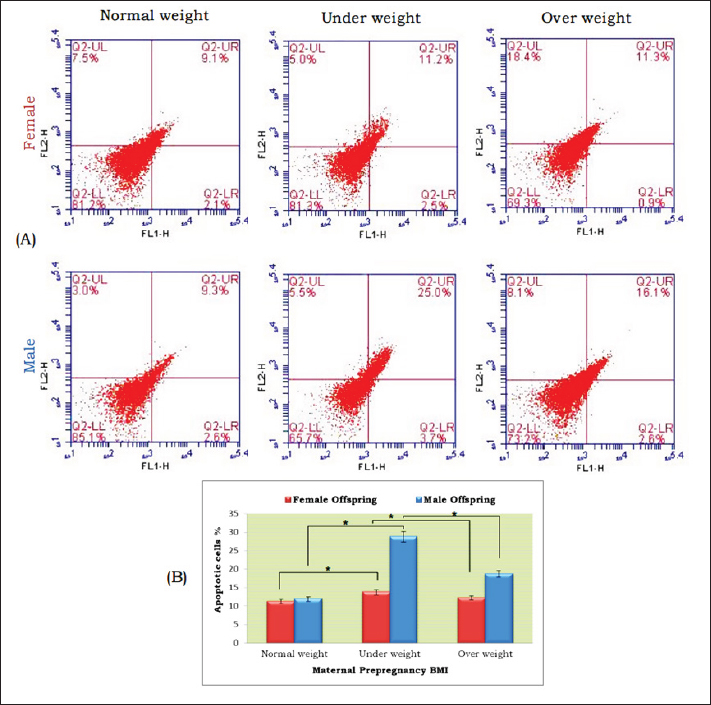
Fig. 6. Apoptosis assay using flow cytometric analysis of annexin-v in hippocampus tissue of a 15-day postnatal offspring in different experimental groups. (A) Representative scatter plots of Annexin-v: Lower left quadrants (LL) indicating viable cells; lower right quadrants (LR) represent the early apoptotic cells; upper left quadrants (UL) indicating the necrotic cells; upper right quadrants (UR) indicating late apoptosis. UR plus LR exhibited the summation of apoptosis. (B) Quantification graph of apoptotic cells percentage of the different experimental groups, star means significant at p < 0.05 comparing between groups.
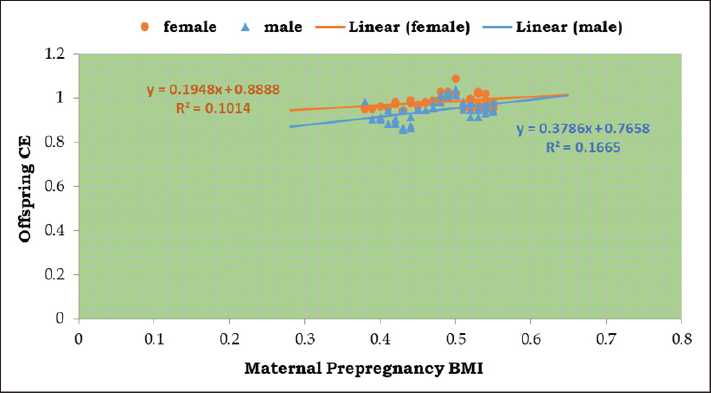
Fig. 7. Linear regression analysis of maternal pre-pregnancy BMI versus offspring cognition equivalence of experimental groups. Both male and female offspring had a gentle slope, the expression is y=0.3786x + 0.7658 in male and y=0.1948x + 0.8888 in female offspring. Male exhibited a higher correlation coefficient (R2=0.1665) than females offspring (R2=0.1014). Abbreviations; BMI, body mass index; CE, cognition equivalent. A common outcome of activating inflammatory signal transduction is the promotion of apoptosis signals (Siegel et al., 2003), which explains the higher incidence of apoptosis in male offspring’s hippocampus than in females; especially in the pre-pregnancy underweight mother group. The cognitive abilities or intelligence of animal species are often measured using Bauchot and Stephan’s cognitive equivalence equation (Bauchot and Stephan, 1966). This equation takes into account both body and brain weights to estimate cognitive brain function. According to the results, the underweight mother-offspring had lower cognitive ability than the overweight mother-offspring, when compared to the normal-weight pre-pregnancy mother-offspring. These findings may relate to the reactive astrocytosis noted in the histopathological and immunostaining changes because astrocytes play a crucial role in synapse development, maturation, and regulation (Cresto et al., 2019). If astrocytes are not functioning correctly, it can lead to damage to neurons and cognitive deficits (Verkhratsky et al., 2023) by releasing GABA which reduces the spike probability of the perforant-path-to-dentate-granule-cell synapse (Chun et al., 2020), which is consistent with poorer performance on some neurocognitive function assessments in infants at 2 years of age (Helderman et al., 2012; Reynolds et al., 2014; van der Burg et al., 2015) and lower scores on intelligence tests at 10 years of age (Jensen et al., 2017) as well as increased anxious and ADHD-like traits (Sullivan et al., 2014; Menting et al., 2019) compared to those born to mothers with normal-BMI. ConclusionAccording to the results of this study, compared to offspring born to pre-pregnancy normal-weight mothers, offspring of pre-pregnancy underweight mothers had increased levels of inflammatory and apoptotic markers in the hippocampus than those born to pre-pregnancy overweight mothers. These indicate that there may be a connection between a woman’s pre-pregnancy BMI and her offspring’s cognitive capacities, which calls for more study to gain a deeper knowledge and to create interventions that target the physical health of the mother prior to pregnancy in order to enhance their offspring’s health and cognitive outcomes. AcknowledgmentsThe authors thank the Faculty of Science, Port Said University for providing the necessary facilities for this work. Conflict of interestThe authors declare no conflicts of interest. FundingNo fund. Authors’ contributionsAll authors contributed equally, and all authors read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesBahrami, H., Sadatsafavi, M., Pourshams, A., Kamangar, F., Nouraei, M., Semnani, S., Brennan, P., Boffetta, P. and Malekzadeh, R. 2006. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 6, 158. Basatemur, E., Gardiner, J., Williams, C., Melhuish, E., Barnes, J. and Sutcliffe, A. 2013. Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics. 131, 56–63. Bauchot, R. and Stephan, H. 1966. Donnees nouvelles sur l’encephalisation des insectivores et des prosimiens. Mammalia. 30(1), 160–196. Black, R.E., Allen, L.H., Bhutta, Z.A., Caulfield, L.E., de Onis, M., Ezzati, M., Mathers, C. and Rivera, J. 2008. Maternal and child undernutrition study group. maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 371(9608), 243–260. Chun, H., Im, H., Kang, Y.J., Kim, Y., Shin, J.H., Won, W., Lim, J., Ju, Y., Park, Y.M., Kim, S., Lee, S.E., Lee, J., Woo, J., Hwang, Y., Cho, H., Jo, S., Park, J.H., Kim, D., Kim, D.Y., Seo, J.S., Gwag, B.J., Kim, Y.S., Park, K.D., Kaang, B.K., Cho, H., Ryu, H. and Lee, C. J. 2020. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci., 23(12), 1555–1566. Cresto, N., Pillet, L.-E., Billuart, P. and Rouach, N. 2019. Do Astrocytes play a role in intellectual disabilities? TINS, 42(8), 518–527. Cunha, F. da S., Dalle Molle, R., Portella, A.K., Benetti, C. da S., Noschang, C., Goldani, M. Z. and Silveira, P.P. 2015. Both food restriction and high-fat diet during gestation induce low birth weight and altered physical activity in adult rat offspring: the “similarities in the inequalities” model. PLoS One, 10(3), e0118586. Dearden, L. and Balthasar, N. 2014. Sexual dimorphism in offspring glucose-sensitive hypothalamic gene expression and physiological responses to maternal high-fat diet feeding. Endocrinology 155(6), 2144–2154. Engelbregt, MJ., van Weissenbruch, M.M., Popp-Snijders, C., Lips, P. and Delemarre-van de Waal, H.A. 2001. Body mass index, body composition, and leptin at onset of puberty in male and female rats after intrauterine growth retardation and after early postnatal food restriction. Pediatr. Res. 50(4), 474–478. Ezzati, M., Lopez, A.D., Rodgers, A., Vander Hoorn, S. and Murray, C.J.L. 2002 Comparative risk assessment collaborating group. Selected major risk factors and global and regional burden of disease. Lancet (London, England), 360(9343), 1347–1360. Finucane, M.M., Stevens, G.A., Cowan, M.J., Danaei, G., Lin, J.K., Paciorek, C.J., Singh, G.M., Gutierrez, H.R., Lu, Y., Bahalim, A.N., Farzadfar, F., Riley, L.M. and Ezzati, M. 2011. Global burden of metabolic risk factors of chronic diseases collaborating group (body mass index). National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet (London, England), 377(9765), 557–567. Fishman, S.M., Caulfield, L.E., de Onis, M., Blössner, M., Hyder, A.A., Mullany, L. and Black, R.E. 2004. Childhood and maternal underweight. In: Ezzati, M., Lopez, A.D., Rodgers, A. and Murray, C.J.L. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva, Switerzland: WHO. 1, 39–161. Gaccioli, F., Lager, S., Powell, T. and Jansson, T. 2013. Placental transport in response to altered maternal nutrition. J DOHaD, 4(2), 101–115. Gillies, G.E., Murray, H.E., Dexter, D. and McArthur, S. 2004. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol. Biochem. Behav. 78(3), 513–522. Glendining, K.A., Fisher, L.C. and Jasoni, C.L. 2018. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology, 96, 132–141. Grissom, N.M., Lyde, R., Christ, L., Sasson, I.E., Carlin, J., Vitins, A.P., Simmons, R.A. and Reyes, T.M. 2014. Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(4), 801–810. Gross, S.M., Gary, T.L., Browne, D.C. and LaVeist, T.A. 2005. Gender differences in body image and health perceptions among graduating seniors from a historically black college. J. Natl. Med. Assoc., 97(12), 1608–1619. Haslam, D.W. and James, W.P.T. 2005. Obesity. Lancet (London, England), 366(9492), 1197–1209. Helderman, J.B., O’Shea, T.M., Kuban, K.C., Allred, E.N., Hecht, J.L., Dammann, O., Paneth, N., McElrath, T.F., Onderdonk, A. and Leviton, A. 2012. ELGAN study Investigators. antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 129(3), 494–502. Hossain, P., Kawar, B. and El Nahas, M. 2007. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 356(3), 213–215. James, W.P.T., Jackson-Leach, R., Mhurchu, C.N., Kalamara, E., Shayeghi, M., Rigby, N.J., Nishida, C. and Rodgers, A. 2004. Overweight and obesity (high body mass index). In: Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Eds., Ezzati, M., Lopez, A.D., Rodgers, A. and Murray, C.J.L. Geneva, Switerzland: WHO. 1, 497–596. Jensen, E.T., van der Burg, J.W., O’Shea, T.M., Joseph, R.M., Allred, E.N., Heeren, T., Leviton, A., Kuban, K.C.K. 2017. Extremely low gestational age newborns study investigators. The relationship of maternal prepregnancy body mass index and pregnancy weight gain to neurocognitive function at age 10 years among children born extremely preterm. J. Pediatr. 187, 50–57. Li, K., Li, J., Zheng, J. and Qin, S. 2019. Reactive astrocytes in neurodegenerative diseases. Aging dis. 10(3), 664–675 Li, X., Andres, A., Shankar, K., Pivik, R.T., Glasier, C.M., Ramakrishnaiah, R.H., Zhang, Y., Badger, T.M. and Ou, X. 2016. Differences in brain functional connectivity at resting state in neonates born to healthy obese or normal-weight mothers. Int. J. Obes. 40(12), 1931–1934. Menting, M.D., van de Beek, C., Mintjens, S., Wever, K.E., Korosi, A., Ozanne, S.E., Limpens, J., Roseboom, T.J., Hooijmans, C. and Painter, R.C. 2019. The link between maternal obesity and offspring neurobehavior: a systematic review of animal experiments. Neurosci. Biobehav. Rev. 98, 107–121. Naef, L., Moquin, L., Dal Bo, G., Giros, B., Gratton, A. and Walker, C.D. 2011. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience, 176, 225–236. Niculescu, M.D. and Lupu, D.S. 2009. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Develop. Neurosci. 27(7), 627–633. ProCon.org. 2020. Global Obesity Levels. 2020. Available via https://obesity.procon.org/global-obesity-levels/ Reynolds, L.C., Inder, T.E., Neil, J.J., Pineda, R.G. and Rogers, C.E. 2014. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J. Perinatol. 34(9), 688–692. Robinson, M., Zubrick, S.R., Pennell, C.E., Van Lieshout, R.J., Jacoby, P., Beilin, L.J., Mori, T.A., Stanley, F.J., Newnham, J.P. and Oddy, W.H. 2013. Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. JDOHaD, 4(1), 42–48. Ronsmans, C., Holtz, S. and Stanton, C. 2006. Socioeconomic differentials in caesarean rates in developing countries: a retrospective analysis. Lancet (London, England), 368(9546), 1516–1523. Salihagic Kadic, A. and Predojevic, M. 2012. Fetal neurophysiology according to gestational age. Semin. Fetal Neonat. M. 17(5), 256–260. Salihagic Kadic, A., Predojevic, M. and Kurjak, A. 2009. Advances in Fetal Neurophysology. In: Pooh, R.K. and Kurjak, A. (eds.). Fetal Neurology. New Delhi, India: Jaypee Brothers, 161–221. Salzwedel, A.P., Gao, W., Andres, A., Badger, T.M., Glasier, C.M., Ramakrishnaiah, R.H., Rowell, A.C. and Ou, X. 2019. Maternal adiposity influences neonatal brain functional connectivity. Front. Hum. Neurosci. 12, 514. Shivers, K.Y., Amador, N., Abrams, L., Hunter, D., Jenab, S. and Quiñones-Jenab, V. 2015. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine, 72(2), 121–129. Siegel, R.M., Muppidi, J., Roberts, M., Porter, M. and Wu, Z. 2003. Death receptor signaling and autoimmunity. Immunol. Res. 27(2–3), 499–512. Spann, M.N., Scheinost, D., Feng, T., Barbato, K., Lee, S., Monk, C. and Peterson, B.S. 2020. Association of maternal prepregnancy body mass index with fetal growth and neonatal thalamic brain connectivity among adolescent and young women. JAMA Netw. Open. 3(11), e2024661. Steyn, N.P., Mann, J., Bennett, P.H., Temple, N., Zimmet, P., Tuomilehto, J., Lindström, J., and Louheranta, A. 2004. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 7(1A), 147–165. Sullivan, E.L., Nousen, E.K. and Chamlou, K.A. 2014. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 123, 236–242. Tozuka, Y., Kumon, M., Wada, E., Onodera, M., Mochizuki, H. and Wada, K. 2010. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 57(3), 235–247. Valera, E.M., Brown, A., Biederman, J., Faraone, S.V., Makris, N., Monuteaux, M.C., Whitfield-Gabrieli, S., Vitulano, M., Schiller, M. and Seidman, L.J. 2010. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am. J. Psychiatry, 167(1), 86–94. van der Burg, J.W., Allred, E.N., Kuban, K., O’Shea, T.M., Dammann, O. and Leviton, A. 2015. Maternal obesity and development of the preterm newborn at 2 years. Acta Paediatr, 104(9), 900–903. Vasile, F., Dossi, E. and Rouach, N. 2017. Human astrocytes: structure and functions in the healthy brain. Brain Struct. Funct. 222(5), 2017–2029. Verkhratsky, A., Butt, A., Li, B., Illes, P., Zorec, R., Semyanov, A., Tang, Y. and Sofroniew, M.V. 2023. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 8(1), 396. Vucetic, Z., Kimmel, J., Totoki, K., Hollenbeck, E. and Reyes, T.M. 2010. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology, 151(10), 4756–4764. Wan, W., Faber, R.J. and Fung, A. 2003. Perceived impact of thin female models in advertising: a cross-cultural examination of third person perception and its impact on behaviors. Asia Pac. J. Mark. Logist. 15(1/2), 51–73. Weesner, F.M. 1968. General zoological microtechniques. Baltimore, MD: Williams and Wilkins Company; Calcutta, India: Scientific Book Agency. WHO. 2000. Obesity: preventing and managing the global epidemic. Geneva, Switerzland: World Health Organization, 2000 (WHO Technical Report Series No. 894). WHO. 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva, Switerzland: World Health Organization, 1995 (Technical Report Series No. 854). Wright, T., Langley-Evans, S.C., and Voigt, J.P. 2011. The impact of maternal cafeteria diet on anxiety-related behaviour and exploration in the offspring. Physiol. Behav. 103(2), 164–172. Yu, Z., Han, S., Zhu, J., Sun, X., Ji, C. and Guo, X. 2013. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One, 8(4), e61627. Zhang, Y., Li, N., Li, C., Zhang, Z., Teng, H., Wang, Y., Zhao, T., Shi, L., Zhang, K., Xia, K., Li, J. and Sun, Z. 2020. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl. Psychiatry, 10(1), 4. | ||
| How to Cite this Article |
| Pubmed Style Greash ZA, Elmajdoub LO, Fayad E, Almaaty AHA. Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Vet. J.. 2024; 14(11): 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 Web Style Greash ZA, Elmajdoub LO, Fayad E, Almaaty AHA. Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. https://www.openveterinaryjournal.com/?mno=215694 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.21 AMA (American Medical Association) Style Greash ZA, Elmajdoub LO, Fayad E, Almaaty AHA. Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Vet. J.. 2024; 14(11): 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 Vancouver/ICMJE Style Greash ZA, Elmajdoub LO, Fayad E, Almaaty AHA. Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 Harvard Style Greash, Z. A., Elmajdoub, . L. O., Fayad, . E. & Almaaty, . A. H. A. (2024) Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Vet. J., 14 (11), 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 Turabian Style Greash, Zahraa A., Layla Omran Elmajdoub, Eman Fayad, and Ali H. Abu Almaaty. 2024. Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Veterinary Journal, 14 (11), 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 Chicago Style Greash, Zahraa A., Layla Omran Elmajdoub, Eman Fayad, and Ali H. Abu Almaaty. "Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat." Open Veterinary Journal 14 (2024), 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 MLA (The Modern Language Association) Style Greash, Zahraa A., Layla Omran Elmajdoub, Eman Fayad, and Ali H. Abu Almaaty. "Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat." Open Veterinary Journal 14.11 (2024), 2924-2935. Print. doi:10.5455/OVJ.2024.v14.i11.21 APA (American Psychological Association) Style Greash, Z. A., Elmajdoub, . L. O., Fayad, . E. & Almaaty, . A. H. A. (2024) Possible relationship between pre-pregnancy maternal body mass index and offspring hippocampus: An experimental study in albino Wistar rat. Open Veterinary Journal, 14 (11), 2924-2935. doi:10.5455/OVJ.2024.v14.i11.21 |