| Research Article | ||
Open Vet. J.. 2025; 15(3): 1253-1263 Open Veterinary Journal, (2025), Vol. 15(3): 1253-1263 Research Article A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit modelsDwi Utari Rahmiati1,2*, Gunanti Gunanti2, Deni Noviana2, Raden Harry Soehartono2, Eva Harlina31Postgraduate student of Veterinary Biomedical Sciences Study Program, School of Veterinary Medicine and Biomedical Sciences, IPB University, Dramaga, Indonesia 2Division of Surgery and Radiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Dramaga, Indonesia 3Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Dramaga, Indonesia *Corresponding Author: Dwi Utari Rahmiati. School of Veterinary Medicine and Biomedical Sciences, IPB University. Email: dwi-ut [at] apps.ipb.ac.id Submitted: 05/12/2024 Accepted: 22/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
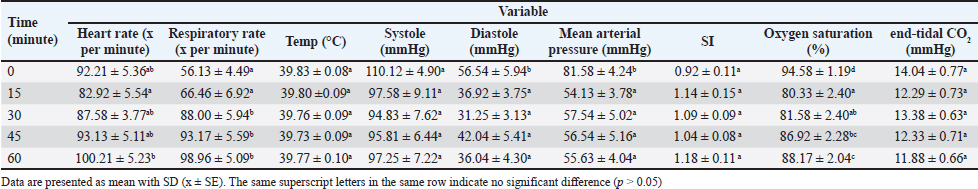
AbstractBackground: Hemorrhagic shock is a life-threatening condition resulting from acute blood loss, leading to compromised tissue perfusion and organ dysfunction. Currently, the guidelines for categorizing and managing hemorrhagic shock in pets are based on protocols developed for humans. Aim: This study employed New Zealand White rabbits as an animal model to systematically evaluate the physiological and biochemical responses to fixed-volume hemorrhage, aiming to establish its role in inducing shock and significant physiological alterations. Methods: A total of 21 New Zealand White rabbits, weighing 2–3 kg, were subjected to controlled hemorrhage by withdrawing 30%–35% of their total blood volume via the auricular artery using a 24-G IV catheter over 15 minutes. Parameters were assessed at baseline and 45 minutes post-induction. Results: Hemorrhage induced significant increases in heart rate and respiratory rate, reflecting compensatory mechanisms to maintain perfusion during shock. The mean arterial pressure and blood pressure significantly declined, consistent with hemorrhagic shock. Oxygen saturation initially decreased but partially recovered over time. All hematological variables decreased. Coagulopathy was indicated by prolonged prothrombin time and activated partial thromboplastin time. Elevated lactate levels indicate a shift to anaerobic metabolism due to hypoxia. The increased levels of interleukin-10 and tumor necrosis factor-alpha suggested an adaptive anti-inflammatory response to mitigate excessive inflammation. Conclusion: Fixed-volume hemorrhage in New Zealand White rabbits induces the physiological changes characteristic of hemorrhagic shock, providing valuable insights into the pathophysiological response to acute blood loss. Keywords: Animal model, Coagulation, Fixed volume, Hemorrhage, Inflammation. IntroductionHemorrhagic shock is one of the leading causes of death from acute trauma in humans and animals. Rapid and significant blood loss disrupts tissue perfusion, causing vital organs such as the brain, kidneys, and liver to receive insufficient oxygen, leading to organ dysfunction and death (Kislitsina et al., 2019; Dutton et al., 2023). Although interventions such as fluid resuscitation and blood transfusion are already in use, mortality rates due to hemorrhagic shock remain high (Knight et al., 2023). In veterinary medicine, studies on managing hemorrhagic shock in companion animals like dogs and cats, are still limited, with many approaches adopted from human medical practices. Most data on hemorrhagic shock management in companion animals are derived from human medical literature. However, there are critical differences in the physiological responses between humans and animals, making animal-specific research essential. For instance, the hemodynamic and metabolic mechanisms in dogs and cats may differ from those in humans, meaning that therapies effective for humans may not yield the same results in animals (Tucker et al., 2022). Therefore, the use of animal models is crucial for gaining a deeper understanding of the pathophysiology of hemorrhagic shock in animals (Fülöp et al., 2013). Animal models are often used to study naturally occurring diseases in companion animals and to test therapeutic interventions before their wider application in veterinary clinics. For example, New Zealand White rabbits have been used in various studies to model hemorrhagic shock due to their physiological responses to bleeding, which are applicable in preclinical research for companion animals (Fowler and Franch, 1957; Schweinburg et al., 1959). Several bleeding models used in animal research include fixed-volume, fixed-pressure, and uncontrolled hemorrhage. This study applied fixed-volume hemorrhage induction to measure the body’s compensatory response to blood loss over a specific period. The advantage of this model is its ability to illustrate the hemodynamic response of animals to a particular volume of blood loss, ease of execution, and minimal trauma (Lomas-Niera et al., 2005; Moochhala et al., 2009). This approach can serve as a basis for establishing standard levels of hemorrhagic shock in animals, particularly companion animals, as an expected outcome of this research. Another benefit of the fixed-volume model is its ability to predict clinical prognosis based on more accurate physiological changes (Frankel et al., 2007). Previous studies by Frankel et al. (2007) and Pfeifer et al. (2013) used the fixed-volume method but used mice and pigs as animal models, respectively. Mice and pigs are less suitable as models for companion animals, particularly dogs. Mice and dogs have different dopamine responses in renin-angiotensin release as natural vasoconstrictors (Morris et al., 2024), whereas pigs and dogs exhibit different hemodynamic responses to synthetic vasoconstrictors. In dogs, these preparations more significantly affect myocardial contractility, whereas in pigs, they influence peripheral resistance (Tune et al., 2020). Other studies by Rezende-Neto et al. (2010), Luo et al. (2015), Sun et al. (2017), and Wang et al. (2021) used rabbits but applied fixed-pressure or uncontrolled hemorrhagic shock methods. To date, no study has used rabbits as animal models with fixed-volume hemorrhagic shock induction, nor has any study provided comprehensive data to study physiological responses in companion animals. Materials and MethodsThis study used 21 New Zealand White rabbits weighing 2–3 kg. The treatment began with inhalation anesthesia induction using 3%–4% isoflurane. Hemorrhagic shock was induced by removing 30%–35% of the total blood volume (60 ml x body weight) via the auricular artery using a 24-G intravenous catheter. Blood withdrawal was performed by repeated aspiration until the target volume was reached, and the procedure was completed within 15 minutes. Clinical parameters, including heart rate (HR), respiratory rate (RR), temperature, systolic pressure, diastolic pressure, mean arterial pressure (MAP), oxygen saturation (SPO2), and end-tidal CO2 volume (ETCO2), were observed every 15 minutes until the 60 minutes of shock using a patient monitor (Infunix Purescope IP-4050), Blood Pressure Monitor (Contec08A-VET), and Capnograph (Contec® CA10M). Hematology parameters, prothrombin time (PT), activated partial thromboplastin time (aPTT), lactate acid, interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) were measured by comparing baseline (0 minute) to 60 minutes of shock. Hematology parameters were analyzed using a hematology analyzer (Mindray BC-2800Vet), PT, and aPTT with a QuickVet® Specialty Analyzer™ and QuickVet® PT/aPTT Coag Combo Test™. Lactate levels were measured using the Edge® Lactate Analyzer, and IL-10 and TNF-α levels were assessed using ELISA (Elabscience®). The research data were statistically analyzed using one-way ANOVA followed by Duncan’s test with a 95% confidence interval. The data were presented as means and displayed in table form. Ethical approvalAll procedures conducted in this study were approved by the ethics committee of the School of Veterinary Medicine and Biomedical Sciences of IPB University approval number: 033/KEH/SKE/III/2023. ResultsClinicalThe induced bleeding had an effect on variable values at the 0 minute mark post-induction, with a decrease across all variables except RR (Table 1). Some variables demonstrated similar changes from 0 to 60 minutes. The HR variable showed a decrease only at the 0 minute mark, followed by an increase that nearly returned to the baseline value at 0 minute. The temperature and ETCO2 values followed a similar pattern, decreasing but not far from the 0 minute value. Systolic pressure and SpO2 exhibited fluctuating values, generally trending upward toward the 0 minute level. Diastolic pressure and MAP showed fluctuating changes but tended to decrease, remaining far from the 0 minute value. The RR variable is the only one that demonstrates an increase from 15 to 60 minutes. HematologyThe results indicate significant changes in various hematological parameters of the animal models experiencing hemorrhagic shock (Table 2), as denoted by different letters (a and b). All hematological variables had decreased values. Blood CoagulationThe PT variable showed an increase from 18.38 ± 0.14 at minute 0 to 23.33 ± 2.60 at minute 60, indicating a significant prolongation in blood clotting time (Table 3). The differences marked by different letters (a and b) suggest this change is statistically significant. This increase may indicate a disruption of the coagulation process. For the aPTT parameter, although there was an increase from 165.55 ± 12.80 at minute 0 to 223.59 ± 24.55 at minute 60, this change might not be statistically significant, as indicated by the letter “a” (Table 3). Table 1. Clinical parameters before and after induction of fixed volume hemorrhagic shock in New Zealand White rabbits.
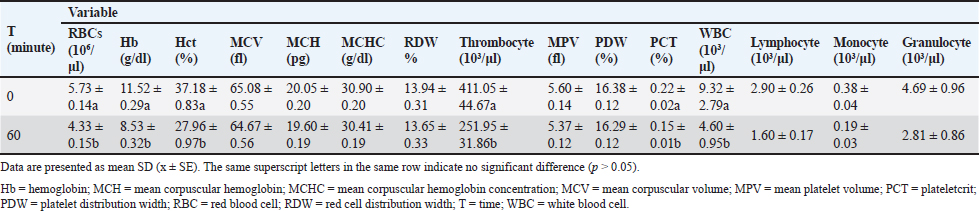
Table 2. Hematology parameters before and after fixed-volume hemorrhagic shock induction in New Zealand White rabbits.
Table 3. Prothrombin time (PT), activated partial thromboplastin time (aPTT), and lactate levels before and after fixed-volume hemorrhagic shock induction in New Zealand White rabbits.
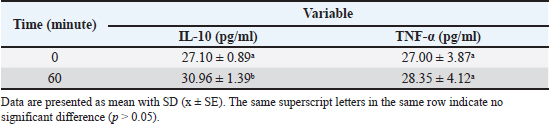
Table 4. Interleukin-10 (IL-10) and tumor necrosis factor-alpha (TNF-α) levels before and after fixed-volume hemorrhagic shock induction in New Zealand White rabbits.
Inflammatory mediatorsTable 4 presents the levels of IL-10 and TNF-α in New Zealand White rabbits before (0 minute) and after fixed-volume hemorrhagic shock induction (60 minutes). The IL-10 level increased significantly from 27.10 ± 0.89 pg/ml at 0 minute to 30.96 ± 1.39 pg/ml at 60 minutes, indicating a stronger anti-inflammatory response following hemorrhagic shock induction. In contrast, the TNF-α level did not differ significantly between 0 minute (27.00 ± 3.87 pg/ml) and 60 minutes (28.35 ± 4.12 pg/ml), indicating that hemorrhagic shock did not significantly affect TNF-α release during this period. These findings suggest that in this hemorrhagic shock model, the anti-inflammatory response mediated by IL-10 is more dominant than the pro-inflammatory response triggered by TNF-α within the first 60 minutes after induction. DiscussionThe changes induced by hemorrhagic shock (30%–35%) in this study are similar to those observed in previous research (Rahmiati et al., 2023). Despite differences in the blood withdrawal site and volume extracted, the clinical effects were comparable. An increase in HR after shock induction occurs due to baroreflex receptor activation, which triggers the renin-angiotensin system and adrenaline release. This physiological response aims to increase the HR and ensure tissues and cells receive an adequate blood supply. Additionally, this response helps maintain blood pressure and optimizes vital organ perfusion, which is critical in emergencies like hemorrhagic shock (Schultz and McConachie, 2015). The increase in the RR involves more specific cellular mechanisms. Blood loss leads to an unmet oxygen demand in tissues, causing mitochondria to shift from aerobic to less efficient anaerobic metabolism for adenosine triphosphate (ATP) production. During this process, pyruvate is produced and converted to lactic acid to regenerate nicotinamide adenine dinucleotide (NAD+), thereby maintaining partial cellular respiration despite oxygen scarcity. This shift to anaerobic metabolism results in lactic acid accumulation, potentially causing blood pH to drop (acidosis). To compensate, the body increases its respiration rate to expel carbon dioxide (CO2) and reduce blood acidity. This hyperventilation response aims to create respiratory alkalosis by removing CO2 from the blood (Convertino et al., 2019). This compensatory mechanism helps maintain acid-base balance and ensures tissue oxygenation despite hemorrhagic shock (Hooper and Armstrong, 2022). Temperature changes showed a declining trend but remained close to the baseline. The gradual decrease in temperature results from the bleeding process itself. In the acute phase, blood flow to the skin and mucosal surfaces diminishes, potentially reducing metabolism and increasing heat loss, potentially leading to hypothermia. Body temperature regulation involves balancing heat production and loss. In mammals, heat is a byproduct of metabolism. The mechanisms for maintaining this balance include convection, conduction, evaporation, and radiation (Henderson et al., 2000). In rabbits, heat loss through radiation and convection primarily occurs via the ears (Yuan et al., 2022). In other species, heat loss pathways may vary (Jordan, 1995). Systolic pressure changes tended to fluctuate, with a decrease followed by an increase toward baseline at minute 0. Systolic pressure is influenced by HR. A decrease in HR typically lowers systolic pressure (Priestley et al., 2019). Baroreflex receptor activation-induced increase in HR raises systolic pressure. Unlike systolic pressure, diastolic pressure responded differently, showing fluctuating declines until minute 60, which is far from the baseline value. This could result from the acute hemorrhagic shock response, involving positive modulation of chronotropy (sinoatrial node conductance), dromotropic (atrioventricular node conductance), and negative lusitropic (relaxation), marked by HR elevation (Ranjan and Gulati, 2023). During acute hemorrhagic shock, the priority is to pump blood rapidly to tissues, quickly filling myocardial vasculature during systole. Conversely, during diastole, slower ventricular vascular filling increases the risk of diastolic dysfunction. Several studies have found that hemorrhagic conditions can impair myocardial contractility and cause diastolic dysfunction (Suzuki et al., 1995). Elansary et al. (2022) demonstrated that the severity of hemorrhagic shock is correlated with greater left ventricular dysfunction and reduced myocardial blood flow. According to D’Annunzio et al. (2012), diastolic pressure below 40 mm Hg indicates subendocardial ischemia. The MAP changes mirrored those of diastolic pressure. MAP is influenced by cardiac output and systemic vascular resistance. Blood volume loss reduces vascular lumen pressure and cardiac output. Physiologically, rapid blood loss lowers MAP during the first 10 minutes and gradually increases afterward, provided no recurrent bleeding occurs (Frankel et al., 2007). In this study, the body seemed unable to compensate for blood loss by increasing MAP, possibly due to the physiological limits of MAP elevation after hemorrhage. Scully et al. (2016) found that MAP elevation after hemorrhage occurs within the first 15 minutes after bleeding, with a modest increase of around 20 mm Hg compared with the initial 30 mm Hg decrease. Changes in HR, systolic pressure, diastolic pressure, and MAP can predict cardiac function. However, these variables are best evaluated in combination. For instance, HR was associated with systolic pressure, MAP, or diastolic pressure. Acute arterial pressure drops are usually counteracted by sympathetic nervous system activity, although compensation can sometimes become maladaptive. Therefore, ratios such as HR to systolic pressure (shock index, SI) or HR to MAP can help identify underlying dysfunctions. SI is a good predictor of mortality, although it has low sensitivity in geriatric and obstetric patients (Kamikawa and Hayashi, 2020). A higher SI indicates more severe shock. The SI in this study was 1.18 ± 0.11. In humans, SI-based shock classifications are as follows: Class I (no shock): SI < 0.6; Class II (mild shock) : SI ≥ 0.6 to < 1.0; Class III (moderate shock) : SI ≥ 1.0 to < 1.4; and Class IV (severe shock) : SI ≥ 1.4 (Mutschler et al., 2013). However, these values may not be directly applicable to animals. Further evaluation of clinical parameters is necessary to establish shock classification in animals. Unlike the SI, the HR-to-diastolic pressure ratio (diastolic index, DAI) predicts circulatory dysfunction during vasodilation (Ospina-Tascón et al., 2020). DAI values were not analyzed in this study. Based on these results, the HR increase did not compensate for the diastolic pressure drop, likely due to a gradual reduction in vascular tone (Ospina-Tascón et al., 2020; Han et al., 2022). DAI is commonly used to predict sepsis severity or post-resuscitation conditions (Han et al., 2022). As previously mentioned, a drop in diastolic pressure indicates myocardial contractility dysfunction, which requires confirmation through ejection fraction assessment via ultrasound (Carrara et al., 2018). This was not performed in the present study, representing a limitation. Comparatively, myocardial contractility dysfunction usually appears 2 to 5 hours post-shock (Meng et al., 2005; Carrara et al., 2018). Compensation during shock without subsequent myocardial dysfunction is typically observed with hemodynamic changes, such as systolic and MAP decreases, while diastolic pressure is maintained. This finding differs from that of this study, suggesting that shock induction likely causes myocardial contractility dysfunction, which is potentially reversible. SPO2 changes mirrored systolic pressure patterns, with end-study values < 90%. In humans, SPO2 < 90% indicates poor prognosis and severe shock (Qi et al., 2020). SPO2 reductions result from decreased blood volume and hemoglobin (Hb), lowering blood oxygen levels. SPO2 reflects microcirculation, tissue perfusion, and oxygenation (Raux et al., 2006). Factors like acidic pH, increased CO2, and red blood cell (RBC) glycolysis affect Hb’s oxygen affinity post-shock (Fecher et al., 2021). ETCO2 reflects exhaled CO2 levels at the end of expiration, indicating the sufficiency of CO2 transport in the blood. In this study, ETCO2 changes followed temperature patterns, with slight decreases from baseline. This variable is closely linked to RR. The study noted that RR increases with hyperventilation to create respiratory alkalosis by expelling CO2, neutralizing H+ accumulation, and counteracting pH decreases. Respiratory alkalosis is reflected by simultaneous ETCO2 decreases (Convertino et al., 2019). The clinical changes observed in this study are consistent with the patterns reported in hemorrhagic shock induction via carotid or femoral artery catheterization (Luo et al., 2015; Wang et al., 2021; Sun et al., 2017;). This demonstrates that the New Zealand White rabbit model, induced using a fixed-volume and minimally invasive method in this study, can exhibit clinical changes similar to those observed with the fixed-pressure method, which typically results in greater trauma. The induction of hemorrhage through blood withdrawal resulted in the loss of blood components, including RBCs, platelets, and WBCs (white blood cells) (Constable et al., 2016). A decrease in Hb occurred immediately as the RBC count decreased, and a similar decrease in Hct was observed as blood components were reduced. In this study, the reduction in all hematological variables ranged from 22% to 50%. This downward trend was observed for almost all variables except for the percentage of lymphocytes and granulocytes. The normal hematological ranges for NZW rabbits, the RBC, Hb, packed cell volume (PCV), mean corpuscular hemoglobin concentration, platelet, and WBC values at 60 minutes were below the normal range (Moore et al., 2021). This condition indicates anemia, thrombocytopenia, and leukopenia, collectively known as pancytopenia. Anemia caused by trauma or hemorrhage can persist for several weeks to months. This condition resembles chronic inflammatory anemia and is characterized by low reticulocyte counts, high erythropoietin levels, and dysfunctional iron regulation (Kelly et al., 2021). Furthermore, anemia may persist longer due to changes in RBC size and shape resulting from shock-induced oxidative stress (Berezina et al., 2001). Factors such as oxygen free radicals, toxins, ATP depletion, changes in intracellular ionic composition, and complement activation reduce RBC deformability and alter their morphology (Zaets et al., 2003). Variables like mean corpuscular volume and red cell distribution width are used to analyze RBC size and shape changes, but in this study, they showed minimal variation. Studies on the morphological changes in RBCs in response to hemorrhage vary widely. According to Schumacher (1992), RBC shape changes occur 1–2 weeks after hemorrhage, whereas Berezina et al. (2001) observed such changes within minutes. The extent of RBC morphological changes depends on the severity and duration of hypotension (Berezina et al., 2001). Hb levels follow a similar trend as RBC counts, with decreases observable within minutes. In humans, Hb levels below 10 g/dl within 30 minutes post-hemorrhage can indicate the presence of significant bleeding sources (Bruns et al., 2007; Figueiredo et al., 2018). Thrombocytopenia following trauma or hemorrhage is associated with consumptive processes and dilutional effects. After injury, platelets are extensively utilized for clot formation to halt bleeding, thereby reducing their availability in circulation. Dilutional effects can occur even before resuscitation because hypotension induces fluid reabsorption to maintain blood flow. This reabsorption leads to the dilution of cellular components in the microvasculature, a process known as autotransfusion (Woodcock and Woodcock, 2012; Michel et al., 2020; Munoz et al., 2020). WBC differential counts all decreased compared with baseline values. This result differs from those of Gaylor et al. (1969) and Hawksworth et al. (2012), who reported increased neutrophil and lymphocyte counts post-hemorrhage. The discrepancy may arise from differences in blood collection timing post-hemorrhage and the physical factors affecting the vascular lumen. Neutrophil mobilization from marginal to circulating pools occurs within minutes to hours (Tvedten and Raskin, 2011). However, according to Suwa et al. (2000), biphasic neutrophilia occurs at approximately 3 and 9 hours post-induction of inflammatory mediators. In this study, blood was collected 1 hour after hemorrhage, likely before peak neutrophil levels were reached. Normally, lymphocyte counts increase in response to hypoxia (Kokura et al., 2000) under the influence of inflammatory mediators, including those induced by post-hemorrhagic hypoxia. Moreover, monocyte mobilization is triggered by inflammatory mediators (Qin et al., 2020). These responses occur within minutes to hours and are correlated with increased vascular permeability that allows neutrophils, lymphocytes, and monocytes to circulate (Jian et al., 2019; Qin et al., 2020). However, in this study, these responses were likely not observed within the limited observation period. Lactate levels also showed a significant increase from 27.25 ± 5.05 at minute 0 to 63.67 ± 6.49 at minute 60. This significant difference, indicated by different letters (a and b) (Table 3), suggests an increase in lactate production, possibly due to elevated anaerobic metabolic activity or hypoxic conditions. These results indicate a significant metabolic disturbance alongside coagulation process disruption, as seen in the increase in PT. Overall, these changes demonstrate a significant physiological response over time. PT and aPTT are tests used to detect abnormalities in blood coagulation, each evaluating different pathways: PT assesses the extrinsic pathway, while aPTT evaluates the intrinsic pathway (Ford and Maazafeero, 2012). In this study, an increase in PT values or prolonged clotting time was observed. Generally, coagulation abnormalities are indicated when the PT or aPTT values exceed 1.5 times the upper limit of the reference interval (Condrey et al., 2020). In this study, the 0 minute value was used as the reference. The increase observed 45 minutes after induction was 1.3 times the 0 minute value. Although this does not indicate a coagulation disorder, it does show a statistically significant difference compared with the 0 minute PT value. Prolonged PT is associated with the occurrence of hypocoagulability (Moore et al., 2021; Tuan et al., 2021). During trauma or bleeding, exposed tissue factors initiate thrombin and clot formation. Platelets are activated through cellular signaling involving collagen, von Willebrand factor (vWF), and glycoproteins, which enhance coagulation and deplete fibrinogen, and factor V. Fibrinolysis also occurs when plasminogen is converted into plasmin. This leads to hypocoagulation and hyperfibrinolysis in post-trauma or hemorrhagic patients (Loscalzo & Schafer, 2003; Shaz et al., 2011). In this study, aPTT values also showed an increase or prolonged clotting time. Similar to the PT values, the aPTT increased by approximately 1.3 times from 0 minute, although it was not significantly different from the 0 minute mark. The slight prolongation in PT and aPTT suggests a moderate decrease in procoagulant (Moore et al., 2021). Physiologically, a decline in procoagulant may accompany increased thrombin, marking clot formation at required injury sites. Excessive thrombin formation plays a crucial role in delayed hypercoagulability in patients with trauma or hemorrhage. In severe trauma and unresolved bleeding, circulating thrombin accumulates, shifting the state to hypercoagulability, which is termed trauma-induced coagulopathy (TIC) (Dunbar and Chandler, 2009; Woolley et al., 2020). Lactate levels are commonly monitored to assess tissue perfusion status. Lactate is produced by most tissues, and its highest production occurs in the muscles. Under normal conditions, lactate is quickly metabolized by the liver and, to a lesser extent, the kidneys. Aerobically, pyruvate is produced via glycolysis and enters the Krebs cycle, whereas anaerobically, lactate is the final glycolysis product, serving as a substrate for gluconeogenesis (Andersen et al., 2013). In this study, lactate levels increased 2.3 times at 45 minutes post-induction compared with the 0 minute mark, showing a statistically significant increase. Hemorrhagic shock leads to reduced blood volume, affecting the cellular level. Microcirculation suffers from reduced tissue perfusion, subsequently inducing anaerobic metabolism and acid production, causing acidosis (Munoz et al., 2020). Acidosis, in turn, impacts blood coagulation (Engström et al., 2006). Acidosis causes plasma protein dysfunction and rapid fibrinogen degradation, disrupting nearly all coagulation stages under this condition (Savioli et al., 2021). In humans, pH below 7.4 can lead to platelet shape and structural changes, reduced factor activity, and impaired thrombin production (Martini et al., 2005). It also decreases fibrinogen concentration, increases fibrinogen degradation (due to fibrinolysis and elevated factor XIII), and does not affect fibrinogen production while promoting a pro-inflammatory response mediated by platelets (Etulain et al., 2012). Bicarbonate administration to correct acidosis is not correlated with TIC reversal (Martini et al., 2006). Animal studies and in vitro experiments have shown that acidosis inhibits fibrin polymerization, decreases factor V and IX activity, reduces platelet aggregation, increases fibrinogen consumption, lowers platelet count, reduces thrombin formation, weakens clot strength, and produces abnormal conventional coagulation test results (Martini et al., 2005; Martini and Holcomb, 2007). As hemorrhagic shock progresses, hypercoagulability occurs due to prothrombotic changes and the cessation of fibrinolysis, which increases organ damage by producing thrombi and clogging microvascular circulation, ultimately leading to organ failure (Moore et al., 2021). Both IL-10 and TNF-α showed an increase compared to the 0 min mark (Table 4). This change is consistent with Bahrami et al. (1997) and Schneider et al. (2004). In the post-hemorrhagic shock mechanism, TNF-α and IL-10 play crucial roles in regulating the inflammatory response. TNF-α is one of the first pro-inflammatory cytokines released after injury, initiating the early inflammatory response and stimulating the release of other cytokines like IL-1 and IL-6 (Bahrami et al., 2017). Although essential for initiating tissue repair, excessive TNF-α elevation can lead to further tissue damage and increase the risk of multiple organ failure (Kirchhoff et al., 2009; Villarroel et al., 2023). Conversely, IL-10 functions as an anti-inflammatory cytokine, controlling and suppressing the inflammatory response by inhibiting the production of TNF-α and IL-6 (Schneider et al., 2004). In the early phase following hemorrhagic shock, IL-10 acts to balance the pro-inflammatory effects triggered by TNF-α to prevent uncontrolled inflammation (Schwacha et al., 2001). However, excessive release of IL-10 can also have adverse effects because it may cause immunosuppression, increasing the body’s susceptibility to secondary infections (Ayala et al., 1994). The balance between TNF-α and IL-10 is critical in determining post-hemorrhagic shock outcomes, as an excessively high or low inflammatory response can lead to serious complications, such as organ failure or infection (Giannoudis et al., 2000). ConclusionResearch on fixed-volume hemorrhage in New Zealand White rabbits demonstrates significant physiological responses characteristic of hemorrhagic shock, providing valuable insights into the body’s pathological adaptation to acute blood loss. This model demonstrates how blood pressure, HR, oxygenation, and tissue perfusion are affected during controlled hemorrhage, simulating a shock state similar to human responses. These findings highlight the usefulness of New Zealand White rabbits as a translational model for hemorrhagic shock research in companion animals. The results of this study can be used to establish more detailed shock level standards for companion animals in accordance with clinical criteria and blood profiles. AcknowledgmentsThe authors would like to express their sincere gratitude to the Veterinary Teaching Hospital, including the Division of Surgery and Radiology and the Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, for providing the research facilities and location for this study. Appreciation is also extended to PT Zoetis Indonesia for their valuable technical support. Conflict of interestThe authors declare no conflict of interest. FundingThe authors gratefully acknowledge the financial assistance provided by the Center for Higher Education Funding (BPPT) and the Indonesia Endowment Fund for Education (LPDP), as stated in decree number 01802/J5.2.3/BPI.06/9/2022. Authors’ contributionsDUR: general study design, data collection, data interpretation, manuscript drafting. G: design of shock induction in animal models. DN: interpretation of clinical data. RHS: reviewing manuscript drafting. EH: interpretation of pathological immune response. Data availabilityAll related data is presented in the text. ReferencesAndersen, L.W., Mackenhauer, J., Roberts, J.C., Berg, K.M., Cocchi, M.N. and Donnino, M.W. 2013. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 88(10), 1127–1140. Ayala, A., Lehman, D.L., Herdon, C.D. and Chaudry, I.H. 1994. Mechanism of enhanced susceptibility to sepsis following hemorrhage: interleukin-10 suppression of T-cell response is mediated by eicosanoid-induced interleukin-4 release. Arch. Surg. 129(11), 1172–1178. Bahrami, S., Yao, Y.M., Leichtfried, G., Redl, H., Marzi, I. and Schlag, G. 1997. Significance of TNF in hemorrhage-related hemodynamic alterations, organ injury, and mortality in rats. Am. J. Physiol. Heart Circ. Physiol. 272(5), H2219–H2226. Bahrami, A., Jafarmadar, M., Redl, H., Bahrami, S. and Jiang, J.X. 2017. TNF-α release capacity is suppressed immediately after hemorrhage and resuscitation. Chin. J. Traumatol. 20(4), 207–211. Berezina, T.L., Zaets, S.B., Kozhura, V.L., Novoderzhkina, I.S., Kirsanova, A.K., Deitch, E.A. and Machiedo, G.W. 2001. Morphologic changes of red blood cells during hemorrhagic shock replicate changes of aging. Shock 15(6), 467–470. Bruns, B., Lindsey, M., Rowe, K., Brown, S., Minei, J.P., Gentilello, L.M. and Shafi, S. 2007. Hemoglobin drops within minutes of injuries and predicts need for an intervention to stop hemorrhage. J. Trauma Acute Care Surg. 63(2), 312–315. Carrara, M., Babini, G., Baselli, G., Ristagno, G., Pastorelli, R., Brunelli, L. and Ferrario, M., 2018. Blood pressure variability, heart functionality, and left ventricular tissue alterations in a protocol of severe hemorrhagic shock and resuscitation. J. Appl. Physiol. 125(4), 1011–1020. Condrey, J.A., Flietstra, T., Nestor, K.M., Schlosser, E.L., Coleman-McCray, J.D., Genzer, S.C., Welch, S.R. and Spengler, J.R. 2020. Prothrombin time, activated partial thromboplastin time, and fibrinogen reference intervals for inbred strain 13/n guinea pigs (Cavia porcellus) and validation of low volume sample analysis. Microorganisms 8(8), 1127. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Grünberg, W. 2016. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. Amsterdam, Netherlands: Elsevier Health Sciences. Convertino, V.A., Lye, K.R., Koons, N.J. and Joyner, M.J. 2019. Physiological comparison of hemorrhagic shock and V˙ O2max: a conceptual framework for defining the limitation of oxygen delivery. Exp. Biol. Med (Maywood). 244(8), 690–701. D’Annunzio, V., Donato, M., Fellet, A., Buchholz, B., Arciuch, V.G.A., Carreras, M.C., Valdez, L.B., Zaobornyj, T., Morales, C., Boveris, A. and Poderoso, J.J. 2012. Diastolic function during hemorrhagic shock in rabbits. Mol. Cell Biochem. 359(1–2), 169–176. Dunbar, N.M. and Chandler, W.L. 2009. Transfusion practice: thrombin generation in trauma patients. Transfusion 49(12), 2652–2660. Dutton, R.P., Grissom, T.E., Herbstreit, F. and Pivalizza, E.G. 2023. Trauma anesthesiology. Anesth. Analg. 136(5), 827–828. Elansary, N.N., Stonko, D.P., Treffalls, R.N., Abdou, H., Madurska, M.J. and Morrison, J.J. 2022. Class of hemorrhagic shock is associated with progressive diastolic coronary flow reversal and diminished left ventricular function. Front. Physiol. 13, 1033784 Engström, M., Schott, U., Nordstrom, C.H., Romner, B. and Reinstrup, P. 2006. Increased lactate levels impair the coagulation system—a potential contributing factor to progressive hemorrhage after traumatic brain injury. J. Neurosurg. Anesthesiol. 18(3), 200–204. Etulain, J., Negrotto, S., Carestia, A., Pozner, R.G., Romaniuk, M.A., D’Atri, L.P., Klement, G.L. and Schattner, M. 2012. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. J. Thromb. Haemost. 107(1), 99–110. Fecher, A., Stimpson, A., Ferrigno, L. and Pohlman, T.H. 2021. The pathophysiology and management of hemorrhagic shock in the polytrauma patient. J. Clin. Med. 10(20), 4793. Figueiredo, S., Taconet, C., Harrois, A., Hamada, S., Gauss, T., Raux, M., Duranteau, J. and Traumabase Group Arie Attias Sylvain Ausset Mathieu Boutonnet Gilles Dhonneur Olivier Langeron Catherine Paugam-Burtz Romain Pirracchio Bruno Riou Guillaume de St Maurice Bernard Vigué. 2018. How useful are hemoglobin concentration and its variations to predict significant hemorrhage in the early phase of trauma? A multicentric cohort study. Ann. Intensive Care. 8, 1–10. Ford, R.B. and Mazzaferro, E.M. 2011. Laboratory diagnosis and test protocols. In Kirk & Bistner’s handbook of veterinary procedures and emergency treatment, St. Louis, Missouri, USA. p: 551. Fowler, N.O. and Franch, R. 1957. Mechanism of pressor response to l-norepinephrine during hemorrhagic shock. Circ. Res. 5(2), 153–156. Frankel, D.A., Acosta, J.A., Anjaria, D.J., Porcides, R.D., Wolf, P.L., Coimbra, R. and Hoyt, D.B. 2007. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J. Surg. Res. 43(2), 276–280. Fülöp, A., Turóczi, Z., Garbaisz, D., Harsányi, L. and Szijártó, A. 2013. Experimental models of hemorrhagic shock: a review. Eur. Surg. Res. 50(2), 57–70. Gaylor, M.S., Chervenick, P.A. and Boggs, D.R. 1969. Neutrophil kinetics after acute hemorrhage. Proc. Soc. Exp. Biol. Med. 131(4), 1332–1336. Giannoudis, P.V., Smith, R.M., Perry, S.L., Windsor, A.J., Dickson, R.A. and Bellamy, M.C. 2000. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. J. Intensive Care Med. 26, 1076–1081. Han, C., Lee, J.H. and Korean Hypothermia Network Investigators. 2022. Heart rate and diastolic arterial pressure in cardiac arrest patients: a nationwide, multicenter prospective registry. PLoS One 17(9), e0274130. Hawksworth, J.S., Graybill, C., Brown, T.S., Gillern, S.M., Wallace, S.M., Davis, T.A., Elster, E.A. and Tadaki, D.K. 2012. Lymphocyte depletion in experimental hemorrhagic shock in Swine. J. Inflamm (Lond). 9, 1–12. Henderson, R.A., Whitehurst, M.E., Morgan, K.R. and Carroll, R.G. 2000. Reduced metabolic rate accompanies the hemorrhage-induced hypothermia in conscious rats. Resuscitation 44(2), 129–138. Hooper, N. and Armstrong, T.J. 2022. Hemorrhagic shock. In StatPearls [Internet]. StatPearls Publishing. Rockville Pike,Maryland, USA. Jian, Z., Liu, R., Zhu, X., Smerin, D., Zhong, Y., Gu, L., Fang, W. and Xiong, X. 2019. The involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 10, 2167. Jordan, D. 1995. Temperature regulation in laboratory rodents. J. Anat. 186(Pt 1), 228. Kamikawa, Y. and Hayashi, H. 2020. Equivalency between the shock index and subtracting the systolic blood pressure from the heart rate: an observational cohort study. BMC Emerg. Med. 20, 1–8. Kelly, L.S., Darden, D.B., Fenner, B.P., Efron, P.A. and Mohr, A.M. 2021. The hematopoietic stem/progenitor cell response to hemorrhage, injury, and sepsis: a review of pathophysiology. Shock 56(1), 30–41. Kirchhoff, C., Biberthaler, P., Mutschler, W. E., Faist, E., Jochum, M. and Zedler, S. 2009. Early down-regulation of the pro-inflammatory potential of monocytes is correlated to organ dysfunction in patients after severe multiple injury: a cohort study. J. Crit. Care. 13, 1–11. Kislitsina, O.N., Rich, J.D., Wilcox, J.E., Pham, D.T., Churyla, A., Vorovich, E.B., Ghafourian, K. and Yancy, C.W. 2019. Shock–classification and pathophysiological principles of therapeutics. Curr. Cardiol. Rev. 15(2), 102–113. Knight, C.D., Bebarta, V., Meledeo, M.A., Ross, E., Wu, X., Bynum, J., Schauer, S., Getz, T. and April, M. 2023. A narrative review of prehospital hemorrhagic shock treatment with non-blood product medications. Transfusion 63, S256–S262. Kokura, S., Wolf, R.E., Yoshikawa, T., Ichikawa, H., Granger, D.N. and Yee Aw, T. 2000. Endothelial cells exposed to anoxia/reoxygenation are hyperadhesive to T-lymphocytes: kinetics and molecular mechanisms. Microcirculation 7(1), 13–23. Lomas-Niera, J.L., Perl, M., Chung, C.S. and Ayala, A. 2005. Shock and hemorrhage: an overview of animal models. Shock 24(Suppl 1), 33–39. Loscalzo, J. and Schafer, A.I. (Eds.). 2003. Thrombosis and hemorrhage. Philadelphia, PA: Lippincott Williams & Wilkins. Luo, Z., Wang, P., Zhang, A., Zuo, G., Zheng, Y. and Huang, Y. 2015. Evaluation of the microcirculation in a rabbit hemorrhagic shock model using laser Doppler imaging. Plos One 10(2), e0116076. Martini, W.Z., Pusateri, A.E., Uscilowicz, J.M., Delgado, A.V. and Holcomb, J.B. 2005. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J. Trauma Acute Care Surg. 58(5), 1002–1010. Martini, W.Z., Dubick, M.A., Pusateri, A.E., Park, M.S., Ryan, K.L. and Holcomb, J.B. 2006. Does bicarbonate correct coagulation function impaired by acidosis in swine? J. Trauma Acute Care Surg. 61(1), 99–106. Martini, W.Z. and Holcomb, J.B. 2007. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann. Surg. 246(5), 831–835. Meng, X., Ao, L., Song, Y., Raeburn, C.D., Fullerton, D.A. and Harken, A.H. 2005. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-α receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288(3), R600–R606. Michel, C.C., Woodcock, T.E. and Curry, F.R.E. 2020. Understanding and extending the Starling principle. Acta Anaesthesiol. Scand. 64(8), 1032–1037. Moochhala, S., Wu, J. and Lu, J. 2009. Hemorrhagic shock: an overview of animal models. Front. Biosci. 14(12), 4631–4639. Moore, E.E., Moore, H.B., Kornblith, L.Z., Neal, M.D., Hoffman, M., Mutch, N.J., Schöchl, H., Hunt, B.J. and Sauaia, A. 2021. Trauma-induced coagulopathy. Erratum in: Nat. Rev. Dis. Primers. 7(1), 30. Morris, C.J., Rolf, M.G., Starnes, L., Villar, I.C., Pointon, A., Kimko, H. and Di Veroli, G.Y. 2024. Modelling hemodynamics regulation in rats and dogs to facilitate drugs safety risk assessment. Front. Pharmacol. 15, 1402462. Munoz, C., Aletti, F., Govender, K., Cabrales, P. and Kistler, E.B. 2020. Resuscitation after hemorrhagic shock in the microcirculation: targeting optimal oxygen delivery in the design of artificial blood substitutes. Front. Med. 7, 585638. Mutschler, M., Nienaber, U., Münzberg, M., Wölfl, C., Schoechl, H., Paffrath, T., Bouillon, B., Maegele, M. and TraumaRegister DGU®. 2013. The shock index revisited–a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU®. Crit. Care. 17(4), R172. Ospina-Tascón, G.A., Teboul, J.L., Hernandez, G., Alvarez, I., Sánchez-Ortiz, A.I., Calderón-Tapia, L.E., Manzano-Nunez, R., Quiñones, E., Madriñan-Navia, H.J., Ruiz, J.E. and Aldana, J.L. 2020. Diastolic shock index and clinical outcomes in patients with septic shock. Ann. Intensive Care. 10, 1–11. Pfeifer, R., Lichte, P., Schreiber, H., Sellei, R.M., Dienstknecht, T., Sadeghi, C., Pape, H.C. and Kobbe, P. 2013. Models of hemorrhagic shock: differences in the physiological and inflammatory response. Cytokine 61(2), 585–590. Priestley, E.M., Inaba, K., Byerly, S., Biswas, S., Wong, M.D., Lam, L., Benjamin, E. and Demetriades, D. 2019. Pulse pressure as an early warning of hemorrhage in trauma patients. J. Am. Coll. Surg. 229(2), 184–191. Qi, J., Ding, L., Bao, L. and Chen, D. 2020. The ratio of shock index to pulse oxygen saturation predicting mortality of emergency trauma patients. PLoS One 15(7), e0236094. Qin, X., Akter, F., Qin, L., Cheng, J., Guo, M., Yao, S., Jian, Z., Liu, R. and Wu, S. 2020. Adaptive immunity regulation and cerebral ischemia. Front. Immunol. 11, 689. Rahmiati, D.U., Noviana, D., Soehartono, H. and Harlina, E. 2023. Syok Indeks sebagai Penduga Tingkat Syok Hewan Model Kelinci New Zealand White pada Kejadian Syok Hemoragi. J Vet. 24(4), 422–430. Ranjan, A.K. and Gulati, A. 2023. Controls of central and peripheral blood pressure and hemorrhagic/hypovolemic shock. J. Clin. Med. 12(3), 1108. Raux, M., Thicoïpé, M., Wiel, E., Rancurel, E., Savary, D., David, J.S., Berthier, F., Ricard-Hibon, A., Birgel, F. and Riou, B. 2006. Comparison of respiratory rate and peripheral oxygen saturation to assess severity in trauma patients. Intensive Care Med. 32, 405–412. Rezende-Neto, J.B., Rizoli, S.B., Andrade, M.V., Lisboa, T.A. and Cunha-Melo, J.R. 2010. Rabbit model of uncontrolled hemorrhagic shock and hypotensive resuscitation. Braz. J. Med. Biol. Res. 43(12), 1153–1159. Savioli, G., Ceresa, I.F., Caneva, L., Gerosa, S. and Ricevuti, G. 2021. Trauma-induced coagulopathy: overview of an emerging medical problem from pathophysiology to outcomes. Medicines 8(4), 16. Schneider, C.P., Schwacha, M.G. and Chaudry, I.H. 2004. The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim. Biophys. Act. 1689(1), 22–32. Schultz, W. and McConachie, I. 2015. Vital signs after haemorrhage–caution is appropriate. Trends Anaesth. Crit. Care. 5(4), 89–92. Schumacher, L.C. 1992. Anemia of blood loss. In Clinical hematology, principle, procedure, correlation. Eds., Lotspeich-Steininger, C.A., Stiene-Martin, E.A. and Koepke, J.A. Hagerstown, MD: JB Lippincott Company, pp: 280–281. Schwacha, M.G., Schneider, C.P., Bland, K.I. and Chaudry, I.H. 2001. Resistance of macrophages to the suppressive effect of interleukin-10 following thermal injury. Am. J. Physiol. Cell Physiol. 281(4), C1180–C1187. Schweinburg, F.B., Smiddy, F.G. and Fine, J. 1959. The granulocytopenic response in hemorrhagic shock. J. Clin. Invest. 38(4), 673–680. Scully, C.G., Daluwatte, C., Marques, N.R., Khan, M., Salter, M., Wolf, J., Nelson, C., Salsbury, J., Enkhbaatar, P., Kinsky, M. and Kramer, G.C. 2016. Effect of hemorrhage rate on early hemodynamic responses in conscious sheep. Physiol. Rep. 4(7), e12739. Shaz, B.H., Winkler, A.M., James, A.B., Hillyer, C.D. and MacLeod, J.B. 2011. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J. Trauma Acute Care Surg. 70(6), 1401–1407. Sun, W., Shao, Z., Xu, H., Qiu, W. and Sun, J. 2017. Application of pulsed arterial resuscitation in a rabbit model of hemorrhagic shock. Ulus. Travma Acil Cerrahi Derg. 23(6), 445–451. Suwa, T., Hogg, J.C., English, D. and Van Eeden, S.F. 2000. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J. Physiol. Heart Circ Physiol. 279(6), H2954–H2960. Suzuki, K., Ogino, R., Nishina, M. and Kohama, A. 1995. Effects of hypertonic saline and dextran 70 on cardiac functions after burns. Am. J. Physiol. 268(2 Pt 2), H856–H864. Tvedten, H. and Raskin, R.E. 2011. Leukocyte disorders. Willard M.D. and Tvedten H. In Small animal clinical diagnosis by laboratory methods, St. Louis, Missouri, USA. pp: 63. Tuan, T.A., Ha, N.T.T., Xoay, T.D., My, T.T.K., Nghiem, L.T. and Dien, T.M. 2021. Hypocoagulable tendency on thromboelastometry associated with severity and anticoagulation timing in pediatric septic shock: a prospective observational study. Front. Pediatr. 9, 676565. Tune, J.D., Baker, H.E., Berwick, Z., Moberly, S.P., Casalini, E.D., Noblet, J.N., Zhen, E., Kowala, M.C., Christe, M.E. and Goodwill, A.G. 2020. Distinct hemodynamic responses to (pyr) apelin-13 in large animal models. Am. J. Physiol. Heart Circ Physiol. 318(4), H747–H755. Tucker, C., Winner, A., Reeves, R., Cooper, E.S., Hall, K., Schildt, J., Brown, D. and Guillaumin, J. 2022. Resuscitation patterns and massive transfusion for the critical bleeding dog—a multicentric retrospective study of 69 cases (2007–2013). Front. Vet. Sci. 8, 788226. Villarroel, J.P.P., Guan, Y., Werlin, E., Selak, M.A., Becker, L.B. and Sims, C.A. 2013. Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression. J. Trauma Acute Care Surg. 75(1), 24–31. Wang, L., Lou, J., Cao, J., Wang, T., Liu, J. and Mi, W. 2021. Bicarbonate Ringer’s solution for early resuscitation in hemorrhagic shock rabbits. Ann. Transl. Med. 9(6), 462. Woodcock, T.E. and Woodcock, T. 2012. Revised starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 108(3), 384–394. Woolley, T., Gwyther, R., Parmar, K., Kirkman, E., Watts, S., Midwinter, M., Lucca, J.D. and Hunt, B.J. 2020. A prospective observational study of acute traumatic coagulopathy in traumatic bleeding from the battlefield. Transfusion 60, S52–S61. Yuan, H., Liu, C., Wang, H., Wang, L. and Sun, F. 2022. Optimization and comparison of models for core temperature prediction of mother rabbits using infrared thermography. Infrared Phys. Technol. 120, 103987. Zaets, S.B., Berezina, T.L., Morgan, C., Kamiyama, M., Spolarics, Z., Xu, D.Z., Deitch, E.A. and Machiedo, G.W. 2003. Effect of trauma-hemorrhagic shock on red blood cell deformability and shape. Shock 19(3), 268–273. | ||
| How to Cite this Article |
| Pubmed Style Rahmiati DU, Gunanti G, Noviana D, Soehartono RH, Harlina E. A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Vet. J.. 2025; 15(3): 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 Web Style Rahmiati DU, Gunanti G, Noviana D, Soehartono RH, Harlina E. A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. https://www.openveterinaryjournal.com/?mno=218464 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.17 AMA (American Medical Association) Style Rahmiati DU, Gunanti G, Noviana D, Soehartono RH, Harlina E. A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Vet. J.. 2025; 15(3): 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 Vancouver/ICMJE Style Rahmiati DU, Gunanti G, Noviana D, Soehartono RH, Harlina E. A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 Harvard Style Rahmiati, D. U., Gunanti, . G., Noviana, . D., Soehartono, . R. H. & Harlina, . E. (2025) A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Vet. J., 15 (3), 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 Turabian Style Rahmiati, Dwi Utari, Gunanti Gunanti, Deni Noviana, Raden Harry Soehartono, and Eva Harlina. 2025. A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Veterinary Journal, 15 (3), 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 Chicago Style Rahmiati, Dwi Utari, Gunanti Gunanti, Deni Noviana, Raden Harry Soehartono, and Eva Harlina. "A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models." Open Veterinary Journal 15 (2025), 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 MLA (The Modern Language Association) Style Rahmiati, Dwi Utari, Gunanti Gunanti, Deni Noviana, Raden Harry Soehartono, and Eva Harlina. "A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models." Open Veterinary Journal 15.3 (2025), 1253-1263. Print. doi:10.5455/OVJ.2025.v15.i3.17 APA (American Psychological Association) Style Rahmiati, D. U., Gunanti, . G., Noviana, . D., Soehartono, . R. H. & Harlina, . E. (2025) A comprehensive overview of fixed-volume hemorrhage effects in New Zealand White rabbit models. Open Veterinary Journal, 15 (3), 1253-1263. doi:10.5455/OVJ.2025.v15.i3.17 |