| Research Article | ||
Open Vet. J.. 2024; 14(12): 3336-3344 Open Veterinary Journal, (2024), Vol. 14(12): 3336-3344 Research Article Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family LabridaeNajiah M. Alyamani*Department of Biology, College of Science, University of Jeddah, Jeddah, Saudi Arabia *Corresponding Author: Najiah M. Alyamani. Department of Biology, College of Science, University of Jeddah, Jeddah, Saudi Arabia. Email: nmalyamani [at] uj.edu.sa Submitted: 26/08/2024 Accepted: 24/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
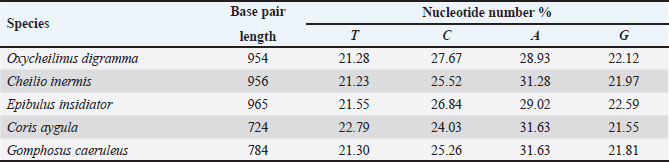
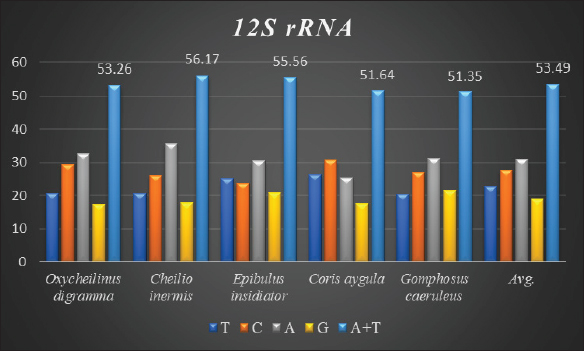
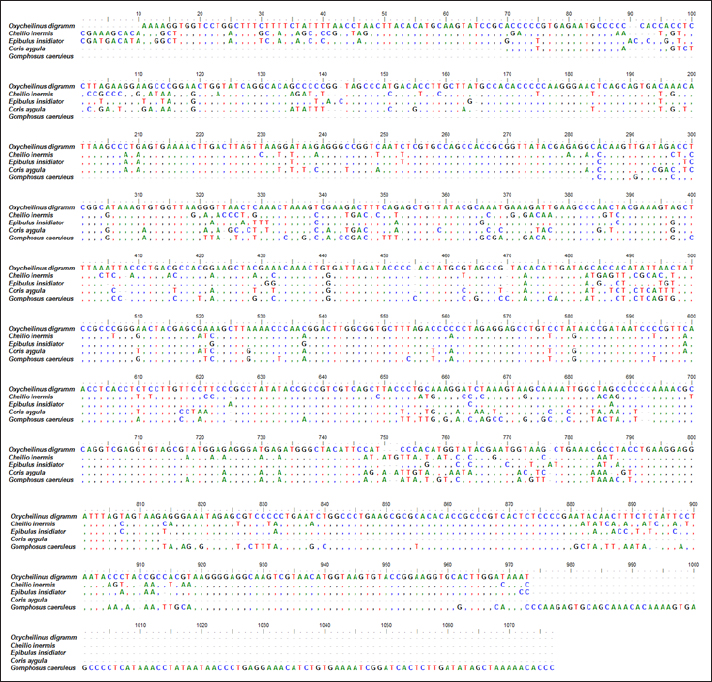
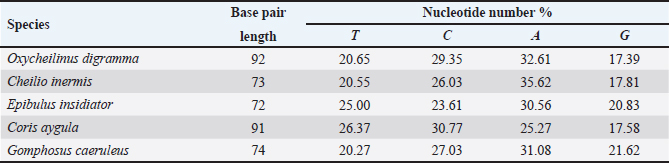
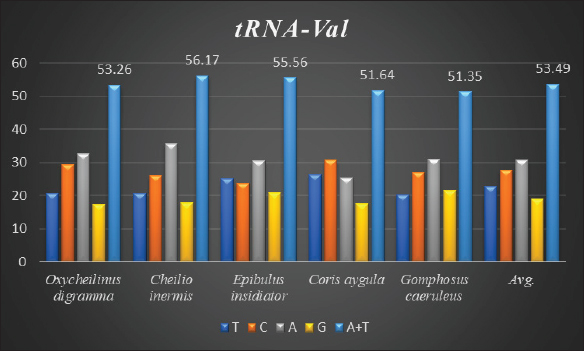
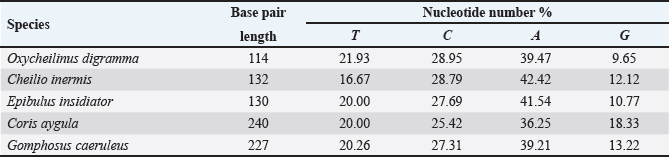
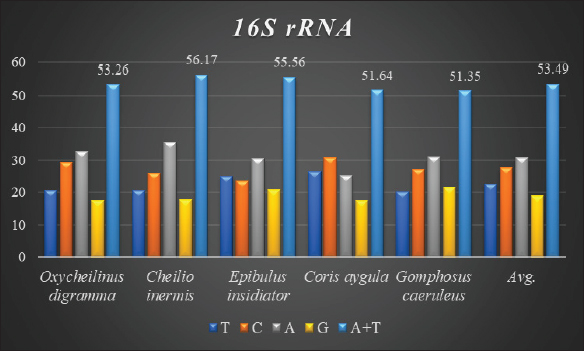
AbstractBackground: Mitochondrial genomes play a key role in molecular biology research by providing essential information about evolutionary links, population history, and genetic diversity. Aim: The aim of this investigation was to produce a partial sequence of 12S rRNA and 16S rRNA genes, as well as a complete sequence of tRNA-Val gene in some species of family Labridae. Methods: Five species of labrid fishes (Oxycheilinus digramma, Cheilio inermis, Epibulus insidiator, Coris aygula, and Gomphosus caeruleus) belonging to Family Labridae were collected from the Red Sea, thereafter, taken to a laboratory for morphological identification in accordance with. Using forward and reverse primers, genome DNA was amplified through polymerase chain reaction. Results: The tRNA-Val gene’s entire sequence, the 12S rRNA gene’s partial sequence, and the 16S rRNA gene’s partial sequence were all submitted to GenBank/NCBI with accession numbers (PP962382.1—PP962386.1). The sequences’ outcomes showed that the average A + T values were higher than the C + G values. Conclusion: The partial sequences of 12S RNA and 16S RNA, and the whole sequence of the tRNA-Val gene, were arranged so that, the 12S RNA and 16S RNA have been distinguished by the tRNA-Val gene. Keywords: Mitochondrial 12S rRNA gene, 16S rRNA gene, tRNA-Val gene, Labridae. IntroductionFound in nearly all eukaryotic species, mitochondria are fundamental components of cells because they control apoptosis, aging, energy metabolism, and several illnesses (Sergi et al., 2019). For systematic research, mitochondrial DNA is an important molecular marker. Its simple structure, quick rate of evolution, large number of copies, and simplicity of isolation make it commonly utilized. Because of these features, mtDNA is a useful and efficient tool for analyzing phylogenetic patterns and genetic links (Mishmar et al., 2019). In molecular biology research, mitochondrial genomes are crucial because they offer vital details regarding genetic variety, population history, and evolutionary links (Boore, 1999). They are widely used in the identification, categorization, and analysis of species, allowing for the discovery of the evolutionary links between species and assisting in the creation of a genus’s evolutionary tree (Machado et al., 2016). Furthermore, the analysis of gene flow, patterns of migration, and genetic variation amongst species is made possible by mitochondrial genomes (Sun et al., 2021). Studies on mitochondrial genomes have yielded extensive knowledge about patterns of population dynamics, molecular evolution, and adaptive mechanisms across a diverse range of animals (Zhong et al., 2022; Ding et al., 2023; Li et al., 2023; Palacios-Barreto et al., 2023; Plancarte and Solórzano, 2023; Zhou et al., 2023). Mitochondrial genomes’ tiny size, high substitution rate, lack of recombination, and massive copy number have made them crucial for molecular evolution and worldwide genetic barcoding efforts for species identification (Brown et al., 1979; Wang et al., 2016; Zhang et al., 2021). Due to processing-related constraints in morphological identification, molecular data is required (Muñoz-Colmenero et al., 2015; Pardo et al., 2016). Fish mitochondrial DNA, just like in other vertebrates, is arranged as closed circular, extranuclear, double-stranded molecules made up of light (L) and heavy (H) strands (Xiao and Zhang, 2000; Satoh et al., 2016). Fish mitochondrial DNA normally has a size of 15–18 kb and contains 13 PCGs (protein-coding genes), 22 tRNAs (transfer RNA), two rRNAs (ribosomal RNA), and one control region (D-loop) (Brown, 2008; Satoh et al., 2016). Since bioinformatics research and high-production DNA sequencing techniques have developed swiftly in recent years, fish mitochondrial genomes are becoming more and more successfully sequenced and identified (Zhang et al., 2023). Analysis of mitochondrial genomes frequently aids in our understanding of speciation and adaptive divergence (Crampton-Platt, 2016). The Red Sea coasts represent one of the greatest levels of endemism and diversity among the coral reef fishes globally (Alwany and Stachowitsch, 2007). On tropical reefs around the world, the Labridae family of fish, better known as wrasses, is one of the most abundant and noticeable fish families. Furthermore, the colors, shapes, and sizes of wrasses are remarkably diverse, and they frequently show notable differences, even within the same species (Parenti and Randall, 2011). Members of the Labridae family exhibit a wide variety of trophic behaviors, they are important members of reef communities as herbivores, planktivores, piscivores, durophages, feeders of ectoparasites, and eaters of different invertebrates linked with the reef (Randall, 1983; Lieske and Myers, 1994; Floeter et al., 2007; Khalaf Allah, 2013; AL-Zahaby, 2015; Sampaio et al., 2016; Pradhan and Mahapatra, 2017). The aim of this investigation was to produce a partial sequence of 12S rRNA and 16S rRNA genes, as well as a complete sequence of tRNA-Val gene in some species of family Labridae. For forthcoming studies aimed at understanding the evolutionary history and geneic diversity of the family Labridae, this sequence data will be an important genomic resource. Materials and MethodsSamples collectionFive species of labrid fishes (Oxycheilinus digramma, Cheilio inermis, Epibulus insidiator, Coris aygula, and Gomphosus caeruleus) belong to Family Labridae were collected from the Red Sea, thereafter, taken to a laboratory for morphological identification in accordance with (Randall, 1982). Individual muscle tissues were separated and kept at −20°C until genomic DNA was extracted. DNA isolationFollowing the manufacturer’s instructions, each fish’s genomic DNA was isolated from its muscular tissues using the DNA Mini kit (Qiagen, Germany). PCR conditionsGenomic DNA amplification through the polymerase chain reaction (PCR) was carried out using forward and reverse primers according to (Wang et al., 2000). One μl of forward and reverse primers, genomic DNA, and 22 ml of PCR master mix were used in each PCR reaction, with a final reaction volume of 50 μl. A four-minute initial denaturation at 95°C was followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for thirty seconds, and extension at 72°C for 10 minutes in the PCR process The 1.3% agarose gel stained with ethidium bromide was used to visualize the PCR results. PCR product sequencings and sequence alignmentsAfter PCR amplification, each species produced a single band during the agarose gel electrophoresis. Macrogen (Seoul, South Korea) performed the DNA sequencing. The sequences of 12S rRNA gene, tRNA-Val gene, and 16S rRNA genes were uploaded to GenBank/NCBI to receive the accession numbers. MEGA version 7.0 18 (Kumar et al., 2016) was used to align the sequences. ResultsThe tRNA-Val gene’s entire sequence, the 12S rRNA gene’s partial sequence, and the 16S rRNA gene’s partial sequence were all submitted to GenBank/NCBI with accession numbers (PP962382.1—PP962386.1). Sequence variation using partial sequence of small subunit ribosomal RNA geneThe nucleated sequence lengths using a partial sequence of small subunit ribosomal RNA gene in five species of labrid fishes (Oxycheilinus digramma, Cheilio inermis, Epibulus insidiator, Coris aygula, and Gomphosus caeruleus) were ranged from 724 bp and 965 bp. (Table 1). In all samples, the A + T ratio of the 12S rRNA is more than the C + G (Fig. 1). There were 668, 297, and 103 conserved sites, variable sites, and parsimony informative sites, respectively, among the 1077 bp that made up the final alignments (Fig. 2). Sequence variation using complete sequence of tRNA-Val geneThe lengths of a nucleated sequence of tRNA-Val gene in five species of labrid fishes (Oxycheilinus digramma, Cheilio inermis, Epibulus insidiator, Coris aygula, and Gomphosus caeruleus) were ranged from 72 bp and 92 bp. (Table 2). In all samples, the A + T ratio of the tRNA-Val is more than the C + G (Fig. 3). There were 27, 56, and 10 conserved sites, variable sites, and parsimony informative sites, respectively, among the 103 bp that made up the final alignments (Fig. 4). Table 1. Nucleotide frequencies of partial sequence of 12S rRNA gene in five species of labrid fishes.
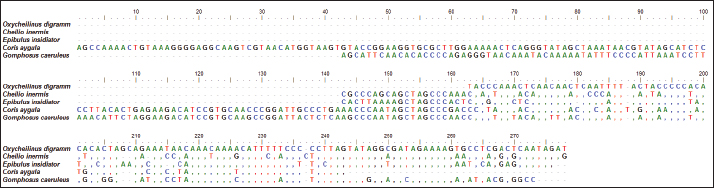
Fig. 1. The average partial sequence of the 12S rRNA gene and nucleotide frequencies in five species of labrid fishes. Sequence variation using partial sequence of large subunit ribosomal RNA geneThe lengths of the nucleated sequence using a partial sequence of 16S rRNA gene in five species of labrid fishes (Oxycheilinus digramma, Cheilio inermis, Epibulus insidiator, Coris aygula, and Gomphosus caeruleus) were ranged from 114 bp and 240 bp (Table 3). In all samples, the A + T ratio of the 16S rRNA is more than the C + G (Fig. 5). There were 112, 122, and 8 conserved sites, variable sites, and parsimony informative sites, respectively, among the 278 bp that made up the final alignments (Fig. 6). DiscussionDue to its high copy number within the cell, ease of separation from the nuclear genome, short size, and quick rate of mutation accumulation, the mitochondrial genome has been extensively used in evolutionary and population genetics research (Moritz et al., 1987; Sotelo et al., 1993; Unseld et al., 1995). Mitochondrial DNA has many characteristics including the absence of introns, limited recombination, uniparental inheritance (mostly in animal phyla), and increased rate of evolution (Galtier et al., 2009; Tiwary et al., 2016). The fundamental idea behind using molecular markers to investigate biodiversity in fishes is the analysis of nucleotide variations (Noikotr et al., 2013; Saad and Abd El-Sadek, 2017; Saad, 2019). In this study, the used primers (Wang et al., 2000) generated PCR fragments containing 12S rRNA, tRNAVAL, and 16S rRNA. This gene order is common throughout all vertebrate’s mitochondrial genomes with only minor variations in length (Wang et al., 2000). As well as the mitochondrial genome of Halichoeres nigrescens was 17,252 bp long and comprised two rRNA genes, thirteen protein-coding genes, twenty-two tRNA genes, and one large non-coding region. Halichoeres nigrescens shares the same arrangement of mitochondria genes as other common fishes (Shi et al., 2018). Like that, the complete mitochondrial of the fish Thalassoma lunare was 17,073 bp in length. The complete mitochondrial sequence had 12S RNA and 16S RNA, which were separated by tRNA-Val gene and situated between tRNA-Phe and tRNA-Leu (Yukai et al., 2019). Also, the complete mitochondrial genome of Iniistius trivittatus was inserted into the NCBI database (MG976729) with 16,820 bp in length. The 12S rRNA gene was situated between the tRNAPhe and tRNAVal genes, and the 16S rRNA gene was situated between the tRNAVal and tRNALeu genes (Liu et al., 2020). Likewise, the gene order of the Pseudocheilinus hexataenia mitochondrion (17,111 bp, GenBank accession no. MZ357706) was identical to those of all known wrasse mitogenomes (Nam et al., 2022). Our results revealed that the 12S rRNA, tRNA-Val, and 16S rRNA genes were encoded on the H-strand, this was similar to that observed for other Labridae studies. Similar to, Qi et al. (2013) who observed that the 12S rRNA, tRNAVal, and the 16S rRNA genes of Cheilinus undulatus were encoded on the H-strand. Likewise, Liu et al. (2020) studied the mitochondrial genome of Iniistius trivittatus and mentioned that the 12S rRNA, tRNAVal, and the 16S rRNA genes were encoded on the H-strand. Also, Wang et al. (2023) reported that the mitochondrial sequence of Cheilinus trilobatus was 17,292 bp in length, and 12S rRNA, tRNAVal, and 16S rRNA genes were encoded on the H-strand.
Fig. 2. Multiple sequence alignment of the partial sequence of 12S rRNA gene in five species of labrid fishes. Table 2. Nucleotide frequencies of complete sequence of tRNA-Val gene in five species of labrid fishes.
Fig. 3. The average partial sequence of the tRNA-Val gene and nucleotide frequencies in five species of labrid fishes.
Fig. 4. Multiple sequence alignment of the partial sequence of tRNA-Val gene in five species of labrid fishes. Table 3. Nucleotide frequencies of partial sequence of 16S rRNA gene in five species of labrid fishes.
In all understudied species, our analysis of the 12S rRNA gene indicated a higher A + T composition than the C + G. This is consistent with several studies. Norazila and Patimah (2002) applied the 12S rRNA/tRNA-Val gene on three varieties (normal, green, and yellow) of the tiger barb (Puntius tetrazona). Sivaraman et al. (2009) characterized the 12S rRNA gene in four Cyprinid species. Widayanti et al. (2021) examined the genetic diversity and phylogenetic reconstruction of the Indonesian catfish (baung fish) using the 12S rRNA gene. Similarly, Mahrous and Allam (2022) found similar findings during their study on eleven catfish species using the 12S rRNA gene. Likewise, Aziz et al. (2024) observed similar results during their study on some species of the family Apogonidae using 12S rRNA gene sequencing.
Fig. 5. The average partial sequence of the 16S rRNA gene and nucleotide frequencies in five species of labrid fishes.
Fig. 6. Multiple sequence alignment of the partial sequence of 16S rRNA gene in five species of labrid fishes. In many fish’s studies, the barcoding and identification of fishes have traditionally used the 16S rRNA gene system since it is simpler to amplify and sequence (Miglietta et al., 2009; Moura et al., 2011; Rosas et al., 2018; Saad, 2019). When compared to C + G, the whole 16S rRNA gene exhibits A + T abundance (Bo et al., 2013). In All our samples the A + T ratio of the 16S rRNA is more than the C + G, this was consistent with research on fish by Lakra et al. (2009) and Singh et al. (2015), which discovered high A + T levels in their study on fishes. As well as Basheer et al. (2015) during the study on Rastrelliger species found the C + G content of 16S rRNA was shorter than the A + T. Also, Mar’ie and Allam (2019) found a high A + T proportion compared to C + G in two puffer fish. ConclusionIn this study, we used forward and reverse primers (Wang et al., 2000) to amplify tRNA-Val gene’s entire sequence, the 12S rRNA gene’s partial sequence, and the 16S rRNA gene’s partial sequence. The sequences’ outcomes showed that the average A + T values were higher than the C + G values. These sequences were arranged so that, the 12S RNA and 16S RNA have been distinguished by the tRNA-Val gene. AcknowledgmentsThe author would like to thank the University of Jeddah. Conflict of interestThe author declares that there is no conflict of interest. FundingNot applicable. Authors’ contributionsThere is one author in this manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAlwany, M.A. and Stachowitsch, M. 2007. Distribution and diversity of six common reef fish families along the Egyptian coast of the Red Sea. J. Fish. Aquat. Sci. 2(1), 1–16. Al-Zahaby, M.A. 2015. Biological studies on the reproductive cycle of broomtail wrasse, Cheilinus lunulatus inhabiting coral reef in the Red Sea, M.Sc. Thesis, Zool. Dep., Fac. Sci. Cairo, Egypt; Al–Azhar University, pp: 207. Aziz, M.M. Abu Almaaty, A.H. and Allam, M. 2024. Phylogenetic inference of some species of the family apogonidae using 12S rRNA sequence Egypt. J. Aquat. Biol. Fish. 28(4), 55–65. Basheer, V.S., Mohitha, C., Vineesh, N. and Divya, P.R., Gopalakrishnan, A. and Jena, J.K. 2015. Molecular phylogenetics of three species of the genus Rastrelliger using mitochondrial DNA markers. Mol. Biol. Rep. 42(4), 873–879. Bo, Z., Xu, T., Wang, R., Jin, X. and Sun, Y. 2013. Complete mitochondrial genome of the Bombay duck Harpodon nehereus (Aulopiformes, Synodontidae). Mitochondrial DNA 24(6), 660–662. Boore, J.L. 1999. Animal Mitochondrial Genomes. Nucleic Acids Res. 27, 1767–1780. Brown, W.M., George, M. Jr. and Wilson, A.C. 1979. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 76(4), 1967–1971. Brown, K.H. 2008. Fish mitochondrial genomics: sequence, inheritance and functional variation. J. Fish. Biol. 72, 355–374. Crampton-Platt, A., Yu, D.W., Zhou, X. and Vogler, A.P. 2016. Mitochondrial metagenomics: Letting the genes out of the bottle. GigaScience 5, 15. Ding, W.L., Xu, H.Z., Wu, Z.P., Hu, L.Z., Huang, L., Yang, M.S. and Li, L.L. 2023. The mitochondrial genomes of the Geometroidea (Lepidoptera) and their phylogenetic implications. Ecol. Evol. 13(2), e10188. Floeter, S.R., Krohling, W., Gasparini, J.L., Ferreira, C.E.L. and Zalmon, I.R. 2007. Reef fish community structure on coastal islands of the southeastern Brazil: the influence of exposure and benthic cover. Environ. Biol. Fishes, 78, 147–160. Galtier, N., Nabholz, B., Glémin, S. and Hurst, G.D. 2009. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol. Ecol. 18(22), 4541–4550. Khalaf Allah, H.M.M. 2013. Morphological adaptations of digestive tract according to food and feeding habits of the broomtail wrasse, Cheilinus lunulatus. Egypt. J. Aquat. Biol. Fish. 17(1), 123–141. Kumar, S., Stecher, G. and Tamura, K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1870–1874. Lakra, W.S., Goswami, M. and Gopalakrishnan, A. 2009. Molecular identification and phylogenetic relationships of seven Indian Sciaenids (Pisces: Perciformes, Sciaenidae) based on 16S rRNA and cytochrome c oxidase subunit I mitochondrial genes. Mol. Biol. Rep. 36(5), 831–839. Li, J.X., Chen, Y.L., Liu, Y.L., Wang, C., Li, L. and Chao, Y.H. 2023. Complete mitochondrial genome of Agrostis stolonifera: insights into structure, codon usage, repeats, and RNA editing. BMC Genomics. 24, 446. Lieske, E. and Myers, R. 1994. Collins pocket guide to coral reef fishes: indopacific and caribbean. New York, NY: Harper Collins. Liu, D., Zhang, Y., Zhang, M., Yang, J. and Tang, W. 2020. Complete mitochondrial genome of Iniistius trivittatus and unique variation in two observed inserts between rRNA and tRNA genes in wrasses. BMC Evol. Biol. 20(1), 125. Machado, D.J., Lyra, M.L. and Grant, T. 2016. Mitogenome assembly from genomic multiplex libraries: comparison of strategies and novel mitogenomes for five species of frogs. Mol. Ecol. Resour. 16, 686–693. Mahrous, N.S. and Allam, M. 2022. Phylogenetic relationships among some catfishes assessed by small and large mitochondrial rRNA sequences. Egypt. J. Aquat. Biol. Fish. 26(6), 1069 –1082. Mar’ie, Z.A. and Allam, M. 2019. Molecular phylogenetic linkage for nile and marine puffer fishes using mitochondrial DNA sequences of cytochrome b and 16S rRNA. Egypt. J. Aquat. Biol. Fish. 23(5), 67–80. Miglietta, M.P., Schuchert, P. and Cunningham, C.W. 2009. Reconciling genealogical and morphological species in a worldwide study of the family Hydractiniidae (Cnidaria, Hydrozoa). Zool. Scr. 38(4), 403–430. Mishmar, D., Levin, R., Naeem, M.M. and Sondheimer, N. 2019. Higher order organization of the MtDNA: beyond mitochondrial transcription Factor A. Front. Genet. 10, 1285. Moritz, C., Dowling, T.E. and Brown, W.M. 1987. Evolution of animal mitochondrial DNA: relevance for population biology and systematics. Annu. Rev. Ecol. Syst. 18, 269–292. Moura, C.J., Cunha, M.R., Porteiro, F.M. and Rogers, A.D. 2011. The use of the DNA barcode gene 16S mRNA for the clarification of taxonomic problems within the family Sertulariidae (Cnidaria, Hydrozoa). Zool. Scr. 40(5), 520–537. Muñoz-Colmenero, M., Klett-Mingo, M., Díaz, E.; Blanco, O., Martínez, J.L. and GarciaVazquez, E. 2015. Evolution of hake mislabeling niches in commercial markets. Food Control. 54, 267–274. Nam, S.-E., Eom, H.-J., Park, H.S. and Rhee, J.-S. 2022. Complete mitochondrial genome of the six-line wrasse Pseudocheilinus hexataenia (Labriformes, Labridae). Mitochondrial DNA B Resour. 7(1), 167–169. Noikotr, K., Chaveerach, A., Pinthong, K., Tanomtong, A., Sudmoon, R. and Tanee, T. 2013. RAPD and barcode analyses of groupers of the genus Epinephelus. Genet. Mol. Res. 12(4), 5721–5732. Norazila, K.S. and Patimah, I. 2002. Mitochondrial 16S and 12S rRNA/tRNA-Val Gene Analysis in Tiger Barbs (Puntius tetrazona). J. Biol. Sci. 2(11), 754-756. Palacios-Barreto, P., Mar-Silva, A.F., Bayona-Vasquez, N.J., Adams, D.H. and Díaz-Jaimes, P. 2023. Characterization of the complete mitochondrial genome of the Brazilian cownose ray Rhinoptera brasiliensis (Myliobatiformes, Rhinopteridae) in the western Atlantic and its phylogenetic implications. Mol. Biol. Rep. 50(5), 4083–4095. Pardo, M.Á., Jiménez, E. and Pérez-Villarreal, B. 2016. Misdescription incidents in seafood sector. Food Control. 62, 277–283. Parenti, P. and Randall, J.E. 2011. Checklist of the species of the families Labridae and Scaridae: an update. Smithiana Bull. 13, 29–44. Plancarte, D.C. and Solórzano, S. 2023. Structural and gene composition variation of the complete mitochondrial genome of Mammillaria huitzilopochtli (Cactaceae, Caryophyllales), revealed by de novo assembly. BMC Genomics. 24, 509. Pradhan, A. and Mahapatra, B.K. 2017. First record of the two-spot razorfish, Iniistius bimaculatus (Perciformes: Labridae) from Digha, north-east coast of India. Cuadernos de Investigación UNED Res. J. 9(1), 115–118. Qi, X.Z., Yin, S. W., Luo, J. and Huo, R. 2013. Complete mitochondrial genome sequence of the humphead wrasse, Cheilinus undulatus. Genet. Mol. Res. 12(2), 1095–1105. Randall, J.E. 1983. Red sea reef fish. Randall, J.E. (ed.). London, UK: Immel Publishing Limited, pp: 192. Randall, J.E. 1982. The diver’s guide to red sea reef fishes. London, UK: Publishing Limited. Rosas, U., Menendez, F., Cornejo, R., Canales, R. and Velez-Zuazo, X. 2018. Fish DNA barcoding around large marine infrastructure for improved biodiversity assessment and monitoring. Mitochondrial DNA A DNA Mapp. Seq. Anal. 29(8), 1174–1179. Saad, Y.M. 2019. Analysis of 16S mitochondrial ribosomal DNA sequence variations and phylogenetic relations among some Serranidae fishes. S. Afr. J. Anim. Sci. 49(1), 80–89. Saad, Y.M. and Abd El-Sadek, H.E. 2017. The efficiency of cytochrome oxidase subunit 1 gene (cox1) in reconstruction of phylogenetic relations among some crustacean species. World Academy of Science, Engineering and Technology, Int. J. Animal Vet. Sci. 11(7), 515–520. Sampaio, C.L.S., Neto, J.S. and Costa, T.L.A. 2016. Hogfish, Lachnolaimus maximus (Labridae) confirmed in the south-western Atlantic Ocean. J. Fish Biol. 89(3), 1873–1879. Satoh, T.P., Miya, M., Mabuchi, K. and Nishida, M. 2016. Structure and variation of the mitochondrial genome of fishes. BMC Genomics 17(1), 719. Sergi, D., Naumovski, N., Heilbronn, L.K., Abeywardena, M., O’Callaghan, N., Lionetti, L. and Luscombe-Marsh, N. 2019. Mitochondrial (dys) function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 10, 532. Shi, W., Chen, S. and Yu, H. 2018. The complete mitochondrial genome sequence of Halichoeres nigrescens (Labriformes: Labridae). Mitochondrial DNA B Resour. 3(2), 1048–1049. Singh, A.K., Kumar, R., Singh, M., Mishra, A.K., Chauhan, U.K., Baisvar, V.S., Verma, R., Nagpure, N.S. and Kushwaha, B. 2015. Mitochondrial 16S rRNA gene-based evolutionary divergence and molecular phylogeny of Barilius spp. Mitochondrial DNA A DNA Mapp. Seq. Anal. 26(1), 41–47. Sivaraman, G.K., Barat, A., Kapila, R., Nagappa, K. and Mahanta, P.C. 2009. Molecular phylogeny of cyprinid fishes of India using 12S rRNA gene sequences. The IUP J. Genet. Evol. 2(4), 43–53. Sotelo, C.G., Piñeiro, C., Gallardo, J.M. and Pérez-Martin, R.I. 1993. Fish species identification in seafood products. Trends Food Sci. Technol. 4(12), 395–401. Sun, C.-H., Liu, H.-Y., Xu, N., Zhang, X.-L., Zhang, Q. and Han, B.-P. 2021. Mitochondrial genome structures and phylogenetic analyses of two tropical characidae fishes. Front. Genet. 12, 627402. Tiwary, C., Badhul Haq, M.A., Vaitheeswari, S., Kalaiselvi, M., Sikder, M.N.A. and Min, W. W. 2016. DNA barcoding and intra species analysis of the ember parrot fish Scarus rubroviolaceus using mtCO1. IRA-Int. J. Appl. Sci. 5(2), 91–109. Unseld, M., Beyermann, B., Brandt, P. and Hiesel R. 1995. Identification of the species origin of highly processed meat products by mitochondrial DNA sequences. PCR Methods Appl. 4(4), 241–243. Wang, K., Li, X., Ding, S., Wang, N., Mao, M. Wang, M. and Yang, D. 2016. The complete mitochondrial genome of the Atylotus miser (Diptera: Tabanomorpha: Tabanidae), with mitochondrial genome phylogeny of lower Brachycera (Orthorrhapha). Gene. 586(1), 184–196. Wang, H.-Y., Tsai, M.-P., Tu, M.-C. and Lee, S.-C. 2000. Universal primers for amplification of the complete mitochondrial 12S rRNA gene in vertebrates. Zool. Stud. 39(1), 61–66. Wang, T., Li, Y., Ma, Q., Liu, Y., Xiao, Y., Wu, P., Lin, L. and Li, C. 2023. The complete mitochondrial genome of Cheilinus trilobatus (Perciformes: Labridae). Mitochondrial DNA B Resour. 8(1), 73–75. Widayanti, R., Kusumaastuti, K.A., Novi, J.M., Adani, F.K., Gultom, C.R.P., Prastiti, A.D., Nugroho, H.A. and Pakpahan, S. 2021. Genetic variation and phylogenetic analysis of Indonesian indigenous catfish (baung fish) based on mitochondrial 12S rRNA gene. Vet. World. 14(3), 751–757. Xiao, W.H. and Zhang, Y.P. 2000. Genetics and evolution of mitochondrial DNA in fish. Acta Hydrobiol. Sin. 24, 384–391. Yukai, Y., Xiaolin, H., Heizhao, L., Tao, L., Wei, Y. and Zhong, H. 2019. The complete mitochondrial genome of Thalassoma lunare (Labriformes, Labridae). Mitochondrial DNA B Resour. 4(2), 3147–3148. Zhang, K., Zhu, K., Liu, Y., Zhang, H., Gong, L., Jiang, L., Liu, L., Lu, Z. and Liu, B. 2021.Novel gene rearrangement in the mitochondrial genome of Muraenesox cinereus and the phylogenetic relationship of Anguilliformes. Sci. Rep. 11(1), 2411. Zhang, R., Zhu, T. and Luo, Q. 2023. The complete mitochondrial genome of the freshwater fish Onychostoma ovale (Cypriniformes, Cyprinidae): genome characterization and phylogenetic analysis. Genes. 14, 1227. Zhong, C., Jin, J., Zhou, R.R., Liu, H., Xie, J., Wan, D., Xiao, S.G. and Zhang, S.H. 2022. Comparative analysis of the complete mitochondrial genomes of four cordyceps fungi. Ecol. Evol. 12(4), e8818. Zhou, S., Zhi, X., Yu, R., Liu, Y. and Zhou, R. 2023. Factors contributing to mitogenome size variation and a recurrent intracellular DNA transfer in Melastoma. BMC Genomics. 24, 370. | ||
| How to Cite this Article |
| Pubmed Style Najiah M. Alyamani. Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Vet. J.. 2024; 14(12): 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 Web Style Najiah M. Alyamani. Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. https://www.openveterinaryjournal.com/?mno=217238 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.18 AMA (American Medical Association) Style Najiah M. Alyamani. Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Vet. J.. 2024; 14(12): 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 Vancouver/ICMJE Style Najiah M. Alyamani. Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 Harvard Style Najiah M. Alyamani (2024) Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Vet. J., 14 (12), 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 Turabian Style Najiah M. Alyamani. 2024. Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Veterinary Journal, 14 (12), 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 Chicago Style Najiah M. Alyamani. "Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae." Open Veterinary Journal 14 (2024), 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 MLA (The Modern Language Association) Style Najiah M. Alyamani. "Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae." Open Veterinary Journal 14.12 (2024), 3336-3344. Print. doi:10.5455/OVJ.2024.v14.i12.18 APA (American Psychological Association) Style Najiah M. Alyamani (2024) Partial and complete sequence of small and large subunit ribosomal RNA genes, tRNA-Val gene in some species of family Labridae. Open Veterinary Journal, 14 (12), 3336-3344. doi:10.5455/OVJ.2024.v14.i12.18 |