| Research Article | ||
Open Vet. J.. 2024; 14(12): 3309-3316 Open Veterinary Journal, (2024), Vol. 14(12): 3309-3316 Research Article Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon blackJemy Caesar1, Widjiati Widjiati2, Eduardus Bimo Aksono Herupradoto2, Moh. Sukmanadi2, Sri Pantja Madyawati2, Hani Plumeriastuti2 and Epy Muhammad Luqman2*1Bachelor of Veterinary Medicine Study Program, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Epy Muhammad Luqman. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: epy-m-l [at] fkh.unair.ac.id Submitted: 22/08/2024 Accepted: 05/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
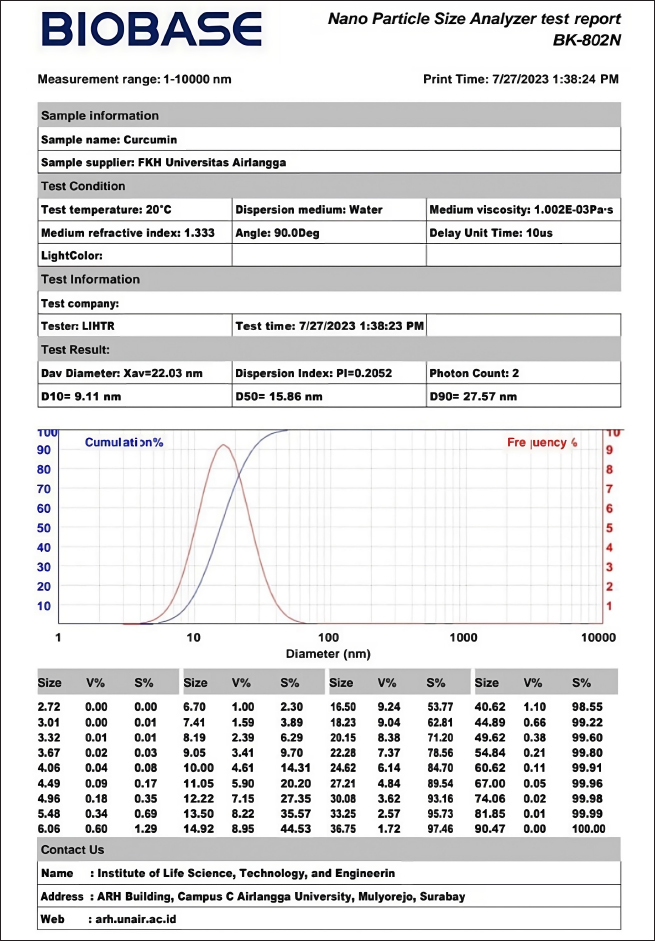
AbstractBackground: Continuous exposure to carbon black could affect the reproductive health of female animals, especially in the process of folliculogenesis. Curcumin nanoparticles are expected to maintain reproductive organ fertility through exposure to pollution containing carbon. Aim: The purpose of this research is to investigate the effect of curcumin nanoparticles on the number of preantral and antral ovarian follicles in white rats (Rattus norvegicus) exposed to carbon black. Methods: This study used 30 female rats divided into five groups. The negative (K−) and positive (K+) control groups were given aquades + Na-CMC 0.5% orally, while the treatment group received different doses of curcumin nanoparticles, namely, in the P1 (50 mg/kgBW), P2 (100 mg/kgBW), and P3 (150 mg/kgBW) groups, via oral administration. After that, K+, P1, P2, and P3 were exposed to carbon black with a concentration of 1064 mg/m3 for 6 hours/day for 30 days. The ovarian organ would then be made histopathological preparations to examine the number of preantral and antral follicles with HE staining. Results: The results showed a significant difference (p < 0.05). The average number of preantral follicles in the K− group (27.17 ± 6.37), K+ (10.33 ± 6.22), P1 (26.17 ± 5.98), P2 (19.17 ± 3.71), and P3 (23.50 ± 10.36) (p < 0.05) along with the average number of antral follicles in the K− group (18.50 ± 8.89), K+ (9.17 ± 2.14), P1 (17.67 ± 7.45), P2 (16.00 ± 5.30), and P3 (9.50 ± 5.09). Conclusion: The conclusion of this study shows that curcumin nanoparticles with different doses could affect and maintain preantral and antral ovarian follicles in white rats exposed to carbon black. It was also found that the dose of the P1 group (50 mg/kgBW) had the highest effectiveness in maintaining the number of ovarian follicles. The results of this study demonstrate the therapeutic potential of curcumin nanoparticles as an affordable drug. Keywords: Affordable medicines, antral follicular, carbon black, curcumin nanoparticles, preantral follicular. IntroductionAir pollution is one of the problems in the field of health in humans and animals. The source of this air pollution can come from various daily activities such as industrial activities, motor vehicles, and housing, besides that this air pollution can also come from nature, such as forest fires, volcanic eruptions, and natural gas (Hendrawan et al., 2018). Air pollution that comes from various sources can be in the form of carbon monoxide, nitrogen oxide, sulfur oxide, hydrocarbon, and other combustion products called particulate matter (PM). The main component of PM is made up of carbon particles. There are two terms related to the naming of these carbon particles, namely, black carbon (soot) and carbon black. The term “soot” is used to describe carbon particles from unwanted by-products from urban, industrial, and diesel vehicle waste particles with impure carbon content. In addition to black carbon (soot), there is the term carbon black, which is often used to describe carbon produced for commercial and controlled purposes such as in the rubber, printing, and painting industries for commercial use, with a pure carbon content of up to 97% (Niranjan et al., 2017). Carbon black spread around the world reaches 8.1 million metric tons per year, 90% comes from rubber production, 9% is used as pigment, and the remaining 1% is used as raw materials from various types of equipment. Continuous exposure to carbon black can affect the reproductive health of female animals, especially in the process of folliculogenesis. Foliculogenesis is the process responsible for follicle development and the release of one or more eggs at specific intervals during the reproductive cycle. Folliculogenesis is divided into two follicle formations. The first follicles are called preantral follicles or gonadotropin-independent follicles, which are characterized by oocyte growth and differentiation. The second follicle is called the antral follicle or gonadotropin-dependent, which is characterized by the rapid expansion of the follicle itself (Simon et al., 2020). Research by Luderer et al. (2022) concluded that exposure to carbon black resulted in a reduction in the number of follicles that developed induced cell death due to DNA damage and increased cell apoptosis. Exposure to PM in the form of carbon black on the female reproductive system shows a decrease in fertility, an increase in irregular estrous cycles, a reduction in the number of follicles, miscarriage, and reproductive cancer (Veras et al., 2009). The adverse impact of exposure to carbon black on the reproductive health of female animals can be reduced, and one of the ways is done by using turmeric as an herbal medicine therapy. Turmeric contains many active substances, one of which is antioxidants. The most important antioxidant component in turmeric is curcuminoids (Itokawa et al., 2008). Curcumin has important groups that support its role as antioxidants, such as phenolic hydroxyl groups and β-diketone groups. Phenolic hydroxy groups play a role in capturing free radicals in the early stages of antioxidant mechanisms, while β-diketone groups play a role in capturing free radicals in later stages. In addition, the β-diketone group also plays an important role in the properties of curcumin as an effective metal binder (Priyadarsini, 2014). The use of curcumin in various diseases has been widely proven by researchers. However, curcumin has low oral bioavailability, low solubility, and easy degradation, which makes it difficult to apply in clinical applications. To improve the work of curcumin in dosage form, currently, many forms of curcumin have been developed in the form of nanoparticles (Ochekpe et al., 2009). Some of the advantages of nanoparticles are being able to penetrate intercellular spaces and increase the bioavailability of drugs with low solubility (Buzea et al., 2007). The primary aim of this study was to investigate the potential protective effects of curcumin nanoparticles against the adverse effects of carbon black exposure on ovarian folliculogenesis in white rats. The objective was structured to not only contribute to the existing knowledge on the impact of environmental pollutants on reproductive health but also to explore the therapeutic potential of curcumin nanoparticles as affordable medicines. The novelty of this study lies in its exploration of the protective effects of curcumin nanoparticles against carbon black-induced changes in ovarian folliculogenesis. While previous research had extensively documented the antioxidant and anti-inflammatory properties of curcumin, its application in the form of nanoparticles to combat environmental pollutants affecting reproductive health was relatively unexplored. Materials and MethodsMaterialsA rectangular plastic cage measuring 60 × 40 × 15 cm with wood shavings as bedding was used. A gavage tube combined with a 1-cc tuberculin syringe, glass bottles, digital scales, cotton buds, plastic trays, aluminum foil, latex gloves, and a carbon black inhalation chamber were also utilized. Surgical instruments included surgical scissors, anatomical tweezers, underpads, organ pots, 5-cc syringes, test tubes, label paper, glass slides, cover glasses, and 1.5-ml Eppendorf tubes. The research materials consisted of nanocurcumin (Curcuma longa powder extract Curcumin 8.20354-Sigma, Aldrich made into solution for each treatment group) and carbon black powder, brand Vulcan N330. Ketamine was used as anesthesia during surgery. To prepare histopathological slides of mouse ovaries, materials, such as 0.9% physiological NaCl, 10% formalin, 70% alcohol, xylol, liquid paraffin, and haematoxylin eosin stain, were used. Preparation and characterization of nanocurcuminCharacterization of nanocurcumin describes how nanocurcumin was prepared and its particle size verified to ensure that it is within the nanorange. The average size of the nanocurcumin particles was determined using dynamic light scattering, where 1 mg of nanocurcumin powder (Curcumin 8.20354-Sigma, Aldrich) was dissolved in 10 ml of distilled water for the analysis. In addition, scanning electron microscopy (EX-250 System, Horiba, Kyoto, Japan) was utilized, involving the spreading of the nanoparticle dispersion on carbon tape followed by drying with a nitrogen stream. The sample was subsequently coated with a gold layer under vacuum conditions to enhance imaging (Kumar et al., 2021). The data pertaining to the size of the curcumin nanoparticles employed are presented in Figure 1. The test was conducted using the Nano Particle Size Analyzer BK-802N, with a measurement range of 1–10,000 nm. The test results showed a particle distribution with an average diameter (Xav) of 22.03 nm and a dispersion index of 0.2052. Key particle size distributions were measured as follows: D10=9.11 nm (indicating that 10% of the particles are smaller than this size), D50=15.86 nm (the median particle size, with 50% of the particles being smaller than this size), D90=27.57 nm (indicating that 90% of the particles are smaller than this size) (Fig. 1). In addition, the cumulative distribution and frequency graph depict the size distribution of the curcumin particles. The particle sizes range from very small diameters to about 90 nm, although the majority of the particles fall below 30 nm. From these results, it can be concluded that the curcumin tested possesses relatively uniform nano-sized particles, with a distribution range predominantly between 9 and 28 nm, indicating promising potential for applications that require nano-sized particles, such as in pharmaceuticals.
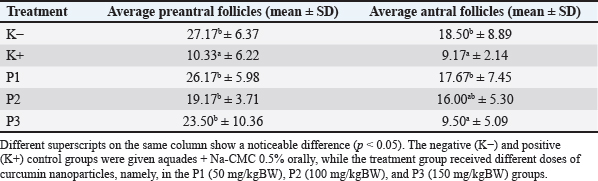
Fig. 1. Nanoparticle size analyzer test. MethodsTreatment on 30 female white rats was randomly divided into five groups, namely, K(–), K(+), P(1), P(2), and P(3) groups. The negative control group (K–) was given aquadest peroral aquadest + Na-CMC 0.5% for 30 days without exposure to carbon black. The positive control group (K+) was given aquades + Na-CMC 0.5% peroral and then given perinhalation carbon black exposure at a dose of 1064 mg/m3 for 6 hours/day for 30 days. Treatment group 1 (P1) was given 50 mg/kgBB of peroral curcumin nanoparticles and then given exposure to perinhaled carbon black at a dose of 1064 mg/m3 for 6 hours/day for 30 days. Treatment group 2 (P2) was given curcumin nanoparticles of 100 mg/kgBB perorally and then given exposure to perinhaled carbon black at a dose of 1064 mg/m3 for 6 hours/day for 30 days. Treatment group 3 (P3) was given curcumin nanoparticles of 150 mg/kgBB orally and then given perinhalation exposed to carbon black at a concentration of 1064 mg/m3 for 6 hours/day over 30 days. The determination of the dose for the administration of curcumin nanoparticles to white rats refers to a study conducted by Konatham (2020), namely, curcumin nanoparticles with doses of 50 mg/kgBW, 100 mg/kgBW, and 150 mg/kgBW. The implementation of the research began with the adaptation of female rats for 7 days and grouping. On the eighth day, carbon black exposure treatment began to be given to K(+), P1, P2, and P3. The treatment of experimental animals was carried out for 30 days, and on the 31st day, the rats were weighed and euthanized using ketamine at a dose of 0.1 ml/kgBB intraperitoneally. After the anesthesia of white rats is operated on by making an incision in the abdominal area, then the ovarian organs are removed and inserted into an organ pot in which there is 10% formalin. The ovarian organ will then be made histopathological preparations to examine the number of preantral and antral follicles with HE staining. The calculation of the number of preantral and antral follicles was carried out using a light microscope with 40× magnification and optilab. Observations were carried out by combining the histological preparation starting from the top-left corner of the preparation and then moving spirally to the lower right to get the five best fields of view. Data analysisData analysis in this study was carried out using the ANOVA test with statistical product and service solution computer software. If there is a real difference between the treatments (p < 0.05), it is followed by the Duncan test to find out the differences in each treatment group. Ethical approvalThis research has received an ethics certificate from the Ethics Commission of the Faculty of Veterinary Medicine, Universitas Airlangga (No.2. KEH.171.11.2024). The research conducted is experimental research. This study uses a post-test-only control group design with a simple random design treatment. This research was carried out using 30 female rats at the age of 2 months. ResultsBased on the research that has been carried out, the average number of preantral and antral follicles is obtained. Examination of ovarian histopathology preparation data was carried out to calculate the number of preantral follicles and antral follicles from each group by looking at each tail from five fields of view using a microscope and raster image application. Average preantral folliclesIn Table 1, it can be seen that the average number of preantral follicles in the K(−) group that was only given aquadest + Na-CMC 0.5%, which was 27.17 showed a significantly different result (p < 0.05) compared to the K(+) group who was given exposure to carbon black of 1064 mg/m3, which showed an average of 10.33. On average, each treatment group was given curcumin nanoparticles with a certain dose before exposure to carbon black 1064 mg/m3, and it was obtained that the P1 group had the highest average number of preantral follicles, which was 26.17, while the P2 group had the lowest average number of preantral follicles, which was 19.17 and P3 23.50. The average histopathological features of the preantral follicles can be seen in Figure 2. Average antral folliclesBased on Table 1, the average number of antral follicles in the K(−) group that was only given aquadest + Na-CMC 0.5%, which was 18.50 ± 8.89, showed a significantly different result (p < 0.05) compared to the K(+) group exposed to carbon black of 1064 mg/m3, which was 9.17 ± 2.14. On average, each treatment group was given curcumin nanoparticles with a certain dose before exposure to carbon black 1064 mg/m3, and it was seen that the P1 group had the highest average number of antral follicles, which was 17.67 ± 7.45, while the P3 group had the lowest average number of antral follicles, which was 9.50 ± 5.09, and the P2 group was 16.00. K(+) was not significantly different (p > 0.05) from the P3 group. This shows that the administration of carbon black in the K(+) group has a real effect on the number of antral follicles and in the P3 group that is exposed to carbon black and the dose of curcumin nanoparticles of 150 mg/kgBB gives similar results to the K(+) group. The P2 group did not differ significantly (p > 0.05) from the K(+) and P3 groups. In addition, P2 is not significantly different from the (K−) and P1 groups. In the data obtained, it can be seen that P1 does not differ significantly (p > 0.05) from K(−), showing that the dose used in P1 can give an average value that is close to the K(−) value. The mean histopathology of antral follicles can be seen in Figure 3. Table 1. Average and standard deviation of preantral and antral follicles exposed to carbon black and given curcumin nanoparticles.
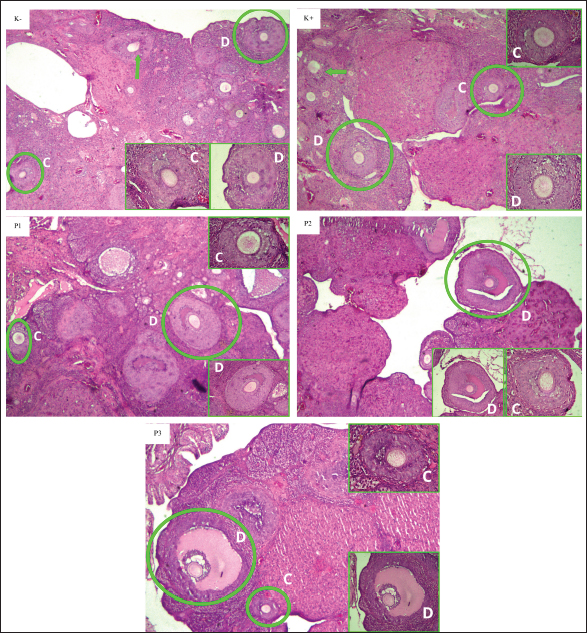
Fig. 2. Histopathological description of preantral ovarian follicles of white rats (R. norvegicus) given curcumin nanoparticles as a prevention of exposure to carbon black in the K−, K+, P1, P2, and P3 groups with 40× magnification HE staining. The circles and arrows of yellow color indicate the preantral follicles [primary and secondary follicles (A and B) observed]. In box shows the primary follicle (A) and the secondary follicle (B). The negative (K–) and positive (K+) control groups were given aquades + Na-CMC 0.5% orally, while the treatment group received different doses of curcumin nanoparticles, namely, in the P1 (50 mg/kgBW), P2 (100 mg/kgBW), and P3 (150 mg/kgBW) groups. DiscussionStatistically, the data in Table 1 shows that the K(−) group and the K(+) group are significantly different from each other. The K(−) group has an average number of preantral follicles 27.17 and antral 18.50, while in the K(+) group, the average number of preantral follicles was obtained 10.33 and antral 9.17. This indicates that exposure to carbon black can inhibit the development of follicles, causing inflammation and apoptosis in follicle cells. A decrease in the number of follicles has been as stated by Luderer et al. (2022), and carbon black can affect folliculogenesis and increase the risk of infertility. The use of curcumin as an antioxidant in this study had a beneficial influence on the development of follicles after exposure to carbon black. A study conducted by Sahebkar et al. (2015) showed that curcumin compounds provided significant reductions in oxidative stress markers, including superoxide dismutase, catalase, glutathione peroxidase, and lipid peroxide. In addition, curcumin reduces the response of proteins involved in inflammatory processes, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-2, IL-6, IL-8, and IL-12 (Anthwal et al., 2014).
Fig. 3. Histopathological description of the antral follicles of the ovaries of white rats (R. norvegicus) given curcumin nanoparticles as a prevention of exposure to carbon black in the K−, K+, P1, P2, and P3 groups with 40× magnification HE staining. The circles and green arrows indicate the antral follicles [tertiary and de graaf follicles (C and D) observed]. In box shows the tertiary follicle (C) and follicle de Graaf (D). The negative (K−) and positive (K+) control groups were given aquades + Na-CMC 0.5% orally, while the treatment group received different doses of curcumin nanoparticles, namely, in the P1 (50 mg/kgBW), P2 (100 mg/kgBW), and P3 (150 mg/kgBW) groups. Curcumin nanoparticles can activate transcription agents, namely, nuclear-related factor 2, which plays an important role in the transcription of phase II detoxification enzymes such as glutathione S-transferase, NAD(P)H Quinone Oxidoreductase 1, and glutamylcystein synthetase. These phase II detoxification enzymes function to protect cells from free radicals, especially from the reactive oxygen species (ROS) group (Reyes et al., 2013). Under normal circumstances, Nrf2 is inactivated in the cytosol by binding to Kelch-like ECH-associated protein 1 (González-Reyes et al., 2013). By activating Nrf2, curcumin helps modulate cellular antioxidant responses, increase the production of phase II detoxification enzymes, and reduce oxidative stress (Ghafouri-Fard et al., 2022). However, in the P3 group, there was a decrease in the average antral follicle, which was far from normal conditions. This can be affected because exposure to carbon black can affect the work of hormones such as FSH, which plays a role in stimulating follicle development. The highest dose in group P3 was less effective. This is related to the pharmacokinetics of nanoparticles, biodistribution, and side effects related to their clearance (Wei et al., 2018). The clearance of nanoparticles from the body depends on many factors, including particle size, shape, material, and surface modification (Poon et al., 2019). According to Pinlaor et al (2021), curcumin nanoparticles with a particle size of 400–1,000 nm after dispersion in water can be eliminated by the reticuloendothelial system or mononuclear phagocyte system. Macrophages will phagocytose curcumin nanoparticles and release them into the bloodstream in smaller sizes. According to Wei et al. (2018), higher doses of curcumin nanoparticles can prolong the elimination process in several organs, including the ovary, leading to tissue damage through the production of ROS enhanced by inflammatory cells. Overall, higher doses can cause an imbalance in mice, including excessive ROS production, increased production of the pro-oxidant cytokine IL6, and decreased antioxidant enzymes. In addition, curcumin can inhibit steroidogenesis or the formation of steroid hormones in females. Curcumin could inhibit the activity of key enzymes in the steroidogenesis pathway, such as the cytochrome P450 enzyme involved in the synthesis of cholesterol to pregnenolone, the initial step in the formation of all steroids (Rodríguez Castaño et al., 2019). Several previous studies have reported that curcumin may cause a pronounced antifertility effect in female rats, in the form of decreased levels of FSH and LH hormones and blocking the estrus cycle. This study was designed as a preliminary investigation into the potential protective effects of curcumin nanoparticles. We anticipated future studies to include a comprehensive hormonal profile analysis, including FSH and LH, once these initial results confirmed the protective effect of the nanoparticles. The use of curcumin nanoparticle doses in the P3 group at a dose of 150 mg/kgBW or higher than the treatment group may cause disturbances or ineffectiveness in follicle development. Although curcumin nanoparticles have the side effect of inhibiting steroidogenesis, some studies explain that high doses of curcumin nanoparticles are still not able to cause toxicity. The research conducted by Sandhiutami et al. (2022) showed that mice fed curcumin nanoparticles with the lowest dose of 175 mg/kgBW and the highest dose of 5.000 mg/kgBW for 14 days did not show any death. ConclusionBased on the results of the research that has been obtained, it can be concluded that the administration of curcumin nanoparticles can maintain the number of antral and preantral follicles of white rats (R. norvegicus) exposed to carbon black. The treatment group showed good effectiveness in maintaining the development of preantral and antral follicles with the best dose obtained in the P1 group with a dose of 50 mg/kgBW. AcknowledgmentsThis manuscript is based on research funded by the Internal Fund of the Faculty of Veterinary Medicine, Universitas Airlangga, under the Excellent Basic Research scheme. The author expresses gratitude to the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia, as well as the Faculty of Medicine, Universitas Airlangga, for providing financial support and research facilities. Conflict of interestThe authors state that they have no conflicts of interest to disclose. FundingThis manuscript was the result of research funded by the Internal Fund of the Faculty of Veterinary Medicine, Universitas Airlangga, with the Excellent Basic Research scheme with contract number: 1504/UN3.FKH/PT.01.03/2024. Authors’ contributionJC, WW, EBAH, MS, SPM, HP, and EML were responsible for the design of this study. These researchers also conducted field surveys and collected samples. All authors participated in the examination of samples in the laboratory. The final manuscript was compiled, reviewed, revised, and approved by all contributing authors. Data availabilityThe information backing the discoveries of this research is not openly accessible due to sensitivity concerns but can be obtained from the corresponding author upon reasonable inquiry. ReferencesAnthwal, A., Thakur, B.K., Rawat, M.S., Rawat D.S., Tyagi, A.K. and Aggarwal, B.B. 2014. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-α-induced NF-κB activation. BioMed. Res. Int. 2014, 524161. Buzea, C., Blandino, I.I.P. and Robbie, K. 2007. Nanomaterial and nanoparticles: sources and toxicity. Biointerphases. 2(4), MR170–MR172. Ghafouri-Fard, S., Shoorei, H., Bahroudi, Z., Hussen, B.M., Talebi, S.F., Taheri, M. and Ayatollahi, S.A. 2022. Nrf2-related therapeutic effects of curcumin in different disorders. Biomolecules 12(1), 82. González-Reyes, S., Guzmán-Beltrán, S., Medina-Campos, O.N. and Pedraza-Chaverri, J. 2013. Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid. Med. Cell Longev. 2013, 801418. Hendrawan, V.F., Wulansari, D. and Oktanella, Y. 2018. Effectiveness of chlorogenic acid supplementation on VEGF serum and placental MAP kinase expression in carbon black-Exposed pregnant Rattus norvegicus. Res. J. Pharm. Technol. 11(5), 1830–1834. Itokawa, H., Shi, Q., Akiyama, T., Morris-Natschke, S.L. and Lee, K.H. 2008. Recent advances in the investigation of curcuminoids. Chinese Med. 3, 11. Konatham, S. 2020. Liposomal delivery of curcumin to liver. Turk. J. Pharm. Sci. 7(2), 89–98. Kumar, V., Kumar, R. and Jain, V.K. 2021. Preparation and characterization of nanocurcumin based hybrid virosomes as a drug delivery vehicle with enhanced anticancerous activity and reduced toxicity. Sci. Rep. 11, 368. Luderer, U., Lim, J., Ortiz, L., Nguyen, J.D., Shin, J.H., Allen, B.D., Liao, L.S., Malott, K., Perraud, V., Wingen, L.M., Arechavala, R.J., Bliss, B., Herman, D.A. and Kleinman, M.T. 2022. Exposure to environmentally relevant concentrations of ambient fine particulate matter (PM2.5) depletes the ovarian follicle reserve and causes sex-dependent cardiovascular changes in apolipoprotein E null mice. Part. Fibre. Toxicol. 19(1), 5–15. Niranjan, R. and Thakur, A.K. 2017. The toxicological mechanisms of environmental soot (black carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front. Immunol. 8, 763. Ochekpe, N.A., Olorunfemi, P.O. and Ngwuluka, N.C. 2009. Nanotechnology and drug delivery part 2: nanostructure for drug delivery. Trop. J. Pharm Res. 8(3), 275–287. Pinlaor, S., Jantawong, C., Intuyod, K., Sirijindalert, T., Pinlaor, P., Pairojkul, C., Charoensuk, L., Sitthirach, C., Vaeteewoottacharn, K., Puthongking, P. and Priprem A. 2021. Solid dispersion improves release of curcumin from nanoparticles: potential benefit for intestinal absorption. Mater. Today Commun. 26, 1–9. Priyadarsini, K.I. 2014. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 19, 20091–20112. Poon W., Zhang Y.N., Ouyang B., Kingston B.R., Wu J.L.Y., Wilhelm S. and Chan W.C.W. 2019. Elimination pathways of nanoparticles. ACS Nano. 13(5), 5785–5798. Rodríguez Castaño, P., Parween, S. and Pandey, A.V. 2019. Bioactivity of curcumin on the cytochrome P450 enzymes of the steroidogenic pathway. Int. J. Mol. Sci. 20(18), 4606. Sahebkar A., Serban M.-C., Ursoniu S. and Banach M. 2015. Effect of curcuminoids on oxidative stress: a systematic review and meta-analysis of randomized controlledtrials. J. Funct. Foods. 6, 898–909. Sandhiutami, N.M.D., Dewi, R.S., Khairani, S. and Widyadari, S.A.M. 2022. Safety evaluation of the development of curcumin nanoparticle formula in mice and in-vitro antioxidant potential safety evaluation of the development of curcumin nanoparticle formula in mice and in-vitro antioxidant potential. Indonesia J. Pharm. Sci. 20(1), 63–72. Simon, L.E, Kumar, T.R and Duncan, F.E. 2020. In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables. Biol. Reprod. 103(3), 455–470. Veras, M.M., Damaceno-Rodrigues, N.R., Guimarães Silva, R.M., Scoriza, J.N., Nascimento, S.P.H. and Garcia, C. 2009. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ. Res. 109, 536–543. Wei Y., Quan L., Zhou C. and Zhan Q. 2018. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomed. (Lond).13(12), 1495–1512. | ||
| How to Cite this Article |
| Pubmed Style Caesar J, Widjiati W, Herupradoto EBA, Sukmanadi M, Madyawati SP, Plumeriastuti H, Luqman EM. Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Vet. J.. 2024; 14(12): 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 Web Style Caesar J, Widjiati W, Herupradoto EBA, Sukmanadi M, Madyawati SP, Plumeriastuti H, Luqman EM. Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. https://www.openveterinaryjournal.com/?mno=216652 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.15 AMA (American Medical Association) Style Caesar J, Widjiati W, Herupradoto EBA, Sukmanadi M, Madyawati SP, Plumeriastuti H, Luqman EM. Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Vet. J.. 2024; 14(12): 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 Vancouver/ICMJE Style Caesar J, Widjiati W, Herupradoto EBA, Sukmanadi M, Madyawati SP, Plumeriastuti H, Luqman EM. Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 Harvard Style Caesar, J., Widjiati, . W., Herupradoto, . E. B. A., Sukmanadi, . M., Madyawati, . S. P., Plumeriastuti, . H. & Luqman, . E. M. (2024) Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Vet. J., 14 (12), 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 Turabian Style Caesar, Jemy, Widjiati Widjiati, Eduardus Bimo Aksono Herupradoto, Moh. Sukmanadi, Sri Pantja Madyawati, Hani Plumeriastuti, and Epy Muhammad Luqman. 2024. Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Veterinary Journal, 14 (12), 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 Chicago Style Caesar, Jemy, Widjiati Widjiati, Eduardus Bimo Aksono Herupradoto, Moh. Sukmanadi, Sri Pantja Madyawati, Hani Plumeriastuti, and Epy Muhammad Luqman. "Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black." Open Veterinary Journal 14 (2024), 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 MLA (The Modern Language Association) Style Caesar, Jemy, Widjiati Widjiati, Eduardus Bimo Aksono Herupradoto, Moh. Sukmanadi, Sri Pantja Madyawati, Hani Plumeriastuti, and Epy Muhammad Luqman. "Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black." Open Veterinary Journal 14.12 (2024), 3309-3316. Print. doi:10.5455/OVJ.2024.v14.i12.15 APA (American Psychological Association) Style Caesar, J., Widjiati, . W., Herupradoto, . E. B. A., Sukmanadi, . M., Madyawati, . S. P., Plumeriastuti, . H. & Luqman, . E. M. (2024) Effect of curcumin nanoparticles on the number of preantral and antral follicles of white rats (Rattus norvegicus) exposed to carbon black. Open Veterinary Journal, 14 (12), 3309-3316. doi:10.5455/OVJ.2024.v14.i12.15 |