| Research Article | ||
Open Vet. J.. 2024; 14(11): 3055-3062 Open Veterinary Journal, (2024), Vol. 14(11): 3055-3062 Research Article Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake ManzalaHadeer Abd El-hak Rashed1, Layla Omran Elmajdoub2, Eman Fayad3* and Ali Hussein Abu Almaaty11Zoology Department, Faculty of Science, Port Said University, Port Said, Egypt 2Zoology Department, Faculty of Science, Misurata University, Misurata, Libya 3Department of Biotechnology, College of Sciences, Taif University, Taif, Saudi Arabia *Corresponding Author: Eman Fayad. Department of Biotechnology, College of Sciences, Taif University, Taif, Saudi Arabia. Email: e.esmail [at] tu.edu.sa Submitted: 13/09/2024 Accepted: 16/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Oreochromis aureus is a member of the tilapia family that is considered one of the main food sources in different countries all over the world. Aim: Thus, studying the different pathogens infecting tilapia species is still one of the interesting search points. Methods: One hundred and eighty specimens of O. aureus were collected from Lake Manzala and transported directly in iceboxes to the laboratory of the faculty of Science at Port Said University. The fish were categorized into two groups depending on their sex. 0.1 g of muscles and the internal organs were examined. Statistical epidemiological parameters were used based on the sex of the fish. The histological changes of the most infected organs of each syndrome were detected. Results: Diplopstomiasis was more prevalent in females than in males. On the contrary, acanthamoebiasis was more abundant in males than females. Muscles and the liver were the most infected organs in diplopstomiasis and acanthamoebiasis, respectively. Muscles suffered from severe deformations in the bundles, while there were many granulomatous areas in the liver and there were inflammatory immune cellular infiltrations in both tissues. Conclusion: Besides diplopstomiasis, acanthamoebiasis was observed in all examined organs of the study samples, with different distributions and severity in these organs. Further investigations were recommended for more understanding of these pathological syndromes and their relationship to their host. Keywords: Diplopstomiasis, Acanthamoebiasis, Severity, Distribution, Histopathology. IntroductionOreochromis aureus, commonly known as blue tilapia, is a species of the Cichlidae family. The members of this family are mainly known as tilapia (Cichlids). Tilapia is native to western and northern Africa, the Middle East, Central and South America, and southern India (William, 2015). Studying the prevalence and potential effect of parasites infecting fish can be essential for explaining the patterns related to the dynamics of fish populations and community structure (Quist et al., 2007). Parasites can also commercially affect fisheries (Timi and Mackenzie, 2015). For many countries, tilapia is one of the main food sources, so studying the different pathogens infecting tilapia is still one of the most interesting research points for many researchers (Rashied et al., 2016; Abiyu et al., 2020; Al Malki et al., 2021; Ayoub et al., 2021). Tilapia species are exposed to either single or multiple pathogens such as bacterial, parasitic, or mixed infections that lead to diseases and mortalities (Shoemaker et al., 2008). Understanding the prevalence and potential effect of pathogens in fish can contribute to explaining the patterns related to the dynamics of fish populations and community structure (Quist et al., 2007). Black-spot syndrome, or diplopstomiasis, is a condition characterized by the presence of black spots on the fins and body (Elmer et al., 2019). The syndrome is caused by Neascus-metacercaria, the larval stage of a diplostomid trematode that encysts in fish as an intermediate host (Moema et al., 2013; Achatz et al., 2019). These black spots are visible melanocytes that form around the metacercariae cysts in fish species (Duru et al., 1981). The presence of amoeboid organisms about fish has been reported since the beginning of the 20th century. In some cases, these descriptions were limited to the observation only or even the isolation of amoebae from fish, without a clear description of the specific relationship with the fish or the specific tissue (Martínez-Lara et al., 2021). There were few data available recording the presence of Acanthamoeba sp. in fish species because they are amphizoic species, i.e., free-living forms that can colonies fish under certain specific conditions (Bruno et al., 2006). This study is aimed at evaluating the occurrence of two harmful pathological syndromes infecting one of the most common fish species, which is considered one of the main food sources all over the world. Furthermore, focusing on their histopathological effects on their hosts. Materials and MethodsStudy areaOreochromis aureus source was Lake Manzala, which is the biggest coastal lake in Egypt; it is located in the northeast quadrant of the Nile Delta, between 31.2743° or 31° 16’ 27.5” north latitude and 32.2317° or 32° 13’ 54” east longitude. It is bounded to the north by the Mediterranean Sea, to the east by the Suez Canal, to the northwest by Damietta Province, and to the southwest by Dakahlia Province. The Lake is 35 km in length and 30 km in width (Rashad and Abdel-Azeem, 2010) (Fig. 1). Sampling of the specimensOne hundred eighty specimens of O. aureus were collected in May and July of 2024. The samples were then transported directly in iceboxes to the laboratory of the faculty of Science at Port Said University to be examined. The fish species identification was made according to the morphological descriptions of Boschung and Mayden (2004). Fish were categorized into two groups depending on their sex. Sampling for parasiteAfter the dissection of the examined specimens, a constant weight of 0.1 g from each organ (muscles, liver, kidneys, gonads, and digestive tract) (Fig. 2) was prepared for microscopic examination to detect the presence of diplopstomiasis and acanthamoebiasis. The specimens were washed in 0.9% NaCl for the removal of blood to facilitate the microscopic examination. The degree of the detected pathogens was categorized according to Table 1. Parasites occurrenceBush et al. (1997) provided the formula for the parasitological indicators, represented by the prevalence, abundance, and intensity. The percentage of infected samples is referred to as the prevalence. The average number of parasites detected in the evaluated samples is indicated by the term “mean abundance.” Conversely, mean intensity denotes the average number of parasites found in all the infected fish. Besides, parasite occurrence was calculated based on the fish sex. Histopathological studyFor the histological examination, specimens were fixed in 10% buffered formalin. Sections were stained with hematoxylin and eosin according to the standard laboratory procedures (Bancroft and Stevens, 1990). Fish sex was detected based on the microscopic examination of the gonads.
Fig. 1. Satellite view of Lake Manzala.
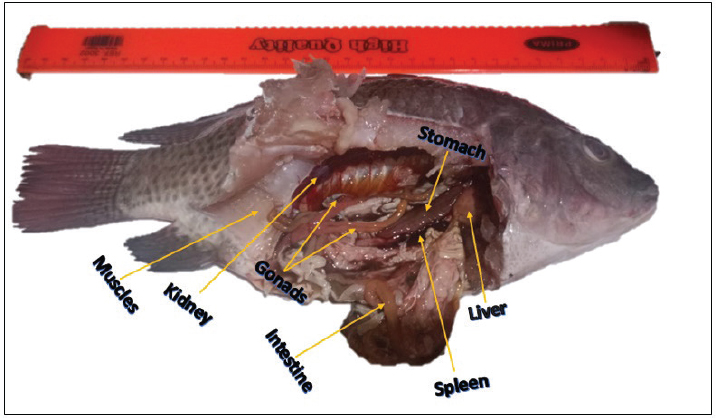
Fig. 2. Photograph of the visceral cavity of O. aureus. Table 1. Pathogen severity.
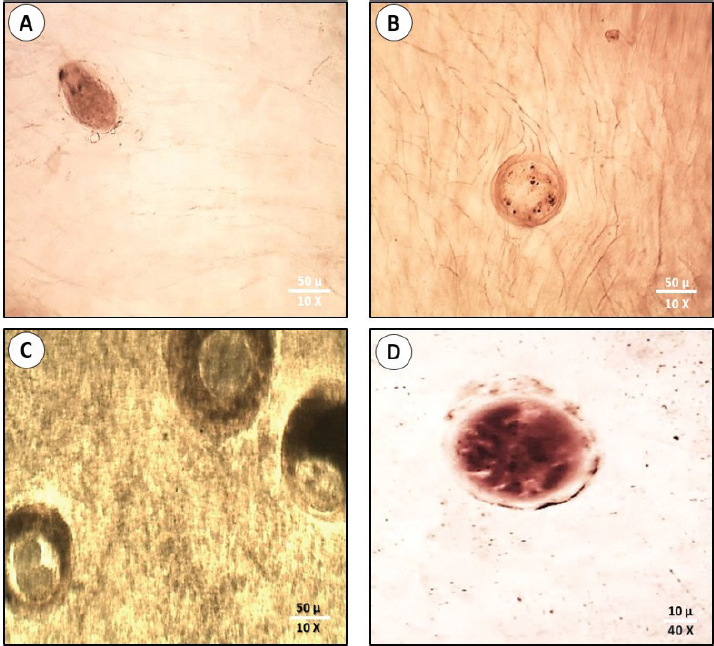
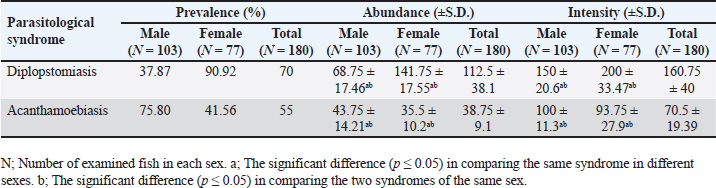
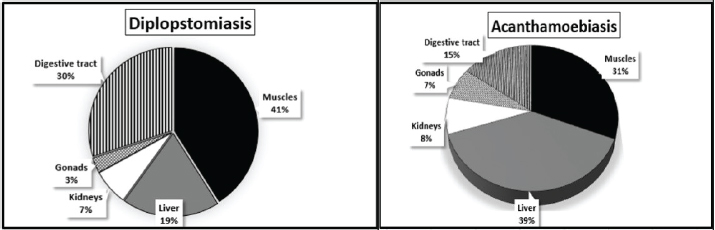
Statistical analysisThe intensity abundance and pathogen severity results were expressed as the mean ± standard deviation (S.D.). The analysis of variance by T-test was done using the Statistic Program Sigma Stat, version 20 to test significant differences between varied sexes or syndromes for the dominance of parasitic infection. A p-value of p ≤ 0.05 was considered statistically significant. Ethical approvalThis study was confirmed by the research ethical committee of the Faculty of Science, Suez Canal University (protocol number: REC222/2023). ResultsFrom the examined samples, some infections with metacercariae belonging to the Heterophyidae family were recorded. These Heterophyidae pathogens were not abundant in O. aureus fish collected from the study area. Hence, we focused on the most dominant pathological syndromes represented by diplopstomiasis, and acanthamoebiasis. The diplopstomiasis cyst was found in an oval to spherical form with a granulated grey appearance and two dark spots (eyes pot). In this study, diplopstomiasis was observed in the tissues at three levels according to the accumulation of the host’s cyst surrounding the pathogen. In Figure 3A, there was no encystation yet with a free pathogen, the degree of encystation increased to a moderate level (Fig. 3B), and finally, the highest degree of encystation was observed in Figure 3C. Acanthamoebiasis was distinguished by its rounded cyst and radical internal composition (Fig. 3D). The occurrence of the two pathogens is summarized in Table 2. Diplopstomiasis recorded a higher incidence in the total values of the prevalence of 70%, abundance 112.5 ± 38.1, and intensity of 160.75 ± 40 than that of acanthamoebiasis which recorded 55%, 38.75 ± 9.1, and 70.5 ± 19.39, respectively. In both syndromes, the occurrence was affected according to the sex of the sample. Diplopstomiasis was more prevalent in females 90.92% than male hosts 37.87%. Otherwise, acanthamoebiasis was more abundant in males than females recording a prevalence of 75.80%, and 41.56%, respectively. The distribution percentage of the two parasites in the different tissues (muscles, liver, kidneys, gonads, and the digestive tract) is shown in Figure 4. The highest distribution of diplopstomiasis was recorded in the muscles at 41%, followed by the digestive tract at 30%. Moreover, acanthamoebiasis was distributed within the different tissues, with the highest recorded value in the liver at 39%, followed by the muscles. The lowest distribution values of both parasites were recorded in the gonads.
Fig. 3. Diplopstomiasis and Acanthamoebiasis forms in the examined fish. Diplopstomiasis pathogen free of cyst (A). Diplopstomiasis with moderate encystation (B). Diplopstomiasis with severe encystation (C). Acanthamoebiasis cyst (D). Table 2. Incidence of the parasitological syndromes according to host sex.
Fig. 4. Percentage of pathogens distribution in different organs.
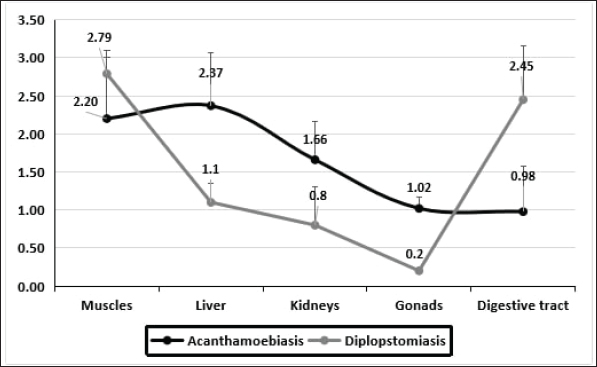
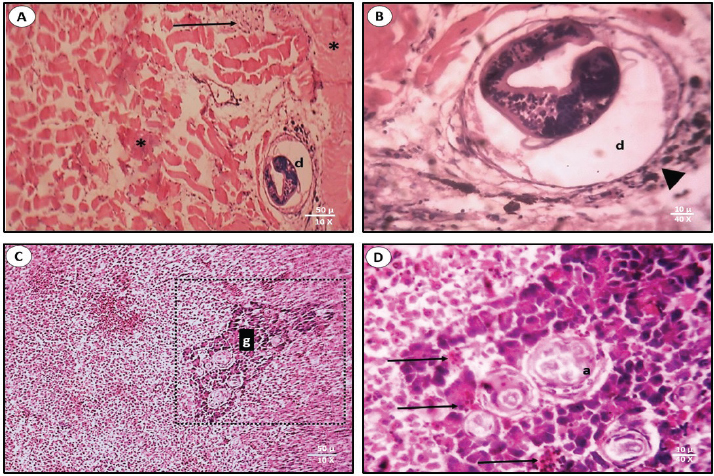
Fig. 5. The average of the parasitic syndromes severity in each organ. According to the number of infections in all studied samples, the severity of the infections with the two syndromes is shown in Figure 5. The sequence from the severe to the mild infection of the two pathogens in the different tissues influenced the distribution percentage from the highest to the lowest. The highest infection of diplopstomiasis was recorded in the muscles at 2.79 ± 0.60, while in acanthamoebiasis, the highest average was recorded in the liver at 2.37 ± 0.70. This section focuses on the histopathological lesions of both pathogens on the most affected tissue. Thus, muscles and liver tissues were examined for diplopstomiasis, and acanthamoebiasis, respectively. Diplopstomiasis causes deformations in muscle fiber bundles with inflammatory cellular infiltration (Fig. 6A). Diplopstomiasis was found in the form of encysted metacercariae of the parasite (Fig. 6A). Increasing the magnification power reflects the black pigmented melanin surrounding the cyst (Fig. 6B). Acanthamoebiasis caused severe observed damage in the area surrounding the parasitic cyst in the liver section (Fig. 6C). This deformed area, granuloma, is darkly stained and also characterized by cellular infiltration (Fig. 6D).
Fig. 6. Sections of the parasitic syndromes in the most impacted tissues. Diplopstomiasis in muscle fibers (A&B). Acanthamoebiasis in the liver tissue (C&D). d: diplopstomiasism, a: acanthamoebiasis, g: granuloma. Black asterisk: damaged fibrous area; black arrowhead: melanin granules; black arrow: cellular infiltration. DiscussionThe laboratory diagnosis and the detection of the prevalence of the different lesions that happen in a certain region aid in guiding the pathologist toward the etiological diagnosis (Junior et al., 2021). Therefore, in this research, we focus on two pathogens in the study area, hoping for further future studies correlated with the control of these parasites. Pathogen dominance in fish varies by sex due to hormonal influences, immune response differences, and behavior. Males’ riskier behaviors also increase their exposure to pathogens. Furthermore, physiological differences and some pathogens’ preferences for specific sexes contribute to distinct infection patterns, illustrating the complex interactions affecting fish health (Poulin, 1996; Gbankoto et al., 2001; Barber and Scharsack, 2010; Klein and Flanagan, 2016). There were not any available results recorded previously that revealed the presence of diplopstomiasis in O. aureus samples from the study area. The females (p < 0.001) were more susceptible to the diplopstomiasis than males. This is agreed with a recent study performed on the same parasite but in different species, Oreochromis niloticus (Charo-Karisa et al., 2021). On the other hand, acanthamoebiasis was more abundant (p < 0.04) in males than in females. Both syndromes were recorded in specific organs of the examined samples (Bruno et al., 2006; Charo-Karisa et al., 2021). At present, the pathogens are found in all tissues. This may be due to the differences in species or study areas with different environmental parameters, which affect immunity and hence the degree of infection in the hosts (McCormick, 2011; Larsen et al., 2018). Muscles recorded the highest values in the percentage of infection among all examined fish and also the average infection severity in the case of diplopstomiasis helminthic infection. This agrees with Radwan et al. (2022), who found that muscles recorded the highest values of parasitic infection of helminths in Oreochromis niloticus. Although the most elevated values of the protozoal infection acanthamoebiasis were recorded in the liver, the infection was also recorded in the rest of the tissues. This disagrees with Bruno et al. (2006), who reported the infection in the liver tissues only. The lowest recorded values of percentage of pathogen infection were recorded in the gonads, followed by the kidneys in both syndromes. According to our knowledge, there is no specific reason for the causes in the case of gonads. The low values in the kidneys may be due to their acting as the main organ of the immune system in bony fish (Zapata et al., 2006). Immune cells are found over the entire kidney as the anterior kidney has the highest concentration of developing B-lymphoid cells (Meseguer et al., 1995). The degree of infection and the type of organ affected can influence the number of histopathological responses. These responses can range from encapsulation of the parasite by host immune cells to acute and chronic inflammation and finally necrosis (Secombes, and Chappell, 1996). Regarding diplopstomiasis, there were malformations in the muscle bundles that in turn affected the arrangement of these bundles. This is due to the settling of the metacercaria of the parasitic trematode in between. There was a cellular infiltration of the immune cells and chromatophores responsible for the formation of dark spot syndrome. These observations agree with Charo-Karisa et al. (2021) and Flores-Lopes and Thomaz (2011). A granuloma can be known as a mass of granulation cells that are regularly stimulated in response to infections, inflammation, or the presence of foreign material and constitutes a strategy of the immune system to isolate pathogens or subject’s incapable of being eliminated (Martínez-Lara et al., 2021). The infection resulted in a severe granuloma composed of layers of cells and fibers surrounding the parasitic cyst. The area of granulomatous reaction is heavily stained and can be observed. Bruno et al. (2006) recorded the presence of trophozoites of Acanthamoeba sp. in the liver sections of Cyprinus carpi. ConclusionDiplopstomiasis was more prevalent in females while acanthamoebiasis was more abundant in males. Both syndromes were observed in all examined organs in the study samples, with different distributions and severity in these organs. Further investigations were recommended for more understanding of these pathological syndromes and their relation to their host and how to overcome these pathogens. AcknowledgmentThe authors would like to thank Port Said University. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo fund. Authors’ contributionAll authors contributed equally. All authors read, revised, and approved the final manuscript. Data availabilityReferencesAbiyu, M., Mekonnen, G. and. Hailay, K. 2020. Prevalence of internal nematode parasites of Nile tilapia. Oreochromis niloticus. J. Aquac. Res. Dev. 11, 582–589. Achatz, T.J., Curran, S.S., Patitucci, K.F., Fecchio, A. and Tkach, V.V. 2019. Phylogenetic affinities of Uvulifer Spp. (Digenea: Diplostomidae) in the Americas with description of two new species from Peruvian Amazon. J. Parasitol. 105, 704–717. Al Malki, S., Mohammadein, A., Ramadan, A.M., Abd El-Atti, M. and Elraey, S.M.A. 2021. Drastic parasitic infestations among cultured tilapias at El-Abbassa fish farms, Egypt, with respect to stressors of abiotic factors. Egypt. J. Aquat. Biol. Fish. 25(3), 281–295. Ayoub, H.F., Eman, Y., Tohamy, E.Y., Salma, H.M. and Mohamed, S.S. 2021. Isolation, identification and antimicrobial profile of Aeromonas spp., Pseudomonas spp. and Vibrio spp. from the Nile Tilapia, Oreochromis niloticus in fish farms. Egypt. J. Aquat. Biol. Fish. 25(3), 171–185. Bancroft, J.D. and Stevens, G.A. 1990. Theory and practice of histological techniques, 2nd edition. London, UK: Churchill Livingstone. Barber, I. and Scharsack, J.P. 2010. The three-spined stickleback–Schistocephalus solidus system: an experimental test of evolutionary hypotheses.Parasitol. 137(5), 411–424. Boschung, H.T. and Mayden, R.L. 2004. Fishes of Alabama. Washington, D.C.: Smithsonian Books, pp: 736. Bruno, D.W., Nowak, B. and Elliott, D.G. 2006. Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Dis. Aquat. Organ. 70(1-2), 1–36. Bush, A.O., Lafferty, K.D., Lotz, J.M. and Shostak, A.W. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83, 575–583. Charo-Karisa, H., Ali, S.E., Marijani, E., Ibrahim, N.A., Trinh, T.Q., Chadag M.V. and Benzie, J.A. 2021. Genetic parameters for black spot disease (diplopstomiasis) caused by Uvulifer sp. infection in Nile tilapia (Oreochromis niloticus L.). Aquac. 532, 736039. Duru, C., Johnson A.D. and Blouin, E.1981. Neascus pyriformis chandler, 1951 (trematoda: Diplostomatidae), redescription and incidence in fishes from Brule Creek, South Dakota. Proc. Helminthol. Soc. Wash. 48, 177–183. Elmer, F.E., Kohl, Z.F., Johnson P.T.J. and Peachey, R.B.J. 2019. Black spot syndrome in reef fishes: using archival imagery and field surveys to characterize spatial and temproal distribution in the Caribbean. Coral. Reefs. 38(6), 1303–1315. Flores-Lopes, F. and Thomaz, A.T. 2011. Assessment of environmental quality through analysis of frequency of the black spot disease in an assemblage of fish, Guaíba Lake, RS, Brazil. Braz. J. Biol. 71, 915–923. Gbankoto, A., Pampoulie, C., Marques A. and Sakiti, G.N. 2001. Occurrence of myxosporean parasites in the gills of two tilapia species from Lake Nokoué (Bénin, West Africa): effect of host size and sex, and seasonal patterns of infection. Dis. Aqua. Organ. 44, 217–222. Junior, J.A.F., Cardoso, S.P., da Fonseca, N.D.S., Nascimento, K.A., Rodrigues, F., da Rocha, G.C., Macêdo, J.T.S.A. and Pedroso, P.M.O. 2021. Parasitic lesions in fish in the federal district, Brazil. Acta. Sci. Vet. 49, 1818. Klein, S.L. and Flanagan, K.L. 2016. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638. Larsen, A.K., Nymo, I.H., Sørensen, K.K., Seppola, M., Rødven, R., Jiménez de Bagüés, M.P., Al Dahouk S. and Godfroid, J. 2018. Concomitant temperature stress and immune activation may increase mortality despite efficient clearance of an intracellular bacterial infection in Atlantic cod. Front. Microbiol. 9, 2963. Martínez-Lara, P., Martínez-Porchas, M., Gollas-Galván, T., Hernández-López J. and Robles-Porchas, G.R. 2021. Granulomatosis in fish aquaculture: a mini review. Rev. Aquac. 13(1), 259–268. McCormick, S.D. 2011. Hormonal control of metabolism and ionic regulation: the hormonal control of osmoregulation in teleost fish. In encyclopedia of fish physiology: from genome to environment. Ed. A.P. Farrell, Cambridge, UK: Academic Press, pp: 1466–1473. Meseguer, J., lopez-Ruiz, A. and Garcia-Ayala, A. 1995. Reticulo-endothelial stroma of the head-kidney from the seawater teleost gilthead seabream (Sparus aurata L.): an ultrastructural and cytochemical study. Anat. Rec. 241(3), 303–309. Moema, E.B.E., King, P.H., Rakgole, J.N. and Baker, C. 2013. Descriptions of diplostomid metacercariae (Digenea: Diplostomidae) from freshwater fishes in the Tshwane area. J. Vet. Res. 80, 1–7. Poulin, R. 1996. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 147(2), 287–295. Quist, M.C., Bower, M.R. and Hubert, W.A. 2007. Infection by a black spot-causing species of Uvulifer and associated opercular alterations in fishes from a high-desert stream in Wyoming. Dis. Aquat. Organ. 78(2), 129–136. Radwan, M., Abbas, M.M.M., Afifi, M.A.M., Mohammadein, A. and Al Malki, J.S. 2022. Fish parasites and heavy metals relationship in wild and cultivated fish as potential health risk assessment in Egypt. Front. Environ. Sci. 10, 890039. Rashad, H.M. and Abdel-Azeem, A.M. 2010. Lake Manzala, Egypt: A bibliography. Assiut Univ. J. Bot. 39(1), 253–289. Rashied, H.A., Abu Almaaty, A.H., Hassan, E.A. and Soliman, F.M. 2016. Comparative study of the effect of biological factors on helminthes occurrence in Oreochromis niloticus and Tilapia zilli from Lake Manzala, Port Said, Egypt. Egypt. Acad. J. Biol. Sci., 8(1), 25–32. Secombes, C.A. and L.H. Chappell, 1996. Fish immune responses to experimental and natural infection with helminth parasites. Annu. Rev. Fish. Dis. 6, 167–177. Shoemaker, C.A., Xu, D.H., Klesius P.H. and Evans, J.J. 2008. Concurrent infections (parasitism and bacterial disease) in tilapia, in proceedings of the 8th international symposium on tilapia in aquaculture, Cairo, Egypt, pp: 1365–1375. Timi, J.T. and Mackenzie, K. 2015. Parasites in fisheries and mariculture. Parasitol. 142(1), 1–4. William, C.H., 2015. Evaluation of blue tilapia (Oreochromis aureus) for Duckweed (Lemna minor) Control in South Carolina’s Private Waters. Ph.D. Dissertation, Clemson Univ., Clemson, SC, USA. Zapata, A., Diez, B., Cejalvo, T., Gutierrez, T., de Frias, C. and Cortes, A. 2006. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 20(2), 126–136; doi: 10.1016/j.fsi.2004.09.005. | ||
| How to Cite this Article |
| Pubmed Style Rashed HAE, Elmajdoub LO, Fayad E, Abu-almaaty AH. Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Vet. J.. 2024; 14(11): 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 Web Style Rashed HAE, Elmajdoub LO, Fayad E, Abu-almaaty AH. Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. https://www.openveterinaryjournal.com/?mno=219659 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.34 AMA (American Medical Association) Style Rashed HAE, Elmajdoub LO, Fayad E, Abu-almaaty AH. Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Vet. J.. 2024; 14(11): 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 Vancouver/ICMJE Style Rashed HAE, Elmajdoub LO, Fayad E, Abu-almaaty AH. Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 Harvard Style Rashed, H. A. E., Elmajdoub, . L. O., Fayad, . E. & Abu-almaaty, . A. H. (2024) Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Vet. J., 14 (11), 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 Turabian Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu-almaaty. 2024. Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Veterinary Journal, 14 (11), 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 Chicago Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu-almaaty. "Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala." Open Veterinary Journal 14 (2024), 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 MLA (The Modern Language Association) Style Rashed, Hadeer Abd El-hak, Layla Omran Elmajdoub, Eman Fayad, and Ali Hussein Abu-almaaty. "Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala." Open Veterinary Journal 14.11 (2024), 3055-3062. Print. doi:10.5455/OVJ.2024.v14.i11.34 APA (American Psychological Association) Style Rashed, H. A. E., Elmajdoub, . L. O., Fayad, . E. & Abu-almaaty, . A. H. (2024) Diplopstomiasis and acanthamoebiasis syndromes in the different organs of Oreochromis aureus collected from Lake Manzala. Open Veterinary Journal, 14 (11), 3055-3062. doi:10.5455/OVJ.2024.v14.i11.34 |