| Research Article | ||
Open Vet. J.. 2024; 14(11): 3017-3025 Open Veterinary Journal, (2024), Vol. 14(11): 3017-3025 Research Article Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus)Felician Lestari Wongkar1, Yos Adi Prakoso2 and Agustina Dwi Wijayanti3*1Master Program in Veterinary Sciences, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Department of Pharmacology, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, Surabaya, Indonesia 3Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Agustina Dwi Wijayanti, Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: wagustinadwi [at] gmail.com Submitted: 28/08/2024 Accepted: 23/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
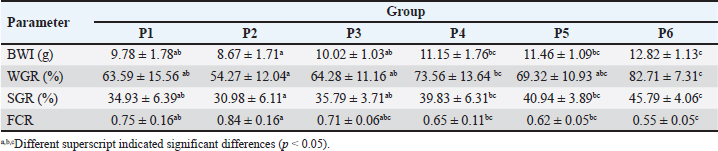
AbstractBackground: Disease prevention in cultivating ranchu goldfish (Carassius auratus) can be prevented by giving immunostimulants in the food. One immunostimulant can come from herbs such as calabash fruit (Crescentia cujete L.). Aim: This study aims to analyze the potential of calabash fruit extract (BFE) on the hematological profile of ranchu goldfish. Methods: This study used 30 ranchu goldfish, which were divided into six treatments, as follows: P1=standard food; P2=food + 0.5% CMC Na; P3=food + 0.1% POWER C®; P4=food + 0.5% BFE; P5=food + 0.3% BFE; and P6=food + 0.6% BFE. The treatment was performed for 28 days with a feeding frequency of 2 times a day. On the 28th day, fish performance was measured. The blood was collected for hematology and immunohistochemistry against antibody anti-cyclooxygenase-2 (COX-2) and CD4+. The data were statistically analyzed using SPSS with a confidence level of 95%. Results: The group P6 indicated the highest growth performance compared to the other groups (p < 0.05). This group also showed a significant elevation of heterophils compared to the others (p < 0.05). In contrast, the levels of Hb, PCV, MCV, MCH, MCHC, WBC, lymphocytes, monocytes, and thrombocytes did not differ from each other (p > 0.05). Interestingly, there is a decrease in C-reactive protein and immunoreactivity of COX-2 and an elevation of CD4+ following the increase in BFE concentration (p < 0.05). Conclusion: It can be concluded that BFE affects the performance and hematological profile of ranchu goldfish and has the potential as an immunostimulant. Keywords: Calabash fruit extract, CD4+, COX-2, Hematology, Ranchu goldfish. IntroductionRanchu goldfish (Carassius auratus) farming has increased over the past decade (Anjur et al., 2021). However, ranchu goldfish farming can face many problems, especially diseases caused by viruses and bacteria. Several diseases including carp pox viral disease, furunculosis, motile Aeromonas septicemia and etcetera. Those diseases cause significant economic losses of approximately 400 million USD in 2018 globally (Evan and Putri, 2021). Poor milieu conditions such as polluted water conditions, extreme temperature changes, and inadequate feed nutrition result in immunosuppression, decreased production, and economic losses (Canosa and Bertucci, 2023). These conditions can be prevented with immunostimulants. Immunostimulants can increase fish growth performance because they can improve fish immunology (Sarsembayeva et al., 2021). Immunostimulant-rich antioxidants potentially reduce the oxidative stress from the cyclooxygenase (COX) pathway. Antioxidants from immunostimulants can also suppress inflammation in the cyclooxygenase-2 (COX-2) pathway (Wang et al., 2016a). Decreasing COX in fish also has an effect on increasing muscle mass (Srivastava and Reddy, 2021), controlling metabolism, and suppressing tissue damage during oxidative stress (Shastak and Pelletier, 2023). Immunostimulants containing antioxidants can stimulate innate and adaptive immunity and also fish hematology (Anjusha et al., 2019). Immunostimulant-rich antioxidants can be obtained from herbs. Calabash fruit (Crescentia cujete L.) is one of the herbs that can be used as an immunostimulant. In previous studies, calabash fruit extract (BFE) can act as a preservative for vegetables (Magno et al., 2021), an antioxidant and anti-inflammatory agent based on an in-silico study (Gonzales et al., 2022), and can help maintain water quality through its coagulant function (Boakye et al., 2024). Furthermore, BFE contains bioactive compounds such as carotenoid, phenolic, alkaloid, pectin, tannin, flavonoid, terpenoid, and α-tocopherol (Rahmaningsih and Andriani, 2020). These compounds can also serve as antioxidants. Previous studies on rainbow trout have demonstrated that antioxidants from α-tocopherol found in herbs can lead to increased levels of erythrocytes, leukocytes, hematocrit, hemoglobin, lysozyme activity, IgM, and T cell activity expressing CD4+ (Khara et al., 2016). Despite these benefits, the use of BFE in aquaculture remains limited and requires to be clarified. However, due to its potential, using BFE is expected to reduce inflammation in farmed fish by decreasing COX-2 expression and increasing CD4+ lymphocytes. Therefore, this study aims to explore the potential of BFE on the ranchu goldfish, specifically by evaluating fish performance, hematology, serology, and immunohistochemistry against CD4+ and COX-2. Materials and MethodsHerbal preparation and determination of α-tocopherol by high-performance liquid chromatography (HPLC)Calabash fruit (1.5 months, yellowish green color, and ± 40 cm of diameters) was obtained from the garden of Wijaya Kusuma University Surabaya. Calabash fruit was dried and macerated with 70% ethanol (1:4). Maceration was carried out three times (3 × 24 hours), and the macerate was evaporated at 69°C using a Soxhlet evaporator (Buchi R-100; Soxhlet, Buchi, Indonesia). Evaporation was carried out until a thick extract was obtained. The thick extract was stored at 4°C (Wilujeng et al., 2023). The α-tocopherol of the extract was then determined using HPLC (Shimadzu version 6.1, Japan). This procedure was performed following the procedure in the previous study (Darnet et al., 2011). The BFE was found to consist α-tocopherol at 41.37 ± 0.98 mg/kg. Coating fish pelletsA total of 1 kg of feed was coated using 0.15%, 0.3%, and 0.6% BFE concentrations (Medagoda et al., 2023). BFE of 0.15 ml, 0.3 ml, and 0.6 ml, respectively, were dissolved in 100 ml distilled water and then mixed into 1 kg of fish feed. After that, the feed was sprayed using 0.5% CMC Na. Feed was dried for 1 hour at room temperature (25°C and 70% humidity) (Nikiforov-Nikishin et al., 2022). TreatmentA total of 30 ranchu goldfish (3 months, 15.82 ± 1.20 g) was used in the study were divided into six treatment groups. The groups were separated as follows: P1=standard feed; P2=feed + 0.5% CMC Na; P3=feed + 0.1% POWER C® (Haiku, Japan); P4=feed + 0.15% BFE; P5=feed + 0.3% BFE; and P6=feed + 0.6% BFE. The concentration selection in this study refers to a previous study by Ortuño et al. (2003). The pellet used in this study was from Saki-Hikari Fancy Goldfish®, Kyorin, Japan. Fish were kept in an aquarium measuring 60 × 29.5 × 36.5 cm. The water was maintained with 0.1% salt concentration, 6.5–7.5 pH, and water temperature at 28°C–31°C. The treatment was carried out for 28 days. The frequency of feeding was twice a day at 08.00 am and 4.00 pm, with the amount of feed was 2% of the fish BW/day. During the study, the water change was conducted weekly. Half of the aquarium’s water volume was disposed of, and an equal amount of clean water containing 0.1% salt was added. Ranchu goldfish performanceThe observed performance of ranchu goldfish is body weight increase (BWI), weight gain ratio (WGR), feed conversion ratio (FCR), and specific growth rate (SGR). The formula used in this study was following Saheli et al. (2021) as follows: BWI=Wt - W0 Note: BWI=body weight increase (g), Wt=average weight of fish at the end of the study (g), W0=average weight of fish at the end of the study (g). WGR=100 × [(Wt-W0) / W0] Note: WGR=weight gain ratio (%), Wt=average weight of fish at the end of the study (g), W0=average weight of fish at the end of the study (g). FCR=F ÷ (Wt - W0) Note: FCR=feed conversion ratio, F=total amount of feed fed (g), Wt=final fish weight (g), W0=initial fish weight (g). SGR=100 × Ln Wt - Ln W0 ÷ t Note: SGR=specific growth rate (%), Wt=final fish weight (g), W0=initial fish weight (g), t=maintenance time (days). Blood sample examinationA blood sample was collected on the final day, 28th day. First, the fish was immersed using cold water (4°C). After that, the fish were reclined laterally and the blood was collected via lateral vein using a disposable syringe (26G, Onemed, Indonesia) containing ethylenediaminetetraacetic acid. Blood was stored at 4°C and tested for several parameters, including: total erythrocytes, total leukocytes, hemoglobin, packed cell volume, MCV, MCH, MCHC, leukocyte differential, and thrombocytes. Hematology profile analysis was performed using an automatic hemoanalyzer (Wheisman AC310, Indonesia). Differential leukocytes were counted manually using blood smears stained with Giemsa (Witeska et al., 2022). C-reactive proteinThe blood was then centrifuged and the plasma was tested against C-reactive protein (CRP). The CRP was tested following the previous study by Kanaparthy et al. (2012). ImmunohistochemistryFor immunohistochemistry, the blood was inserted into a microcapillary. The blood was centrifuged and the microcapillary was broken using a diamond pen. The buffy coat was then smeared on the object glass. The slide was then dehydrated using graded alcohol, xylol, and tap water (Prakoso et al., 2021). Blood specimens were stained against mouse antibody anti-COX-2 (Cox-2 antibody 29, catalogue number sc-19999; Santa Cruz Biotechnology Inc.) and mouse antibody anti-CD4+ (RTU-CD4-1F6, catalogue number PA0427; Novocastra). The staining procedure was conducted following the previous study (Prakoso et al., 2020). MorphometryAll leucocytes that are visible on the blood smear were recorded and were counted until 100 of cells. The counted cells were all leucocytes either expressing or not expressing COX-2 or CD4+. The number of leucocytes which is immunoreactive against COX-2 or CD4+ were multiplied by the total leukocyte and absolute results were obtained. The leucocyte analysis was done using magnification at 1000×. Slides were also photographed and reported qualitatively. Data analysisData analysis in this study was performed using SPSS software version 26 (IBM Statistic, USA). Prior to analysis, the normality and homogeneity of data were tested using Kolmogorov-Smirnov and Levene tests, respectively. Normally distributed and homogeneous data were analyzed using analysis of variance followed by a post hoc test using Bonferroni. Data that were not normally distributed and/or not homogeneous were analyzed using the Kruskal-Wallis test, followed by the Mann-Whitney U test. The confidence level used was 95%. Ethical approvalThis study was approved by the Ethical Committee for Research on Animals, Faculty of Dentistry, Universitas Airlangga, Surabaya. The ethical approval registration number is 1259/HRECC.FODM/XI/2023. The ethical clearance committee performed monitoring and evaluation before and during the experiment. ResultsThe results showed a significant increase in BWI in group P6 compared to P1 (p < 0.05). Group P6 showed a significant increase of WGR compared to groups P1, P2, and P3 (p < 0.05). The SGR in groups P4, P5, and P6 significantly increased than those in groups P1, P2, and P3. All treatment groups using BFE have better FCR values compared to the other groups (p < 0.05), however, group P6 indicated the most significant result (p < 0.05) (Table 1). Group P6 indicated a significant effect on the red blood cell (RBC) compared to the other groups (p < 0.05). The highest heterophil value was shown by group P2 and the lowest occurred in group P6 (p < 0.05). Meanwhile, the administration of BFE did not affect several parameters including, hemoglobin, MCV, MCH, MCHC, WBC, lymphocytes, monocytes, and thrombocytes (p > 0.05) (Tables 2 and 3). The results showed that there is an effect of BFE administration on the level of CRP, CD4+, COX-2, and CD4+/COX-2 ratio (p < 0.05) (Figs. 1 and 2). Moreover, the level of CRP in group P6 indicated the lowest value compared to other groups (p < 0.05). Immunoreactivity of CD4+ in all treatment groups using either vitamin C or BFE indicated a significant elevation compared to groups P1 and P2 (p < 0.05). In contrast, the immunoreactivity of COX-2 in P3, P4, P5, and P6 was lower than those in groups P1 and P2, and the lowest was group P6 (p < 0.05) (Table 4). DiscussionIn this study, there was a significant increase in growth performance (BWI, WGR, and SGR) of ranchu goldfish, along with the increasing concentration of BFE on the feed. This is suspected because of α-tocopherol compounds inside the BFE. As mentioned previously on the methodology, this study found that BFE consist α-tocopherol at 41.37 ± 0.98 mg/kg. This result is in accordance with Niu et al. (2014), which states that Scophthalmus maximus fish has maximum growth after utilization of α-tocopherol at higher doses. The increase in growth performance due to α-tocopherol can trigger superoxide dismutase and catalase (CAT) activity and reduce malondialdehyde (MDA) levels in the serum and fish muscle (Rahman et al., 2023). The oxidative stability of feed containing α-tocopherol compounds can improve fish growth performance. The requirement for α-tocopherol compounds in feed increases with higher lipid levels and when there is a high concentration of unsaturated fatty acids in the feed (Lu et al., 2016). FCR is an essential indicator of feed quality. A lower FCR value indicates better feed quality (Shaha et al., 2022; Rohani et al., 2023). In this study, the FCR value decreased as the concentration of BFE increased. The compound α-tocopherol has been found to reduce the FCR value of feed. This is because α-tocopherol prevents unsaturated fatty acids in feed and fish tissue and helps maintain regular metabolic activity (Pereira et al., 2022). Table 1. Mean and standard deviation results of ranchu goldfish performance.
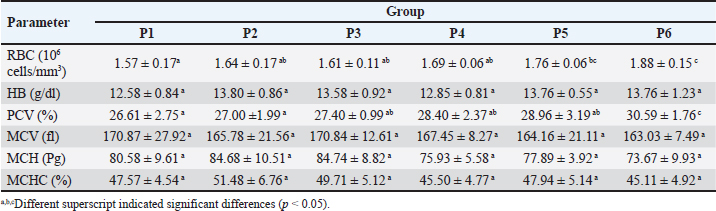
Table 2. Mean and standard deviation of hematology of ranchu goldfish.
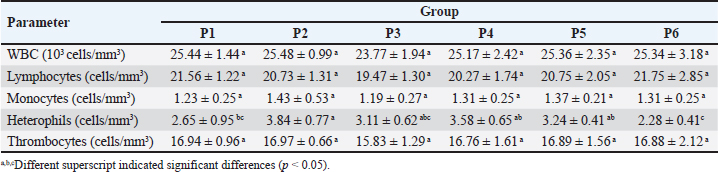
Table 3. Mean and standard deviation of leucocytes and differential leucocytes count of ranchu goldfish.
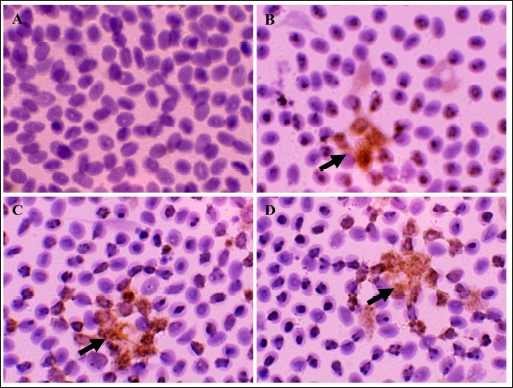
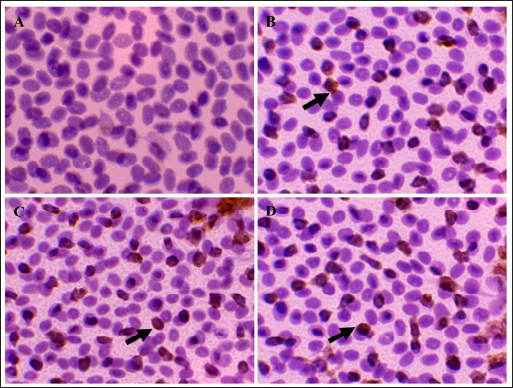
Fig. 1. Immune-expression of CD4+ on cell tube block of ranchu goldfish blood. No CD4+ immune-expression was found in the control without primary antibody (A); CD4+ immuneexpression (arrows) on the surface of lymphocytes of group P2 fed with CMC-Na (B); CD4+ group P3 fed with Power C® (C); group P6 fed with 0.6% BFE (D). Immunohistochemistry of anti-CD4+ antibody, 1000×.
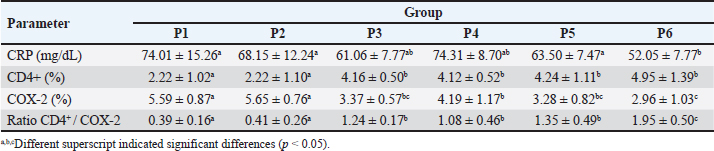
Fig. 2. Immune-expression of COX-2 on cell tube block of ranchu goldfish blood. No COX-2 immune-expression was found in the control without primary antibody (A); COX-2 immuneexpression (arrows) on the nucleus of leucocytes of group P2 fed with CMC-Na (B); group P3 fed with Power C® (C); group P6 fed with 0.6% BFE (D). Immunohistochemistry of antiCOX-2 antibody, 1000×. Moreover, the BFE is also influencing on the RBC level in ranchu goldfish. A previous study by Sharifzadeh et al. (2015) described that α-tocopherol compounds can increase erythropoiesis in hematopoietic tissues. α-tocopherol can also prevent phospholipid peroxidation in the erythrocyte membrane, thus maintaining the structural and functional integrity of fish’s blood cells (El-Sayed and Izquierdo, 2022). In contrast, the PCV level did not significantly increase, although it is trending upward in the treated group. The relatively low PCV value in the untreated group indicates that the fish are suffering from oxidative stress, which shows high MDA values (Sharma and Chadha, 2021). In this study, the WBC, lymphocytes, and monocyte values did not show significant changes except for heterophiles. The heterophils in P6 showed the lowest level, contrasting with the group without therapy. The occurrence of heterophils elevation concomitantly depresses the number of circulatory lymphocytes in the early stage of inflammation during vitamin E deficiency (de Andrade Belo et al., 2014; Klak et al., 2024). The high levels of BFE in feed supplementation can help maintain the stability of leucocytes, lymphocytes, monocytes, and heterophils (Mazini et al., 2022). Compounds of α-tocopherol were found to increase the levels of lymphocytes and monocytes in goldfish. The differences in the results of these studies are believed to be due to variations in the concentration of α-tocopherol used. CD4+ is a type of protein found on the surface of T-cells (Ashfaq et al., 2019). An immunohistochemical examination (Fig. 1) revealed high levels of CD4+ in group P6 compared to P2 and P3. CD4+ lymphocytes are vital in coordinating the immune response by serving as helper cells (Iqbal et al., 2023). Statistically, there was a significant increase in CD4+ values in P3, P4, P5, and P6 compared to P1 and P2, although there was no significant increase in lymphocyte values. The compound α-tocopherol can enhance LAT phosphorylation, which aids in recruiting adaptor and effector proteins crucial for modulating the early stages of Th-cell activation, thereby increasing the percentage of CD4+ (Tang et al., 2024). Consequently, while the total lymphocyte count did not increase, the expression of CD4+ did. Table 4. Mean and standard deviation of CRP and immune-expression of CD4+, COX-2 and ratio of CD4+/COX-2 results of ranchu goldfish.
COX-2 is a biomarker for inflammation; an increase in COX-2 indicates inflammatory processes (Olsen et al., 2012). In this study, the COX-2 value in the P1 group was significantly higher than in P6 (Fig. 2). This was due to the α-tocopherol compound. α-tocopherol can reduce the activity of COX-2 and inhibit the synthesis of prostaglandin E2 (PGE2) (Li et al., 2023). Inhibition of PGE2 synthesis reduces the inflammatory response, especially heterophils, which act as the immune system’s first line (Roy et al., 2017). This correlates with the study, indicating a significant decrease in heterophils in the P6-treated group using the highest BFE concentration. A decrease in the value of heterophils reduces the formation of oxidative stress (Guo et al., 2023). Furthermore, the increased immunoreactivity of CD4+ and the decreased levels COX-2 work together to raise the CD4+/COX-2 ratio. The rationale behind this parameter is that a higher CD4+/COX-2 ratio indicates better immunity and vice versa. This is similar to the findings of Ashfaq et al. (2019), who described that elevated expression of CD4+ promotes immunity in teleost fish, while depletion of COX-2 improves the quality of the fish’s immune system. Additionally, Wang et al. (2016b) added that COX-2, involved in prostaglandin synthesis, is a crucial factor in promoting stress in Larmichthys crocea. A decrease in heterophils determines that fewer cytokines are produced, so hepatocyte cells are not stimulated to produce acute-phase proteins. CRP is the acute-phase protein produced during the initial inflammation (Charlie-Silva et al., 2019; Bello-Perez et al., 2020). CRP, an acute-phase protein, helps bacterial agglutination, phagocytosis, and modulation of inflammation during infection (Li et al., 2022). In this study, the CRP results in the P6 group were lower than in P1. Increased immunity helps fish avoid oxidative stress, thus increasing the growth rate. Moreover, Rahman et al. (2023) described that the right concentration of α-tocopherol compounds improves immunity, stress tolerance, and body performance. This study suggests that BFE can be utilized in aquaculture as an immunostimulant through simple methods, such as food coating. However, further research is needed to explore its other positive effects and potential toxicity in aquaculture to ensure the biosafety of using BFE. Enhancing BFE in aquaculture could involve developing new ready-to-use formulations that are easier for farmers to apply. ConclusionThis study demonstrates that coating fish feed with 0.6% BFE significantly enhances the performance and immunity of ranchu goldfish. Notable improvements were observed in growth parameters, including final weight, absolute weight gain, and specific growth rate. Additionally, hematological benefits were evident, with increased RBC counts and CD4+ lymphocytes, alongside reductions in heterophils, CRP, and COX-2 levels, indicating reduced inflammation and oxidative stress. Further research is needed to explore the potential of BFE as a supplement in fish feed to combat various fish diseases and fish species in real-world or laboratory settings and also the effects of long-term utilization of BFE. AcknowledgmentsThe authors acknowledge the Universitas Gadjah Mada for providing the financial support for this study through Final Project Recognition Program, with grant number: 5286/UN1.P1/PT.01.03/2024. Moreover, the laboratory technicians from Integrated Laboratory, University of Muhammadiyah Sidoarjo were acknowledged for their support in conducting the study. Conflict of interestThe authors have no conflict of interest. FundingThis study received financial support from Universitas Gadjah Mada through Final Project Recognition Program, with grant number: 5286/UN1.P1/PT.01.03/2024. Authors’ contributionFLW and ADW designed the study. FLW, YAP, and ADW performed the animal experimentation. FLW formulated, prepared, and preserved the tested compound. FLW and YAP performed data analysis and interpretation. FLW, YAP, and ADW equally contributed to the drafting, revising, and finalizing of the submitted paper. All the authors approved the current version of the paper. Data availabilityAll the data in this study are available within the manuscript. ReferencesAnjur, N., Sabran, S.F., Daud, H.M. and Othman, N.Z. 2021. An update on the ornamental fish industry in Malaysia: Aeromonas hydrophila-associated disease and its treatment control. Vet. World. 14(5), 1143–1152. Anjusha, K.V., Mamun, M.A.A., Dharmakar, P. and Shamima, N. 2019. Effect of medicinal herbs on hematology of fishes. Int. J. Current. Microbiol. Appl. Sci. 8(9), 2371–2376. Ashfaq, H., Soliman, H., Saleh, M. and El-Matbouli, M. 2019. CD4: a vital player in the teleost fish immune system. Vet. Res. 50, 1–11. Bello-Perez, M., Pereiro, P., Coll, J., Novoa, B., Perez, L. and Falco, A. 2020. Zebrafish C-reactive protein isoforms inhibit SVCV replication by blocking autophagy through interactions with cell membrane cholesterol. Sci. Rep. 10(1), 566. Boakye, A., Attiogbe, F. and Emahi, I. 2024. Crescentia cujete fruit shell as green and efficient coagulant for water purification. Clean. Water. 1, 100009. Canosa, L.F. and Bertucci, J.I. 2023. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 14, 1109461. Charlie-Silva, I., Klein, A., Gomes, J.M., Prado, E.J., Moraes, A.C., Eto, S.F., Fernandes, D.C., Fagliari, J.J., Junior, J.D.C., Lima, C. and Lopes-Ferreira, M. 2019. Acute-phase proteins during inflammatory reaction by bacterial infection: fish-model. Sci. Rep. 9(1), 4776. Darnet, S., Serra, J.L., da Cruz Rodrigues, A.M. and da Silva, L.H.M. 2011. A high-performance liquid chromatography method to measure tocopherols in assai pulp (Euterpe oleracea). Food Res. Int. 44(7), 2107–2111. de Andrade Belo, M.A., de Moraes, F.R., Yoshida, L., da Rosa Prado, E.J., de Moraes, J.R. E., Soares, V.E. and da Silva, M.G. 2014. Deleterious effects of low level of vitamin E and high stocking density on the hematology response of pacus, during chronic inflammatory reaction. Aquaculture. 422, 124–128. El-Sayed, A.F.M. and Izquierdo, M. 2022. The importance of vitamin E for farmed fish—A review. Rev. Aqua. 14(2), 688–703. Evan, Y. and Putri, N.E. 2021. Status of aquatic animal health in Indonesia. In (Aya, F.A., de la Peña, L.D., Salayo, N.D. and Tendencia, E.A. (Eds.) [Conference paper]. Aquaculture Department, Southeast Asian Fisheries Development Center, pp: 138–146. Gonzales, A., Sevilla, U.T.A., Tsai, P. and Huang, S.K. 2022. Antioxidant and anti-inflammatory activities of bioactive compounds from Crescentia cujete L. leaves and fruit–A review. Int. J. Adv. Appl. Sci. 9, 64–70. Guo, K., Zhang, R., Luo, L., Wang, S., Xu, W. and Zhao, Z. 2023. Effects of thermal stress on the antioxidant capacity, blood biochemistry, intestinal microbiota and metabolomic responses of Luciobarbus capito. Antioxidants 12(1), 198. Iqbal, K.J., Majeed, H., Iqbal, K.J., Asghar, M., Azmat, H., Fatima, M., Khan, N., Baboo, I., Tehseen, A., Ali, W., Saeed, U., Khizar, A., Fatima, A., Nisa, S. and Davies, S.J. 2023. Administration of vitamin E and C enhances immunological and biochemical responses against toxicity of silver nanoparticles in grass carp (Ctenopharyngodon idella). PLoS One. 18(4), e0284285. Kanaparthy, A., Kanaparthy, R. and Niranjan, N. 2012 Evaluation of serum C-reactive protein levels in subjects with aggressive and chronic periodontitis and comparison with healthy controls. Dent. Res. J. 9(3), 261–265. Khara, H., Sayyadborani, M. and Sayyad-Borani, M. 2016. Effects of α-tocopherol (vitamin E) and ascorbic acid (vitamin C) and their combination on growth, survival and some haematological and immunological parameters of Caspian brown trout, Salmo trutta caspius juveniles. Turkish J. Fish Aquatic. Sci. 16(2), 385–393. Klak, K., Maciuszek, M., Pijanowski, L., Marcinkowska, M., Homa, J., Verburg-van Kemenade, B.M L., Rakus, K. and Chadzinska, M. 2024. Evolutionarily conserved mechanisms regulating stress-induced neutrophil redistribution in fish. Front. Immunol. 15, 1330995. Li, M., Gou, D., Gong, P., Di, W., Wang, L., Ding, J., Chang, Y. and Zuo, R. 2023. An Investigation on the effects of dietary vitamin e on juvenile sea urchin (Strongylocentrotus intermedius): Growth, intestinal microbiota, immune response, and related gene expression. Biology. 12(12), 1523. Li, Q., Jiang, B., Zhang, Z., Huang, Y., Xu, Z., Chen, X., Cai, J., Huang, Y. and Jian, J. 2022. CRP involved in Nile tilapia (Oreochromis niloticus) against bacterial infection. Biology. 11(8), 1149. Lu, Y., Liang, X.P., Jin, M., Sun, P., Ma, H.N., Yuan, Y. and Zhou, Q.C. 2016. Effects of dietary vitamin E on the growth performance, antioxidant status and innate immune response in juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture 464, 609–617. Magno, I., Esmail, R., Lim, M., Padua, J., Reyes, K., Tugom, L., Valdez, A. and Abusama, H. 2021. Antibacterial effect of calabash (Crescentia Cujete) leaf and fruit extract on preservation of lettuce (Lactuca Sativa) leaves with Escherichia coli. AJSE. 2(1), 91–94. Mazini, BS.M., Martins, G.P., de Castro Menezes, L.L. and Guimarães, I.G. 2022. Nutritional feed additives reduce the adverse effects of transport stress in the immune system of Tambaqui (Colossoma macropomum). Fish Shellfish Immunol. Rep. 3, 100051. Medagoda, N., Chotikachinda, R., Hasanthi, M. and Lee, K.J. 2023. Dietary supplementation of a mixture of nucleotides, β-glucan and vitamins c and e improved the growth and health performance of olive flounder, Paralichthys olivaceus. Fish. 8(6), 302. Nikiforov-Nikishin, A., Smorodinskaya, S., Kochetkov, N., Nikiforov-Nikishin, D., Danilenko, V., Bugaev, O., Vatlin, A., Abrosimova, N., Antipov, S., Kudryavtsev, A. and Klimov, V. 2022. Effects of three feed additives on the culturable microbiota composition and histology of the anterior and posterior intestines of zebrafish (Danio rerio). Animals. 12(18), 2424. Niu, H., Jia, Y., Hu, P., Meng, Z. and Lei, J. 2014. Effect of dietary vitamin E on the growth performance and nonspecific immunity in sub-adult turbot (Scophthalmus maximus). Fish Shellfish Immunol. 41(2), 501–506. Olsen, R.E., Svardal, A., Eide, T. and Wargelius, A. 2012. Stress and expression of cyclooxygenases (COX1, COX2A, COX2B) and intestinal eicosanoids, in Atlantic salmon, Salmo salar L. Fish Physiol. Biochem. 38, 951–962. Ortuño, J., Esteban, M.A. and Meseguer, J. 2003. The effect of dietary intake of vitamins C and E on the stress response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 14(2), 145–156. Pereira, R., Costa, M., Velasco, C., Cunha, L.M., Lima, R.C., Baião, L.F., Batista, S., Marques, A., Sá, T., Campos, D.A., Pereira, M., Jesus, D., Fernández-Boo, S., Costas, B., Pintado, M. and Valente, L.M.P. 2022. Comparative analysis between synthetic vitamin E and natural antioxidant sources from tomato, carrot and coriander in diets for market-sized Dicentrarchus labrax. Antioxidants 11(4), 636. Prakoso, Y.A., Hidayah, N., Rini, C.S. and Kurniasih, K. 2021. Dynamic change of blood profile in rat models with acute skin injury artificially infected with methicillin-resistant Staphylococcus aureus. Vet. World. 14(8), 2085. Prakoso, Y.A., Rini, C.S., Rahayu, A., Sigit, M. and Widhowati, D. 2020. Celery (Apium graveolens) as a potential antibacterial agent and its effect on cytokeratin-17 and other healing promoters in skin wounds infected with methicillin-resistant Staphylococcus aureus. Vet. World 13(5), 865–871. Rahman, H., Alam, M.A., Flura, M.M. and Lupa, S.T. 2023. Effects of vitamin E supplemented feed on growth performance of fish: A review. J. Aqua. Fish. 7(070), 2. Rahmaningsih, S. and Andriani, R. 2020. Potential analysis of majapahit fruit powder (Crescentia cujete L) as shrimp immunostimulants using the insilico method. Material Sci. Eng. 874(1), 012002. Rohani, M.F., Tarin, T., Hasan, J., Islam, S.M.M. and Shahjahan, M. 2023. Vitamin E supplementation in diet ameliorates growth of Nile tilapia by upgrading muscle health. Saudi J. Biol. Sci. 30(2), 103558. Roy, S., Kumar, V., Kumar, V. and Behera, B. K. 2017. Acute-phase proteins and their potential role as an indicator for fish health and in the diagnosis of fish diseases. Protein Pept. Lett. 24, 1–13. Saheli, M., Islami, H.R., Mohseni, M. and Soltani, M. 2021. Effects of dietary vitamin E on growth performance, body composition, antioxidant capacity, and some immune responses in Caspian trout (Salmo caspius). Aqua. Rep. 21, 100857. Sarsembayeva, N.B., Akkozova, A.S., Abdigaliyeva, T.B., Abzhalieva, A.B. and Aidarbekova, A.B. 2021. Effect of feed additive “Ceobalyk” on the biological and microbiological parameters of African sharptooth catfish (Clarias gariepinus). Vet. World 14(3), 669–677. Shaha, A., Talukdar, A. and Das, M. 2022. Effect of vitamin E supplemented fish feed on growth performance of brood gangetic Mystus (Mystus cavasius). Int. J. Fish Aqua. Stud. 10(4), 62–68. Sharifzadeh, S.A., Khara, H. and Ghobadi, S. 2015. Effects of vitamins E and riboflavin (B2) and combinations of them on the hematological parameters of common goldfish, Cyprinus goldfishio L., fingerlings. Fish Aqua. Life. 23(2), 107–111. Sharma, P., and Chadha, P. (2021). Bisphenol A induced toxicity in blood cells of freshwater fish Channa punctatus after acute exposure. Saudi J. Biol. Sci. 28(8), 4738–4750. Shastak, Y. and Pelletier, W. 2023. Captivating colors, crucial roles: Astaxanthin’s antioxidant impact on fish oxidative stress and reproductive performance. Animals. 13(21), 3357. Srivastava, B. and Reddy, P.B. 2021. Haematological profile in fish as an effective and sensitive index in aquatic pollution. Nimitmai. Rev. J. 3(2), 19–27. Tang, J., Li, G., Chen, D., Jiang, L., Huang, B., Jiang, P., Zhang, C. and Qin, X. 2024. Effect of vitamin E on energy metabolism indicators and gill tissue structure of crucian carp (Carassius auratus) under cooling stress. Sci. Rep.14(1), 19484. Wang, T., Mai, K. and Ai, Q. 2016a. A review of cyclooxygenase-2 role in fish. Austin. J. Nutr. Metab. 3, 1037. Wang, T., Yan, J., Xu, W., Ai, Q. and Mai, K. 2016b. Characterization of cyclooxygenase-2 and its induction pathways in response to high lipid diet-induced inflammation in Larmichthys crocea. Sci. Rep 6. 19921. Wilujeng, S., Wirjaatmadja, R. and Prakoso, Y.A. 2023. Effects of extraction, fermentation, and storage processes on the levels of choline derived from calabash fruit (Crescentia cujete L.). J. Res. Pharm. 27(2), 620–626. Witeska, M., Kondera, E., Ługowska, K. and Bojarski, B. 2022. Hematological methods in fish–Not only for beginners. Aquaculture. 547, 737498. | ||
| How to Cite this Article |
| Pubmed Style Wongkar FL, Prakoso YA, Wijayanti AD. Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Vet. J.. 2024; 14(11): 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 Web Style Wongkar FL, Prakoso YA, Wijayanti AD. Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). https://www.openveterinaryjournal.com/?mno=217648 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.30 AMA (American Medical Association) Style Wongkar FL, Prakoso YA, Wijayanti AD. Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Vet. J.. 2024; 14(11): 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 Vancouver/ICMJE Style Wongkar FL, Prakoso YA, Wijayanti AD. Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 Harvard Style Wongkar, F. L., Prakoso, . Y. A. & Wijayanti, . A. D. (2024) Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Vet. J., 14 (11), 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 Turabian Style Wongkar, Felician Lestari, Yos Adi Prakoso, and Agustina Dwi Wijayanti. 2024. Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Veterinary Journal, 14 (11), 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 Chicago Style Wongkar, Felician Lestari, Yos Adi Prakoso, and Agustina Dwi Wijayanti. "Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus)." Open Veterinary Journal 14 (2024), 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 MLA (The Modern Language Association) Style Wongkar, Felician Lestari, Yos Adi Prakoso, and Agustina Dwi Wijayanti. "Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus)." Open Veterinary Journal 14.11 (2024), 3017-3025. Print. doi:10.5455/OVJ.2024.v14.i11.30 APA (American Psychological Association) Style Wongkar, F. L., Prakoso, . Y. A. & Wijayanti, . A. D. (2024) Effects of Crescentia cujete (L.) fruit extract on the profile of hematology of ranchu goldfish (Carassius auratus). Open Veterinary Journal, 14 (11), 3017-3025. doi:10.5455/OVJ.2024.v14.i11.30 |