| Research Article | ||
Open Vet. J.. 2024; 14(11): 2950-2959 Open Veterinary Journal, (2024), Vol. 14(11): 2950-2959 Research Article Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomyJosé Goicochea-Vargas1,2*, Marisol Warthon-Medina3,4, Wilson Rondón-Jorge1, Fidel Acosta-Pachorro1, Marcelo Ratto-Fuster5, Mauricio Silva-Jiménez6, Ximena Valderrama-Linares7, Iain Richards4 and Max Salvatierra-Alor21Department of Surgery and Reproductive Biotechnology, Faculty of Veterinary Medicine and Zootechnics, University Nacional Hermilio Valdizán, Huánuco, Perú 2Department of Molecular Biotechnology, Central Laboratory Unit, University Nacional Hermilio Valdizán, Huánuco, Perú 3University of Central Lancashire (UCLan), School of Veterinary Medicine, Preston, UK 4Norfolk and Norwich University Hospital, NHS Foundation Trust, Norwich, UK 5Institute of Animal Science, Faculty of Veterinary Sciences, University Austral de Chile, Chile 6Department of Veterinary Sciences and Public Health, Faculty of Natural Resources, University Católica de Temuco, Temuco, Chile 7Department of Agricultural and Veterinary Sciences, University Viña del Mar, Viña del Mar, Chile *Corresponding Author: José Goicochea-Vargas. Department of Surgery and Reproductive Biotechnology, Faculty of Veterinary Medicine and Zootechnics, University Nacional Hermilio Valdizán, Huánuco, Perú. Email: jgoicochea [at] unheval.edu.pe Submitted: 19/08/2024 Accepted: 10/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
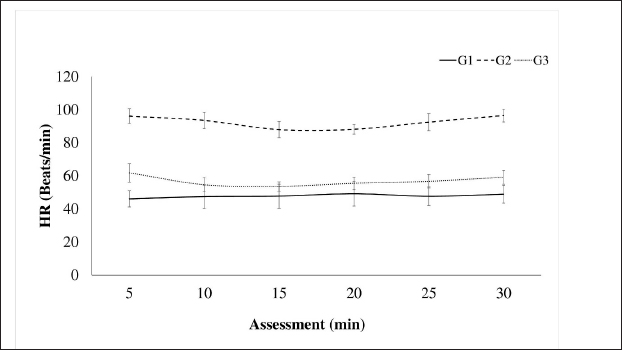
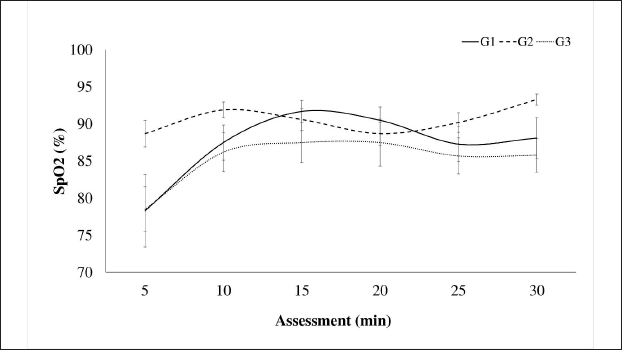
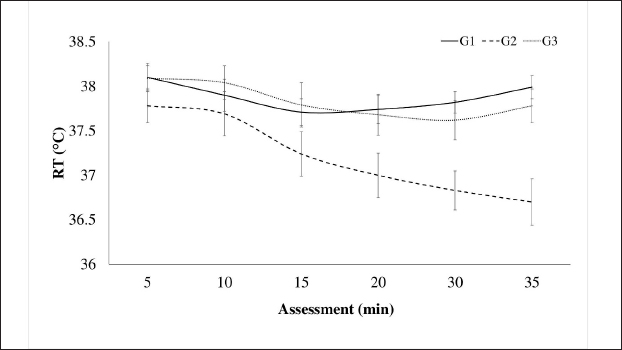
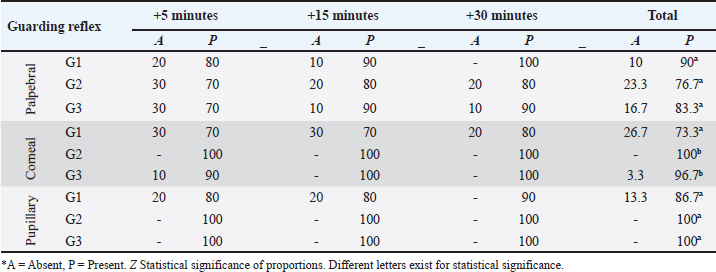
AbstractBackground: The limited and detailed literature on total intravenous anesthesia (TIVA), as well as the clinical indications for unilateral ovariectomy in llamas, are not well-defined. Therefore, it is necessary to understand the anesthetic events and the surgical intervention in this species. Aim: The objective of this study was to evaluate the intraoperative physiological and clinical parameters in llamas undergoing unilateral ovariectomy, under three protocols of TIVA. Methods: The study was conducted with 30 healthy female llamas. Three groups were considered G1: Pre-anaesthetic - xylazine i/v, Induction: ketamine. Maintenance: fentanyl and ketamine (FLK) (fentanyl + lidocaine + ketamine) by continuous infusion. G2: Pre-anaesthetic - midazolam + tramadol + ketamine i/v. Induction – propofol. Maintenance, boluses of 20% of the total induction dose were added if required. G3: Pre-anaesthetic - midazolam + xylazine i/v. Induction: ketamine. Maintenance: with 20% of the total induction. Physiological parameters were evaluated at 5, 15, and 30 minutes. Results: The heart rate, respiratory rate, arterial oxygen saturation (SpO2), and rectal temperature (RT) of the groups showed significant differences at some point in the evaluation, presenting a degree of bradycardia and bradypnea in G1 and G2. A drop in SpO2 was seen at minute 5 in all groups, because of respiratory depression post-anesthetic induction, subsequently, the values increased, with better saturation in G2. RT remained similar in the groups until minute 20, then there was a significant decrease in G2. G1 showed significant fluctuations for SpO2 and G2 for RT. The majority of animals from G1 and G3 maintained marked relaxation of the abdomen and limbs, anal sphincter (G3), and mandible (G1). Salivation and tearing were an infrequent sign in G2. All animals from G3 presented some degree of sensitivity to the abdominal wall. The average duration of the surgery was 14 minutes, and the shortest anesthetic recovery time occurred in G2 (28 minutes). Conclusion: The animals of the three groups showed significant differences in their physiological and clinical parameters, in response to the agents used. The G1 protocol: xylazine, ketamine y FLK. Demonstrated better physiological and hemodynamic stability with an acceptable level of surgical anesthesia. Keywords: Xylazine, Ketamine, Propofol, Surgery, South American camelids. IntroductionSouth American camelids, particularly llamas (Llama glama) and alpacas (Lama pacos), are species with particular morphological, physiological, and reproductive characteristics that are of particular interest to farming communities and to the scientific world (Charry et al., 2003). Some clinical examinations or minor surgeries can be performed on llamas and alpacas using containment methods, administration of sedatives, local anesthesia, or a combination of all three (Abrahamsen, 2009). However, in the case of major surgeries such as abdominal obstruction or ovariectomy, it is necessary to use an adequate protocol of general anesthesia to avoid noxious stimuli. One of the main challenges that both professionals and researchers face is to achieve non-traumatic and less stressful handling during the procedures to be carried out. In this context, it is important to have an effective analgesic and anesthetic strategy that avoids stress, injury, and trauma during the surgical act. Further consideration has to be given to the cardiovascular and respiratory depressant effects caused by the drugs and possibly potentiated by the gastrointestinal anatomy of these species (Pawson and Forsyth, 2004; Cruz et al., 2009). Two studies of anesthesia protocols in alpacas using propofol and a combination of ketamine/diazepam (Del Álamo et al., 2015; Taylor et al., 2017) have been reported, also a study in llamas using a combination of xylazine, ketamine, and halothane (Gavier et al., 1988) and the effect of propofol on cardiopulmonary parameters in llamas has also been described. (Duke et al., 1997). In a recent study (Taylor et al., 2017) a prospective randomized crossover design with six male alpacas, and three total intravenous anesthesia (TIVA) protocols were evaluated. These protocols consisted of a) ketamine plus diazepam, b) propofol, and c) ketamine plus propofol. The constants evaluated were heart rate (HR), respiratory rate (RR), mean arterial pressure, end-tidal CO2, and hemoglobin oxygen saturation, recorded every 2 minutes after induction. There were no significant differences in cardiopulmonary variables between the three anesthesia protocols, nor in the duration of anesthesia. Mild hypoxemia and hypercapnia were also similar between protocols. In another study (Espezua et al., 2015) the use of xylazine and ketamine combined with fentanyl in alpacas induced moderate anesthesia and acceptable analgesia. Therefore, the aim of this study was to evaluate three TIVA in adult llamas undergoing unilateral ovariectomy, including xylazine, ketamine, and FKL(G1), tramadol, midazolam, ketamine and propofol (G2) or midazolam, xylazine, and ketamine (G3). Materials and MethodsThirty clinically healthy female llamas, were selected by suitability, not pregnant or lactating, aged between 4 and 6 years old and weighing 72.70 ± 13.32 kg, were selected at the Kotosh research station, Hermilio Valdizán National University, Huánuco, Peru (8°21′47″ South Latitude - 76°18′ 56″ West Longitude and 1,800 m above sea level). Llamas were kept in separate pens and had access to pellet concentrate supplemented with forage alfalfa (Medicago sativa) and water ad libitum. A prospective randomized study of three independent samples (n=10 llamas/group) was proposed and the animals were randomly assigned according to protocols. G1: Pre-anaesthetic - xylazine (0.2 mg/kg) intravenously, Induction - ketamine (5 mg/kg) Maintenance with fentanyl and ketamine (FLK) (0.15 mg of fentanyl, 100 mg of lidocaine and 20 mg of ketamine in 100 ml of 0.9% saline solution) by continuous infusion at 3 ml/kg/hour. G2: Pre-anaesthetic - midazolam (0.2 mg/kg) tramadol (2 mg/kg) ketamine (0.5 mg/kg) i/v. Induction - propofol (4 mg/kg). Maintenance, boluses of 20% of the total induction dose were added if required. G3: Pre-anaesthetic - midazolam (0.4 mg/kg) + xylazine (0.2 mg/kg) i/v. Induction - ketamine (5 mg/kg). Maintenance with 20% of the total induction dose. The drugs were administered to G1 and G2 via the intrajugular route through a catheter No. 20 G, 1.1 Ø, and L 35 mm, while G3 was directly without a catheter (Fig. 5). Animals were restrained in a standing position (Fig. 5A), prior to administration, the injection site was trichotomized and aseptically prepared. The catheter was secured with a Roman sandal suture and adhesive tape (Fig. 5A). Before surgery, animals were fasted for 12 hours (solid food) and 10 hours (liquid). Unilateral ovariectomy was performed through the right or left flank approach depending on the presence of the preovulatory follicle, detected by rectal or transrectal ultrasonography (7.5 MHz linear transducer) (Aloka, SSD-500, International Clinics), and under the coxal tuberosity, with an incision no larger than 30 mm. A Snook hook was used to retract the ovary and the polydiamine seal to obliterate the vascular pedicle, and the flank wall was sutured with No. 2 polydiamine using a modified Figure 8 stitch (Fig. 6). During the postoperative period, the animals received 5 days of antibiotic therapy with ceftiofur which also acted as a prevention of infection, and meloxicam as pain management (0.5 mg/kg as a single dose). Anaesthetic monitoringIntra-surgical monitoring of physiological parameters was performed every 5 minutes for six readings. HR and functional hemoglobin oxygen saturation in arterial blood were evaluated with the NONIN 9847V-Vet multipara Gmeter monitor. RR was measured by observing the abdominal and thoracic movement per minute, and rectal temperature (RT) was taken with a mercury thermometer over 1 minute. Clinical parameters were recorded at 5, 15, and 30 minutes. The sensitivity of the abdominal wall was initially evaluated in response to the painful reaction caused by the incision in the skin and muscles of the pelvic area, the following manoeuvres were caused by applied pressure with Kocher forceps, under the criteria present or absent. Abdominal relaxation was assessed by the tension offered to digital pressure, the relaxation of the lips by the firmness offered to traction; the relaxation of the anal sphincter in response to the degree of opening, and tension to the introduction of the thermometer; the relaxation of the jaw and of extremities due to resistance to the opening movement and extension, respectively, using the criteria of null, mild and marked. The corneal and palpebral protection reflexes were assessed in response to tactile stimulation, with the pupillary reflex assessed through light stimulation, under the present-absent criterion. Salivation and tearing were considered null, mild, and moderate, depending on the degree of secretion. Surgical duration was recorded, and measured from the beginning of the approach to the closure of the skin. Exit from the anesthetic plane was determined by the presence of interdigital sensitivity and retraction of the limb in response to the pressure caused by the Kocher clamp. The pressure was placed on interdigital space for the assessment of the nociception and anesthetic recovery was evaluated from the moment the anesthetic plane is achieved until the return of motor skills and four-leggedness with or without incoordination. Statistical analysisThe statistical analysis was carried out using the IBM SPSS Statistics v.26 program (IBM Corp, USA), having parametric data, it was carried out with a one-way ANOVA, using the General Linear Model with the comparison test of Tukey’s honestly significant difference (HSD) means, the distribution of non-parametric data was analyzed using Kruskal Wallis and the proportions using the Z test. The analyses were performed with a confidence level of 95% (p < 0.05). Ethical approvalThe experimental procedures were reviewed and approved by the bioethics committee of the University and were performed according to the animal care protocols established by the same institution. ResultsThe cardiopulmonary measurements are presented in Figures 1 and 2. The HR in G1: 47.82 ± 2.49 (beats/minute), G3: 56.88 ± 12.99 (beats/minute), RR G1: 29.08 ± 7.31 (RR/minute), and G3: 25.17 ± 6.38 (RR/minute), remaining within physiological parameters, unlike G2, with no differences in evaluation times (p ≥ 0.05). The SpO2 percentage (Fig. 3) was higher in G2 (90.57% ± 0.58%), different from groups G1 and G3 (p=0.001), with no variations in the evaluation times (p ≥ 0.05), except in G1 where at minute 5 the lowest percentage was detected (78.30% ± 4.88%). In Figure 4, RT is shown, remaining within the physiological range in G1 (37, 86°C ± 0.06°C) and G3 (37.83°C ± 0.84°C) without variations over time (p < 0.05), unlike G2, where a decrease from minute 15 (p=0.007) was observed. The protection reflexes as indicative of the degree of central nervous system (CNS) depression during the anesthetic plane are shown in Table 1. The pupillary reflex was present in 100% of the llamas in groups G2 and G3, and 86.7% in G1. Likewise, the palpebral reflex in 90% (G1), 83.3% (G3), and 76.7% (G2), with no statistical difference in the proportions (p ≥ 0.05). However, the corneal reflex was appreciated in 100% of the animals (G2) and 96.7% (G3), different from 73.3% (G1).
Fig. 1. Intraoperative HR in llamas undergoing unilateral ovariectomy, according to analgesic-anaesthetic protocols.
Fig. 2. Intraoperative RR in llamas subjected to unilateral ovariectomy, according to analgesic-anaesthetic protocols.
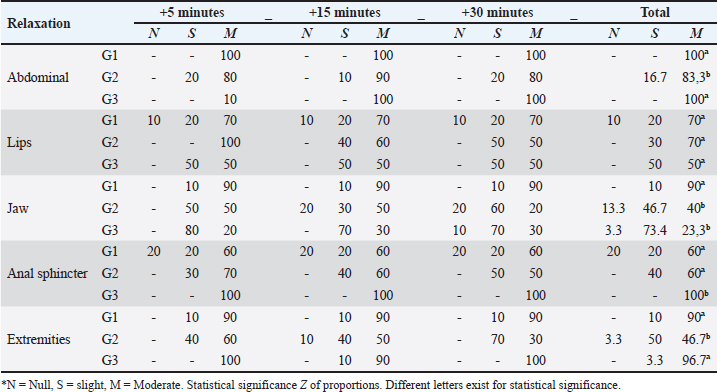
Fig. 3. Intraoperative oxygen saturation (SpO2) in llamas undergoing unilateral ovariectomy, according to analgesic-anaesthetic protocols. Table 2 shows the degree of abdominal relaxation, lips, jaw, anal sphincter, and extremities during the intraoperative period, with abdominal relaxation being “marked” in 100% of the animals corresponding to G1 and G3. Regarding the relaxation of the lips in the “marked” category, there were no differences between groups. However, the animals of G1 and G3 presented a higher percentage of relaxation in the extremities, likewise, G1 in the mandible and G3 in the anal sphincter.
Fig. 4. Intraoperative RT in llamas subjected to unilateral ovariectomy, according to analgesic-anaesthetic protocols.
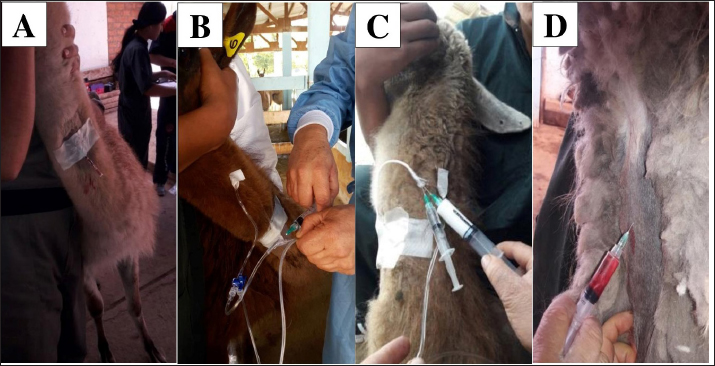
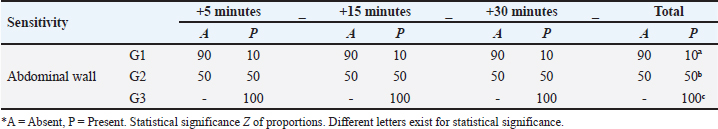
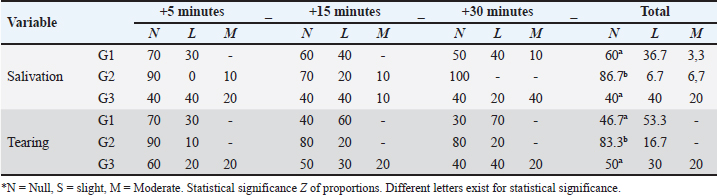
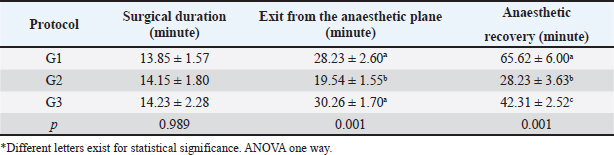
Fig. 5. Catheterization and anesthetic induction of the experimental groups. A: Intrajugular catheterization in llamas. B: Anesthetic induction with ketamine and FLK intraoperatively (G1). C: Anesthetic induction with ketamine and maintenance with propofol (G2). D: Anesthetic Induction with Xylazine-ketamine (G3). The sensitivity of the abdominal wall to the incision (Table 3) was absent in 90% of the llamas of G1 and 50% of G2. However, in G3 100% presented sensitivity. The highest percentage of G2 llamas did not present salivation or tearing (Table 4), with no differences between G1 and G3. The results related to the duration of unilateral ovariectomy (Table 5), there were no significant differences between groups: G1 (13.85 ± 1.57 minute), G2 (14.15 ± 1.80 minute) and G3 (14, 23 ± 2.28 minute) (p=0.989). While in the recovery of interdigital sensitivity, as an indication of the animals leaving the anesthetic plane, this was lower in G2 (19.54 ± 1.55 minute) with respect to G1 (28.23 ± 2.60 minute) and G3 (30.26 ± 1.70 minute) (p=0.001). In the anesthetic recovery until achieving motor skills with less incoordination, there were differences in G2 (19.54 ± 1.55 minute) with respect to G1 (28.23 ± 2.60 minute) and G3 (30.26 ± 1.70 minute) (p=0.001).
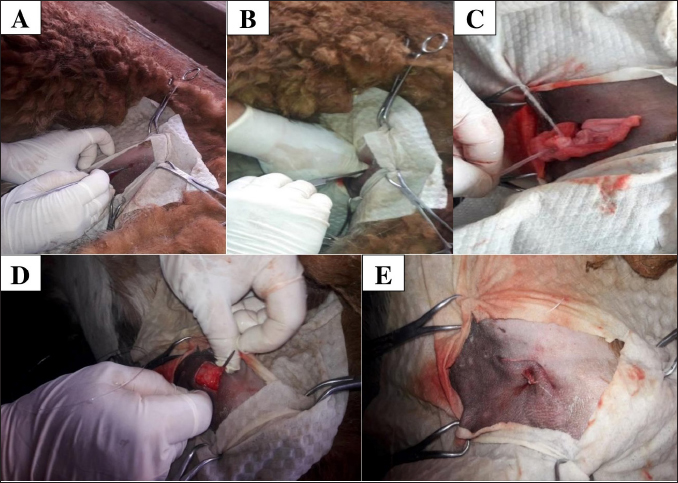
Fig. 6. Surgical intervention for unilateral ovariohysterectomy in llamas A: Flank approach about 2 to 3 cm below the iliac tuberosity, with an incision no longer than 30 mm. B: Insert the Hook to remove the uterine horn along with the corresponding ovary. C: After removing the ovary, proceed to form the ovarian pedicle using a polyamide ligature. D: Close the abdominal wall with a modified figure-8 suture, using a double-curved needle and No. 2 polyamide. E: Once the surgery is completed, a single suture can be seen encompassing skin—peritoneum on both sides—muscle layer on both sides, and finally skin. Table 1. Percentage of llamas (%) with protection reflexes in the intra-surgical period of unilateral ovariectomy, according to TIVA analgesia and anaesthesia protocols.
Table 2. Percentage of llamas (%) with degrees of relaxation in the intra-surgical period of unilateral ovariectomy, according to TIVA analgesia and anaesthesia protocols.
Table 3. Percentage of llamas (%) with algogenic sensitivity of the abdominal wall in the intra-surgical period of unilateral ovariectomy, according to TIVA analgesia and anaesthesia protocols.
Table 4. Percentage of llamas (%) with salivation and tearing in the intra-surgical period of unilateral ovariectomy, according to TIVA analgesia and anesthesia protocols.
Table 5. Post-surgical evaluation of TIVA analgesia and anaesthesia protocols in llamas undergoing unilateral ovariectomy (Mean ± SE).
DiscussionThe most relevant physiological parameters observed in G2 were tachycardia and bradypnea, whereas in groups G1 and G3, these parameters remained within normal ranges, similar to what has been reported in alpacas and vicuñas (Urquieta et al., 1992; Trujillo, 2018). The functional hemoglobin saturation percentage in arterial blood (SpO2) in all three groups stayed above the critical value. In contrast, RT in all three groups showed values below the normal minimum. Improved muscle relaxation and deep flank analgesia were observed in G1. HR results in G1 showed a slight bradycardia, which was attributed to the cardiovascular effects of xylazine that reduce cardiac output (Abrahamsen, 2009). This effect was further enhanced by lidocaine, which inhibits myocardial excitability and was administered along with FLK (Laredo and Cantalapiedra, 2001). In G2, the observed tachycardia was induced by the sympathomimetic properties of ketamine, which increased HR, systemic arterial pressure, and cardiac output (Kaka et al., 2016). Conversely, in G3, HR remained within normal ranges, with no significant changes post-induction, similar to what is observed in alpacas (Raggi et al., 1994). Regarding RR, G1 exhibited values similar to those observed in alpacas using a continuous infusion protocol with ketamine, xylazine, and fentanyl (Trujillo, 2018). In G2, bradypnea was recorded, which could be attributed to the effects of propofol and ketamine (Taylor et al., 2017), known for their vagotonic effects and tendency to induce transient apnea post-induction a phenomenon previously reported by Abrahamsen (2009) and Otero (2012). In G3, RR remained within normal ranges but showed a slight tendency towards bradypnea, as reported in vicuñas under anesthesia protocols using xylazine and ketamine (Urquieta et al., 1992). Oxygen saturation (SpO2) levels in G1 were comparable to those observed in alpacas undergoing abdominal surgery by Trujillo (2018), maintaining acceptable limits (88%–90%), although slightly lower than in G2, which presented better oxygenation (94%). This difference could be attributed to the respiratory depressant effect of the xylazine-ketamine combination (G1, G3). Nevertheless, all groups maintained SpO2 values above the critical threshold (60%) throughout the intraoperative period (Cantalapiedra and Cruz, 2001) RT showed a slight decrease in G2, a consequence of the depression of thermoregulatory centers caused by propofol, which affected physiological compensation mechanisms at the hypothalamic level, altering thermoregulation (Sessler, 2010). Nevertheless, temperature remained slightly below normal ranges (38.3°C–39°C), similar to what occurs in alpacas subjected to short surgical procedures (Espezua et al., 2015) Regarding protective reflexes, G1 showed a slight reduction in protective reflexes, such as pharyngeal and anal reflexes, due to the effects of ketamine, which is consistent with previous reports on its use in alpacas and vicuñas (Sumano and Ocampo, 2006; Sinner and Graf, 2008). In G2, corneal and pupillary reflexes remained present, partly due to the use of midazolam and propofol, which preserve certain reflexes while inducing adequate relaxation (Sánchez et al., 2018). In G3, the presence of xylazine and ketamine also caused a moderate reduction in reflexes, similar to that observed in G1, although with less anesthetic depth. Abdominal relaxation and sensitivity were best achieved in G1, facilitating surgical access and reducing operative time. The combination of midazolam, xylazine, and ketamine provided adequate muscle relaxation (Laredo and Cantalapiedra, 2001; Otero, 2012). Furthermore, G1 demonstrated deep analgesia, especially in the flank area, due to the use of continuous infusion of fentanyl, lidocaine, and ketamine, which maximized analgesic effects. This contrasts with G2 and G3, where bolus administration did not achieve the same level of visceral analgesia (Del Álamo et al., 2015). Salivation and tearing were primarily observed in G1 and G3, as a consequence of ketamine and xylazine administration, which increased exocrine gland secretion (Gross et al., 2013). In G2, salivation was less pronounced due to the reduced ketamine dose (0.5 mg/kg), consistent with other studies in vicuñas using alpha-2-agonist and dissociative anesthetic protocols (Urquieta et al., 1992). The recovery period was faster in G2 compared to G1 and G3, with the animals reaching quadrupedal position in a shorter time and showing less incoordination, attributable to the faster hepatic metabolism of propofol compared to ketamine and xylazine (Gross et al., 2013). This advantage makes G2 preferable in procedures that require rapid and stable recovery. G1 proved to be the most effective protocol, demonstrating advantages in cardiovascular stability, muscle relaxation, and deep analgesia, which facilitated surgical procedures and enhanced postoperative recovery. Although minor bradycardia and respiratory depression were observed, the anesthetic effects remained within acceptable clinical limits. The continuous infusion of FLK allowed for superior pain management and muscle relaxation compared to G2 and G3, where bolus administrations of ketamine and propofol did not achieve comparable outcomes. ConclusionIn conclusion, the three evaluated protocols showed significant differences in their physiological and clinical parameters. The G1 protocol showed better physiological and hemodynamic stability, and acceptable level of surgical anesthesia. For G2 and G3, the values of HR and RT were similar. However, G2 presents advantages in SpO2 as well as in the degree of analgesia. Despite the environmental concerns about the use of volatile agents, TIVA presents a useful alternative for safe and effective surgical procedures. AcknowledgmentsThe authors thank the National University Hermilio Valdizan – Peru, for providing them with the facilities to use the facilities of the Kotosh Production Center for the maintenance of the animals during the experiment. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe publication of this research was funded by the FONDECYT – REGULAR project No. N°1160934 of the Government of Chile. Authors’ contributionsJGV and MRF participated in the design and conception of the study. WRJ, FAP, and MSJ, managed the anesthesia and collected the required data. JGV and MRF performed the surgical intervention, FAP and MRF analyzed the collected data and JGV, MWM, MRF, XVL, MSA, and IR wrote the manuscript. All authors reviewed and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAbrahamsen, E.J. 2009. Chemical restraint, anesthesia, and analgesia for camelids. Vet. Clin. Food Anim. 25(2), 455–494. Cantalapiedra, A.G. and Cruz, I.J. 2001. Monitorización anestésica en pequeños animales. Consult. Difus. Vet. 9(77), 104. Charry, A., Kemp, R. and Lawrie, J.W. 2003. Alpacas and ecosystems management. In 14th Congress, International Farm Management Association, Perth, Western Australia, Aug 10–15, 2003, pp 24365. Cruz, J., Giraldo, C., Fernández, E. and Tovar, O. 2009. Farmacología y uso clínico de la ketamina. Rev. CES. 4(1), 68–79. Del Álamo, A.M., Mandsager, R.E., Riebold, T.W. and Payton, M.E. 2015. Evaluation of intravenous administration of alfaxalone, propofol, and ketamine-diazepam for anesthesia in alpacas. Vet. Anaesth. Analg. 42(1), 72–82. Duke, T., Egger, C.M., Ferguson, J.G. and Frketic, M.M. 1997. Cardiopulmonary effects of propofol infusion in llamas. Am. J. Vet. Res. 58, 153–156. Espezua, O.F., Chipayo, Y.G. and Olazabal, J.L. 2015. Anestesia total intra venosa de la combinación xilacina, ketamina y fentanilo para canulación del primer compartimento en alpacas. Rev. Ivestig. Altoandin. 17(1), 41–46. Gavier, D., Kittleson, M.D., Fowler, M.E., Johnson, L.E., Hall, G. and Nearenberg, D. 1988. Evaluation of a combination of xylazine, ketamine, and halothane for anesthesia in llamas. Am. J. Vet. Res. 49(12), 2047–2055. Gross, E.M., Giuliano, A.E., Raffe, M.R., Carpenter, E.R., Carroll, L.G., Martin, D.D., Marretta, M.S., Pettifer, R.G., Grubb, L.T., Hardie, M.E., Lukasik, M.V., Cornick-Seahorn, L.J., Grimm, B.J. and Marks L.S. 2013. Consideraciones anestésicas para intervenciones especiales En Anestesia y Analgesia en pequeñas especies. Edit. El manual Moderno, S.A. de AC. 18, 466. Kaka, U., Saifullah, B., Abubakar, A.A., Goh, Y.M., Fakurazi, S., Kaka, A., Behan, A.A., Ebrahimi, M. and Chen, H.C. 2016. Serum concentration of ketamine and antinociceptive effects of ketamine and ketamine-lidocaine infusions in conscious dogs. BMC Vet. Res. 12(1), 198. Laredo, F. and Cantalapiedra, A. 2001. Técnicas de anestesia general inyectable; TIVA. Consult. Difus. Vet. 9(77), 51–61. Otero, P.E. 2012. Protocolos de anestésicos y manejo del dolor en pequeños animales: reporte de casos. 1ra. Ed. Buenos Aires, Argentina: Inter-Medica, vol. 4, p: 269. Pawson, P. and Forsyth, S. 2004. Agentes anestésicos. In Farmacología clínica en pequeños animales, 1st ed. Eds., Maddison, P.J., Page, S. and Church, D. Buenos Aires, Argentina: Intermédica, pp: 73–86. Raggi, L.A., Crossley, J., Coppia, S. and Ferrando, G. 1994. Caracteristicas fisiológicas de la alpaca (Lama pacos) sometida a manejo extensivo en el altiplano chileno. Archivos de Zootecnia. 43(163), 201–206. Sánchez, C.I., Torralbo, D.D. and Soto, M.M. 2018. Anestesia en procedimientos de mínima invasión En Técnicas de mínima invasión en pequeños animales. Edit. Multimedica Ediciones Veterinarias. 4, 8. Sessler, D.I. 2010. Temperature monitoring. In Anesthesia, 7th ed. Ed. Miller, R.D. Philadelphia, PA: Churchill Livingstone, pp: 1533–1556. Sinner, B. and Graf, B.M. 2008. Ketamine. In Modern anesthetics: handbook of experimental pharmacology. Eds., Schüttler, J. and Schwilden, H, vol. 182, pp: 313–333. Sumano, H.S. and Ocampo, L. 2006. Farmacología veterinaria. 3rd ed. México: McGraw-Hill. pp: 1084. Taylor, S.D., Baird, A.N., Weil, A.B. and Ruple, A. 2017. Evaluation of three intravenous injectable anesthesia protocols in healthy adult male alpacas. Vet. Rec. 181(12), 322. Trujillo, L.A. 2018. Parámetros fisiológicos y clínicos, durante la anestésia disociativa y bajo mantenimiento con fentanilo, lidocaina y ketamina (Flk), en cirugía abdominal de alpacas (Vicugna pacos). B. S. Tesis, UNHEVAL, Huánuco, Perú. Urquieta, B.M., Schiappacasse, F.M., Raggi, S.L., Martínez, P.R., James, G. and Ferguson. 1992. Sedación, inmovilización y anestesia con xilacina-ketamina en vicuña (Vicugna vicugna). Avances en Ciencias Veterinarias. 7(2). | ||
| How to Cite this Article |
| Pubmed Style Goicochea-vargas J, Warthon-medina M, Rondón-jorge W, Acosta-pachorro F, Ratto-fuster M, Silva-jiménez M, Valderrama-linares X, Richards I, Salvatierra-alor M. Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Vet. J.. 2024; 14(11): 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 Web Style Goicochea-vargas J, Warthon-medina M, Rondón-jorge W, Acosta-pachorro F, Ratto-fuster M, Silva-jiménez M, Valderrama-linares X, Richards I, Salvatierra-alor M. Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. https://www.openveterinaryjournal.com/?mno=216150 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.23 AMA (American Medical Association) Style Goicochea-vargas J, Warthon-medina M, Rondón-jorge W, Acosta-pachorro F, Ratto-fuster M, Silva-jiménez M, Valderrama-linares X, Richards I, Salvatierra-alor M. Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Vet. J.. 2024; 14(11): 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 Vancouver/ICMJE Style Goicochea-vargas J, Warthon-medina M, Rondón-jorge W, Acosta-pachorro F, Ratto-fuster M, Silva-jiménez M, Valderrama-linares X, Richards I, Salvatierra-alor M. Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 Harvard Style Goicochea-vargas, J., Warthon-medina, . M., Rondón-jorge, . W., Acosta-pachorro, . F., Ratto-fuster, . M., Silva-jiménez, . M., Valderrama-linares, . X., Richards, . I. & Salvatierra-alor, . M. (2024) Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Vet. J., 14 (11), 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 Turabian Style Goicochea-vargas, José, Marisol Warthon-medina, Wilson Rondón-jorge, Fidel Acosta-pachorro, Marcelo Ratto-fuster, Mauricio Silva-jiménez, Ximena Valderrama-linares, Iain Richards, and Max Salvatierra-alor. 2024. Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Veterinary Journal, 14 (11), 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 Chicago Style Goicochea-vargas, José, Marisol Warthon-medina, Wilson Rondón-jorge, Fidel Acosta-pachorro, Marcelo Ratto-fuster, Mauricio Silva-jiménez, Ximena Valderrama-linares, Iain Richards, and Max Salvatierra-alor. "Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy." Open Veterinary Journal 14 (2024), 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 MLA (The Modern Language Association) Style Goicochea-vargas, José, Marisol Warthon-medina, Wilson Rondón-jorge, Fidel Acosta-pachorro, Marcelo Ratto-fuster, Mauricio Silva-jiménez, Ximena Valderrama-linares, Iain Richards, and Max Salvatierra-alor. "Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy." Open Veterinary Journal 14.11 (2024), 2950-2959. Print. doi:10.5455/OVJ.2024.v14.i11.23 APA (American Psychological Association) Style Goicochea-vargas, J., Warthon-medina, . M., Rondón-jorge, . W., Acosta-pachorro, . F., Ratto-fuster, . M., Silva-jiménez, . M., Valderrama-linares, . X., Richards, . I. & Salvatierra-alor, . M. (2024) Physiological and clinical parameters under different protocols of total intravenous anesthesia in llama (Lama glama) undergoing unilateral ovariectomy. Open Veterinary Journal, 14 (11), 2950-2959. doi:10.5455/OVJ.2024.v14.i11.23 |