| Research Article | ||
Open Vet. J.. 2024; 14(11): 3037-3046 Open Veterinary Journal, (2024), Vol. 14(11): 3037-3046 Research Article Etiological and histomorphological studies on early chick mortality in broiler chicken in Kashmir, IndiaPradeep Kumar Yadav1, Showkat Ahmad Shah1*, Majid Shafi1, Shayaib Ahmad Kamil1, Masood Saleem Mir2, Mudasir Ali Rather3, Ajaz Ahmad Ganaie4 and Zahoor Ahmad Wani51Veterinary Pathology, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Srinagar, India 2Associate Director Research, Directorate of Research, SKUAST-K, Srinagar, India 3Veterinary Public Health, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Srinagar, India 4SMS, KVK, Pulwama, India 5Veterinary Parasitology, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Srinagar, India *Corresponding Author: Showkat Ahmad Shah. Veterinary Pathology, Faculty of Veterinary Sciences and Animal Husbandry, SKUAST-K, Srinagar, India. Email: vetshowkat [at] skuastkashmir.ac.in Submitted: 03/09/2024 Accepted: 19/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
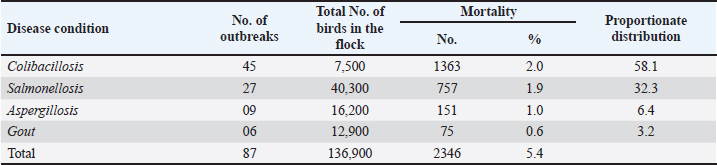
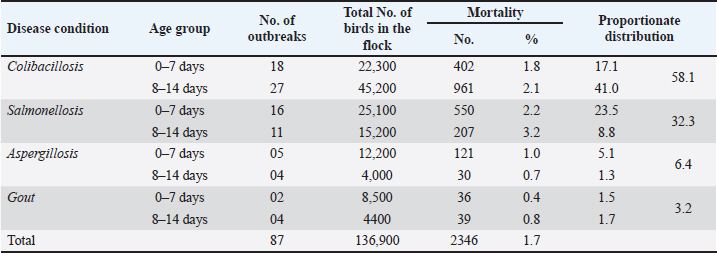
AbstractBackground: Early chick mortality (ECM) is one of the most important problems of the poultry industry that causes severe economic losses to the farmers. The chick mortality varies in different geographical locations and its etiological factor also varies. Aim: The aim of the present work was to isolate and identify various etiological agents responsible for causing ECM in broilers, and study the overall occurrence and pathology of various disease conditions responsible for causing ECM in broilers. Methods: The study included clinical and laboratory investigations vis-a-vis ECM. A total of 2,346 broiler chickens under the age group of 2 weeks from around 87 outbreaks were necropsied and examined for the presence of lesions corresponding to different disease conditions. Representative samples (heart, liver, intestine, lung, and spleen) were collected in a sterile Petri dish for bacterial and fungal isolation and stored at 4°C till inoculation in nutrient broth, followed by a collection of tissue samples (heart, liver, lung, spleen, intestines, and kidney) in 10% buffered formalin for histopathological examination. Results: The overall mortality in the flocks was 1.7%, with 1.6% mortality in the first week and 1.8% in the second week of life. Colibacillosis was responsible for causing the highest ECM of 2.01% followed by salmonellosis (1.9%), aspergillosis (0.9%), and gout (0.6%). The case prevalence of colibacillosis, salmonellosis, aspergillosis, and gout was seen as 58.1%, 32.3%, 6.4%, and 3.2%, respectively. Colibacillosis (2.1%), salmonellosis (3.2%), and gout (1.7%) were responsible for causing higher mortality (3.2%) in the second week of life of broilers, aspergillosis in the first week (1.0%) and gout caused similar percentage of mortalities in the first and second week of life. Microscopic changes were predominately characterized by congestion, hemorrhage, infiltration by various inflammatory cells especially heterophils, the focal granulomatous reaction in aspergillosis, and urates in the tubular parenchyma in the form of pink amorphous radiating material surrounded by a narrow zone of inflammatory cells in cases of visceral gout. Conclusion: Salmonellosis, colibacillosis, aspergillosis, and gout were diseases mainly responsible for ECM in broilers. Colibacillosis was responsible for causing the highest ECM followed by aspergillosis, salmonellosis, and gout. Keywords: Early chick mortality, Colibacillosis, Salmonellosis, Aspergillosis, Broilers. IntroductionChick mortality presents a significant economic challenge for poultry farmers and is a major concern. Early chick mortality (ECM) can be attributed to various factors, some of which originate in the hatchery where the chicks are hatched before being transported to their respective farms. Diseases are a primary cause of mortality in young chicks, often linked to the source of the day-old chicks (Appiah, 2018). Records of mortality during the first 7 days of brooding have been used to assess the quality of chicks in the broiler industry. Apart from diseases, mortality in day-old chicks can also be associated with poor management, inadequate brooding temperatures, and heat stress (Chou et al., 2004). First-week mortalities can account for up to 50% of total flock losses (Yassin et al., 2009; Olsen et al., 2012). Early mortality rates are often indicative of the overall performance of the flock, leading to contracts between hatcheries and broiler farmers that frequently include an adjusted cost per chick based on their performance during the first week (Yassin et al., 2009). Chick survival during the initial period has been reported to be linked to the broiler breeder farm and hatcheries, with particular emphasis on the management of the breeder flock. (Renema et al., 2008; Olsen et al. 2012). Bacterial infections, primarily Escherichia coli, were responsible for approximately 50% of layer flock mortalities during the first week. (Olsen et al., 2012). Omphalitis and/or yolk sac infections, sometimes accompanied by septicemia, were reported. These infections may originate from breeder hens with salpingitis, where the yolk sac becomes infected in ovo, or from contamination in the hatchery environment (Vandekerchove et al., 2004; Mokady et al., 2005). Over the past 40 years, the market age of broiler chickens has decreased by roughly 1 day per year, a trend that continues and underscores the importance of growth during the first week of life, which now represents 16% of the broiler’s lifespan (Gyles, 1989). Newly hatched chicks are highly susceptible to infection during the first week post-hatch due to their lack of established gastrointestinal flora, making early exposure to opportunistic pathogens particularly dangerous. Consequently, the first few days of life are critical for broiler chicks (Henderson et al., 1999). The poultry sector has great importance in Kashmir at the economic and health levels. Economically, many farmers depend on this sector as one of the main sources of their income. In J and K, chick mortality during the early weeks poses a great economic challenge to poultry farmers and is a matter of great concern. The chick mortality varies in different geographical locations and its etiological factor also varies. The present study was therefore aimed to isolate and identify various etiological agents responsible for causing ECM in broilers and study the overall occurrence and pathology of various disease conditions responsible for causing ECM in broilers. Materials and MethodsA total of 87 outbreaks of ECM that occurred in central districts (Srinagar, Budgam, and Ganderbal) were screened. The outbreaks suspected of various infectious and non-infectious disease conditions responsible for causing ECM in broiler chicken were identified based on the history, clinical signs, and lesions, after following a thorough post-mortem examination of birds. The history of each suspected flock also included flock size, mortality, and total number of birds per outbreak. Representative samples (heart, liver, intestine, lung, and spleen) from 2,346 dead birds were collected in a sterile Petri dish for bacterial and fungal isolation and stored at 4°C till inoculation in nutrient broth, followed by a collection of tissue samples (heart, liver, lung, spleen, intestines, and kidney) in 10% buffered formalin for histopathological examination. The occurrence of ECM was evaluated by considering total flock strength and the number of mortalities occurring in the first 2 weeks of life. Isolation and identificationE. coli Representative samples from intestines were inoculated into nutrient broth and incubated at 37°C for 24 hours. The bacterial growth in the nutrient broth was re-inoculated on MacConkey agar plates (HI Media, Mumbai, India), and the plates were incubated at 37°C for 24 hours. The lactose fermenting colonies on MacConkey plates were re-inoculated on Eosin Methylene Blue agar (HI Media, Mumbai, India). The Escherichia coli colonies typically showing metallic sheen were transferred to the nutrient agar slants and stored at 4°C for further characterization. Identification of isolates was further carried out using standard morphological and biochemical tests including Grams staining and IMViC tests and characterization of Enterobacteriaceae. SalmonellaThe representative samples were initially inoculated into Tetrathionate broth; after overnight incubation, the cultures were allowed to grow on Brilliant green agar (BGA). Salmonella produced pink, small circular, and smooth colonies on BGA. From the pure culture of BGA, Gram’s staining was performed to observe Salmonella organism. Under the compound light microscope, the organism was identified as Gram-negative, rod-shaped organisms. The pink colonies that were identified as Gram-negative rods were characterized for Salmonella by inoculating on Xylose lysine desoxycholate agar (XLDA) plates. On XLDA, the Salmonella isolates produced red colonies with black centers. Identification of isolates was further carried out using standard morphological and biochemical tests including Grams staining and IMViC tests. The pure cultures from selective media plates were characterized biochemically and gave the following results typical for Enterobacteriaceae family. Aspergillus spp.The suspected samples were impressed directly on Sabouraud dextrose agar supplemented with chloramphenicol (0.05 mg/ml). Cultures were incubated aerobically at 25°C and 37°C for 5–7 days. Whitish-grey colonies revealed Aspergillus fumigatus. Wet mount slides were prepared using lacto phenol blue staining method for identification on a morphological basis. Duplicate histopathological sections from selected cases were stained using PAS-Alcian blue staining techniques for the demonstration of fungi (Luna, 1968). Non-infectious cases: The cases of visceral gout were screened and preliminarily diagnosed on the basis of gross lesions. Representative tissue samples fixed in 10% NBF were processed by paraffin embedding technique for microtomy and 4–5 µm thick sections were prepared and stained by routine Haematoxylin and Eosin (HandE) for detailed histopathological studies as described by Luna (1968). Parallel sections from tissue samples fixed in absolute alcohol were also stained by De Galantha method for gout. Pathoanatomical studiesThe carcasses were subjected to systematic necropsy examination. Various visceral organs were thoroughly examined for gross lesions true to colibacillosis, salmonellosis, aspergillosis, and gout-causing ECM. The organs were examined for changes in color, consistency, size, and type of lesions. Tissue samples of various organs were collected in 10% buffered formalin for histopathological examination and processed by routine paraffin embedding technique. The sections were stained with Harri’s hematoxylin and eosin technique for routine examination, and Alcian Blue PAS technique for demonstration of neutral and acidic mucopolysaccharides (Luna, 1968). For the demonstration of urates in the tissue sections, de Galantha stain was used. Special stain PAS-Alcian Blue was used for the demonstration of fungus in the tissue section. (Luna, 1968). ResultsIn the present study, a total of 2,346 broiler chickens under the age group of 2 weeks from around 87 outbreaks were necropsied and examined for the presence of lesions corresponding to different disease conditions. Salmonellosis, Colibacillosis, Aspergillosis, and gout were the diseases diagnosed on the basis of history, clinical signs, gross pathognomonic lesions, and isolation of specific pathogens from the organs of affected dead birds. Occurrence of ECM among commercial broiler chickenThe overall occurrence of disease affections and the age-wise distribution of diseases up to 2 weeks of age among commercial broiler chicken is shown in Tables 1 and 2. A total of 2,346 broiler chickens under the age group of 2 weeks from around 87 outbreaks were necropsied and examined for the presence of lesions corresponding to different disease conditions. Salmonellosis, Colibacillosis, Aspergillosis, and gout were the diseases diagnosed on the basis of history, clinical signs, gross pathognomonic lesions, and isolation of specific pathogens from the organs of affected dead birds. The overall occurrence of disease affections and the age-wise distribution of diseases up to 2 weeks of age among commercial broiler chicken is shown in Tables 1 and 2. Colibacillosis was seen as the main contributor of mortality (58.1%) in the broilers chicks followed by salmonellosis (32.3%), aspergillosis (6.4%), and visceral gout (3.2%). Prevalence of salmonella in chicks was recorded as 32.3% with 23.5% in the age groups of 0–7 days and 8.8% in 8–14 days. Aspergillosis was reported with a prevalence rate of 6.4% in the age groups of 0–7 days and 1.3% in the age group of 8–14 days. Gout revealed a case prevalence of 3.2% in chicks up to 2 weeks of age. The proportionate mortality due to gout was higher at 1.7% in the age groups of 8–14 days followed by 1.5% in the age groups of 0–7 days. Table 1. Overall mortality and prevalence of various disease conditions causing ECM among broiler chicken.
Table 2. Age group wise mortality and prevalence of various disease conditions causing ECM among broiler chicken.
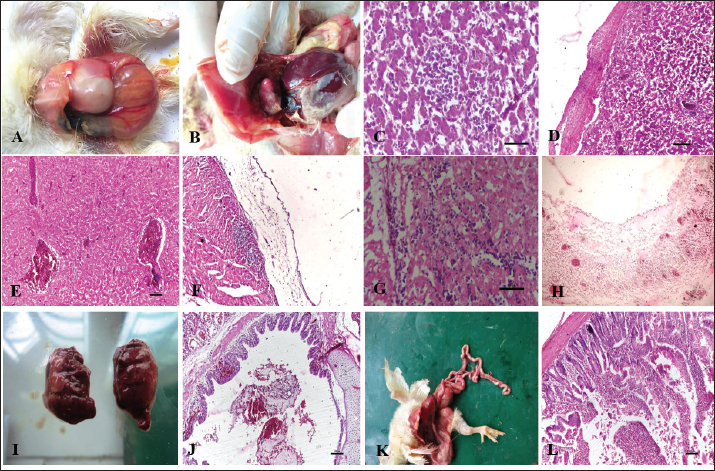
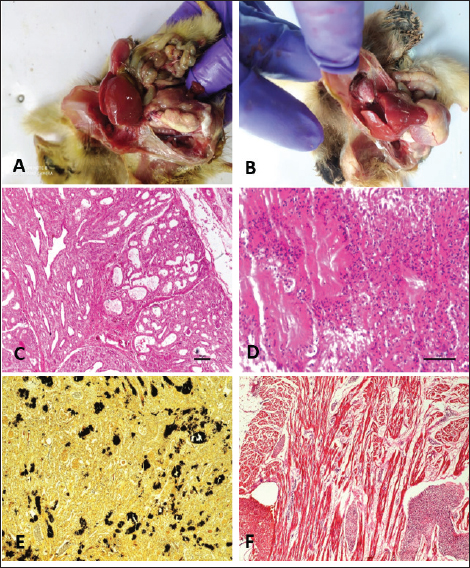
Gross and histopathologyColibacillosis: Chicks that had died of colibacillosis revealed a distended yolk sac resulting in a pendulous abdomen, congestion of the superficial blood vessels, and inflammation of the sac (Fig. 1A). The contents of the sac were thick and inspissated in severe cases. On microscopic examination, the yolk sac revealed marked vascular congestion and leucocyte infiltration. The liver was typically covered by a layer of fibrin seen adherent to most parts of the liver (Fig. 1B). Histopathological examination of the liver revealed cellular swelling, individualization of hepatocytes along with distortion of hepatic cords, focal areas of cellular infiltration (Fig. 1C) and thickened capsule with presence of large amount of fibrin and heterophils (Fig. 1D). Liver sections also revealed severe vascular and sinusoidal congestion (Fig. 1E). Gross examination of heart revealed adherent fibrin layer giving a typical “bread and butter” appearance. Microscopically, thickened pericardium with fibrinous exudate and heterophilic infiltration (Fig. 1F), congestion and hemorrhages in the myocardium, and degeneration of myocardium with severe leucocyte infiltration were predominant lesions seen in the heart (Fig. 1G). Air sacs were cloudy and covered with thin to thick layers of fibrin depending on the severity of the infection with caseous exudate on the surface in advanced stages. Histopathologically they revealed a thickened air sac membrane with leucocytic infiltration consisting predominantly of heterophils (Fig. 1H). Grossly, the lungs revealed mild congestion, oedema, and consolidation in severe cases (Fig. 1I). Microscopic changes in the lungs were characterized by acute bronchopneumonic changes with the presence of exudates in bronchioles and parabronchi along with infiltration of leucocytes predominantly heterophils (Fig. 1J). In all cases of Colibacillosis, congestion of the intestines was a constant finding and it ranged from mild to severe depending upon the nature of the outbreak (Fig. 1K). Histopathologically, congestion of blood vessels, goblet cell hyperplasia, mild to moderate infiltration of heterophils in lamina propria of intestinal villi, degeneration, necrosis, and desquamation of lining epithelium were frequently observed in intestines (Fig. 1K).
Fig. 1. Gross and Histopathological changes in chicks affected with colibacillosis. A: Pendulous abdomen resulting from distended yolk sac. B: Thin fibrin layer attached to liver and heart. C: Cellular swelling, individualization of hepatocytes along with distortion of hepatic cords. (H.E) D: Thick fibrin layer attached to mildly thickened liver capsule along with infiltration by heterophils. (H.E.) E: Liver section showing severe vascular and sinusoidal congestion. (H.E) F: Section of heart from colibacillosis affected chicken revealing thickened pericardium along with infiltration by heterophils. (H.E.) G: Section of heart from colibacillosis affected chick revealing myocardial degeneration along with infiltration in myocardium. (H.E.) H: Section of air sac revealing necrosis, edema and infiltration. (H.E) I: Lungs from colibacillosis affected birds revealing varying degrees of congestion. J: Section of Lung revealing presence of hemorrhagic exudate in primary bronchus. (H.E) K: Broiler chick affected with colibacillosis revealing congestion of intestines. L: Section of intestine revealing degeneration and desquamation of villus epithelium and heterophils infiltration. (H.E). Salmonellosis: Grossly bronze discoloration of the liver was noticed with numerous necrotic greyish patches and reddish hemorrhagic foci distributed uniformly on the surface (Fig. 2A). Microscopic lesions in liver included diffuse coagulative necrosis of hepatocytes and focal area of heterophil infiltration (Fig. 2B). In some cases, isolated foci of necrosis with infiltration of leucocytes mostly in portal triads and perivascular areas was noticed (Fig. 2C). Grossly in heart, elevated greyish white nodular areas were observed on the myocardium more predominant on the ventricular region (Fig. 2D). Microscopic lesions in heart included thickening of pericardium (Fig. 2E) and necrotic focal areas in myocardium with infiltration of mononuclear cells (Fig. 2F). Grossly, the intestines appeared congested with hemorrhages mostly on the mucosal surface. In severe cases, swollen and foam-filled caeca was seen. (Fig. 2G) Microscopically, mucosal congestion, degeneration, and desquamation of lining epithelium and mild to moderate infiltration of heterophils and mononuclear cells in the lamina propria of the villi were noticed (Fig. 2H, I).
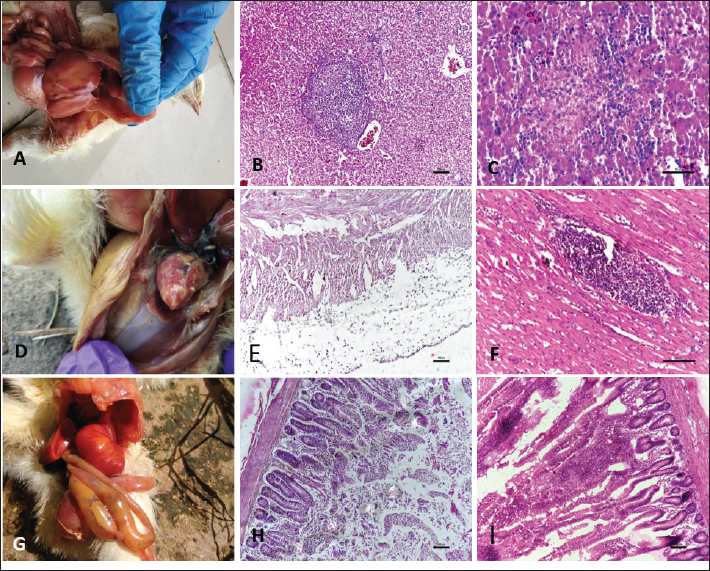
Fig. 2. Gross and Histopathological changes in chicks affected with Salmonellosis. A: Bronze discoloration of liver. B: Diffuse coagulative necrosis of hepatocytes and focal area of heterophil infiltration. (H.E) C: Focal necrosis of hepatocytes along with heterophil infiltration (H.E) D: Broiler chick affected with salmonella revealing elevated greyish nodules on heart E: Section of heart revealing thickened pericardium along with infiltration by heterophils. (H.E) F: Focal infiltration of myocardium by degenerated heterophils. (H.E.) G: Broiler chick affected with salmonella revealing swollen and foam filled caeca. H: Section of intestine from salmonella affected chick revealing degeneration and desquamation of lining epithelium. (H.E) I: Fusion of villi and infiltration in lamina propria. (H.E).
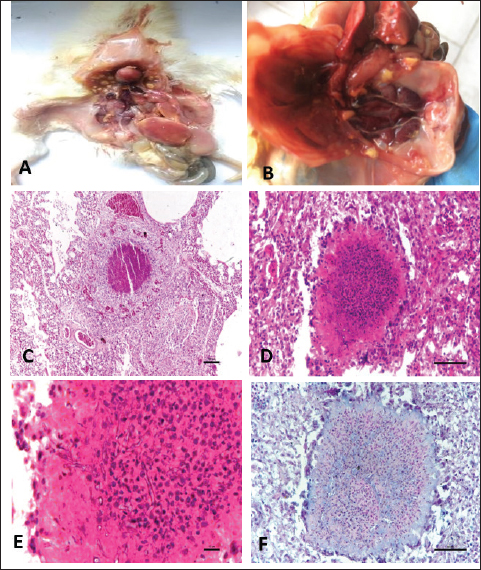
Fig. 3. Gross and Histopathological changes in chicks affected with Aspergillosis. A: Chick affected with aspergillosis revealing white caseous nodules scattered throughout lungs. B: Chick affected with aspergillosis revealing white caseous nodules scattered on abdominal serosa C: Focal granuloma development in the parabronchi. Also note the heterophil infiltration around the necrotic area. (H.E) D: Section of lung from aspergillosis affected chick revealing focal granuloma consisting of central necrotic area mixed with heterophils and surrounded by giant cells. Also note the fungal hyphae in the central necrotic area. (H.E) E: Central necrotic area revealing presence of degenerated heterophils and fungal hyphae more clearly. (H.E) F: Revealing presence of fungal hyphae in the central necrotic area. (PAS). Aspergillosis: Lung and the air sacs were seen mostly affected by the fungus with small white caseous nodules observed scattered throughout the air sacs and lungs (Fig. 3A, B). Histopathologically, focal granulomatous reaction was frequently observed in the lungs. The granulomas were characterized by a central necrotic area with infiltration of heterophils, macrophages, epithelioid cells, and giant cell formation. (Fig. 3C, D, E, and F). Gout: Gout resulted in severe gross lesions in the kidneys marked by paleness of both the kidneys and the appearance of ureters filled with urates (Fig. 4A). Heart was the second most affected organ affected with urate deposition mainly on the pericardium giving a plaster-like appearance (Fig. 4B). Microscopic lesions in kidneys were characterized by focal to diffuse cystic dilation of renal tubule, intertubular hemorrhages, distortion of tubular parenchyma with desquamation of tubular epithelium (Fig. 4C), tubular degeneration, deposits of urates in the tubular parenchyma in the form of pink amorphous radiating material surrounded by a narrow zone of inflammatory cells and occasional infiltration of heterophils and mononuclear cells. (Fig. 4D). The urate deposits gave a positive reaction with De-Galantha stain and appeared as black needle-shaped crystals (Fig. 4E). Lesions in the heart were mainly restricted to pericardium consisting of variable degrees of congestion, hemorrhages, oedema, and thick masses of urate deposition as homogeneous pinkish amorphous material (Fig. 4F). Lesions in the liver were characterized by focal to diffuse areas of coagulative necrosis and urate crystal deposition in hepatic parenchyma.
Fig. 4. Gross and Histopathological changes in chicks affected with Visceral gout. A: Chick affected with systemic gout revealing white chalky Urate deposits on kidneys. B: Chick affected with systemic gout revealing white chalky Urate deposits on heart. C: Section of kidney from gout affected chick revealing cystic dilatation of renal tubules (H.E) D: Section of kidney from gout affected chick revealing urate crystal deposition as pink radiating amorphous material surrounded by a narrow zone of inflammatory cells more clearly. (H.E) E: Section of kidney from gout affected chick revealing black coloured urate crystals. (De Galantha’s Stain) F: Section of heart from gout affected chick revealing degenerated myocytes, leucocytic infiltration and mild urate deposition. (H.E). DiscussionEarly chick mortality in chicks is considered as one of the major problems of the poultry industry caused by various disease conditions like Omphalitis, colibacillosis, enteritis, salmonellosis, aspergillosis, visceral gout, and some unidentified miscellaneous conditions. In the present study, colibacillosis was seen as the main contributor of mortality (58.1%) in the broiler chicks followed by salmonellosis (32.3%), aspergillosis (6.4%) and visceral gout (3.2%). The results strengthen the fact that colibacillosis is one of the principal causes of morbidity and mortality in the poultry industry. Earlier studies also reported colibacillosis as the predominant disease causing mortality in young chicks (Amin et al., 2017; Amin et al., 2020; Shah et al., 2020; Wani et al., 2020). Prevalence of salmonella in chicks was recorded as 32.3% with 23.5% in the age groups of 0–7 days and 8.8% in 8–14 days. The reasons for the higher prevalence of salmonellosis in chicks is because of immature immune system, weaker gut flora, rapid disease progression, higher susceptibility to environmental stress, and the vertical transmission of the disease. Similar findings were also reported by Alam et al. (2003) and Sikder et al. (2005) who observed a 23.8% and 23.5% prevalence of salmonellosis in chicks, respectively. However, the lower prevalence rate of 5.88% of Salmonella was reported by Jinu et al. (2014) from chicks in Bareilly, Uttar Pradesh. This difference is due to geographical variation. Aspergillosis was reported with a prevalence rate of 6.4% in the age groups of 0–7 days and 1.3% in the age group of 8–14 days. The reasons for the higher prevalence of aspergillosis in young chicks could be because of an underdeveloped immune system, smaller respiratory tract, weaker physical barriers, exposure to contaminated environments and inadequate thermoregulation. These findings are in concordance with the previous reports (Malay et al., 2005; Sultana et al., 2015). Gout revealed a case prevalence of 3.2% in chicks up to 2 weeks of age. The proportionate mortality due to gout was higher at 1.7% in the age groups of 8–14 days followed by 1.5% in the age groups of 0–7 days. The findings of our study are in accordance with the observations of Ali et al. (2018) and Yadav et al. (2020) who also reported similar mortalities by gout in broiler chicks. Grossly in cases of colibacillosis, yolk sac was distended resulting in pendulous abdomen, superficial blood vessels of the sac were congested; livers were typically covered by a thin layer of fibrin with some of the birds revealing thick fibrin layer adhered with liver parenchyma and adjacent thoracic cavity giving a characteristic bread and butter appearance to the liver. Histopathological examination of the liver revealed cellular swelling, individualization of hepatocytes along with distortion of hepatic cords, focal areas of cellular infiltration, and thickened capsules with the presence of a large amount of fibrin and heterophils. The gross and histopathological findings in the liver of the cases of colibacillosis were in concordance with the earlier reports (Kumari et al., 2013; Abalaka et al. 2017; Shah et al., 2019). Similarly, the gross and histopathological findings in the heart, intestines, and lungs are in agreement with the earlier studies (Yaqub et al., 2023; Shafi et al., 2024). In the cases of salmonellosis, grossly bronze discoloration of the liver, elevated greyish-white nodules on heart, congested and hemorrhagic intestines were noticed. Histopathological findings were mostly dominated by necrosis, mononuclear cell infiltration, and desquamation of lining epithelium. Similar findings were also reported by Nazir et al. (2012); Kumari et al. (2013); Yadav et al. (2020); Kashani et al. (2021) and Janwari et al. (2022). Aspergillosis resulted in the development of small white nodules on lungs and air sacs which were microscopically characterized by a central necrotic area with infiltration of heterophils, macrophages, epithelioid cells, and giant cell formation. Similar findings were also reported in cases of aspergillosis earlier (Olias et al., 2010; Sultana et al., 2015; Shoukat et al., 2018). Visceral gout resulted in the deposition of chalky white material on kidneys, heart, and other organs which was microscopically characterized by pink amorphous radiating material surrounded by a narrow zone of inflammatory cells and occasional infiltration of heterophils and mononuclear cells. Similar gross and histopathological findings were reported earlier (Ali et al., 2018; Namratha et al., 2019; Yadav et al., 2020; Rafiq et al., 2024). ConclusionIn conclusion, the main contributor to mortality in chicks was colibacillosis followed by salmonellosis, aspergillosis, and gout with overall mortality in the flocks at 1.7%, with 1.6% in the first week and 1.8% in the second week of life. AcknowledgmentThe authors are thankful to the Vice Chancellor and Director Education, SKUAST. Kashmir for providing the necessary facilities for the smooth conduct of this research. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. FundingThe authors declare that no funds, grants, or other support were received for this study and during the preparation of this manuscript. Authors’ contributionsPK Yadav conducted research. S A Shah contributed to conceptualization, methodology, supervision, and writing original draft. M Shafi, S A Kamil, and M S Mir contributed in Histopathological evaluation. M A Rather contributed in the isolation and identification of bacteria. A B Beigh and Z A Wani contributed to data curation and editing. All authors have read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAbalaka, S.E., Sani, N.A., Idoko, I.S., Tenuche, O.Z., Oyelowo, F.O., Ejeh, S.A. and Enem, S.I. 2017. Pathological changes associated with an outbreak of colibacillosis in a commercial broiler flock. Sokoto J. Vet. Sci. 15(3), 95–102. Alam, J., Koike, I., Giasuddin, M. and Rahman, M. 2003. Seroprevalence of poultry diseases in native chickens in Bangladesh. 9th BSVER Annual Scientific Conference, BSVER Publication. No. 24.p. 26. 2. Bangladesh Agricultural University in Mymensingh. Ali R., Kamil, S.A., Amin U., Mir M.S., Shah A., Kashani B., Dar T.A, Shah S.A. and Qureshi S. 2018. Clinicopathology of sodium bicarbonate induced gout in broiler chicken model. Indian J. Vet. Pathol .42(1), 58–63. Amin U., Kamil. S.A.,Wani B.M., Qureshi S., Shah S.A., Dar T.A., Adil S. and Mir M.S. 2020. Haematological and biochemical alterations of broiler chicken affected naturally with colibacillosis. Int. J. Curr. Microbiol. App. Sci. 9(6), 1906–1913. Amin, U., Kamil, S.A., Shah, S.A., Dat, T.A., Mir, M.S., Ali, R., Kashoo, Z.A. and Wani, B.M. 2017. Serotyping and prevalence of avian pathogenic Escherichia coli infection in broilers in Kashmir. Pharma Innov. J. 6(10), 336–338. Appiah, M.O. 2018. An investigation of factors that may influence the occurrence of early chick mortality on some farms in Ghana. Int. J. Anim. Sci. 2(5), 1036. Chou, C.C., Jiang, D.D. and Hung, Y.P. 2004. Risk factors for cumulative mortality in broiler chicken flocks in the first week of life in Taiwan. Br. Poult. Sci. 45, 573–577. Gyles, N.R. 1989. Poultry, people and progress. Poult. Sci. 68, 1–8. Henderson, S.C., Bounous, D.I. and Lee, M.D. 1999. Early events in the pathogenesis of avian Salmonellosis. Infect. Immun. 67, 3580–3586. Janwari A.Q., Mir M.S., Kamil S.A, Kawoosa M.S., Shah S.A., Nabi S., Rather M.A., Kashoo, Z.A. and Farooq, S. 2022. Comparative susceptibility of commercial broiler and Vanraja chicken to Salmonella enterica subsp. enterica serovars Gallinarum infection with ameliorative effect of Allium sativum (Garlic). J. Pharmacogn. Phytochem. 11(1), 204-–207. Jinu, M., Agarwal, R.K., Sailo, B., Wani, M.A., Kumar, A., Dhama, K. and Singh, M.K. 2014. Comparison of PCR and conventional cultural method for detection of Salmonella from poultry blood and faeces. Asian J. Anim. Vet. Adv.9, 690–701. Kashani B., Kamil S.A., Beigh A.B., Shah S.A., Wani B.M, Kawoosa M.S., Maqbool M. and Yadav P.K. 2021. Isolation, identification and molecular characterization of Salmonella enterica subsp. enterica serovar Gallinarum from poultry farms of Central Kashmir, North India. Indian J. Vet. Pathol. 45(1), 1–6. Kumari, D., Mishra, S.K. and Lather, D. 2013. Pathomicrobial studies on Salmonella Gallinarum infection in broiler chickens, Vet. World. 6(10), 725–729. Luna, L.G. 1968. Manual of histologic staining method of armed forces institute of pathology. 3rd ed. New York, NY: Mc Graw Hill Book Company. Malay, M., Das S.C., Basak, D.K., Chatterjee, A. and Sikdar, A. 2005. Outbreaks of aspergillosis in broiler chicks. Indian J. Poult. Sci. 40(3), 308–310. Mokady, D., Gophna, U. and Ron, E.Z. 2005. Extensive gene diversity in septicaemia Escherichia coli strains. J. Clin. Microbiol. 43, 66–73. Namratha, L., Kumar, Y.R. and Lakshman, M. 2019. Pathology of visceral gout in layer chicken. Int. J. Recent Sci. Res. 10(10), 35546–35548. Nazir, S., Kamil, S.A., Darzi, M.M., Mir, M.M., Khan, F.A. and Amare, A. 2012. Pathology of spontaneously occurring salmonellosis in commercial broiler chickens of kashmir valley. J. World Poult. Res. 2(4), 63–69. Olias, P., Hauck, R., Windhaus, H., van der Grinten, E., Gruber, A.D. and Hafez, H.M. 2010. Articular aspergillosis of hip joints in turkeys. Avian Dis. 54, 98–101. Olsen, R.H., Frantzen, C., Christensen, H. and Bisgaard, M. 2012. An investigation on first-week mortality in layers. Avian Dis. 56(1), 51–57. Rafiq M., Goswami P., Yaqub M., Shafi M., Kamil S. A. and Shah S.A. 2024. Nephropathy associated with bacterial diseases in broiler chicken in Kashmir Valley. Indian J. Vet. Pathol. 48(2), 169–175. Renema, R.A., Sikur, V.R., Robinson, F.E., Korver, D.R. and Zuidhof, M.J. 2008. Effects of nutrient density and age at photostimulation on carcass traits and reproductive efficiency in fast- and slow-feathering turkey hens. Poult. Sci. 87, 1897–1908. Shafi M., Shabir S., Rather M.A., Baba O.K., Maqbool B., Nadeem M., Ganaie A.A., Mir M.S., Kamil S.A. and Shah S. A. 2024. Exploring the pathological hallmarks of naturally occurring colibacillosis in broiler chickens reared in North Kashmir. Int. J. Adv. Biochem. Res. 8(2), 229–243. Shah, S.A.,Mir M.S., Kamil, S.A., Shafi, M., Adil, S., Wani, B.M., Goswami, P. and Rather, M.A. 2020. Prevalence and isolation of avian pathogenic Escherichia coli from colibacillosis affected broiler chicken in Kashmir valley. Life Sci. Leaflets. 125, 6–13. Shah, S.A., Mir, M.S., Wani, B.M., Kamil, S.A., Goswami, P., Amin, U., Shafi, M., Rather, M.A. and Beigh, A.B., 2019. Pathological studies on avian pathogenic Escherichia coli infection in broilers. Pharm. Innov. J. 8(7), 68–73. Shoukat, S., Wani, H., Jeelani, R., Ali, U. and Ali, M. 2018. An overview of avian Aspergillosis. Int. J. Avian Wildlife Biol. 3(3), 215–216. Sikder, A.J., Islam, M.A., Rahman, M.M. and Rahman, M.B. 2005. Seroprevalence of salmonella and Mycoplasma gallisepticum infection in the six model farms at Patuakhali district of Bangladesh. Int. J. Poult. Sci. 4, 905–910. Sultana, S., Rashid, S.M.H., Islam, M.N., Ali, M.H., Islam, M.M. and Azam, M.G. 2015. Pathological investigation of avian aspergillosis in commercial broiler chicken at Chittagong district. Int. J. Innov. Appl. Stud. 10(1), 366–376. Vandekerchove, D., De Herdt, P., Laevens, H. and Pasmans, F. 2004. Colibacillosis in caged layer hens: characteristics of the disease and the aetiological agent. Avian Pathol. 33, 117–125. Wani B.M., Kamil S.A., Shah S.A., Shafi M., Shabir M., Kashani B., Hassan M.N. and Goswami P. 2020. Isolation and biochemical characterization of avian pathogenic Escherichia coli from different organs in colibacillosis affected broiler chicken. J. Entomol. Zool. Stud. 8(5),1649–1652. Yadav, B.S., Niyogi, D., Gupta, R.K., Singh, S.V., Saif, M. and Jaiswal, S.K. 2020. Incidence and clinicopathological study of gout in broiler birds of Faizabad and Sultanpur districts of eastern Uttar Pradesh. Explor. Anim. Med. Res. 10(1), 88–91. Yaqub, M., Shah, S.A., Shafi, M.,Rafiq, M., Goswami, P., Kamil, S.A., Mir, M.S. Ganie, A.A. Wani, Z.A. and Beigh A B. 2023. Pathological studies on coccidiosis in broiler and layer chicken. Indian J. Vet. Pathol. 47(2), 122–128. Yassin A. Velthuis A.G.J., Boerjan M. and Reil J.V. 2009. Field study on broilers’ first-week mortality. Poult. Sci. 88(4), 798–804. | ||
| How to Cite this Article |
| Pubmed Style Yadav PK, Shah SA, Shafi M, Kamil SA, Mir MS, Rather MA, Ganaie AA, Wani ZA. Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Vet. J.. 2024; 14(11): 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 Web Style Yadav PK, Shah SA, Shafi M, Kamil SA, Mir MS, Rather MA, Ganaie AA, Wani ZA. Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. https://www.openveterinaryjournal.com/?mno=218452 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.32 AMA (American Medical Association) Style Yadav PK, Shah SA, Shafi M, Kamil SA, Mir MS, Rather MA, Ganaie AA, Wani ZA. Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Vet. J.. 2024; 14(11): 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 Vancouver/ICMJE Style Yadav PK, Shah SA, Shafi M, Kamil SA, Mir MS, Rather MA, Ganaie AA, Wani ZA. Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 Harvard Style Yadav, P. K., Shah, . S. A., Shafi, . M., Kamil, . S. A., Mir, . M. S., Rather, . M. A., Ganaie, . A. A. & Wani, . Z. A. (2024) Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Vet. J., 14 (11), 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 Turabian Style Yadav, Pradeep Kumar, Showkat Ahmad Shah, Majid Shafi, Shayaib Ahmad Kamil, Masood Saleem Mir, Mudasir Ali Rather, Ajaz Ahmad Ganaie, and Zahoor Ahmad Wani. 2024. Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Veterinary Journal, 14 (11), 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 Chicago Style Yadav, Pradeep Kumar, Showkat Ahmad Shah, Majid Shafi, Shayaib Ahmad Kamil, Masood Saleem Mir, Mudasir Ali Rather, Ajaz Ahmad Ganaie, and Zahoor Ahmad Wani. "Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India." Open Veterinary Journal 14 (2024), 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 MLA (The Modern Language Association) Style Yadav, Pradeep Kumar, Showkat Ahmad Shah, Majid Shafi, Shayaib Ahmad Kamil, Masood Saleem Mir, Mudasir Ali Rather, Ajaz Ahmad Ganaie, and Zahoor Ahmad Wani. "Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India." Open Veterinary Journal 14.11 (2024), 3037-3046. Print. doi:10.5455/OVJ.2024.v14.i11.32 APA (American Psychological Association) Style Yadav, P. K., Shah, . S. A., Shafi, . M., Kamil, . S. A., Mir, . M. S., Rather, . M. A., Ganaie, . A. A. & Wani, . Z. A. (2024) Etiological and Histomorphological studies on early chick mortality in broiler chicken in Kashmir, India. Open Veterinary Journal, 14 (11), 3037-3046. doi:10.5455/OVJ.2024.v14.i11.32 |