| Research Article | ||
Open Vet. J.. 2024; 14(11): 2989-2994 Open Veterinary Journal, (2024), Vol. 14(11): 2989-2994 Research Article Ultrastructural morphology of second and third-stage larvae of Toxocara cati inside paratenic host tissueLetitia Amanda Theja1, Kusnoto Kusnoto2*, Martia Rani Tacharina3, Lucia Tri Suwanti2, Mufasirin Mufasirin2, Poedji Hastutiek2, Suhita Aryaloka4, Aswin Rafif Khairullah5, Ikechukwu Benjamin Moses6, Ricadonna Raissa7, Putri Wahyu Mulyaningrum1, Sheila Marty Yanestria8 and Katty Hendriana Priscilia Riwu91Profession Program in Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Master Program of Veterinary Agribusiness, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 7Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 8Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 9Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia *Corresponding Author: Kusnoto Kusnoto. Division of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: kusnoto [at] fkh.unair.ac.id Submitted: 21/08/2024 Accepted: 22/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
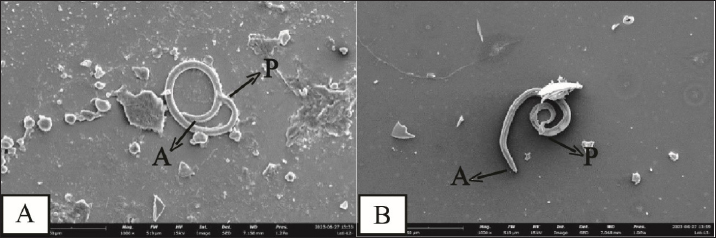
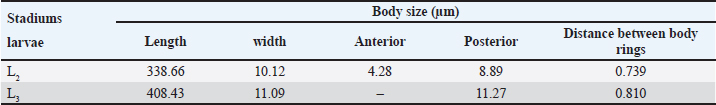
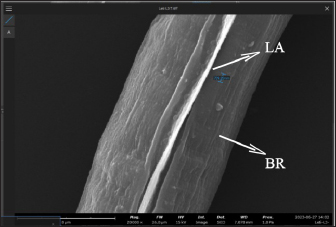
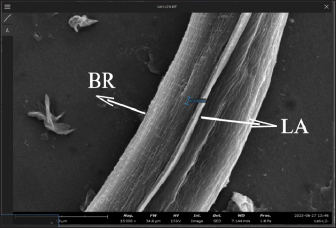
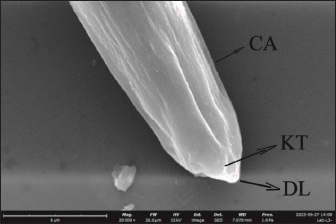
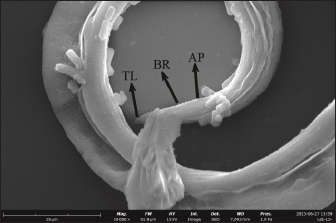
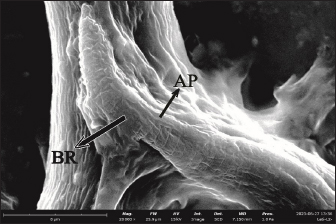
AbstractBackground: Toxocara cati is a known cause of a zoonotic infectious illness called toxocariasis. Parathenic hosts are important as they can transmit larvae 2 (L2) through direct transmission. Scanning electron microscope (SEM) techniques are needed to provide a three-dimensional image of each stage of T. cati larvae. Aim: The aim of this study was to determine the morphology of L2 and L3 T. cati in parathenic host tissue for etiological diagnosis using SEM. Methods: Mice were used as suitable paratenic hosts for this experiment. A total of 786 embryonated eggs (16 eggs/gram body weight) containing L2 were inoculated into pregnant mice at day 11–13 of its gestation period. After delivery, L2 was transmitted to the off-spring. After 14 days, L2 was collected from mice, and L3 was collected from its off-spring. Data were analyzed descriptively based on ultrastructure examination using SEM. Results: SEM examination results indicate that the size of L2 is smaller than L3. Results also showed differences between L2 and L3 based on middle and posterior observations. In the middle of the larval body, the number of L2 body rings was observed to be narrower and more than that of L3. In addition, the distance between L2 body rings was much larger than that of L3. Posteriorly, the tail tip of L3 was more curved than L2. Conclusion: Ultrastructural examination using SEM has the ability to show differences in L2 and L3 body rings of T. cati by observing the middle and posterior parts of its larvae. Keywords: Toxocara cati, Larvae, Scanning electron microscopy, Zoonosis, Parathenic host. IntroductionToxocariasis is a zoonotic infectious illness that can be transmitted from animals to humans. It is a term for diseases caused by Toxocara spp. One form of toxocariasis is Toxocara cati infection which is transmitted from definitive or paratenic hosts to humans (Chen et al., 2018). In order to treat toxocariasis properly, an appropriate diagnostic method is needed. Diagnosis can be made through etiological diagnosis by observing the developmental morphology of each stage of T. cati larvae (Magnaval et al., 2022). Observing the morphology of T. cati larvae usually uses a light microscope, but this method cannot identify them clearly and precisely (Oguz et al., 2018). Some organs of T. cati larvae such as the cervical alae, body ring, and cuticle cannot be seen, so other methods are needed. Observation of larval morphology can be seen more clearly using a scanning electron microscope (SEM) (Dos Santos et al., 2019). SEM techniques are needed to provide a three-dimensional image of each stage of T. cati larvae. Research on larvae 2 (L2) and larvae 3 (L3) of paratenic host tissue using SEM has not been carried out much because previous research only detected L3 migration patterns in paratenic host tissue (Raulf et al., 2021). Cats and kittens can be infected with T. cati, but the incidence of T. cati in kittens is higher than in adult cats (Bonilla-Aldana et al., 2024). Cats can become infected with T. cati by ingesting L2 which then migrates down the portal vein to the liver and lungs. In female animals, L2 does not reach the adult stage. L2 is considered to be dormant in the muscles when the cat is pregnant. L2 develops into L3 when the cat gives birth to kittens, resulting in transmammary transmission or transmission through milk (Castro and Sapp, 2020). Toxocariasis in humans is most often caused by T. cati (Bakhshani et al., 2020). Humans, especially children, can be infected with toxocariasis from the ingestion of T. cati embryonated eggs in the soil (Noordin et al., 2020). Zoonotic transmission through soil (soil transmission) is important because according to Candra et al. (2020), the highest number of worm eggs found is due to the huge percentage of Toxocara spp. eggs observed in the soil of Bangkalan, Madura. Another route of the zoonotic transmission of toxocariasis is through the ingesting of larvae in undercooked meat. In humans, it causes ocular larval migrans (OLMs) and visceral larval migrans (VLMs) (Hare and Franco-Paredes, 2014). VLM has symptoms that vary from asymptomatic to severe pathological conditions that are potentially fatal. In severe pathological conditions, it can cause hepatomegaly, hypergammaglobuline, lung damage, and neurological disorders (Zibaei et al., 2010). OLM can cause inflammation and granulomatous inflammation of the retina, resulting in loss of vision (Morsy, 2020). The migration path of larvae in paratenic hosts is similar to the migration path of definitive hosts. The embryonic larval eggs that are eaten by the paratenic host penetrate the intestinal wall and then migrate through the blood vessels to the liver and lungs (Strube et al., 2013). The larvae lie dormant in organs and muscles; when the paratenic host is eaten by a cat, the larvae develop into adult worms (Maciag et al., 2022). Most researchers believe that T. cati larvae are only transmitted via transmammary, but research from Okada et al. (2021) in which very large doses of embryonated eggs were inoculated into paratenic hosts showed transplancetal transmission. Knowledge of the differences in the ultrastructural morphology of L2 and L3 inside paratenic host tissue is necessary for diagnosis, so as to avoid misidentification of each stage of T. cati larvae. Previous studies majorly focus on the detection and transmission of the L2 and L3 of T. cati using light microscopy with very limited application of electron microscopy which would properly show their morphological structure in comprehensive detail for effective diagnosis of toxocariasis. This study was designed to explore the application of scanning electron microscopy to comprehensively understand the ultrastructural morphology and topography of second and third-stage larvae of Toxocara cati for the accurate diagnosis of toxocariasis, using mice as paratenic hosts. Materials and MethodsStudy area and sample collectionThe research was carried out from February 2024 to July 2024. The research samples used were L2 and L3 T. cati and two pregnant female BALB/C mice (Mus musculus). The mice were purchased and maintained in the Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. L2 T. cati was obtained from embryonated eggs inoculated into mice while L3 was obtained from mouse offspring whose mothers were inoculated with T. cati embryonated eggs. Toxocariasis examination using stool samples that have been taken, fertilization of worm eggs, inoculation on mice, and examination using a light microscope with an optilab camera (Nikon Corporation, Japan) were carried out at the Parasitology Laboratory, Airlangga University. Scanning electron microscopy (Thermo Fisher Scientific Inc, USA) examinations on L2 and L3 samples were carried out at the Institute of Life Sciences, Engineering and Engineering Laboratory, Airlangga University. Worms were obtained from the feces of cats that were identified to have toxocariasis and thereafter stored in storage pots containing phosphate buffer saline (PBS) solution. Worms that have died were surgically removed from the posterior reproductive tract to extract their eggs. On the other hand, those still alive were transferred to Petri dishes containing PBS and 0.9% NaCl solution. The worms were then incubated at 37°C to produce eggs. Eggs were maintained in PBS and NaCl media in a ratio of 1:1 (Suroiyah et al., 2018). Fertilizing worm eggsT. cati worm eggs were cultured with PBS media and the addition of 2–3 drops of 0.5% formalin to kill microorganisms and prevent the growth of egg-disturbing microorganisms. In the media, the eggs will develop into second-stage larvae (L2) (Simarmata et al., 2019). Egg development was routinely observed with a microscope at 100x and 400x magnification. The egg fertilization process was carried out for 21–28 days at room temperature. After fertilizing the eggs, they were placed in another tube and washed using distilled water. Embryoated eggs were deposited for 3–4 hours, collected by removing the supernatant, and thereafter observed using a light microscope. Examination of mouse organsAdult mice were infected using embryonated eggs with second-stage larvae. The dose of infected eggs was 16 embryonated eggs/gram of body weight. Female mice that had given birth were allowed to breastfeed for 14 days and dissected at the end of the 14th day to obtain L2 tissue. Mice pups that had been breastfeeding for 14 days were dissected to obtain L3 tissue. Before surgery, adult mice were euthanized by neck dislocation, while child mice were euthanized by neck decapitation. The examination was carried out by placing the somatic muscles and viscera (lungs, liver, and digestive tract) in a 1% trypsin solution, and thereafter, leaving the organs in an incubator at 37°C for 4 hours before observing the L2 and L3 of T. cati obtained by each organ. The number of observed larvae was then counted for each organ. Sample examination using a scanning electron microscope (SEM)The sample preparation procedure was done by first fixing the sample in 3% glutaraldehyde for 3 hours at 4°C, and then centrifuged at 1,200 rpm for 15 minutes. Each sample was washed with phosphate buffer (pH 7.4) at least 3 times for 5 minutes at 4°C, and then centrifugation at 1,500 rpm for 15 minutes. Next, each sample was dehydrated using graded alcohol of 30%, 50%, and 70% for 15–20 minutes at 4°C, then centrifuged at 1,500 rpm for 15 minutes. Dehydration was continued using 80% and 90% alcohol, and centrifuged at 1,500 rpm for 15 minutes. The next step was replacing the liquid with amyl acetate as a preserving liquid until drying time (Pertiwi et al., 2019). L2 and L3 tissue to be dried were pipetted and dropped onto a glass object that had been cleaned using 70% alcohol. Drying was carried out using a critical point drying tool, then coated with pure gold using a vacuum evaporator. Ethical approvalThis research received approval by Animal Care and Use Committee (ACUC), Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya. Certificate number: 1.KEH.118.09.2022. ResultsL2 was found in the somatic muscles and viscera of female mice in a moving condition. The number of larvae found was 82, equivalent to 10.4% of the 786 embryonated eggs inoculated. Of all the organs examined, the highest amount of L2 was in the liver. The mice that were operated on were those that had been breastfeeding for 14 days. Of the 13 mice that were dissected, L3 was found in the viscera and somatic muscles. The larvae found in the mice were in a moving condition. The number of larvae found in the young mice was 14, equivalent to 1.78% of the 786 embryonated eggs inoculated. Of all the organs examined, the highest number of L3 was found in the viscera. SEM provides a morphological image in three dimensions L2 and L3. The results show that there are differences in size and shape at each larval stage. Images of L2 and L3 using SEM are shown in Figure 1. The SEM image results were measured for each part (Table 1). It was observed that L2 had a length of around 338.66 µm and L3 of 408.43 µm (Table 1). The width of L2 is approximately 10.12 µm, while the width of L3 is 11.09 µm (Table 1). On the L2 and L3 sides of the body, there are elongated refractile lines called lateral alae. On all parts of the L2 and L3 body, there is a body ring formation in the form of smooth lines extending transversally (Fig. 2). L2 has a relatively narrow distance between the rings (739.2 nm) compared to L3 (810.92 nm). The body ring on L2 looks faint and smooth compared to L3 which looks clear and prominent. The lateral alae and body ring of L3 can be seen in Figure 3. The anterior part of L3 was not visible because the worm larvae were positioned facing other parts of the body. In the anterior part of L2, formations such as the dorsal lips, cuticle, and cervical alae which have not yet formed completely were observed. The anterior width of L2 was around 4.28 µm. The width of the right cervical alae was around 419.71 nm and the width of the left one was around 520.59 nm. Anterior observation of L2 is shown in Figure 4.
Fig. 1. L2 and L3 T. cati (1000x); (A) L3 in mouse offspring; (B) L2 in adult mice. A=Anterior, P=Posterior. Table 1. Measurements of L2 and L3 T. cati.
Fig. 2. The middle part of L2 of T. cati (20000x). LA=Lateral alae, BR=Body ring.
Fig. 3. The middle part of the L3 of T. cati (20,000x). LA=Lateral alae, BR=Body ring.
Fig. 4. Anterior section of L2 in adult mouse tissue (20,000x). CA=Cervical alae, DL=Dorsal lips, KT=Cuticle. The posterior length of L2 was around 3.31 µm while that of L3 was around 5.31 µm. In the posterior part, the tip of the L2 tail looks sharp and straight while the tip of the L3 tail looks pointed and curved. In L2 and L3, there was an anal pore formation around the terminal bulb. Precloacal papillae and postcloacal papillae could not be seen in SEM observations. The posterior observation showing body ring structures of both L2 and L3 are shown in Figures 5 and 6. DiscussionFrom the present results, the number of L2 found was 82 from a total of 786 inoculated embryonated eggs (10.4%). In mice, L3 tissue was found in somatic muscles and viscera. The number of L3 tissues found was 14 from a total of 786 inoculated embryonated eggs (1.78%). Of all the organs examined, the highest amount of L2 was in the liver, while L3 was in the viscera. This showed that there is transmission of T. cati larvae from mother mice to their offspring. According to Bakshani et al. (2020) who studied the distribution of T. cati larvae in mice, they argued that the low number of larvae could be caused by not all embryonated eggs hatching in the mice’s bodies or the embryonic eggs being defeated by the immune system before they hatch. Using SEM examination in the present study, the anterior part of L2 was observed to be the lip formation. The anterior part of L3 was not observed because the larva was facing other parts of the body. Interestingly, the tapered dorsal lips were clearly observed. This is different from what was found by Pertiwi et al. (2019) who demonstrated that the dorsal lips were blunt in T. canis. A thickened cuticle was also observed at the bottom of the lips. This is in accordance with Abou-El-Naga et al. (2022) who reported that the larvae will continue to thicken due to cuticle growth. The cuticle layer has been previously reported to line the mouth and esophagus (Bowman et al., 1993). On the lateral side from the present work, there is a part of the cervical alae that has not developed completely. Cervical alae can be seen in second-stage larvae but do not develop completely. The cervical alae develop completely at the adult worm stage (Mahdy et al., 2020).
Fig. 5. Posterior section of L2 in adult mouse tissue (10,000x). AP=Anal pore, BR=Body ring, TL=Tail.
Fig. 6. Posterior section of L3 in mouse tissue (20,000x). AP=Anal pore, BR=Body ring, TL=Tail. The present SEM examination revealed that L2 has a smaller body size than L3. This may indicate that larvae grow in every stage. SEM showed that the posterior length of L2 is smaller than L3. The posterior parts of L2 and L3 appear to taper to form a tail. The tail tip of L2 appears flatter while the tail tip of L3 appears curved. Raulf et al. (2021) previously reported that the L3 somatic muscle was extended from the buccal capsule to the tail. This explains why the tails of L2 and L3 were different. Apart from that, there is an anal pore formation around the terminal bulb and a visible body ring. When observed using SEM, no precloacal papillae or postcloacal papillae were visible. Simarmata et al. (2019) found that there were papilla formations on L2 and L3 of T. cati. This happens because the papilla may peel off during the preparation process. Flay et al. (2022) found that the postfixing process using osmium tetroxide of Haemonchus contortus larvae had poor penetration in a short time. As a result, some parts of the larvae were not visible or missing in ultrastructural observations. Ultrastructural observations of the middle part of the larvae can identify lateral alae and body rings at both L2 and L3 in the present study. The L2 body ring tends to look faint compared to the L3 body ring which looks prominent. The distance between the L2 body rings tends to be narrower than L3. The body ring is a thickened cuticle that is visible throughout the larva’s body (Ghorbanzadeh et al., 2021). An increase in the distance between the body ring and a decrease in the body ring can be caused by the loss of the outer layer of the cuticle which forms folds (Radwan et al., 2009). On the lateral side, there are lateral alae formations in the form of lines at L2 and L3. According to Zibaei (2017), the lateral alae are visible as refractile lines along the larval body. The lateral alae usually disappear in the adult phase and the cervical alae will grow to a length of 45 mm (Ziegler and Macpherson, 2019). ConclusionMice infected with T. cati embryonated eggs showed positive results in the presence of L2 tissue and their offspring were positively infected in the presence of L3. Ultrastructural examination using SEM can also show differences in L2 and L3 by observing the middle and posterior parts. In the middle of the larva’s body, it was observed that the distance between the body rings of L2 was narrower than L3, while in the posterior section, the tail tip of L3 was more curved than L2. AcknowledgmentsThe authors thanks to Universitas Airlangga. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research is funded by Rencana Kerja dan Anggaran Tahunan Skema Mandat year 2020 with grant number: 384/UN3.14/PT/2020. Author’s contributionsLAT, PWM, and KK: Conceived, designed, and coordinated the study. MRT and LTS: Designed data collections tools, supervised the field sample and data collection, and laboratory work as well as data entry. MM, KHPR, and PH: Validation, supervision, and formal analysis. SA and ARK: Contributed reagents, materials, and analysis tools. IBM, SMY, and RR: Carried out the statistical analysis and interpretation and participated in the preparation of the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data are available in the manuscript. ReferencesAbou-El-Naga, I.F., El-Nassery, S.M.F. and Sharaf, I.A. 2022. Immunochemical studies of Toxocara canis proteases. Trop. Biomed. 39(3), 315–320. Bakhshani, A., Khodaverdi, M. and Borji, H. 2020. Distribution of Toxocara cati larvae in experimentally infected BALB/c mice. Vet. Parasitol. 285(1), 109220. Bonilla-Aldana, J.L., Espinosa-Nuñez, A.C., Bonilla-Aldana, D.K. and Rodriguez-Morales, A.J. 2024. Toxocara cati infection in cats (Felis catus): a systematic review and meta-analysis. Animals 14(7), 1022. Bowman, D.D., Oaks, J.A. and Grieve, R.B. 1993. Ultrastructure of the infective stage larva of Toxocara canis (nematoda: ascaridoidea). Proc. Helminthol. Soc. Wash. 60(2), 183–204. Candra, D.A., Lastuti, N.D.R., Hidayati, N., Kusnoto, Hastutiek, P. and Bijanti, R. 2020. Differential counting value and determining of leukocytes in chicken after infected with L2 Toxocara cati. J. Parasite Sci. 4(1), 11–16. Castro, P.D.J. and Sapp, S.G. 2020. Role of cats in human toxocarosis. Companion Anim. 26(1), 1–8. Chen, J., Liu, Q., Liu, G.H., Zheng, W.B., Hong, S.J., Sugiyama, H., Zhu, X.Q. and Elsheikha, H.M. 2018. Toxocariasis: a silent threat with a progressive public health impact. Infect. Dis. Poverty 7(1), 59. Dos Santos, Q.M., Dzika, E. and Avenant-Oldewage, A. 2019. Using scanning electron microscopy (SEM) to study morphology and morphometry of the isolated haptoral sclerites of three distinct diplozoid species. PLoS One 14(1), e0211794. Flay, K.J., Hill, F.I. and Muguiro, D.H. 2022. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals (Basel) 12(10), 1238. Ghorbanzadeh, B., Naem, S. and Farshid, A.A. 2021. Microscopic study of mechanoreceptors and chemoreceptors of anterior and posterior ends of Toxocara Canis using scanning electron microscopy and light microscope. Arch. Razi Inst. 76(2), 311–322. Hare, A.Q. and Franco-Paredes, C. 2014. Ocular larva migrans: a severe manifestation of an unseen epidemic. Curr. Trop. Med. Rep. 1(1), 69–73. Maciag, L., Morgan, E.R. and Holland, C. 2022. Toxocara: time to let cati ‘out of the bag’. Trends Parasitol. 38(4), 280–289. Magnaval, J.F., Bouhsira, E. and Fillaux, J. 2022. Therapy and prevention for human toxocariasis. Microorganisms 10(2), 241. Mahdy, O.A., Mousa, W.M., Abdel-Maogood, S.Z., Nader, S.M. and Abdel-Radi, S. 2020. Molecular characterization and phylogenetic analysis of toxocara species in dogs, cattle and buffalo in Egypt. Helminthologia 57(2), 83–90. Morsy, T.A. 2020. Toxocariasis: visceral and ocular larva migrans. J. Egypt. Soc. Parasitol. 50(1), 41–48. Noordin, R., Yunus, M.H., Farrizam, S.N.T. and Arifin, N. 2020. Serodiagnostic methods for diagnosing larval toxocariasis. Adv. Parasitol. 109(1), 131–152. Oguz, B., Ozdal, N. and Deger, M.S. 2018. Genetic analysis of Toxocara Spp. in stray cats and dogs in van Province, Eastern Turkey. J. Vet. Res. 62(3), 291–295. Okada, N., Ooi, H.K. and Taira, K. 2021. Toxocara cati larval migration to mouse fetuses through transplacental infection. Vet. Parasitol. 290(1), 109350. Pertiwi, V.R., Kusnoto, Koesdarto, S., Lastuti, N.D.R., Suwanti, L.T. and Mufasirin. 2019. The differences between second stage larvaeand L2 Toxocara canis on mice tissueby using scanning electron microscopy. Indones. Vet. J. 20(3), 390–396. Radwan, N.A., Khalil, A.I. and El Mahi, R.A. 2009. Morphology and occurrence of species of toxocara in wild mammal populations from Egypt. Comp. Parasitol. 76(1), 273–282. Raulf, M.K., Lepenies, B. and Strube, C. 2021. Toxocara canis and Toxocara cati somatic and excretory-secretory antigens are recognised by C-type lectin receptors. Pathogens 10(3), 321. Simarmata, E.C., Kusnoto, K., Lazuardi, M., Koesdarto, S., Suprihati, E. and Santoso, K.P. 2019. Microscophy identification of Toxocara cati first stage larvae and second stage larvae. J. Parasite Sci. 3(1), 1–4. Strube, C., Heuer, L. and Janecek, E. 2013. Toxocara spp. infections in paratenic hosts. Vet. Parasitol. 193(4), 375–389. Suroiyah, F.A., Hastutiek, P., Yudhana, A., Sunarso, A. and Praja, R.N. 2018. Prevalence of Toxocara cati infection on domestic cat in Banyuwangi district. J. Vet. Med. 1(3), 99–104. Zibaei, M. 2017. Helminth infections and cardiovascular diseases: toxocara species is contributing to the disease. Curr. Cardiol. Rev. 13(1), 56–62. Zibaei, M., Sadjjadi, S.M. and Uga, S. 2010. Experimental Toxocara cati infection in gerbils and rats. Korean J. Parasitol. 48(4), 331–333. Ziegler, M.A. and Macpherson, C.N.L. 2019. Toxocara and its species. CAB Rev. 14(53), 1–27. | ||
| How to Cite this Article |
| Pubmed Style Theja LA, Kusnoto K, Tacharina MR, Suwanti LT, Mufasirin M, Hastutiek P, Aryaloka S, Khairullah AR, Moses IB, Raissa R, Mulyaningrum PW, Yanestria SM, Riwu KHP. Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Vet. J.. 2024; 14(11): 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 Web Style Theja LA, Kusnoto K, Tacharina MR, Suwanti LT, Mufasirin M, Hastutiek P, Aryaloka S, Khairullah AR, Moses IB, Raissa R, Mulyaningrum PW, Yanestria SM, Riwu KHP. Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. https://www.openveterinaryjournal.com/?mno=216482 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.27 AMA (American Medical Association) Style Theja LA, Kusnoto K, Tacharina MR, Suwanti LT, Mufasirin M, Hastutiek P, Aryaloka S, Khairullah AR, Moses IB, Raissa R, Mulyaningrum PW, Yanestria SM, Riwu KHP. Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Vet. J.. 2024; 14(11): 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 Vancouver/ICMJE Style Theja LA, Kusnoto K, Tacharina MR, Suwanti LT, Mufasirin M, Hastutiek P, Aryaloka S, Khairullah AR, Moses IB, Raissa R, Mulyaningrum PW, Yanestria SM, Riwu KHP. Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 Harvard Style Theja, L. A., Kusnoto, . K., Tacharina, . M. R., Suwanti, . L. T., Mufasirin, . M., Hastutiek, . P., Aryaloka, . S., Khairullah, . A. R., Moses, . I. B., Raissa, . R., Mulyaningrum, . P. W., Yanestria, . S. M. & Riwu, . K. H. P. (2024) Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Vet. J., 14 (11), 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 Turabian Style Theja, Letitia Amanda, Kusnoto Kusnoto, Martia Rani Tacharina, Lucia Tri Suwanti, Mufasirin Mufasirin, Poedji Hastutiek, Suhita Aryaloka, Aswin Rafif Khairullah, Ikechukwu Benjamin Moses, Ricadonna Raissa, Putri Wahyu Mulyaningrum, Sheila Marty Yanestria, and Katty Hendriana Priscilia Riwu. 2024. Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Veterinary Journal, 14 (11), 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 Chicago Style Theja, Letitia Amanda, Kusnoto Kusnoto, Martia Rani Tacharina, Lucia Tri Suwanti, Mufasirin Mufasirin, Poedji Hastutiek, Suhita Aryaloka, Aswin Rafif Khairullah, Ikechukwu Benjamin Moses, Ricadonna Raissa, Putri Wahyu Mulyaningrum, Sheila Marty Yanestria, and Katty Hendriana Priscilia Riwu. "Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue." Open Veterinary Journal 14 (2024), 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 MLA (The Modern Language Association) Style Theja, Letitia Amanda, Kusnoto Kusnoto, Martia Rani Tacharina, Lucia Tri Suwanti, Mufasirin Mufasirin, Poedji Hastutiek, Suhita Aryaloka, Aswin Rafif Khairullah, Ikechukwu Benjamin Moses, Ricadonna Raissa, Putri Wahyu Mulyaningrum, Sheila Marty Yanestria, and Katty Hendriana Priscilia Riwu. "Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue." Open Veterinary Journal 14.11 (2024), 2989-2994. Print. doi:10.5455/OVJ.2024.v14.i11.27 APA (American Psychological Association) Style Theja, L. A., Kusnoto, . K., Tacharina, . M. R., Suwanti, . L. T., Mufasirin, . M., Hastutiek, . P., Aryaloka, . S., Khairullah, . A. R., Moses, . I. B., Raissa, . R., Mulyaningrum, . P. W., Yanestria, . S. M. & Riwu, . K. H. P. (2024) Ultrastructural morphology of second and third stage larvae of Toxocara cati inside paratenic host tissue. Open Veterinary Journal, 14 (11), 2989-2994. doi:10.5455/OVJ.2024.v14.i11.27 |