| Research Article | ||
Open Vet. J.. 2024; 14(12): 3345-3354 Open Veterinary Journal, (2024), Vol. 14(12): 3345-3354 Research Article Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua IslandSafika Safika1*, Agustin Indrawati1, Rahmat Hidayat1 and Alif Rahman Rohim Puarada21School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 2Postgraduate Student of Medical Microbiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia *Corresponding Author: Safika Safika. School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia. Email: safika [at] apps.ipb.ac.id Submitted: 03/09/2024 Accepted: 06/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
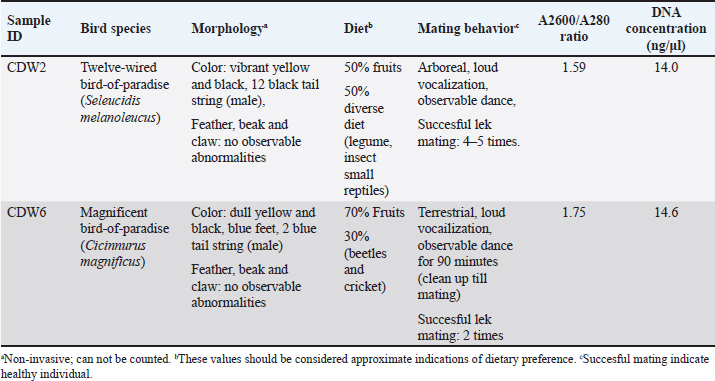
AbstractBackground: Birds-of-paradise, renowned for their stunning plumage and intricate mating rituals, have been extensively studied for their external characteristics. However, the microbial communities inhabiting their digestive tracts remain largely unexplored. The gut microbiome plays a vital role in host health and physiology, influencing digestion, nutrient absorption, and immune function. Understanding the microbiome of birds-of-paradise, particularly in their unique tropical rainforest habitats, may offer valuable insights into their adaptation and overall health. Aim: This study aims to characterize the gut microbiome of birds-of-paradise and to explore the relationship between microbiome and host. Methods: Fecal samples were collected from Jayapura Regency, Indonesia, with non-invasive sampling methods. DNA was extracted using the DNeasy PowerSoil Pro Kit. Shotgun metagenomic sequencing was performed on the MGI DNBSEQ-G400 platform to obtain DNA sequences. DNA sequences were analyzed using DIAMOND followed by MEGAN6 to provide insights into the relative abundance of bacterial taxa within the microbiome. Results: Using Operational Taxonomy Unit analysis we identified 1,398,117 sequences from 5,048,280 initial sequences. Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria, and Acidobacteria were the dominant phyla, with other phyla present in smaller amounts. Burkholderiales, Hyphomicrobiales, Sphingobacteriales, and Enterobacterales were dominant orders, each with specific functional roles. Family and Genus-Level Abundance: Flavobacteriaceae, Comamonadaceae, and Sphingobacteriaceae were dominant families, while Flavobacterium, Delftia, and Pedobacter were dominant genera. Delftia sp., Pedobacter sp., Klebsiella pneumoniae, Achromobacter sp., Bacillus pumilus, Rhizobium sp., and Brevundimonas sp. were among the most abundant species. Conclusion: The microbiome in the gut of birds-of-paradise is characterized by a diverse community of bacteria, fungi, and other microorganisms. The abundance of specific orders, families, and genera varies between samples, suggesting that differences in diet, habitat, or host genetics may influence microbiome composition. The findings reveal a diverse and complex microbial community that likely plays a crucial role in host health and physiology. Keywords: Birds-of-paradise, Microbiome characterization, Microbiome interactions, Shotgun metagenomics. IntroductionBirds-of-paradise, endemic to the islands of Papua and Maluku, have long been the center of attention for biologists and nature lovers. The beauty and uniqueness of this bird, especially in its spectacular mating rituals, has gained global recognition. While the focus of research has largely centered on the striking external and behavioral aspects, such as the beautiful plumage and extraordinary mating dance, we have yet to understand the diversity of microscopic life accompanying birds-of-paradise. Nowadays, the understanding of the microbiota ecosystem within the gut of birds is gaining more and more attention. The gut microbiome, which includes a variety of microorganisms such as bacteria, archaea, viruses, and protozoa, and the complex interactions between them, has been shown to play an important role in the health and physiology of its host (Berg et al., 2020). However, our understanding of this microbiome in birds-of-paradise is too little. The birds-of-paradise’s habitat, which includes dense tropical rainforests and mountains, provides unique challenges and opportunities for its microbiota. The combination of a varied diet, including the consumption of fruits, insects, and nectar, creates a rich and complex environment within the gut; however, the gut microbiome’s adaptation to this diet and its effect on bird health remain unknown (Valdes et al., 2018). Further understanding of the birds-of-paradise microbiome needs to be improved, so this study approached it using the shotgun metagenomic method. This method involves high-speed sequencing of DNA fragments present in bird feces samples. The resulting data is then analyzed using bioinformatics software to identify and characterize the microorganisms present. This approach will provide a comprehensive picture of the diversity, composition, and interactions of the microbiota within the digestive tract of birds-of-paradise. This study may provide new insights into how the microbiome of the digestive tract of birds-of-paradise contributes to their physiological functions, including digestion, nutrient absorption, and even the immune system as in previous studies (Waite and Taylor, 2014, 2015). A better understanding of this microbiome could provide the basis for more effective conservation and management efforts. This study can also provide valuable guidance on the state of health and adaptation of the microbiota when birds return to their natural habitat. It can also provide a richer picture of the biodiversity and biogeography of birds-of-paradise. By understanding the complex interactions between birds, microorganisms, and their environment, we can appreciate their role as important indicators of ecosystem balance in Papua Islands. Materials and MethodsSamplingThe study area was Rhepang Muaif Monitoring Site, Rhepang Muaif Village, Nimbokrang District, Jayapura Regency. Six fecal samples were collected. The identification of the birds-of-paradise for sampling was conducted based on morphology. The sampling method is a non-invasive method as per Fu et al. (2020) and Safika et al. (2023). DNA extractionFecal samples were extracted using the DNeasy PowerSoil Pro kit (Qiagen, Germany) according to the manufacturer’s procedures for the best performance (Shaffer et al., 2022; Puarada et al., 2024). Product qualificationA total of 25 μl of total DNA was taken and then the DNA concentration was measured at the Advanced Research Laboratory (Bogor) using a NanoPhotometer® N60/N50 (Implen, Germany). The A260/A280 ratio was recorded and calculated to determine the purity of the DNA. Qubit fluorometric (Thermo Fisher Scientific, USA) quantitation was then used to assess the extracted DNA quality. Samples with a total DNA concentration of ≥15 ng/μl were selected for sequencing. The threshold for DNA quality typically refers to both the concentration and purity of the extracted DNA, which are crucial for obtaining reliable sequencing data. A specific threshold, such as 15.0 ng/µl, is often set to ensure sufficient DNA is available for successful library preparation and sequencing. A threshold like 15.0 ng/µl ensures enough DNA is present for efficient fragmentation, adapter ligation, and amplification without introducing biases that could occur at lower concentrations (Quince et al., 2017). SequencingA shotgun metagenomic sequencing process targeting the whole genome was conducted on the MGI DNBSEQ-G400 platform. Sample testing included sample integrity and purity. Sample integrity and purity were detected by Agarose Gel Electrophoresis (Concentration of Agarose Gel:1% Voltage:150V, Electrophoresis Time:40 minute). Shotgun metagenomic targeted the whole genome. DNA was randomly fragmented by transposase enzyme. The fragmented genomic DNA was selected by sequencer DNBSEQ-G400 (MGI) purification kit to an average size of 200–500 bp. Fragments were end-repaired and then 3’ adenylated. Adaptors were ligated to the ends of these 3’ adenylated fragments. Polymerase chain reaction (PCR) products were purified MGI purification kit. The double-stranded PCR products were heat-denatured and circularized by the splint oligo sequence. The single-strand circle DNA (ssCir DNA) was formatted as the final library. The library was qualified by QC. The qualified libraries were sequenced by DNBSEQ: ssCir DNA molecule formed a DNA nanoball (DNB). The DNBs were loaded into the patterned nanoarray by using high-density DNA nanochip technology. Finally, pair-end 150 bp reads were obtained by combinatorial Probe-Anchor Synthesis. BioinformaticQC was performed using fastp v0.23.2 for quality control, adapter trimming, filtering by quality, and read trimming and KneadData v0.10.1 was used to perform data filtering of these “contaminant” readings from the host. The data were then assembled using SPAdes genome assembler v3.15.5 and checked with metaQUAST (Quast v5.2.0). Taxonomic classification of the processed sequences is performed using DIAMOND followed by MEGAN6 (Bağcı et al., 2021). These tools provide insights into the relative abundance of bacteria, offering valuable information on microbial community composition. Ethical approvalThis study obtained a permit and a written recommendation letter from Kementerian Lingkungan Hidup dan Kehutanan Direktorat Jenderal Konservasi Sumber Daya Alam dan Ekosistem (Number. AA 308148/SATS/K.4/2023). ResultsSamples characteristicInitial DNA extraction resulted in six samples below the required 15.0 ng/µl concentration threshold. Two samples with the highest concentrations (14.0 and 14.6 ng/µl) and acceptable A260/280 ratios (1.59 and 1.75, respectively) were selected for shotgun metagenomics sequencing on the MGI DNBSEQ-G400 platform (Table 1). Although these concentrations were not optimal, the DNA purity and the platform’s sensitivity to low input amounts suggested sufficient data quality could be obtained. Table 1. Birds-of-paradise sample information.
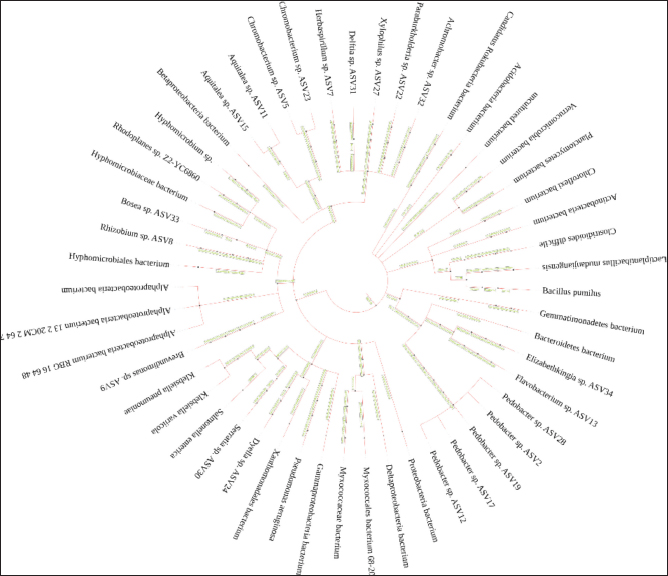
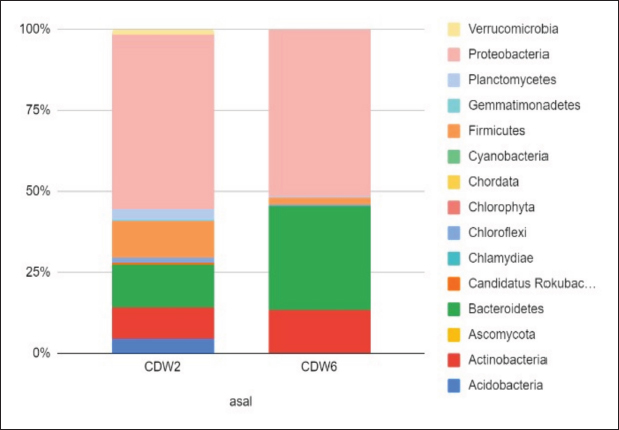
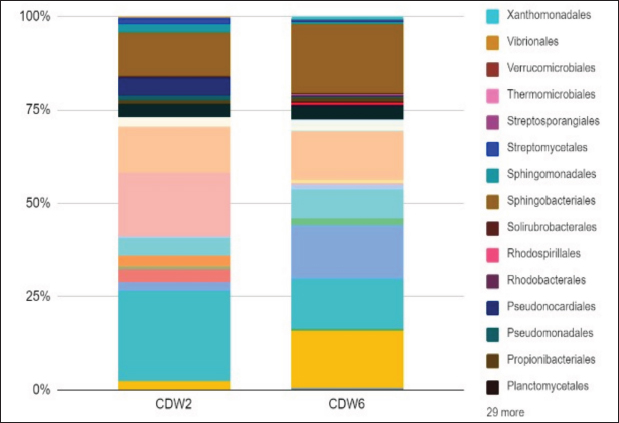
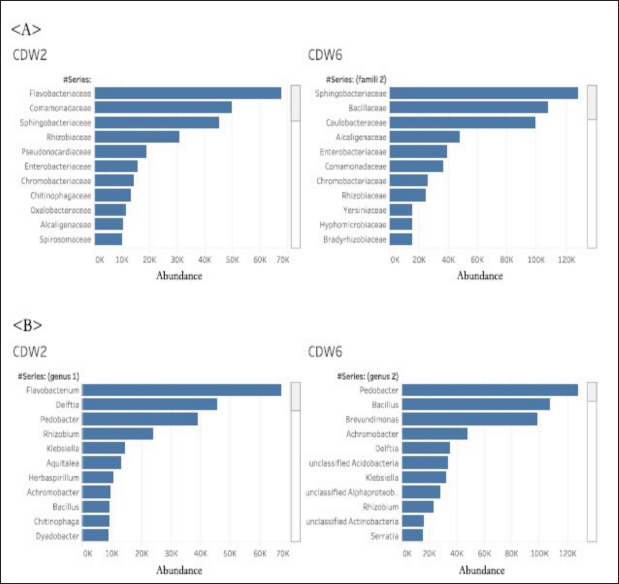
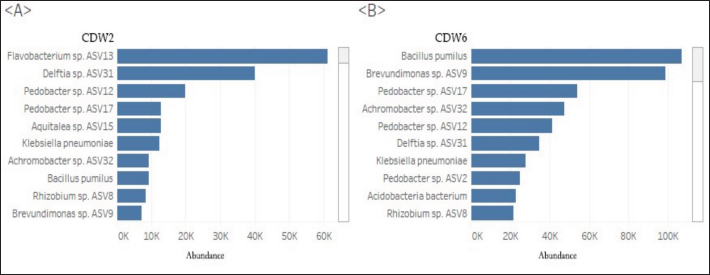
Profile of the bacterial microbiomeThe profile of the bacterial microbiome in the digestive tract of birds of paradise can be seen through Operational Taxonomy Unit (OTU) analysis. Preliminary data analysis showed the presence of bacteria, fungi, algae (viridoplantae), and viruses in the microbiome of the digestive tract of birds of paradise. The analysis results showed OTU has 1,398,117 out of 5,048,280 initial sequences. The arrangement of OTUs in the taxonomic tree can be seen in Figure 1. Bacterial microbiota composition dominated in the gut of birds-of-paradise is the phylum Proteobacter (54.0%, 51.4%), Bacteroidetes (13.3%, 32.1%), Firmicutes (11.4%, 2.2%), Actinobacteria (9.6%, 13.23%), and Acidobacteria (4.4%, 0.1%). Only a few (<1%) were found Candidatus Rokubacteria, Chlamydiae, Chloroflexi, Cyanobacteria, Gemmatimonadetes, Planctomycetes, and Verrucomicrobia. In addition to bacterial microbiota, other microbiota were found, namely Ascomycota (fungi) and Viridiplantae (green algae) in small amounts (Fig. 2). The orders Burkholderiales, Hyphomicrobiales, Sphingobacteriales, and Enterobacterales in the digestive tract of birds of paradise had a dominant abundance in each sample. Other dominant orders were the Flavobacteriales at CDW2, while the Bacillales and Caulobacterales orders were dominant at CDW6 (Fig. 3). Metagenomic analysis of the gut of birds of paradise showed the abundance of the bacterial families Flavobacteriaceae (18.7%), Comamonadaceae (13.7%), and Sphingobacteriaceae (12.4%) in the first sample. The second sample showed the dominance of Sphingobacteriaceae (19.9%), Bacillaceae (16.6%), and Caulobacteraceae (15.4%). Metagenomic analysis at the genus level in the first sample, the dominant bacterial genera were Flavobacterium (20.4%), Delftia (13.8%), and Pedobacter (11.9%). The second sample was dominated by Pedobacter (16.9%), Bacillus (14.2%), and Brevundimonas (13.1%) (Fig. 4). A total of 104 species were successfully sequenced from the samples, 53 species from sample CDW2 (5 unspecified species) and 51 species from CDW6 (18 unspecified species) where 26 of the same species were obtained from both samples (Fig. 5). Most of the microbiome abundance in the digestive tract of birds of paradise from both samples are Delftia sp., Pedobacter sp., Klebsiella pneumoniae, Achromobacter sp., Bacillus pumilus, Rhizobium sp., and Brevundimonas sp. with some differences in the species Flavobacterium sp., Aquitaiea sp. in CDW2 and Acidobacterium sp. in CDW6, respectively. DiscussionThe birds-of-paradise in the northwest lowland of Papua Island represent an extraordinary aspect of avian biodiversity and serve as a symbol of the relationships within rainforest ecosystems. These birds, known for their extraordinary plumage and elaborate mating displays, inhabit diverse ecological niches, ranging from lowland rainforests to mountainous regions in Papua Island and surrounding areas. The twelve-wired Bird-of-Paradise (Seleucidis melanoleucus) is a species distinguished by its physical and behavioral characteristics, particularly exhibited by male individuals during courtship displays. The male 12-wired Bird-of-Paradise is characterized by a glossy black body that shimmers. However, the most striking feature that captures attention is the 12 elongated, slender filaments, commonly referred to as “wires,” that extend from the male’s tail. These distinctive appendages, which are bare and delicate, curl slightly at their tips, creating a breathtaking display during the male’s courtship rituals. Similarly, the Magnificent Bird-of-Paradise (Cicinnurus magnificus) captivates all who encounter it. This small yet extraordinary avian species measures approximately 23–25 cm in length and serves as a testament to the intricate beauty of evolution and the complexities of the natural world. The male’s body glistens with an enchanting array of colors; iridescent yellow-green feathers shimmer alongside striking blue-green and deep black plumage. Among these features, the most visually arresting aspect is the long, elegant tail feathers that trail behind the male, enhancing his regal appearance (Frith and Beehler, 1998).
Fig. 1. Gut microbiome phylogenetic tree of bird-of-paradise in the northwest lowlands of Papua. Each branch represents a microbiome species. The gut microbiome of birds-of-paradise is indeed a remarkable aspect of their biology. The gut microbiome, a complex community of microorganisms including bacteria, fungi, and archaea, is integral to their health and adaptation to these environments. The initial analysis has suggested a “slight variation” in microbial composition between CDW2 and CDW6, but a deeper dive into the data and subsequent analyses could reveal more substantial differences. Both samples shared a core microbiome, consisting of common bacterial taxa found in the gut. This core microbiome could have masked the more specific differences at the species level.
Fig. 2. Relative microbiome abundance of birds-of-paradise at the phylum level. Data were alphabetically. The highest abundance is marked by a line limit (----) and number of abundance.
Fig. 3. Relative microbiome abundance of birds-of-paradise at the order level. The highest abundance is marked by a line limit ( ) and number of abundance. Order with abundance <1% on both sample are catogorized in other.
Fig. 4. Top 10 microbiome abundances of birds-of-paradise in family level (A) and genus level (B). Phylum diversitiesThe gut microbiome of birds-of-paradise is a complex ecosystem teeming with diverse microbial communities. The dominant phyla in the gut microbiome of birds-of-paradise were Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria, and Acidobacteria, accounting for over 90% of the total microbiome. This result is per the research of Wang et al. (2022) which states that the normal flora of some wild birds is dominated by the phylum Proteobacteria and Firmicutes. Waite and Taylor’s (2015) review study showed that Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes are the dominant phylums, characterized by an abundance exceeding 1%. However, in contrast to this study, Proteobacteria was more dominant than Firmicutes and Bacteroidetes. These results suggest that the function of the gut microbiome of wild birds such as healthy birds-of-paradise may be more variable and that the major axes of taxonomic variation in the microbiota do not necessarily capture most of the functional variation. Bodawatta et al. (2018) study of the passerine bird in New Guinea yielded similar results and added that insectivore species were dominated by Firmicutes and omnivores were dominated by Proteobacteria. Proteobacteria were an important part of the birds-of-paradise gut microbiome. This is shown by the percentage of about 54.0% and 51.4% of the entire microbiome in the bird-of-paradise gut. Proteobacteria are known for their metabolic flexibility and role in the nitrogen cycle, with some taxa potentially aiding in immunity (Bradley and Pollard, 2017). Proteobacteria are involved in breaking down complex carbohydrates and synthesizing essential nutrients, which are crucial for the birds’ nutrition, especially given their varied diet of fruits, insects, and small animals (Ali et al., 2022). Some Proteobacteria are also known for their capacity to degrade toxic compounds (Poyntner et al., 2021), which could help these birds handle potentially harmful substances found in their food.
Fig. 5. Top 10 species-level microbiome abundances in samples CDW2 (A) and CDW6 (B). Bacteroidetes specialize in polysaccharide degradation, which contributes to the breakdown of complex carbohydrates in poultry diets. A higher abundance of Bacteroidetes is often associated with fiber-rich diets, reflecting their adaptive role in nutrient utilization. Firmicutes are versatile fermenters and contribute to the extraction of host energy from the diet (Bradley and Pollard, 2017). The abundance ratio of these two phylum (F/B ratio) is still low. In production birds, a high F/B ratio is linked to greater productivity, although this ratio may vary across species and is understudied in wild birds (Lundberg et al., 2021). This could also be due to the different diets of production birds. Birds-of-paradise have a diet of fruits and insects while production birds have their own formulated feed. Orders, families, and genus diversitiesA key finding is the dominance of Burkholderiales, Hyphomicrobiales, Sphingobacteriales, and Enterobacterales orders across various samples. These orders have specific functional roles, including regulating gut homeostasis, degrading complex organic matter, and participating in nitrogen cycling. Diving deeper into the taxonomic hierarchy, we find that Flavobacteriaceae, Comamonadaceae, and Sphingobacteriaceae families are prevalent in the gut microbiome. Flavobacterium, Delftia, Pedobacter, Bacillus, and Brevundimonas are particularly abundant at the genus level. These bacteria are known for their diverse metabolic activities, including the breakdown of complex organic compounds and pollutants (Mondal et al., 2023; Liao et al., 2024). The presence of Sphingobacteriales highlights their role in decomposing plant materials and complex carbohydrates (Kimeklis et al., 2023). There are some variations in the dominance patterns across these levels, consistent findings include the prominence of the families Flavobacteriaceae, Comamonadaceae, and Sphingobacteriaceae. These families were consistently observed to be among the most abundant in both CDW2 and CDW6 samples, supporting their dominance in the gut microbiome of birds-of-paradise. Future research could focus on conducting more detailed comparisons at any level of taxon to gain a deeper understanding of the factors influencing this dominance in birds-of-paradise. Species diversitiesThe presence of specific bacterial species, such as Delftia sp., Pedobacter sp., Klebsiella pneumoniae, Achromobacter sp., Bacillus pumilus, Rhizobium sp., and Brevundimonas sp., further highlights the diversity and functional potential of the gut microbiome. These species contribute to host health by aiding in digestion and nutrient acquisition, although some may cause infections under specific conditions. The bacterial species mentioned represent a wide variety of genera, each with unique characteristics and potential impacts on the microbiome of the digestive tract of birds of paradise. Delftia sp. and Achromobacter sp. are capable of metabolizing a wide range of organic compounds, which may aid in the breakdown of complex dietary components. Delftia sp. is also a normal flora in the cloacal mucosa of condor birds where these bacteria function in degrading biofilms (Jacob et al., 2020). Some species of Delftia sp. such as Delftia acidovorans play an important role in the metabolism of Propinoate, which is one type of carbohydrate. Research by Bilal et al. (2021) showed that this bacterium can help bird immunity under suboptimal environmental conditions. Bacillus pumilus is added as a probiotic to improve the production, immunity, and digestive tract health of production poultry. The normal presence of this bacterium in the digestive tract of birds-of-paradise may affect their immunity. Bacteria such as Rhizobium sp. and Klebsiella pneumoniae can make a positive contribution to the host by fixing nitrogen, potentially increasing nutrient availability in nutrient-poor environments (Milenkov et al., 2011). Brevundimonas sp. has been associated with aquatic environments but discoveries have shown its normal presence in the body mucosa (Leung et al., 2019). Pedobacter species are known for their ability to degrade a wide range of polysaccharides, including cellulose, hemicellulose, and pectin (Cho et al., 2021). This is particularly beneficial for birds-of-paradise, which consume a diet rich in plant matter. Pedobacter species have also been implicated in bioremediation processes, suggesting they may play a role in detoxifying the host bird (Lors et al., 2010). Achromobacter species are versatile biodegraders, capable of metabolizing a wide range of organic compounds, including hydrocarbons, phenols, and pesticides (El-Kurdi et al., 2024). This ability could be beneficial for birds-of-paradise exposed to environmental pollutants. Factors influencing microbiota compositionFor birds-of-paradise that consume significant amounts of fruits or seeds, these bacteria are vital in breaking down plant-based components. The variations observed in the microbiota composition between samples are likely attributed to differences in diet, genetics, and behavior. Their dominance in the avian gut highlights their critical role in efficiently processing and managing the diverse and complex dietary components consumed by birds-of-paradise. There were differences in the microbiota composition of these two samples. These differences can be attributed to the different physiological and metabolic characteristics inherent to each species. Even within the same genus, variations in physiology or metabolic requirements may select certain microbiota communities that are better suited to support host health and nutrition. Small differences in preferences, feeding behavior, or dietary specialization between two species can lead to different microbiota compositions in their gut (Table 1). There is a slight variation in genetic makeup between CDW6 and CDW2, which may also affect their respective microbiomes. CDW2 has a high abundance of the order Flavobacteriales, some of whose functions are to metabolize fiber, carbohydrates, and chitin (Gavriilidou et al., 2020). As observed CDW2 birds have a high-fiber diet with a more diverse diet such as legumes, small reptiles, and insects. Sample CDW6 was dominated by the orders Caulobacterales that function to digest lipids in a high-fat diet and some Bacillales can process diets high in cellulose or hemicellulose as this species tends to eat more fruit than CDW2 (Harirchi et al., 2022; Ignatiou and Pitsouli, 2024). But Bodawatta et al. (2018) also suggest that some species maybe with a specific can have a different microbiome even though they are in the same family. Behavioral differences, including specific mating rituals or social interaction patterns unique to each species, may expose them to different microbiota communities, thereby affecting the gut microbiome. Interactions with the same and other species, as well as with the physical environment during various behaviors, play a role in the acquisition and modulation of the gut microbiome. Understanding these subtle differences and similarities is essential in gaining comprehensive insights into their biology and ecology, supporting informed conservation strategies for these amazing birds-of-paradise (Sun et al., 2022). Implications for conservation and ecological rolesThe microbiome of birds-of-paradise is a complex ecosystem that plays a vital role in their life. Understanding the factors that influence microbiome composition and function is essential for developing effective conservation strategies. The birds-of-paradise’s diet, which likely consists of fruits, insects, and nectar, directly shapes their microbiome. Conserving their food sources and habitats is crucial for maintaining a healthy microbiome and overall health. Additionally, climate change and habitat destruction can disrupt food availability and alter the microbial environment, further impacting the birds’ health. The microbiome acts as a first line of defense against pathogens. Identifying key microbial taxa associated with disease resistance can aid in developing targeted disease prevention strategies. For example, understanding the role of specific bacterial species in immune function can inform the development of probiotics or prebiotics to support microbiome health. The microbiome plays a crucial role in nutrient cycling within the digestive tract. By breaking down complex nutrients into simpler molecules, the microbiome enables the host to absorb essential nutrients. Understanding the microbial functions involved in nutrient degradation and assimilation can help us appreciate the ecological roles of birds-of-paradise in nutrient cycling within their ecosystem. Furthermore, the microbiome may also influence the birds’ ability to detoxify harmful substances present in their environment. The interactions between the microbiome and the host are complex and multifaceted. Investigating these interactions can provide insights into the factors driving microbial community structure and function. This knowledge can inform conservation strategies aimed at maintaining the health and well-being of birds-of-paradise. For example, understanding the factors that influence microbiome diversity can help us identify potential threats to microbiome health and develop strategies to mitigate them. Research into the gut microbiome of birds-of-paradise is still in its early stages, but it holds the potential to uncover fascinating links between these birds’ distinctive ecological adaptations and their microbiota. By unraveling the complexities of this microbial ecosystem, we can develop targeted conservation interventions to protect these exceptional and ecologically important species. Understanding these relationships could provide deeper insights into the evolution and ecology of these extraordinary birds. ConclusionThe dominant phyla in the gut microbiome of birds-of-paradise are Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria, and Acidobacteria, which together account for over 90% of the total microbiome. At the genus level, Flavobacterium, Delftia, Pedobacter, Bacillus, and Brevundimonas are particularly abundant. These bacteria have diverse metabolic activities, including the breakdown of complex organic compounds and pollutants. The factors influencing microbiota composition include diet, genetics, and behavior. Birds-of-paradise that consume significant amounts of fruits or seeds have microbiota communities that are specialized in breaking down plant-based components. Understanding the microbiome is essential for developing effective conservation strategies. Conserving their food sources and habitats is crucial for maintaining a healthy microbiome and overall health. Additionally, climate change and habitat destruction can disrupt food availability and alter the microbial environment, further impacting the birds’ health. Future research should focus on investigating the specific functions of different microbial taxa, the factors that influence microbiome composition, and the interactions between the microbiome and the host. By unraveling the complexities of this microbial ecosystem, we can gain deeper insights into the evolution and adaptation of birds-of-paradise and develop effective conservation strategies to protect them. AcknowledgmentsThe author is grateful to IPB University and PT. Saraswanti Gene Indotech for all the facilities to achieve this study. The author is also grateful to BKSDA Jayapura Mrs. Lusi, Mr. Danial, and other colleagues for their input in this research, especially in field monitoring and bird of paradise behavior, for Mr. Kholiq for the time and knowledge for this research and other people who contributed to this research. Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this article. FundingThis research was funded by PENELITIAN FUNDAMENTAL REGULER (PFR) TAHUN ANGGARAN 2024, The Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia. Contract number: 22081/IT3.D10/PT.01.03/P/B/2024. Authors’ contributionsSS designed the study, interpreted the data, and drafted the manuscript. AI and ARRP were involved in the collection of data and also contributed to manuscript preparation. RH took part in preparing and critically checking this manuscript. All the authors read and approved the manuscript for publication. Data availabilityAll data supporting the findings of this study are available within the manuscript. The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. The data that support the findings of this study are available from the authors. ReferencesAli, Q., Ma, S., La, S., Guo, Z., Liu, B., Gao, Z., Farooq, U., Wang, Z., Zhu, X., Cui, Y., Li, D. and Shi, Y. 2022. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health—a review. Anim. Biosci. 35(10), 1461–1478. Bağcı, C., Patz, S. and Huson, D.H. 2021. DIAMOND+ MEGAN: fast and easy taxonomic and functional analysis of short and long microbiome sequences. Curr. Protoc. 1(3), e59. Berg, G., Rybakova, D., Fischer, D., Cernava, T., Vergès, M.C.C., Charles, T., Chen, X., Cocolin, L., Eversole, K., Corral, G.H. and Kazou, M. 2020. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 1–22. Bilal, M., Si, W., Barbe, F., Chevaux, E., Sienkiewicz, O. and Zhao, X. 2021. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health, and immunity of broiler chickens raised under suboptimal conditions. Poult. Sci. 100(3), 100871. Bodawatta, K.H., Sam, K., Jønsson, K.A. and Poulsen, M. 2018 Comparative analyses of the digestive tract microbiota of New Guinean passerine birds. Front. Microbiol. 9, 1830. Bradley, P.H. and Pollard, K.S. 2017. Proteobacteria explain significant functional variability in the human gut microbiome. Microbiome 5, 1–23. Cho, C.Y., Han, S.R. and Oh, T.J. 2021. Complete genome sequence of Pedobacter sp. PAMC26386 and their low temperature application in arabinose-containing polysaccharides degradation. Curr. Microb. 78(3), 944–953. El-Kurdi, N., El-Shatoury, S., ElBaghdady, K., Hammad, S. and Ghazy, M., 2024. Biodegradation of polystyrene nanoplastics by Achromobacter xylosoxidans M9 offers a mealworm gut-derived solution for plastic pollution. Arch. Microbiol. 206(5), 1–24. Frith, C.B and Beehler, B.M. 1998. The birds of paradise: Paradisaeidae. Oxford, UK: Oxford University Press. Fu, R., Xiang, X., Dong, Y., Cheng, L. and Zhou, L. 2020. Comparing the intestinal bacterial communies of sympatric wintering Hooded Crane (Grus monacha) and domestic goose (Anser anser domesticus). Avian. Res. 11, 1–9. Gavriilidou, A., Gutleben, J., Versluis, D., Forgiarini, F., van Passel, M.W., Ingham, C.J., Smidt, H. and Sipkema, D. 2020. Comparative genomic analysis of Flavobacteriaceae: insights into carbohydrate metabolism, gliding motility and secondary metabolite biosynthesis. BMC Genomics. 21, 1–21. Harirchi, S., Sar, T., Ramezani, M., Aliyu, H., Etemadifar, Z., Nojoumi, S.A., Yazdian, F., Awasthi, M.K. and Taherzadeh, M.J. 2022. Bacillales: from taxonomy to biotechnological and industrial perspectives. Microorganisms 10(12), 2355. Ignatiou, A. and Pitsouli, C. 2024. Host–diet–microbiota interplay in intestinal nutrition and health. FEBS Lett. 598(20), 2482–2517. Jacobs, L., McMahon, B.H., Berendzen, J., Longmire, J., Gleasner, C., Hengartner, N.W., Vuyisich, M., Cohn, J.D., Jenkins, M., Bartlow, A.W. and Fair, J.M. 2020. Correction: California condor microbiomes: bacterial variety and functional properties in captive-bred individuals. PloS one. 15(3), e0230738. Kimeklis, A.K., Gladkov, G.V., Orlova, O.V., Afonin, A.M., Gribchenko, E.S., Aksenova, T.S., Kichko, A.A., Pinaev, A.G. and Andronov, E.E. 2023. The succession of the cellulolytic microbial community from the soil during oat straw decomposition. Int. J. Mol. Sci. 24(7), 6342. Leung, P.H., Subramanya, R., Mou, Q., Lee, K.T.W., Islam, F., Gopalan, V., Lu, C.T. and Lam, A.K.Y. 2019. Characterization of mucosa-associated microbiota in matched cancer and non-neoplastic mucosa from patients with colorectal cancer. Front. Microbiol. 10, 1317. Liao, S.F., Ji, F. and Fan, P. and Denryter, K. 2024. Swine gastrointestinal microbiota and the effects of dietary amino acids on its composition and metabolism. Int. J. Mol. Sci. 25, 1237. Lors, C., Ryngaert, A., Périé, F., Diels, L., & Damidot, D. 2010. Evolution of bacterial community during bioremediation of PAHs in a coal tar contaminated soil. Chemosphere. 81(10), 1263–1271. Lundberg, R., Scharch, C. and Sandvang, D. 2021. The link between broiler flock heterogeneity and cecal microbiome composition. Anim. Microbiome 3, 1–14. Milenkov, M., Thummer, R., Glöer, J., Grötzinger, J., Jung, S. and Schmitz, R.A. 2011. Insights into membrane association of Klebsiella pneumoniae NifL under nitrogen-fixing conditions from mutational analysis. J. Bacteriol. 193(3), 695–705. Mondal, S., Somani, J., Roy, S., Babu, A., Pandey, A.K. 2023. Insect microbial symbionts: ecology, interactions, and biological significance. Microorganisms 11(11), 2665. Poyntner, C., Kutzner, A. and Margesin, R. 2021. Biodegradation potential and putative catabolic genes of culturable bacteria from an alpine deciduous forest site. Microorganisms 9(9), 1920. Puarada, A.R.R., Safika, S. And Indrawati, A. 2024. Exploration of anti-microbial resistant (AMR) genes in the gastrointestinal microbiome of birds of paradise (Paradisaeidae) in Papua, Indonesia. Adv. Anim. Vet. Sci. 12(3), 539–545. Quince, C., Walker, A.W., Simpson, J.T., Loman, N.J. and Segata, N. 2017. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 35(9), 833–844. Safika, S., Indrawati, A., Afiff, U., Hastuti, Y.T., Zureni, Z. and Jati, A. P. 2023. First study on profiling of gut microbiome in wild and captive Sumatran orangutans (Pongo abelii). Vet. World. 16(4), 717–727. Shaffer, J.P., Carpenter, C.S., Martino, C., Salido, R.A., Minich, J.J., Bryant, M., Sanders, K., Schwartz, T., Humphrey, G., Swafford, A.D. and Knight, R. 2022. A comparison of six DNA extraction protocols for 16S, ITS and shotgun metagenomic sequencing of microbial communities. Biotechniques 73(1), 34–46. Sun, F., Chen, J., Liu, K., Tang, M. and Yang, Y. 2022. The avian gut microbiota: diversity, influencing factors, and future directions. Front. Microbiol. 13, 934272. Valdes, A.M., Walter, J., Segal, E. and Spector, T.D. 2018. Role of the gut microbiota in nutrition and health. BMJ. 361, k2179. Waite, D.W. and Taylor, M.W. 2014. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 5, 223. Waite, D.W. and Taylor, M.W. 2015. Exploring the avian gut microbiota: current trends and future directions. Front. Microbiol. 6, 673. Wang, J., Hong, M., Long, J., Yin, Y. and Xie, J. 2022. Differences in intestinal microflora of birds among different ecological types. Front. Ecol. Evol. 10, 920869. | ||
| How to Cite this Article |
| Pubmed Style Safika S, Indrawati A, Hidayat R, Puarada ARR. Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Vet. J.. 2024; 14(12): 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 Web Style Safika S, Indrawati A, Hidayat R, Puarada ARR. Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. https://www.openveterinaryjournal.com/?mno=217463 [Access: January 26, 2026]. doi:10.5455/OVJ.2024.v14.i12.19 AMA (American Medical Association) Style Safika S, Indrawati A, Hidayat R, Puarada ARR. Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Vet. J.. 2024; 14(12): 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 Vancouver/ICMJE Style Safika S, Indrawati A, Hidayat R, Puarada ARR. Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Vet. J.. (2024), [cited January 26, 2026]; 14(12): 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 Harvard Style Safika, S., Indrawati, . A., Hidayat, . R. & Puarada, . A. R. R. (2024) Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Vet. J., 14 (12), 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 Turabian Style Safika, Safika, Agustin Indrawati, Rahmat Hidayat, and Alif Rahman Rohim Puarada. 2024. Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Veterinary Journal, 14 (12), 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 Chicago Style Safika, Safika, Agustin Indrawati, Rahmat Hidayat, and Alif Rahman Rohim Puarada. "Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island." Open Veterinary Journal 14 (2024), 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 MLA (The Modern Language Association) Style Safika, Safika, Agustin Indrawati, Rahmat Hidayat, and Alif Rahman Rohim Puarada. "Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island." Open Veterinary Journal 14.12 (2024), 3345-3354. Print. doi:10.5455/OVJ.2024.v14.i12.19 APA (American Psychological Association) Style Safika, S., Indrawati, . A., Hidayat, . R. & Puarada, . A. R. R. (2024) Characterizing the gut microbiome of birds-of-paradise in the northwest lowland of Papua Island. Open Veterinary Journal, 14 (12), 3345-3354. doi:10.5455/OVJ.2024.v14.i12.19 |