| Research Article | ||
Open Vet. J.. 2024; 14(12): 3317-3326 Open Veterinary Journal, (2024), Vol. 14(12): 3317-3326 Research Article Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cellAhmad Aswin1, Helen Susilowati1, Ira Sari Yudaniayanti2, Lina Susanti2, Diyantoro Diyantoro1,3, Watchareewan Rodprasert4 and Suryo Kuncorojakti1,5*1Research Center for Vaccine Technology and Development, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Clinic, Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Health Science, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia 4Veterinary Stem Cell and Bioengineering Innovation Center, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand 5Division of Veterinary Anatomy, Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Suryo Kuncorojakti. Division of Veterinary Anatomy, Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: suryokuncorojakti [at] fkh.unair.ac.id Submitted: 22/08/2024 Accepted: 02/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
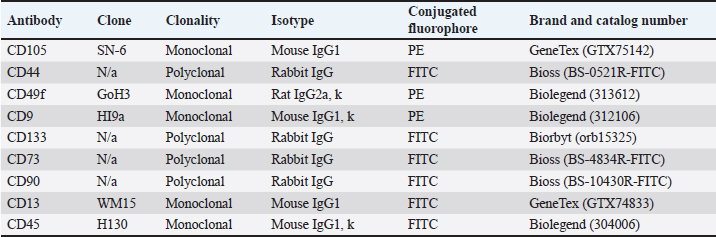
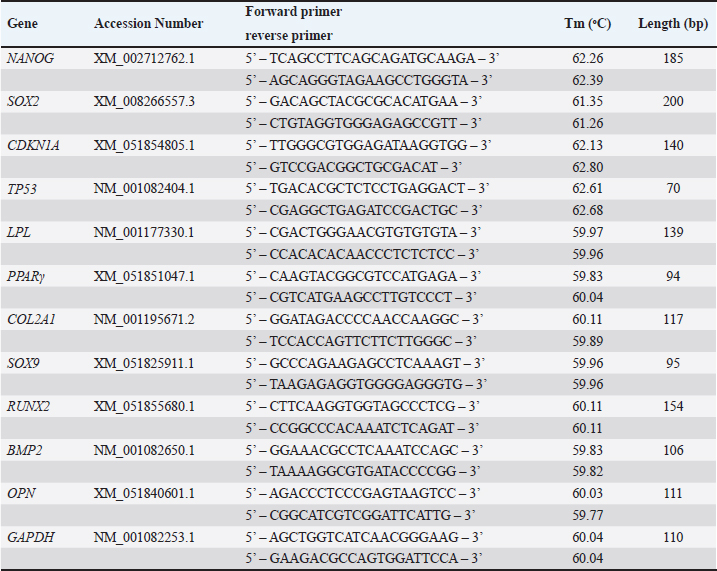
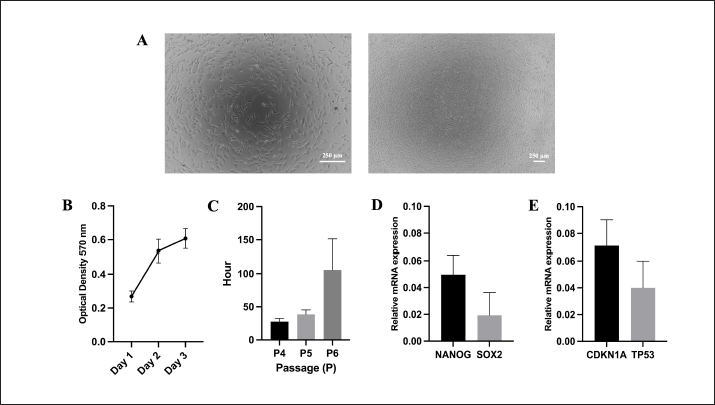
AbstractBackground: As an excellent model for many animal and human diseases, rabbits are the third-most used mammal model after mice and rats. A plethora of studies on the exploration of rabbit mesenchymal stem cells still face discrepancies, especially in the standardization of phenotype and genotype characteristics to support reproducibility in both biomedical and translational research. Aim: This study is aimed to evaluate the characterization and differentiation potential of visceral rabbit adipose-derived mesenchymal stem cells (Rab-ADMSC). Methods: Visceral adipose tissue was obtained from three healthy male White New Zealand rabbits. Cells were further processed and cultivated aseptically. Phenotype and genotype assessments, including morphological observation, proliferation capacity, population doubling time, stemness- and senescence-related genes determination, a set panel of mesenchymal stem/stromal cell (MSC) surface markers evaluation, and multilineage differentiation, were performed in this study. Results: Visceral Rab-ADMSC exhibited fibroblast-like shape morphology and had a plastic adherent ability, expressed stemness- (NANOG, SOX2) and senescence-related (TP53, CDKN1A) markers. Visceral Rab-ADMSC performs high expression of CD9, moderate expression of CD44 and CD49f, dimly expression of CD105, CD90, and CD73, and negative expression of CD13 and CD133 as well as CD45 as a hematopoietic stem cell marker. Despite these discrepancies, visceral Rab-ADMSC maintained its ability to differentiate into osteocytes, adipocytes, and chondrocytes. Conclusion: To recapitulate, visceral Rab-ADMSC reveals the phenotype and genotype characteristics of adult mesenchymal stem cells. The study emphasizes how variations in tissue sources, culture conditions, and techniques can affect the reproducibility and validity of MSC obtained from different specific anatomical depots and species. Thus, the utilization of rabbit MSC as an animal model in biomedical and translational studies should be done with full caution to avoid data misinterpretation. Keywords: Abdominal adipose tissue, Oryctolagus cuniculus, stem cells, regenerative medicine, process innovation. IntroductionRabbit is widely used as an experimental model for human and veterinary medicine because of their ease of handling and relatively economical for daily care. Their size is ideal for surgical procedures and allows for use in a large number of animals, improving the statistical significance. Rabbit is excellent for many applications in biotechnology and translational research, because of their phenotype and genotype similarities with human (Zomer et al., 2018; Tan et al., 2013; Calle et al., 2022). Rabbit adipose mesenchymal stem cells (Rab-ADMSC) have been reported that it has a resemblance in terms of cellular and tissue physiology to human MSC (Zomer et al., 2018). Rabbit adipose mesenchymal stem cells (Rab-ADMSC) have been extensively used to support translational studies in human medicine, such as atherosclerosis, insulin resistance, osteoarthritis, and regenerative medicine (Vachkova et al., 2016; Riester et al., 2017; Tang et al., 2017; Esteves et al., 2018; Schafrum Macedo et al., 2019). Adipose tissues are an excellent alternative source for mesenchymal stem/stromal cells (MSCs) because of their less invasive procedures, less pain, and high-yield cell numbers (Guneta et al., 2016). Adipose tissue-derived MSC obtained from rabbits has been shown to adhere to plastic surfaces and undergo multilineage differentiation (Martinez-Lorenzo et al., 2009; Zomer et al., 2018; Chen et al., 2020; Almaliotis et al., 2021; Tirpakova et al., 2021; Koung Ngeun et al., 2023). Thus, it serves a high potency for the utilization of Rab-ADMSC as an MSC source in supporting biomedical and translational research (Martinez-Lorenzo et al., 2009; Lee et al., 2014; Riester et al., 2017; Zomer et al., 2018). In addition, MSCs obtained from adipose tissues show higher viability, proliferation rate, and differentiation capability compared to bone marrow-derived MSC (Torres et al., 2007; Via et al., 2012; Frese et al., 2016; Koung Ngeun et al., 2023). Rab-ADMSC can be collected from subcutaneous and visceral depots. Although it is harder to collect, visceral Rab-ADMSC showed higher osteogenic potency and high yield compared to subcutaneous depots (Peptan et al., 2006; Jurgens et al., 2008; Cawthorn et al., 2012). Despite a great deal of research on visceral Rab-ADMSC, an inconsistent result has been found primarily due to the lack of characteristic information in cellular and molecular biology basis (Tan et al., 2013). Plethora study on rabbit MSC isolation and characterization revealed that in all studies, the rabbit MSC showed the three-lineage differentiation capability, but the study on MSC surface markers was inconsistent (Lee et al., 2014; Zomer et al., 2018). The obstacle in developing of the standardization rabbit MSC is the difficulties to obtain rabbit-specific antibody provided by the manufacturer. Thus, another approach to assess rabbit MSC surface marker was used, such as gene expression assessment in mRNA level using the RT-qPCR method (Tan et al., 2013; Lee et al., 2014; Tirpakova et al., 2021; Koung Ngeun et al., 2023). According to the International Society for Cellular Therapy (ISCT), the gold standard to evaluate the MSC surface marker should be performed by flow cytometry (Dominici et al., 2006). In addition, there are no guidelines for characterization, and the most recent guideline ISCT only addresses the human mesenchymal stem cells, which may not be valid for multiple species (Wright et al., 2021). Until now, there has been no comprehensive study evaluating the rabbit MSC on both phenotypic and genotypic bases. This study is aimed to establish and validate the Rab-ADMSC characteristics on genotype and phenotype bases. In addition, a set of antibody panels will be used to validate and evaluate the MSC surface marker by flow cytometry as a gold standard. Thus, the results of this study can be used to standardize visceral Rab-ADMSC to ensure reproducibility and avoid inconsistency for further downstream studies in both biomedical and translational studies. Materials and MethodsVisceral Rab-ADMSC isolation and cultureVisceral Rab-ADMSC was isolated from visceral adipose tissues (mesenteric depot) of three healthy male White New Zealand rabbits (2-kg BW on average, 2 old months). Briefly, visceral tissue from rabbit was washed with PBS and then processed with mechanical and enzymatic dissociation methods by TrypLE Express (Gibco, Paisley, UK) for 1 hour under constant stirring at 37 °C. Cells were cultured with alpha minimum essential medium (αMEM) (Gibco, Paisley, UK) and maintained at 37 °C in a humidified incubator with 5% CO2. The medium was supplemented with 10% fetal bovine serum (FBS) (Gibco, New York, NY), 1% penicillin–streptomycin (Gibco, New York, NY), and 1% Amphotericin B (Gibco, New York, NY). After cells achieved 80% confluence, the cells were subcultured. Cells in passages 3–5 were used for all experiments. Cell proliferation assayTo evaluate the proliferation capacity, a number of 5 × 103 cells were plated in 96-well plates and incubated for 24, 48, 72, and 96 hours. The cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and after incubating with 100 μl of MTT solution (0.5 mg/ml in PBS) for an additional 30 minutes at 37 °C, the supernatant solution was removed and 100 μl of DMSO (Sigma Aldrich, Darmstadt, Germany) was added to dissolve the formazan crystal. The absorbance of each well was measured with a plate reader (BIOBASE, Shandong, China) at a 570-nm wavelength. Cell population doubling time (PDT)The cell PDT was obtained by counting the starting and final cells over time (passages 4–6) according to Roth’s algorithm (Zomer et al., 2018). Multilineage differentiation assayFor adipogenic differentiation, 5 × 103 cells were plated onto 24-well plates (NEST, Jiangsu, China) for 14 days in αMEM (Gibco, Paisley, UK) containing 10% FBS (Gibco, New York, NY), 10-mM isobutyl-methylxanthine (Thermo Scientific, Fair Lawn, NJ), 100-mM indomethacin (Sigma Aldrich, MO, USA), 1-mM dexamethasone (Sigma Aldrich, Darmstadt, Germany), and 10-µg/ml insulin (Sigma Aldrich, Darmstadt, Germany). At day 14, the intracellular lipid droplets were stained with Oil Red O (Sigma Aldrich, Darmstadt, Germany) staining, and RT-qPCR was used to assess the gene expression of LPL and PPAR adipogenic mRNA markers. For chondrogenic differentiation, 5 × 103 cells were plated onto 24-well plates (NEST, Jiangsu, China) and treated with a chondrogenic induction medium αMEM (Gibco, Paisley, UK) 10% FBS (Gibco, New York, NY) supplemented with 1× insulin–transferrin–sodium selenite (Sigma Aldrich, Darmstadt, Germany), 40-mg/ml L-proline (Sigma Aldrich, Darmstadt, Germany), 50-mg/ml L-ascorbic acid-2-phosphate (Sigma Aldrich, Darmstadt, Germany), 1-ng/ml transforming growth factor-β1 (Thermo Scientific, CA, USA), and 100-nM dexamethasone (Sigma Aldrich, Darmstadt, Germany) for 21 days. Afterward, the glycosaminoglycan was stained with Alcian Blue (Sigma Aldrich, Darmstadt, Germany) staining, and RT-qPCR was used to quantify the gene expression of COL21A and SOX9 chondrogenic-related markers. For osteogenic differentiation, 3 × 104 cells were plated onto 24-well plates (NEST, Jiangsu, China) and induced with osteogenic induction medium containing αMEM (Gibco, Paisley, UK) supplemented with 10% FBS (Gibco, New York, NY) supplemented with 50-mg/ml L-ascorbic acid-2-phosphate (Sigma Aldrich, Darmstadt, Germany), 10-mM β-glycerophosphate (Sigma Aldrich, Darmstadt, Germany), and 100-nM dexamethasone (Sigma Aldrich, Darmstadt, Germany) for 21 days. The extracellular matrix mineralization was stained with 0.2% Alizarin Red (Merck, Darmstadt, Germany) staining, and RT-qPCR was performed to evaluate the gene expression of RUNX2, BMP2, and OPN osteogenic mRNA markers. ImmunophenotypingVisceral Rab-ADMSC at passages 4 and 5 were harvested and resuspended in PBS at a 1 × 106 cells/ml concentration. The surface markers were evaluated using Attune™ CytPix™ Flow Cytometer (Invitrogen, USA) against specific antibodies: CD105, CD44, CD49f, CD9, CD113, CD73, CD90, CD13, and CD45 (Table 1). All of the staining protocols were performed according to the manufacturer’s instructions. Reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)Total RNA was extracted using RNAsimple Total RNA Kit® (Tiangen, Beijing, China). The quantity of RNA was determined by Qubit (Thermo Scientific, USA). The cDNA was synthesized from 273-ng mRNA of each sample using FastKing gDNA Dispelling RT SuperMix® (Tiangen, Beijing, China) according to the manufacturer’s instructions and was performed using MiniAmp Plus Thermal Cycler (Thermo Scientific, USA), and the cDNA can be kept at −80 °C for further downstream study. Forget-Me-NotTM EvaGreen® qPCR Master Mix (Biotium, CA, USA) was utilized for a real-time quantitative PCR reaction. The RT-qPCR was performed by using QuantStudioTM 5 Real-Time PCR (Thermo Fisher, USA). The set of primers for both target and housekeeping genes are listed in Table 2. Target mRNA was normalized to GAPDH (housekeeping gene) using the formula 2−(∆∆Ct) to determine the relative mRNA expression. Statistical analysisThe results of this study were visualized as a bar chart and analyzed by nonparametric statistic (n=3). The Mann–Whitney U test was used to compare two independent groups. A statistically significant difference was set when the p-value was <0.05. Ethical approvalAll of the protocols involving the animal subject were performed according to the animal bioethics guidelines standard (Council, 2010) and were reviewed and approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine Universitas Airlangga with approval number 1.KEH.112.07.2024. ResultsCell morphology and proliferation capacityMicroscopical observation reveals that the Rab-ADMSC morphology was a spindle-shaped fibroblast-like cell attached to the bottom of container (Fig. 1A). The proliferation rate curve showed an increase in the cell viability within 3 days (Fig. 1B) and the PDT was also increased from 23.53 ± 4.67 and 37.27 ± 8.66 to 105.83 ± 45.54 hours in passages 4 and 5, respectively. The relative mRNA expression of stemness-related marker (NANOG and SOX2) and senescence-related genes (CDKN1A and TP53) was detected by RT-qPCR (Fig. 1D–E) Table 1. List of antibodies.
Table 2. List of primers.
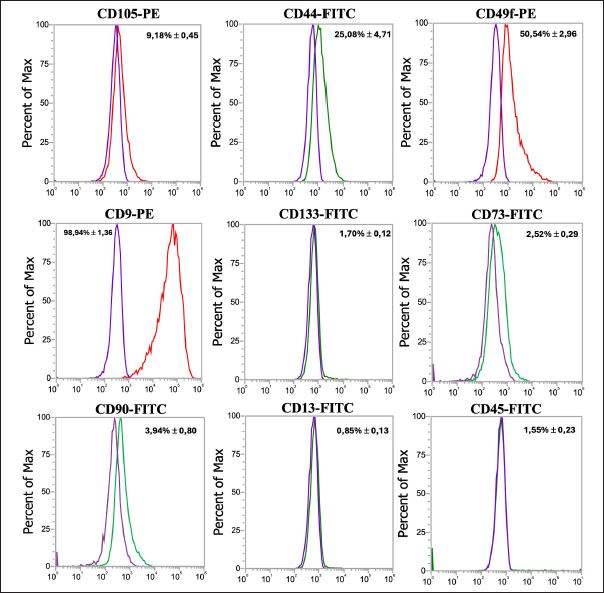
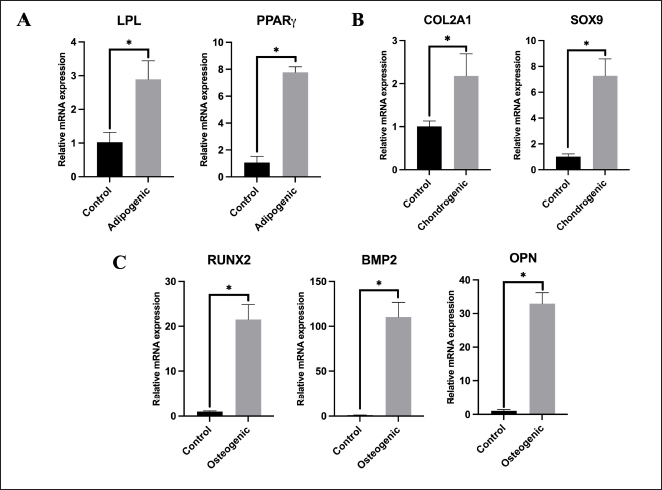
Cell surface marker expressionsPhenotypic analysis of visceral Rab-ADMSC surface markers by flow cytometry revealed that the cells expressed in high positivity of CD9 (98.94 ± 1.36%) and moderately positive of CD44 and CD49f expression 25.08 ± 4.71% and 50.54 ± 2.96%, respectively. A dimly positive of CD105 (9.18 ± 0.45%), CD90 (3.94 ± 0.80%), and CD73 (2.52 ± 0.29%) surface marker was detected in Rab-ADMSC, while negative expression of CD13 (0.85 ± 0.13%) and CD 133 (1.70 ± 0.12%) was detected. The hematopoietic surface marker of CD45 was not expressed in Rab ADMSC. The MSC surface marker expression was represented as the mean (%) ± SD, separately for each marker in Figure 2. Multilineage differentiationIn a multilineage differentiation study of visceral Rab-ADMSC, after the induction, the cells were committed to differentiate into mature adipocytes, chondrocytes, and osteocytes. Phenotypic evaluation by histochemical staining revealed that the differentiated cells are positive in oil red O, Alcian Blue, and Alizarin Red staining (Fig. 3A–C) showing that visceral Rab-ADMSCs were differentiated into adipogenic, chondrogenic, and osteogenic lineage. The genotypic study of three lineage differentiations of visceral Rab-ADMSC was in agreement with the phenotypic results. The expression of gene-related markers for adipogenic (LPL and PPARγ), chondrogenic (SOX9 and COL21A), and osteogenic (OPN, BMP2, and RUNX2) was elevated compared to undifferentiated cells (Fig. 4A–C). DiscussionAccording to the previous study, Rab-ADMSC characteristics might be defined using the same set of parameters as human MSC. The criteria should include plastic adherent behavior, morphological appearances, the expression of specific MSCs surface markers, and the ability for tri-lineage differentiation (Tan et al., 2013). Visceral Rab-ADMSC showed fibroblast-like cells with two processes adhering to the bottom of culture plastic, as described in the previous study (Zomer et al., 2018; Tirpakova et al., 2021; Koung Ngeun et al., 2023). Furthermore, the expression of stemness-related markers (NANOG and SOX2) and senescence-related markers (CDKN1A and TP53) indicates the multipotent, proliferative, and senescence characteristics of Visceral Rab-ADMSC. OCT4 and SOX2 are normally expressed in embryonic and adult stem cells. In the early passage of MSCs, both stemness-related markers are usually expressed at low levels, and as the number of passages increases, those markers eventually decreased. Co-expressing OCT4 and SOX2 in MSCs will improve their capacity for both proliferation and differentiation. Moreover, OCT4 and NANOG are necessary to maintain the pluripotency of embryonic stem cells and MSC properties (Frese et al., 2016). When expanded in vitro, MSCs inevitably undergo senescence and loss of stem cell characteristics, as described by low expression of TP53 and CDKN1A (senescence markers) in the early passage, and tend to increase at late passage (Liu et al., 2020; Gao et al., 2023). TP53 is a precursor of the P53 protein that induces the expression of CDKN1A, which inhibits cell cycle progression (Matveeva et al., 2024). Visceral Rab-ADMSC showed an elevated growth rate day by day, but the PDT increased, simultaneously with the increase of cell passage. The doubling times for rabbit adipose mesenchymal stem cells were previously reported to be 1.6 ± 0.2 days (Tirpakova et al., 2021), 3.5 days (Koung Ngeun et al., 2023), and 1.2 days (Zomer et al., 2018).
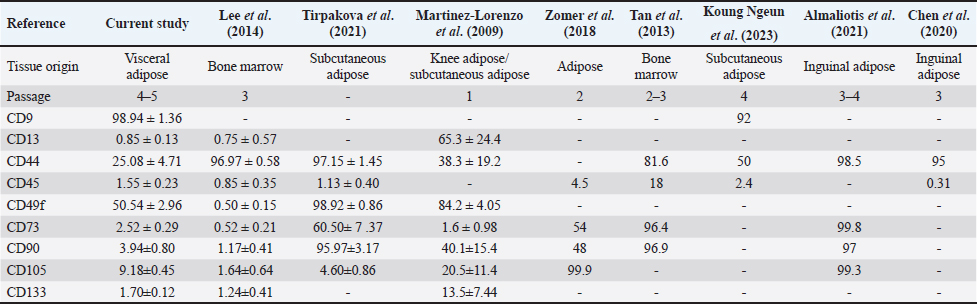
Fig. 1. Cell morphology after 24 hours of culturing in the high magnification and low magnification (100× and 40×, respectively). (A) Proliferation assay using MTT assay. (B) PDT. (C) Stemness. (D) Senescence. (E) related marker quantification by RT-qPCR. This current study’s findings revealed the negative expression of hematopoietic surface marker of CD45 and high expression MSC surface makers of CD9, and these results are in agreement with previous results on rabbit adipose MSC obtained from subcutaneous tissue (Koung Ngeun et al., 2023). CD9 is a key player in intercellular communication and is expressed in human MSCs and exosomes produced from MSCs (Hsieh et al., 2021). The first description of a high proportion of CD9 in rabbits was published by Koung Ngeun et al. (2023). The detailed comparison of the current study on the MSC surface markers study compared to other studies is summarized in Table 3. Contrary to previous studies that reported the high expression of CD105, CD90, and CD73 (Tan et al., 2013; Zomer et al., 2018; Almaliotis et al., 2021), these three surface markers were dimly expressed in the current study. It might have occurred due to the discrepancy of antibody used in this study. Similar results were also shown by the previous study on the low expression of CD13, CD133, CD105, CD90, and CD73 MSC surface markers (Martinez-Lorenzo et al., 2009; Lee et al., 2014). The moderate expression of CD49f and CD44 was still lower compared to the previous study (Martinez-Lorenzo et al., 2009; Lee et al., 2014; Tirpakova et al., 2021). CD49f marker (integrin α6) is associated with cell pluripotency (Tirpakova et al., 2021) and has high expression only at early passage (Martinez-Lorenzo et al., 2009). In this study, the expression of CD49f is moderate, which might be caused by the higher passage compared to a previous study (Martinez-Lorenzo et al., 2009). Nonetheless, it is important to note that the discrepancy in cell surface expression of several markers might be affected by substances released by accessory cells during the earlier stages. In addition, the variation of culture and isolation method, species, and tissue origin may play an important role in the discrepancy of the expression of rabbit MSC surface marker (Martinez-Lorenzo et al., 2009).
Fig. 2. Representative histograms showing the expression of CD45 as a hematopoietic surface marker and CD9, CD44, CD49f, CD13, CD45, CD73, CD90, CD105, and CD133 MSC surface markers. The purple histogram represents isotype control, the green histogram represents FITC-conjugated antibodies, and the red histogram represents PE-conjugated antibodies.
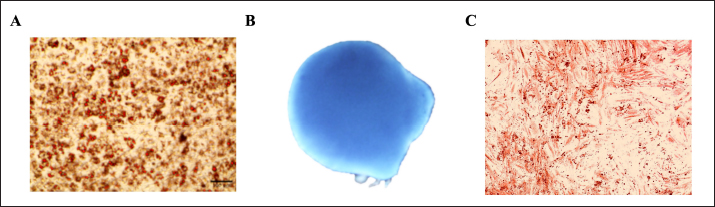
Fig. 3. Multilineage differentiation staining. Adipogenic staining with oil red O revealed red-stained lipid droplets. (A) Chondrogenic staining with Alcian Blue revealed a uniform blue color indicating glycosaminoglycans and cartilage-specific extracellular matrix, (B) whereas osteogenic staining with Alizarin Red revealed a calcium deposit (C).
Fig. 4. Gene expression of multilineage differentiation. (A) Adipogenic-related genes (LPL and PPARγ). (B) Chondrogenic-related genes (COL2A1 and SOX9). (C) Osteogenic-related genes (RUNX2, BMP2, and OPN). The differentiation capability into osteocytes, adipocytes, and chondrocytes is an important requirement for the characteristics of MSCs. According to the prior study, visceral Rab-ADMSCs have tri-lineage differentiation properties, shown by the phenotypic characteristic of the positive histo-chemical specific staining and the elevation of expression-associated genes (Zomer et al., 2018; Tirpakova et al., 2021; Koung Ngeun et al., 2023). Visceral Rab-ADMSC exhibited the upregulation of LPL and PPAR expression as well as red-stained lipid droplets inside the cells after 14-day adipogenic induction. PPARγ plays a crucial role in the development of lipid droplets into adipocytes by downregulating Perilipin and CIDEA. These two factors modulate the activity of lipolytic enzymes and remove triacylglycerol from cells also tuning the size of lipid droplet (Vachkova et al., 2016). The upregulation expression of PPARγ and C/EBPα led to the upregulation expression of adipogenesis-regulated factors such as LPL (Kim et al., 2021). The expression of LPL mRNA as an essential factor of adipogenic differentiation was detected at the initial stage of induction and will be stable in the late induction, resulting in mature adipocytes (Hu et al., 2018). Chondrogenic gene-related marker expressions COL2A1 and SOX9 are upregulated and stained positively with Alcian Blue, which was performed in this study. During chondrogenic induction, at the early stage of the induction, the expression of CoL1 and Fibronectin was upregulated and reached the highest level during the chondrogenic differentiation (middle stage). The expression of CoL1 and Fibronectin was subsequently decreased and replaced by higher COL2A1 expression at the late stage (ECM-related genes) (Tang et al., 2015; Robert et al., 2020). SOX9 is essential for the chondrogenic lineage development of MSCs. This activity is triggered when phosphorylated SOX9 directly binds to gene targets, including Collagen type II, IX, and XI, Aggrecan, and Link Protein, thereby causing their transcriptional activity. SOX9 can be found in both chondroprogenitors and mature chondrocytes, and it will directly upregulate the gene expression, specifically in cartilaginous matrix deposition (Sahu et al., 2020; Yang et al., 2022). Osteogenic differentiation potency was assessed by the upregulation of RUNX2, BMP2, and OPN, as well as calcium deposition was stained after 21-day induction. Hedgehog, Wnt/β-catenin, BMP signaling, hormones, growth factors, and epigenetic regulators were all elevated during the early stages of osteogenesis. The osteogenic key factor of RUNX2 was upregulated during the induction. RUNX2 is a precursor of osteogenic-related marker, essential for the differentiation of osteoblasts and for controlling the expression of other osteogenic genes, such as Col1, OPN, and ALP (Mohamed-Ahmed et al., 2018; Robert et al., 2020). Furthermore, BMP2 promotes the osteogenic development of MSCs by upregulating mitochondrial activity and PGC-1α levels (Li et al., 2022; Koung Ngeun et al., 2023). Table 3. Specific MSC surface marker expression.
ConclusionIn conclusion, the research offers a comprehensive understanding of the characteristic and differentiation potency of visceral Rab-ADMSC, which has a fibroblast-like shape morphology and plastic adherent. Visceral Rab-ADMSC has the proliferative capability as well as expression of senescence- and stemness-related markers such as NANOG, SOX2, CDKN1A, and TP53. The evaluation of mesenchymal stem cell surface markers reveals that the visceral Rab-ADMSC performs high expression of CD9, moderate expression of CD44 and CD49f, low expression of CD105, CD90, and CD73, and negative expression of CD13 and CD133 as well as CD45 as a hematopoietic stem cell marker. The visceral Rab-ADMSC fulfills the phenotype and genotype characterization multilineage differentiation into adipogenic, chondrogenic, and osteogenic lineage. Thus, this study emphasizes the necessity for the establishment of standard protocols to address the discrepancy of phenotype and genotype characterization of rabbit MSC to support reproducibility in biomedical and translational research. AcknowledgmentThe authors would like to thank the Research Center for Vaccine Technology and Development (RCVTD), Institute of Tropical Diseases, Universitas Airlangga, for providing the facilities for this study. This study was supported by grants from the Directorate of Research, Technology and Community Service, Ministry of Education, Culture, Research and Technology Republic of Indonesia through Regular—Fundamental Research Grant (Penelitan Fundamental – Reguler) 2024 with grant number 1712/B/UN3.LPPM/PT.01.03/2024. Conflict of interestThe authors declare that they have no conflict of interest. FundingThis study was supported by grants from the Directorate of Research, Technology and Community Service, Ministry of Education, Culture, Research and Technology Republic of Indonesia through Regular—Fundamental Research Grant (DRTPM, Penelitan Fundamental – Regular) 2024 with grant number 1712/B/UN3.LPPM/PT.01.03/2024. Authors’ contributionAA: performed the experiment, analyzed data, and wrote the manuscript. SK and WR: designed, conceptualized, and supervised the study, and wrote the manuscript. ISY and LS: performed adipose tissue isolation and conceptualized the study. HS and DD: performed visceral Rab-ADMSC processing, maintained cell culture work, and analyzed the data. All authors have read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAlmaliotis, D., Thomas, A., Komnenou, A., Gounari, E., Almpanidou, S., Siempis, T., Papaioannou, N., Koliakos, G., Papakonstantinou, E., Sotiropulos, K. and Karampatakis, V. 2021. Evaluation of clinical and histological outcomes of adipose-derived mesenchymal stem cells in a rabbit corneal alkali burn model. Stem Cells Int. 2021, 6610023. Calle, A., Zamora-Ceballos, M., Barcena, J., Blanco, E. and Ramirez, M.A. 2022. Comparison of Biological Features of Wild European Rabbit Mesenchymal Stem Cells Derived from Different Tissues. Int. J. Mol. Sci. 23(12), 6420. Cawthorn, W.P., Scheller, E.L. and MacDougald, O.A. 2012. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid. Res. 53(2), 227–246. Chen, G., Zhang, W., Zhang, K., Wang, S., Gao, Y., Gu, J., He, L., Li, W., Zhang, C., Zhang, W., Li, M., Hao, Q. and Zhang, Y. 2020. Hypoxia-induced mesenchymal stem cells exhibit stronger tenogenic differentiation capacities and promote patellar tendon repair in rabbits. Stem Cells Int. 2020, 8822609. Council, N.R. 2010. Guide for the care and use of laboratory animals Eighth Edition (Eighth ed.). Washington, DC: The National Academies Press. Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D. and Horwitz, E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4), 315–317. Esteves, P.J., Abrantes, J., Baldauf, H.M., BenMohamed, L., Chen, Y., Christensen, N., Gonzalez-Gallego, J., Giacani, L., Hu, J., Kaplan, G., Keppler, O.T., Knight, K.L., Kong, X. P., Lanning, D.K., Le Pendu, J., de Matos, A.L., Liu, J., Liu, S., Lopes, A.M., Mage, R. 2018. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 50(5), 1–10. Frese, L., Dijkman, P.E. and Hoerstrup, S.P. (2016). Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemother. 43(4), 268–274. Gao, Y., Chi, Y., Chen, Y., Wang, W., Li, H., Zheng, W., Zhu, P., An, J., Duan, Y., Sun, T., Liu, X., Xue, F., Liu, W., Fu, R., Han, Z., Zhang, Y., Yang, R., Cheng, T., Wei, J. an Zhang, X. 2023. Multi-omics analysis of human mesenchymal stem cells shows cell aging that alters immunomodulatory activity through the downregulation of PD-L1. Nat. Commun. 14(1), 4373. Guneta, V., Tan, N.S., Chan, S.K., Tanavde, V., Lim, T.C., Wong, T.C. and Choong, C. 2016. Comparative study of adipose-derived stem cells and bone marrow-derived stem cells in similar microenvironmental conditions. Exp. Cell Res. 348(2), 155–164. Hsieh, C.C., Hsu, S.C., Yao, M. and Huang, D.M. 2021. CD9 upregulation-decreased CCL21 secretion in mesenchymal stem cells reduces cancer cell migration. Int. J. Mol. Sci. 22(4), 1738. Hu, X., Tang, J., Hu, X., Bao, P., Pan, J., Chen, Z. and Xian, J. 2018. MiR-27b impairs adipocyte differentiation of human adipose tissue-derived mesenchymal stem cells by targeting LPL. Cell Physiol. Biochem. 47(2), 545–555. Jurgens, W.J., Oedayrajsingh-Varma, M.J., Helder, M.N., Zandiehdoulabi, B., Schouten, T.E., Kuik, D.J., Ritt, M.J. and van Milligen, F.J. 2008. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 332(3), 415–426. Kim, J.Y., Park, E.J., Kim, S.M. and Lee, H.J. 2021. Optimization of adipogenic differentiation conditions for canine adipose-derived stem cells. J. Vet. Sci. 22(4), e53. Koung Ngeun, S., Shimizu, M. and Kaneda, M. 2023. Characterization of rabbit mesenchymal stem/stromal cells after cryopreservation. Biology (Basel), 12(10), 1312. Lee, T.C., Lee, T.H., Huang, Y.H., Chang, N.K., Lin, Y.J., Chien, P.W., Yang, W.H. and Lin, M.H. 2014. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One 9(11), e111390. Li, Y., Fu, G., Gong, Y., Li, B., Li, W., Liu, D. and Yang, X. 2022. BMP-2 promotes osteogenic differentiation of mesenchymal stem cells by enhancing mitochondrial activity. J. Musculoskelet. Neuronal. Interact. 22(1), 123–131. Liu, J., Ding, Y., Liu, Z. and Liang, X. 2020. Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front. Cell Dev. Biol. 8, 258. Martinez-Lorenzo, M.J., Royo-Canas, M., Alegre-Aguaron, E., Desportes, P., Castiella, T., Garcia-Alvarez, F. and Larrad, L. 2009. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J. Orthop. Res. 27(11), 1499–1507. Matveeva, D., Kashirina, D., Ezdakova, M., Larina, I., Buravkova, L. and Ratushnyy, A. 2024. Senescence-associated alterations in matrisome of mesenchymal stem Cells. Int. J. Mol. Sci. 25(10), 5332. Mohamed-Ahmed, S., Fristad, I., Lie, S. A., Suliman, S., Mustafa, K., Vindenes, H. and Idris, S. B. 2018. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 9(1), 168. Peptan, I.A., Hong, L. and Mao, J.J. 2006. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast. Reconstr. Surg. 117(5), 1462–1470. Riester, S.M., Denbeigh, J.M., Lin, Y., Jones, D.L., de Mooij, T., Lewallen, E.A., Nie, H., Paradise, C.R., Radel, D.J., Dudakovic, A., Camilleri, E.T., Larson, D.R., Qu, W., Krych, A. J., Frick, M.A., Im, H.J., Dietz, A.B., Smith, J. and van Wijnen, A.J. 2017. Safety studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a phase I clinical trial. Stem Cells Transl. Med. 6(3), 910–922. Robert, A.W., Marcon, B.H., Dallagiovanna, B. and Shigunov, P. 2020. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: a comparative transcriptome approach. Front. Cell Dev. Biol. 8, 561. Sahu, N., Budhiraja, G. and Subramanian, A. 2020. Preconditioning of mesenchymal stromal cells with low-intensity ultrasound: influence on chondrogenesis and directed SOX9 signaling pathways. Stem Cell Res. Ther. 11(1), 6. Schafrum Macedo, A., Cezaretti Feitosa, C., Yoiti Kitamura Kawamoto, F., Vinicius Tertuliano Marinho, P., Dos Santos Dal-Bo, I., Fiuza Monteiro, B., Prado, L., Bregadioli, T., Antonio Covino Diamante, G. and Ricardo Auada Ferrigno, C. 2019. Animal modeling in bone research-Should we follow the White Rabbit? Animal Model. Exp. Med. 2(3), 162–168. Tan, S.L., Ahmad, T.S., Selvaratnam, L. and Kamarul, T. 2013. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 222(4), 437–450. Tang, X., Fan, L., Pei, M., Zeng, L. and Ge, Z. 2015. Evolving concepts of chondrogenic differentiation: history, state-of-the-art and future perspectives. Eur. Cell Mater. 30, 12–27. Tang, Y., Pan, Z.Y., Zou, Y., He, Y., Yang, P.Y., Tang, Q.Q. and Yin, F. 2017. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J. Cell Mol. Med. 21(9), 2153–2162. Tirpakova, M., Vasicek, J., Svoradova, A., Balazi, A., Tomka, M., Bauer, M., Makarevich, A. and Chrenek, P. 2021. Phenotypical characterization and neurogenic differentiation of rabbit adipose tissue-derived mesenchymal stem cells. Genes (Basel), 12(3), 431. Torres, F.C., Rodrigues, C.J., Stocchero, I.N. and Ferreira, M.C. 2007. Stem cells from the fat tissue of rabbits: an easy-to-find experimental source. Aesthetic. Plast. Surg. 31(5), 574–578. Vachkova, E., Bosnakovski, D., Yonkova, P., Grigorova, N., Ivanova, Z., Todorov, P., Penchev, G., Milanova, A., Simeonova, G., Stanilova, S. and Georgiev, I. P. 2016. Adipogenic potential of stem cells derived from rabbit subcutaneous and visceral adipose tissue in vitro. In Vitro Cell Dev. Biol. Anim. 52(8), 829–837. Via, A.G., Frizziero, A. and Oliva, F. 2012. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2(3), 154–162. Wright, A., Arthaud-Day, M.L. and Weiss, M.L. 2021. Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species. Front. Cell Dev. Biol. 9, 632717. Yang, X., Tian, S., Fan, L., Niu, R., Yan, M., Chen, S., Zheng, M. and Zhang, S. 2022. Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells. Cancer Cell Int. 22(1), 169. Zomer, H.D., Roballo, K.C., Lessa, T.B., Bressan, F.F., Goncalves, N.N., Meirelles, F.V., Trentin, A.G. and Ambrosio, C.E. 2018. Distinct features of rabbit and human adipose-derived mesenchymal stem cells: implications for biotechnology and translational research. Stem Cells Cloning 11, 43–54. | ||
| How to Cite this Article |
| Pubmed Style Aswin A, Susilowati H, Yudaniayanti IS, Susanti L, Diyantoro D, Rodprasert W, Kuncorojakti S. Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Vet. J.. 2024; 14(12): 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 Web Style Aswin A, Susilowati H, Yudaniayanti IS, Susanti L, Diyantoro D, Rodprasert W, Kuncorojakti S. Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. https://www.openveterinaryjournal.com/?mno=216705 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.16 AMA (American Medical Association) Style Aswin A, Susilowati H, Yudaniayanti IS, Susanti L, Diyantoro D, Rodprasert W, Kuncorojakti S. Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Vet. J.. 2024; 14(12): 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 Vancouver/ICMJE Style Aswin A, Susilowati H, Yudaniayanti IS, Susanti L, Diyantoro D, Rodprasert W, Kuncorojakti S. Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 Harvard Style Aswin, A., Susilowati, . H., Yudaniayanti, . I. S., Susanti, . L., Diyantoro, . D., Rodprasert, . W. & Kuncorojakti, . S. (2024) Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Vet. J., 14 (12), 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 Turabian Style Aswin, Ahmad, Helen Susilowati, Ira Sari Yudaniayanti, Lina Susanti, Diyantoro Diyantoro, Watchareewan Rodprasert, and Suryo Kuncorojakti. 2024. Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Veterinary Journal, 14 (12), 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 Chicago Style Aswin, Ahmad, Helen Susilowati, Ira Sari Yudaniayanti, Lina Susanti, Diyantoro Diyantoro, Watchareewan Rodprasert, and Suryo Kuncorojakti. "Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell." Open Veterinary Journal 14 (2024), 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 MLA (The Modern Language Association) Style Aswin, Ahmad, Helen Susilowati, Ira Sari Yudaniayanti, Lina Susanti, Diyantoro Diyantoro, Watchareewan Rodprasert, and Suryo Kuncorojakti. "Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell." Open Veterinary Journal 14.12 (2024), 3317-3326. Print. doi:10.5455/OVJ.2024.v14.i12.16 APA (American Psychological Association) Style Aswin, A., Susilowati, . H., Yudaniayanti, . I. S., Susanti, . L., Diyantoro, . D., Rodprasert, . W. & Kuncorojakti, . S. (2024) Rabbit visceral adipose stromal cell reveals phenotype and genotype characteristics of adult mesenchymal stem cell. Open Veterinary Journal, 14 (12), 3317-3326. doi:10.5455/OVJ.2024.v14.i12.16 |