| Research Article | ||
Open Vet. J.. 2024; 14(11): 3063-3073 Open Veterinary Journal, (2024), Vol. 14(11): 3063-3073 Research Article Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgeryHelen Mather* and Robin G. StanleyAnimal Eye Care, Melbourne, Australia *Corresponding Author: Helen Mather, Animal Eye Care, Melbourne, Australia. Email: hele.mather [at] gmail.com Submitted: 02/09/2024 Accepted: 17/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
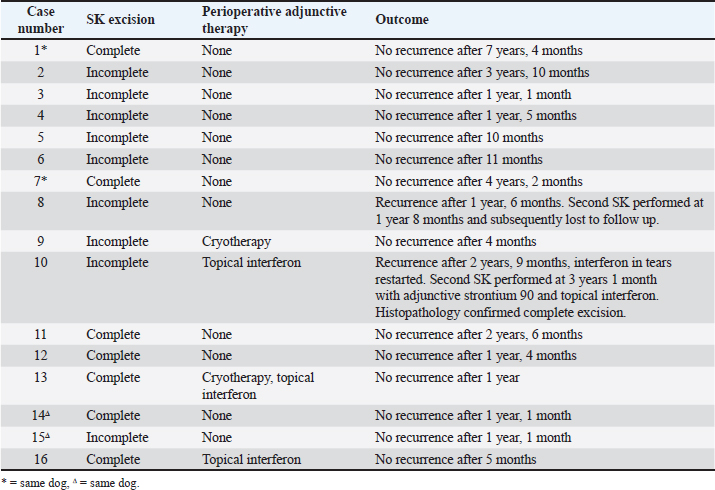
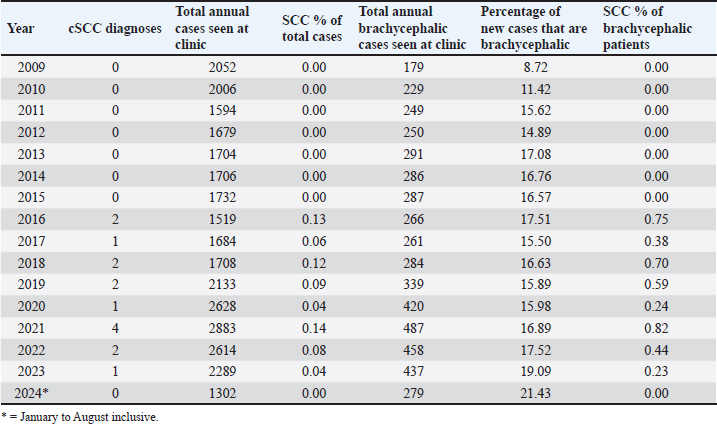
AbstractBackground: Corneal squamous cell carcinoma (cSCC) is a rare neoplasm of dogs that can be treated with various modalities, principally by superficial keratectomy (SK) surgery. It is common to treat cSCC with multiple adjunctive therapies, but this may not always be practical for clinicians, clients, or patients. Aim: This retrospective study describes the signalment of affected dogs, concurrent medical therapy, and success rate of surgical treatment of cSCC with SK surgery alone or in combination with adjunct therapy. Methods: Eligible dogs undergoing SK surgery for histologically confirmed cSCC were selected from medical records (2009-2024) of Animal Eye Care, Melbourne. Records were examined for cSCC recurrence at follow up. Results: Between January 2009 and August 2024, 16 eyes from 14 dogs (5 male; 35.7% (37.5% eyes), 9 female; 64.3% (62.5% eyes) had a confirmed histopathological diagnosis of cSCC following SK surgery. All cases were diagnosed within the last 9 years. There was a notable predilection of brachycephalic breeds (85.7% of dogs; 81.3% of eyes) with pugs the most overrepresented (42.9% of dogs; 37.5% of eyes). The average age at diagnosis was 8.7 years (range 2.1–13.8). Tumor recurrence occurred in two cases following incomplete excision, with no tumor recurrence reported following a second SK surgery. Adjunctive therapy was used in four cases and included cryotherapy and topical interferon alpha-2a. At the time of diagnosis, 12 out of 16 eyes had been treated previously with topical immunomodulatory therapy. Prevalence data varied but peaked in 2021 with 0.14% of total patients and 0.82% of all brachycephalic patients diagnosed with cSCC. Conclusion: Complete excision of cSCC by SK surgery is effective for preventing the recurrence of cSCC in dogs, even in the absence of adjunctive therapies. Dogs with chronic corneal inflammatory conditions, particularly brachycephalic breeds, are at higher risk for developing cSCC. Corneal SCC should be suspected in middle-aged brachycephalic dogs presenting with proliferative, raised, or hyperaemic corneal lesions. Keywords: Canine, Eye, Immunomodulatory, Tacrolimus, Tumor. IntroductionOcular squamous cell carcinoma (SCC) is a rare tumor of the eye of dogs and humans (Gelatt et al., 2021). It is seen most commonly in cattle and horses (Dubielzig, 2016; Gelatt et al., 2021), uncommonly in cats and sheep (Wilcock, 1993), and has been reported in goats (Marà et al., 2005), reindeer (Gonzalez-Alonso-Alegre et al., 2013), and other species (Wilcock 1993; Valentine and Martin, 2007). Publications about corneal SCC (cSCC) in dogs are sparse and are mostly limited to single case reports. In cattle and horses, risk factors for ocular SCC include increased exposure to ultraviolet (UV) radiation (Heeney and Valli, 1985; Tsujita and Plummer, 2010), light pigmentation (Anderson, 1963; Dugan et al., 1991), and breed (Knickelbein et al., 2019, 2020). In the dog proposed risk factors for the development of cSCC include the use of topical immunomodulatory therapies (Dreyfus et al., 2011), hereditary predisposition (Bernays et al., 1999), chronic corneal inflammation (Dreyfus et al., 2011), prior trauma (Latimer et al., 1987), and UV light exposure induced cellular damage (Montiani-Ferreira et al., 2008; Dreyfus et al., 2011) including P53 tumor suppressor gene mutation (Montiani-Ferreira et al., 2008) and papilloma viral infection (Bernays et al., 1999). The incidence of cSCC in dogs appears to be increasing (Dreyfus et al., 2011). Proposed contributors to this increased incidence include the rising popularity of brachycephalic breeds that are prone to conditions causing chronic corneal inflammation and the increasing use of topical immunomodulatory therapies including cyclosporin and tacrolimus in veterinary medicine. The mainstay of treatment of cSCC is surgical excision of affected tissue, most often with superficial keratectomy (SK) (Busse et al., 2008; Montiani-Ferreira et al., 2008; Takiyama et al., 2010). In humans (Yeoh et al., 2022) and animals, specific adjunctive therapies have been utilized alongside SK. These include topical mitomycin C (Karasawa et al., 2008), 5-flurouracil (Overton et al., 2015; Dorbandt et al., 2016), or interferon alpha-2b (Martabano et al., 2024), strontium-90 beta irradiation (Plummer et al., 2007; Nevile et al., 2015), carbon dioxide laser photoablation (Michau et al., 2012), radiofrequency hyperthermia (Fischer et al., 2002), and cryotherapy (Schoster, 1992). It is possible that veterinary clinicians may not always have access to adjunctive therapies, or the ongoing cost may be prohibitive for some owners. It has been reported that in uncomplicated cases surgical excision with wide margins carries a good prognosis (Peiffer et al., 1978) and it, therefore, would not be unreasonable to consider treatment with SK alone. This report presents an analysis of cases of confirmed cSCC presenting to a referral veterinary ophthalmology clinic (Animal Eye Care, Melbourne, Australia) over a 15-year period (January 2009—August 2024). The aims of this study were to retrospectively review the clinical records to assess the efficacy of SK as a treatment for cSCC and to identify the signalment patterns of cSCC. Materials and MethodsCase selectionA retrospective case series study was performed. An electronic search of medical records at a specialist veterinary ophthalmology clinic (Animal Eye Care, Melbourne, Australia) was performed using the keywords “squamous cell carcinoma”, “SCC”, “squamous”, and “carcinoma”, to identify cases of SCC between 1 January 2009 and 31 August 2024. Cases were excluded if minimal follow-up data were available (less than 4 months), if SK surgery was not performed, or if no histopathological diagnosis was available. Data collected from medical records included breed, sex, age, surgeon, surgery details, adjunctive therapies used (if any), date of tumor recurrence (if any), and date of most recent follow-up. Prevalence data were collected by searching medical records for both total initial canine cases seen per year and total initial brachycephalic breed cases seen per year. Presurgical evaluation and treatmentA complete ophthalmic examination was performed at the initial consultation. Each case was examined by a veterinary ophthalmologist before surgery. A presumptive diagnosis was made on the clinical presentation. No cytology or biopsy was performed at the time of consultation. Surgical technique and post-surgical evaluationSurgery was performed by one of four surgeons, including two veterinary ophthalmologists, an ophthalmology resident under direct supervision by a veterinary ophthalmologist and who later became a third veterinary ophthalmologist and a special interest practitioner in ophthalmology under the direct supervision of a veterinary ophthalmologist. Dogs were premedicated with either acepromazine or medetomidine, in combination with either butorphanol or methadone. Anesthesia was induced with alfaxalone (Alfaxan®, Jurox Animal Health, NSW, Australia), and all dogs were intubated and maintained on isoflurane inhalational anesthetic. Patients were given intravenous fluids. The cornea was anesthetized with topical 0.5% proxymetacaine hydrochloride (Alcaine®, Alcon Laboratories Australia Pty Ltd, NSW, Australia). The cornea, conjunctiva, and eyelids were disinfected with 1% povidone-iodine solution. The cornea was regularly irrigated with either a balanced salt solution or the iodine solution during surgery. Using a corneal disc knife (Alcon Laboratories (Australia) Pty Ltd, NSW, Australia), SK surgeries were performed to approximately 20%–50% of the corneal stromal depth with visually clear margins of at least 2 mm, under the magnification of an operating microscope (Topcon OMS-85, Topcon Healthcare Solutions Australia & New Zealand, SA, Australia). At the completion of the surgeries, lateral temporary tarsorraphy sutures were placed using 4/0 nylon (Nylene® N405, Dynek Sutures, South Australia). Excised masses were fixed with 10% neutral buffered formalin and submitted for histopathology (Australian Specialised Animal Pathology Laboratory, Victoria, Australia). Patients were discharged on the day of the procedure. Medical therapies used immediately postoperatively varied at the surgeon’s discretion. Treatments included topical antibiotic therapy (chloramphenicol 0.5% (Chlorsig® Eye Drops, Aspen Australia, NSW, Australia), Ofloxacin 0.3% (Ocuflox® Eye Drops, Allergan Australia, NSW, Australia), or 10,000 IU/g Polymyxin B sulfate, 500 IU/g Bacitracin zinc, 5 mg/g Neomycin sulfate triple antibiotic ointment (Tricin® Eye and Ear ointment, Jurox Animal Health, NSW, Australia)), topical immunomodulatory therapy with calcineurin inhibitors (2 mg/g cyclosporin (Optimmune® Ophthalmic Ointment, MSD Animal Health, NSW, Australia), 0.02% Tacrolimus (Bova Aus, NSW, Australia)) or peginterferon alfa-2a 3MIU in 0.5 ml solution (Roferon-A, Roche Products Pty Ltd, NSW, Australia) diluted to 5,400IU/ml in Blink® Contacts Eye Drops (AMO Australia Pty Ltd, NSW, Australia), an oral anti-inflammatory (carprofen (Rimadyl®, Zoetis, Australia) or 1.5 mg/ml meloxicam suspension (Loxicom, Norbrook Laboratories Australia Pty Ltd, VIC, Australia), systemic antibiotics (doxycycline (Doxy 50/100 Antibiotic Tablets, Dechra Veterinary Products (Australia) Ptd Ltd, NSW, Australia), and gabapentin (WP Gabapentin, Medtas Pty Ltd, NSW, Australia). Ethical approvalThis study describes the findings of a clinical and diagnostic investigation of clinical cases in a private practice setting with non-experimental privately owned animals under the Guidelines for Ethical Research in Veterinary Ophthalmology. Therefore, ethical approval from a committee was not required for publication. ResultsA total of 31,233 new canine patients including 5,002 of brachycephalic breed presented to Animal Eye Care, Melbourne between 1 January 2009 and 31 August 2024. Corneal SCC was suspected in 30 eyes from 28 dogs. Fourteen eyes were excluded from the study because surgery was not pursued (6/14), the eye was enucleated (3/14), histology was not performed following SK surgery (4/16), the eye was lost to follow up 2 months following SK surgery, and histologic confirmation of cSCC (1/14) (Table 1). Corneal SCC was confirmed by histopathology following SK in 16 eyes from 14 dogs, 5 males and 9 females (Table 2). The mean (± standard deviation) age of patients at SCC diagnosis was 9.3 years (± 2.8) with a range of 2.1–13.7 years. In case 8, recurrence was noticed at the original site after 1.5 years (Fig. 1). Case 9 was the youngest dog affected (Fig. 2). The left eye was affected in 8 dogs, the right eye in 4 dogs, and both eyes were affected in 2 dogs. Complete excision of cSCC was confirmed by histology in 7/16 eyes (43.8%). Incomplete excision was suspected in 9/16 eyes (56.2%). 14 out of 16 eyes had no recurrence of tumor after a mean follow-up time of 2 years (± 1.8) with a range of 0.4–7.3 years (Table 3). There was no recurrence of any completely excised tumors. Of the nine cases with suspected incomplete excision, two patients (Case 8 and Case 10) had suspected tumor recurrence (22.2%). The clinical presentation and location of the lesion were the same as in the initial presentation. This case was treated with a second superficial keratectomy although this sample was not submitted for histopathology. In case 10, recurrence was suspected at 2.8 years following initial SK, and repeat SK with adjunct strontium 90 plesiotherapy was performed at 3.1 years. Histopathology confirmed carcinoma in situ with complete excision. There was no recurrence in the remaining 7 cases with suspected incomplete excision after at least 1 year of follow up. Two patients had cSCC diagnosed in both eyes. A Jack Russell Terrier was diagnosed in both eyes simultaneously at 9.7 years of age and a Cavalier King Charles Spaniel was diagnosed in the left eye at 5.1 years of age and in the right eye at 8.2 years of age (Table 2). All study cases were diagnosed within the past 9 years (Table 2). Within this period (January 2016—August 2024), the prevalence ranged from 0% to 0.14% of total canine cases (Table 4). The prevalence among brachycephalic breeds ranged from 0% to 0.82%. In 2009, a total of 179 new brachycephalic patients were seen, or 8.7% of total new cases. In the first 8 months of 2024, a total of 279 new brachycephalic patients were seen, or 21.4% of total new cases. Brachycephalic breeds—Pugs, Bulldogs, and Cavalier King Charles Spaniels, were overrepresented accounting for 81.3% (13/16) of study cases (Table 2). Of note, Pugs accounted for 37.5% (6/16) of all cases, the most prevalent breed diagnosed with cSCC. Table 1. Details of suspected cSCC cases excluded from study and reason for exclusion.
Table 2. Signalment of dogs included in study.
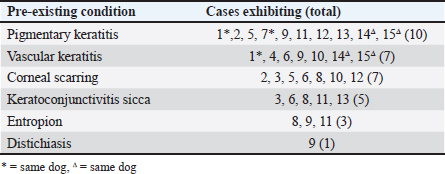
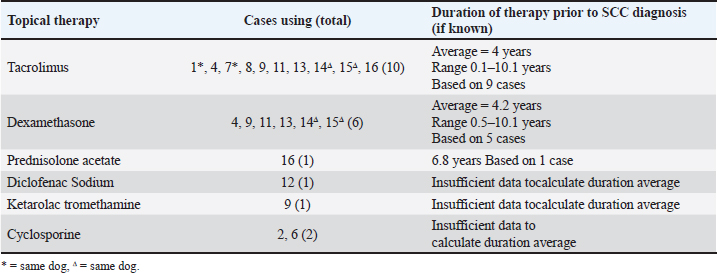
The majority of eyes (15/16) featured a chronic inflammatory condition or prior chronic irritation (Table 5). All Pugs and Cavalier King Charles Spaniels included in the study displayed concurrent pigmentary keratitis making this the most prevalent pre-existing condition in this series. Five dogs had pre-existing keratoconjunctivitis sicca (KCS). One of three non-brachycephalic eyes in the study, a 9.5-year-old Golden Retriever (case 10), had no pre-existing keratitis or KCS recorded, however, had sustained trauma to the eye as a puppy. Most eyes (13/16) included in the study were being treated with long-term topical immunomodulatory or anti-inflammatory therapy prior to SCC diagnosis (Table 6). The most common treatment was tacrolimus (10 eyes), with an average duration of therapy of 4 years prior to a diagnosis of cSCC. Following SK for the confirmed cSCC, adjunctive therapy was used in 4 of the 15 cases (Table 3). Three cases received topical interferon alpha-2a either 2 months before SK surgery (case 16), or following SK surgery (day 1 case 13, day 13 case 10). One of these cases (case 10) had tumor recurrence suspected at 2.8 years postoperatively. Two cases received cryotherapy at the time of surgery and had no recurrence. DiscussionOur study found that 87.5% (14/16) of cases had no tumor regrowth after a mean follow-up time of 2 years (± 1.8). Published reports of canine cSCC in the veterinary literature are increasing but remain relatively rare. In a large-scale retrospective study looking at samples submitted to the Comparative Ocular Pathology Lab of Wisconsin, Dreyfus et al., (2011) found that 10 out of 26 SCC tumors removed by SK recurred at the original site of removal. These authors noted that 7 out of the 10 recurrent cases had incomplete margins after the first keratectomy; however, information regarding the surgical approach was not provided. In our analysis, both cases recur and were incompletely excised based on histology. Interestingly, seven other cases were suspected to have had incomplete excision but had no tumor regrowth during a mean follow-up time of 1.6 years (± 1.2). Corneal SCC has been demonstrated to infiltrate the deep stroma in horses (Kafarnik et al., 2009) and can invade the anterior chamber and periocular tissues in cattle (Martins, 2021). There is only one report of a highly invasive canine cSCC in the literature which demonstrated both exophytic growth and extension deep into the stroma (María Del Mar et al., 2019). Conservative surgical treatment for canine cSCC should always be considered as most published reports indicate that tumors remain exophytic on the surface of the cornea or involve only the superficial stroma, facilitating surgical excision (Karasawa et al., 2008; Takiyama et al., 2010; Dreyfus et al., 2011; Nevile et al., 2015; Dorbandt et al., 2016). Several authors have reported success in treating cSCC with SK alone. Busse et al., (2008) found no recurrence of tumour 25 weeks after SK in a Border Collie with cSCC. Takiyama et al., (2010) reported on two cSCC cases, a pug that after initially having tumor regrowth had no recurrence 18 months after a second SK, and a toy poodle that had no tumor regrowth 15 months after SK. It has previously been reported that in uncomplicated cases of canine cSCC surgical excision with wide margins carries a good prognosis without the use of adjunctive therapies (Peiffer et al., 1978). In humans, the gold standard of ocular surface squamous neoplasia has historically been excision alone (Alvarez et al., 2021), with favorable success rates in uncomplicated cases (Galor et al., 2012). It has been suggested that when human corneal tumors are removed with 2 mm margins adjuvant treatment with cryotherapy is unnecessary (Yan et al., 2011). In contrast, some more recent studies in human medicine show an increasing move toward medical management with chemotherapeutic drugs and immunomodulatory agents (Yeoh et al., 2022).
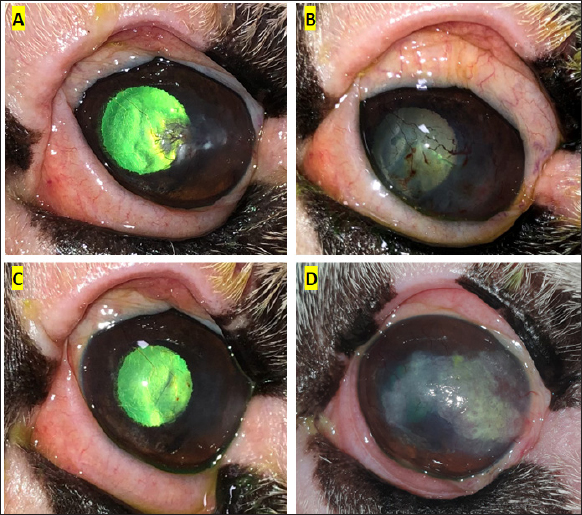
Fig. 1. Clinical progression of case 8. (A) raised opacities with associated vascularisation were seen 7 months prior to initial SK surgery. (B) 8 days following initial SK surgery, the surgical site was epithelialized with prominent corneal vascularisation present. (C) 1 month following initial SK surgery the cornea was clear and only very faint vascularisation remained. (D) 1 year 7 months following initial SK, recurrence of the incompletely excised cSCC was demonstrated. Repeat SK was performed 1 month later. In this study, most cases did not receive adjunctive therapy to treat cSCC. Specific adjunct therapies previously utilized in dogs include strontium-90 beta irradiation (Nevile et al., 2015), topical chemotherapy with 5-flurouracil (Overton et al., 2015) or mitomycin C (Karasawa et al., 2008), and cryotherapy (Ward et al., 1992). Topical 5-flurouracil has been used successfully as a monotherapy to treat a small cSCC in a pug (Dorbandt et al., 2016), and topical mitomycin C has recently been used as a monotherapy to treat cSCC in a cat (Delgado, 2020). Cryotherapy has been utilized to treat ocular surface neoplasms in humans (Galor et al., 2012) and has been used alongside SK surgery in dogs (Latimer et al., 1987; Schoster, 1992; Ward et al., 1992). Topical interferon has the advantage of being well tolerated by the eye (Ghaffari et al., 2021), minimally invasive yet treating the whole cornea (Wilson et al., 2001; Schechter et al., 2002), non-toxic to the ocular surface (Boehm and Huang, 2004), and relatively readily available and affordable. In humans, interferon alpha-2b is used following surgical excision, and topical or perilesional interferon is increasingly being used as a sole therapy (Boehm and Huang, 2004; Ghaffari et al., 2021). Intralesional interferon alpha-2b has recently been used with some success to treat periocular SCC in horses (Martabano et al., 2024). In general, there is a paucity of data on the use of adjunct therapy in treating canine cSCC. Most publications are single case reports or limited case series and therefore inferences on the efficacy of adjunct treatments are difficult to determine. The only existing published large retrospective analysis on canine cSCC did not report on the use of adjunctive therapy following SK (Dreyfus et al., 2011). The current study represents the largest number of confirmed canine cSCC cases known to be successfully treated with SK alone (11/12 eyes) or in combination with adjunctive therapy (3/4 eyes) reported in the literature to date.
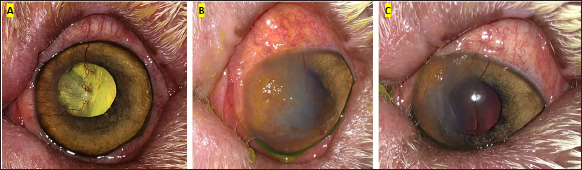
Fig. 2. Clinical progression of case 9. (A) vascularisation and scarring of the dorsomedial cornea were seen 1 year prior to SK surgery. (B) increased opacification and dense, raised vascularisation with an irregular cobblestone appearance was noted a year later, 2 weeks prior to SK surgery. (C) improved corneal clarity and a more discrete, localised, irregular, raised, pink growth was seen 7 days later, 7 days prior to SK surgery, following treatment with topical dexamethasone, topical Polymixin B sulfate/Bacitracin zinc/Neomycin sulfate triple antibiotic ointment, systemic doxycycline and systemic carprofen. Table 3. Treatment and outcome data of included cSCC cases.
Table 4. Cases seen at Animal Eye Care from 1 January 2009–31st August 2024.
Most published cases of cSCC, including the dataset we present here, are recent diagnoses. We found that all included cases of cSCC, and 57.1% (8/14 eyes) of excluded cases were within the last 9 years (Table 2, Table 1). Dreyfus et al., (2011) noted a similar pattern with 23/26 cases being recent diagnoses within 4 years of publication. These authors also reported the diagnosis of cSCC rising as a percentage of cases (0.08% in 2000 to 0.32% in 2007) and suggested a rise in disease prevalence. Whilst our data shows an increased prevalence of cSCC in the last 9 years compared to the prior, it does not show a year-on-year increase in prevalence within the last 9 years. There was no obvious trend observed when considering annual cSCC diagnoses against total annual cases seen (Table 4). While not the case in our data set, the apparent increase in diagnoses by Dreyfus et al., (2011) may be due to more tumors being sent for histology after SK or excisional biopsy in recent years. The data set presented here may be too small to see the true change in the prevalence of cSCC in dogs. A much larger sample size is likely required to see an accurate representation of the rising prevalence of a disease so rare. The number of brachycephalic dogs seen appears to be increasing over time, both in total number of cases and as a percentage of new cases at Animal Eye Care, Melbourne, Australia. The overrepresentation of brachycephalic breeds in this study is similar to that reported by Dreyfus et al. (2011). Most published case reports also involve brachycephalic breeds including Pugs (Takiyama et al., 2010; Overton et al., 2015; Dorbandt et al., 2016), a Japanese Chin (Nevile et al., 2015), an English Bulldog (Montiani-Ferreira et al., 2008), a Lhasa Apso (Bernays et al., 1999), a French Bulldog (María Del Mar et al., 2019) and a Cavalier King Charles Spaniel (Nevile et al., 2015). Brachycephalic breeds are predisposed to chronic inflammatory conditions such as KCS (Kaswan et al., 1989) and pigmentary keratitis (Gelatt et al., 2021; Sebbag and Sanchez, 2023), both of which have been suggested as risk factors for the formation of cSCC (Dreyfus et al., 2011; Labelle and Labelle, 2013). Almost every case included in the current study displayed some form of chronic inflammation, with KCS and pigmentary keratitis featuring heavily (Table 5). The exact reason for the link between chronic keratitis and cSCC formation has not yet been fully explained, but increased cellular proliferation and inflammatory signals have been proposed as mechanisms for tumor development (Dreyfus et al., 2011). It has also been suggested that corneal epithelial metaplasia accompanying pigmentary keratitis may represent a preneoplastic change (Bernays et al., 1999). In line with previous reports, our results suggest that brachycephalic breeds are more at risk of developing canine cSCC (Dreyfus et al., 2011), possibly as a result of their predisposition to chronic inflammatory conditions. Table 5. Pre-existing conditions in cSCC cases.
Table 6. Topical medical treatment used prior to SK surgery and duration of therapy where known.
Some authors have suggested a possible association between canine cSCC development and treatment with topical immunomodulators (Ward et al., 1992; Bernays et al., 1999; Takiyama et al., 2010; Dreyfus et al., 2011). Most dogs in this study were being treated for chronic inflammatory conditions using topical immunomodulatory therapy (calcineuron inhibitors (tacrolimus or cyclosporine) and/or topical steroids (prednisolone acetate or dexamethasone sodium phosphate)) prior to cSCC diagnosis. This finding is consistent with Dreyfus et al. (2011) who found that 16 out of 21 dogs were being treated for KCS with long-duration tacrolimus or cyclosporine. In humans, long-term exposure to cyclosporine was associated with a higher risk of nonmelanoma skin cancer, especially SCC, in psoriasis patients (Paul et al., 2003). Human transplant recipients receiving systemic tacrolimus treatment have been shown to be at higher risk of neoplasia (Rodríguez-Perálvarez et al., 2022). However, more recent research has found no significant increase in the risk of cancer development with the use of topical tacrolimus (Arana et al., 2021; Lam et al., 2021) The authors cannot rule a potential influence of long-term immunomodulatory drug use in this study; however, firm conclusions are impossible to draw as this treatment is utilized for chronic inflammatory conditions that are also proposed risk factors for cSCC development. The study population presented here represents only a fraction of the cases that are treated with topical immunomodulatory therapies for ophthalmic conditions and therefore these findings should not be interpreted as a reason to alter the current standard of care. Exposure to UV light has been shown to contribute to cSCC formation in horses and cattle (Wilcock 1993) and has been suggested as a contributing factor in canine cSCC formation in a case report from Brazil (Montiani-Ferreira et al., 2008). Exposure to UV light can result in a mutation in the p53 tumor suppressor gene, resulting in overexpression frequently associated with ocular SCCs (Teifke and Löhr, 1996; Sironi et al., 1999). The cases included in this study all originated in Melbourne, Australia where the ultraviolet index (UVI) ranges from low (1.8 UVI) in the winter to extreme (10.9 UVI) in peak summer (Australian Radiation Protection and Nuclear Safety Agency 2024). From November through to March UVI remains very high (>7.5 UVI). The anatomy of brachycephalic dog breeds frequently predisposes them to exophthalmos, macropalpebral fissures, and incomplete lid closure which may result in greater cumulative UV exposure. The combination of high UVI and breed-specific morphology may have contributed to cSCC formation in the cases presented here. In Australia, brachycephalics dogs are predominantly kept inside during the hot summer months to minimize the risk of heat stroke. Unfortunately, not enough data were collected on the specific lifestyle of dogs included in this study to draw meaningful inferences, as indoor dogs have a reduced UV exposure risk. Additionally, immunohistochemistry was not performed in our series of cases, so no data on the expression of p53 tumor suppressor gene was available. Interestingly case 10 was diagnosed with a pigmented cSCC with prominent papillomaviral induced cytopathy and papillomaviral PCR testing of the keratectomy sample confirmed concurrent canine papillomavirus 17 infection. Papilloma viral infection has been proposed as a risk factor for cSCC tumorigenesis (Bernays et al., 1999). Pigmented cSCC has not been reported in any other animal in the veterinary literature and canine papillomavirus type 17 has only been reported in one other dog, and in that case was associated with oral SCC (Munday et al., 2015). Corneal SCC remains a seldom reported diagnosis in the veterinary literature and this study is one of only two larger scale retrospective analyses involving multiple cases. Although this study is inherently limited by its retrospective design, there is sufficient evidence to support the suggestion that the pathogenesis of canine cSCC may be influenced by chronic inflammatory conditions and the treatment thereof. This work highlights the importance of considering cSCC as a differential diagnosis in dogs with such conditions, particularly in middle-aged brachycephalic dogs. Complete excision of canine cSCC appears to be curative, and recurrence rates are low even with suspected incomplete excision. This demonstrates the efficacy of SK surgery alone or in combination with adjunctive therapies for the treatment of cSCC in dogs. AcknowledgmentsThe authors would like to thank Dr Marnie Ford, DACVO for editing the manuscript and for providing photographs of the cases that are included in this publication. FundingThis research received no specific grant and was conducted in the absence of any commercial or financial relationships. Authors’ contributionsHelen Mather: Conceptualization, Methodology, Visualisation, Writing—original draft, Writing—review & editing. Robin G Stanley: Conceptualization, Writing—review & editing. Both authors contributed to the writing of, and have approved the final manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request. ReferencesAlvarez, O.P., Zein, M., Galor, A. and Karp, C.L. 2021. Management of ocular surface squamous neoplasia: Bowman Club Lecture 2021. BMJ Open Ophthalmol. 6(1), e000842. Anderson, D.E. 1963. Effects of pigment on bovine ocular squamous carcinoma. Ann. N. Y. Acad. Sci. 100, 436–446. Arana, A., Pottegård, A., Kuiper, J.G., Booth, H., Reutfors, J., Calingaert, B., Lund, L.C., Crellin, E., Schmitt-Egenolf, M., Kaye, J.A., Gembert, K., Rothman, K.J., Kieler, H., Dedman, D., Houben, E., Gutiérrez, L., Hallas, J. and Perez-Gutthann, S. 2021. Long-term risk of skin cancer and lymphoma in users of topical tacrolimus and pimecrolimus: final results from the extension of the cohort study protopic joint European longitudinal lymphoma and skin cancer evaluation (JOELLE). Clin. Epidemiol. 13, 1141–1153. Australian Radiation Protection and Nuclear Safety Agency. 2024. Ultraviolet radiation model. Available via: https://www.arpansa.gov.au/our-services/monitoring/ultraviolet-radiation-monitoring/uv-index-model (Accessed 20 July 2024). Australian government. Bernays, M.E., Flemming, D. and Peiffer, R.L., Jr. 1999. Primary corneal papilloma and squamous cell carcinoma associated with pigmentary keratitis in four dogs. J. Am. Vet. Med. Assoc. 214, 215–217. Boehm, M.D. and Huang, A.J. 2004. Treatment of recurrent corneal and conjunctival intraepithelial neoplasia with topical interferon alfa 2b. Ophthalmology 111, 1755–1761. Busse, C., Sansom, J., Dubielzig, R.R. and Hayes, A. 2008. Corneal squamous cell carcinoma in a Border Collie. Vet. Ophthalmol. 11, 55–58. Delgado, E.C. 2020. Topical chemotherapy with mitomycin C in a feline corneal squamous cell carcinoma. JFMS Open Rep. 6, 2055116920917833. Dorbandt, D.M., Driskell, E.A. and Hamor, R.E. 2016. Treatment of corneal squamous cell carcinoma using topical 1% 5-fluorouracil as monotherapy. Vet. Ophthalmol. 19, 256–261. Dreyfus, J., Schobert, C.S. and Dubielzig, R.R. 2011. Superficial corneal squamous cell carcinoma occurring in dogs with chronic keratitis. Vet. Ophthalmol. 14, 161–168. Dubielzig, R.R. 2016. Tumors of the eye. In: Tumors in Domestic Animals. Ed., Meuten, D.J. Hoboken, NJ: Wiley, pp: 892–922 Dugan S.J., Curtis C.R., Roberts S.M. and Severin G.A. 1991. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 198, 251–256. Fischer, C.A., Lindley, D.M., Carlton, W.C. and Vank Hecke, H. 2002. Tumors of the cornea and sclera. In: Peiffer RL, Simons KB (eds). Ocular Tumors in Animals and Humans, Ames, IA: Iowa State University Press, pp: 149–202. Galor, A., Karp, C.L., Oellers, P., Kao, A.A., Abdelaziz, A., Feuer, W. and Dubovy, S.R. 2012. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology 119, 1974–1981. Gelatt, K.N., Ben-Shlomo, G., Gilger, B.C., Hendrix, D.V., Kern, T.J. and Plummer, C.E. 2021. Veterinary ophthalmology. Newyork, NY: John Wiley & Sons. Ghaffari, R., Barijani, S., Alivand, A., Latifi, G., Ghassemi, H., Zarei-Ghanavati, M. and Djalilian, A.R. 2021. Recombinant interferon Alpha-2b as primary treatment for ocular surface squamous neoplasia. J. Curr. Ophthalmol. 33, 260–265. Gonzalez-Alonso-Alegre, E.M., Rodriguez-Alvaro, A., Martinez-Nevado, E., Martinez-de-Merlo, E.M. and Sanchez-Maldonado, B. 2013. Conjunctival squamous cell carcinoma in a reindeer (Rangifer tarandus tarandus). Vet. Ophthalmol. 16(Suppl 1), 113–116. Heeney, J.L. and Valli, V.E. 1985. Bovine ocular squamous cell carcinoma: an epidemiological perspective. Can. J. Comp. Med. 49, 21–26. Kafarnik, C., Rawlings, M. and Dubielzig, R.R. 2009. Corneal stromal invasive squamous cell carcinoma: a retrospective morphological description in 10 horses. Vet. Ophthalmol. 12, 6–12. Karasawa, K., Matsuda, H. and Tanaka, A. 2008. Superficial keratectomy and topical mitomycin C as therapy for a corneal squamous cell carcinoma in a dog. J. Small. Anim. Pract. 49, 208–210. Kaswan, R.L., Salisbury, M-A. and Ward, D.A. 1989. Spontaneous canine keratoconjunctivitis sicca: a useful model for human keratoconjunctivitis sicca: treatment with cyclosporine eye drops. Arch. Ophthalmol. 107, 1210–1216. Knickelbein, K.E., Lassaline, M.E. and Bellone, R.R. 2019. Limbal squamous cell carcinoma in a rocky mountain horse: case report and investigation of genetic contribution. Vet. Ophthalmol. 22, 201–205. Knickelbein, K.E., Lassaline, M.E., Singer-Berk, M., Reilly, C.M., Clode, A.B., Famula, T.R., Michau, T.M. and Bellone, R.R. 2020. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for ocular squamous cell carcinoma in Belgian horses. Equine Vet. J. 52, 34–40. Labelle, A.L. and Labelle, P. 2013. Canine ocular neoplasia: a review. Vet. Ophthalmol. 16(Suppl. 1), 3–14. Lam, M., Zhu, J.W., Tadrous, M. and Drucker, A.M. 2021. Association between topical calcineurin inhibitor use and risk of cancer, including lymphoma, keratinocyte carcinoma, and melanoma: a systematic review and meta-analysis. JAMA Dermatol. 157, 549–558. Latimer, K., Kaswan, R. and Sundberg, J. 1987. Corneal squamous cell carcinoma in a dog. J. Am. Vet. Med. Assoc. 190, 1430–1432. Marà, M., Di Guardo, G., Venuti, A., Marruchella, G., Palmieri, C., De Rugeriis, M., Petrizzi, L., Simeone, P., Rizzo, C. and Della Salda, L. 2005. Spontaneous ocular squamous cell carcinoma in twin goats: pathological and biomolecular studies. J. Comp. Pathol. 132, 96–100. María Del Mar, L.M., Aloma, M.F., David, V., Elena, M. and Joaquín, O. 2019. Highly invasive and poorly differentiated corneal squamous cell carcinoma in a dog. BMC Vet. Res. 15, 52. Martabano, B.B., Dow, S., Chow, L., Williams, M.M.V., Mack, M.K., Bellone, R. and Wotman, K.L. 2024. Intralesional interferon alpha-2b as a novel treatment for periocular squamous cell carcinoma in horses. PLoS One 19, e0297366. Martins, B.C. 2021. Food and fibre animal ophthalmology, 6th Edition. In: Vet Ophthalmol. Eds., Gelatt KN, Ben-Shlomo G, Gilger, B.C., Hendrix, D.V., Kern, T.J. and Plummer, C.E. Hoboken, NJ: John Wiley and Sons. Michau, T.M., Davidson, M.G. and Gilger, B.C. 2012. Carbon dioxide laser photoablation adjunctive therapy following superficial lamellar keratectomy and bulbar conjunctivectomy for the treatment of corneolimbal squamous cell carcinoma in horses: a review of 24 cases. Vet. Ophthalmol. 15, 245–253. Montiani-Ferreira, F., Kiupel, M., Muzolon, P. and Truppel, J. 2008. Corneal squamous cell carcinoma in a dog: a case report. Vet. Ophthalmol. 11, 269–272. Munday, J.S., Tucker, R.S., Kiupel, M. and Harvey, C.J. 2015. Multiple oral carcinomas associated with a novel papillomavirus in a dog. J. Vet. Diagn. Invest. 27, 221–225. Nevile, J.C., Hurn, S.D., Turner, A.G. and McCowan, C. 2015. Management of canine corneal squamous cell carcinoma with lamellar keratectomy and strontium 90 plesiotherapy: 3 cases. Vet. Ophthalmol. 18, 254–260. Overton, T.L., Allbaugh, R.A., Whitley, D., Ben-Shlomo, G., Griggs, A., Tofflemire, K.L. and Whitley, E.M. 2015. A pulse-dose topical 1% 5-fluorouracil treatment regimen in a young dog with corneal squamous cell carcinoma. Vet. Ophthalmol. 18, 350–354. Paul, C.F., Ho, V.C., McGeown, C., Christophers, E., Schmidtmann, B., Guillaume, J.C., Lamarque, V. and Dubertret, L. 2003. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J. Invest. Dermatol. 120, 211–216. Peiffer, R.L. Jr, Gwin, R.M., Gelatt, K.N., Jackson, W.F., Williams, L.W. and Hill, C.W. 1978. Ciliary body epithelial tumors in four dogs. J. Am. Vet. Med. Assoc. 172(5), 578–583. Plummer, C.E., Smith, S., Andrew, S.E., Lassaline, M.E., Gelatt, K.N., Brooks, D.E., Kallberg, M.E. and Ollivier, F.J. 2007. Combined keratectomy, strontium-90 irradiation and permanent bulbar conjunctival grafts for corneolimbal squamous cell carcinomas in horses (1990–2002): 38 horses. Vet. Ophthalmol. 10, 37–42. Rodríguez-Perálvarez, M., Colmenero, J., González,. A., Gastaca, M., Curell, A., Caballero-Marcos, A., Sánchez-Martínez, A., Di Maira, T., Herrero, J.I., Almohalla, C., Lorente, S., Cuadrado-Lavín, A., Pascual, S., López-Garrido, M., González-Grande, R., Gómez-Orellana, A., Alejandre, R., Zamora-Olaya, J. and Bernal-Bellido, C. 2022. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am. J. Transplant. 22, 1671–1682. Schechter, B.A., Schrier, A., Nagler, R.S., Smith, E.F. and Velasquez, G.E. 2002. Regression of presumed primary conjunctival and corneal intraepithelial neoplasia with topical interferon alpha-2b. Cornea. 21, 6–11. Schoster, J. 1992. Using combined excision and cryotherapy to treat limbal squamous cell carcinoma. Vet. Med. 87, 357–365. Sebbag, L. and Sanchez, R.F. 2023. The pandemic of ocular surface disease in brachycephalic dogs: The brachycephalic ocular syndrome. Vet. Ophthalmol. 26, 31–46. Sironi, G., Riccaboni, P., Mertel, L., Cammarata, G. and Brooks, D.E. 1999. p53 protein expression in conjunctival squamous cell carcinomas of domestic animals. Vet. Ophthalmol. 2, 227–231. Takiyama, N., Terasaki, E. and Uechi, M. 2010. Corneal squamous cell carcinoma in two dogs. Vet. Ophthalmol. 13, 266–269. Teifke, J.P. and Löhr, C.V. 1996. Immunohistochemical detection of P53 overexpression in paraffin wax-embedded squamous cell carcinomas of cattle, horses, cats and dogs. J. Comp. Pathol. 114, 205–210. Tsujita, H. and Plummer, C.E. 2010. Bovine ocular squamous cell carcinoma. Vet. Clin. North Am. Food Anim. Pract. 26. 511–529. Valentine, B.A. and Martin, J.M. 2007. Prevalence of neoplasia in llamas and alpacas (Oregon State University, 2001–2006). J. Vet. Diagn. Invest. 19. 202–204. Ward, D.A., Latimer, K.S. and Askren, R.M. 1992. Squamous cell carcinoma of the corneoscleral limbus in a dog. J. Am. Vet. Med. Assoc. 200, 1503–1506. Wilcock, B.P. 1993. Eye and ear. In: Pathology of domestic animals. Ed., Maxiem M. New York, NY: Elsevier. Wilson, M.W., Czechonska, G., Finger, P.T., Rausen, A., Hooper, M.E. and Haik, B.G. 2001. Chemotherapy for eye cancer. Surv. Ophthalmol. 45, 416–444. Yan, J., Qiu, H. and Li, Y. 2011. Clinicopathological analysis of 39 patients with corneal tumor. Eye Sci. 26, 148–153. Yeoh, C.H.Y., Lee, J.J.R., Lim, B.X.H., Sundar, G., Mehta, J.S., Chan, A.S.Y., Lim, D.K.A, Watson, S.L., Honavar, S.G., Manotosh, R. and Lim, C.H.L. 2022. The management of ocular surface squamous neoplasia (OSSN). Int. J. Mol. Sci. 24(1), 713. | ||
| How to Cite this Article |
| Pubmed Style Mather H, Stanley RG. Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Vet. J.. 2024; 14(11): 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 Web Style Mather H, Stanley RG. Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. https://www.openveterinaryjournal.com/?mno=218278 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.35 AMA (American Medical Association) Style Mather H, Stanley RG. Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Vet. J.. 2024; 14(11): 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 Vancouver/ICMJE Style Mather H, Stanley RG. Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 Harvard Style Mather, H. & Stanley, . R. G. (2024) Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Vet. J., 14 (11), 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 Turabian Style Mather, Helen, and Robin Grant Stanley. 2024. Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Veterinary Journal, 14 (11), 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 Chicago Style Mather, Helen, and Robin Grant Stanley. "Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery." Open Veterinary Journal 14 (2024), 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 MLA (The Modern Language Association) Style Mather, Helen, and Robin Grant Stanley. "Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery." Open Veterinary Journal 14.11 (2024), 3063-3073. Print. doi:10.5455/OVJ.2024.v14.i11.35 APA (American Psychological Association) Style Mather, H. & Stanley, . R. G. (2024) Recurrence rate of corneal squamous cell carcinoma in dogs undergoing superficial keratectomy surgery. Open Veterinary Journal, 14 (11), 3063-3073. doi:10.5455/OVJ.2024.v14.i11.35 |