| Research Article | ||
Open Vet. J.. 2024; 14(11): 3132-3143 Open Veterinary Journal, (2024), Vol. 14(11): 3132-3143 Research Article Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged maresAmal Mahmoud Aboelmaaty1*, Abdalla E. A. Elgharieb2, Hazem Ahmed El-Debaky1, Jamal M. H. Alkhadrawy2,3, Mostafa M. Abou-Ahmed2 and Abdelraouf M. Ghallab21Animal Reproduction and AI Department, Veterinary Research Institute, National Research Centre, Giza, Egypt 2Theriogenology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 3National Center of Animal Health, Ministry of Agriculture, Livestock and Marine Resources, Tripoli, Libya *Corresponding Author: Amal Mahmoud Aboelmaaty. Animal Reproduction and AI Department, Veterinary Research Institute, National Research Centre, Giza, Egypt. Email: am.aly [at] nrc.sci.eg; amalaboelmaaty1 [at] yahoo.com Submitted: 28/09/2024 Accepted: 03/11/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
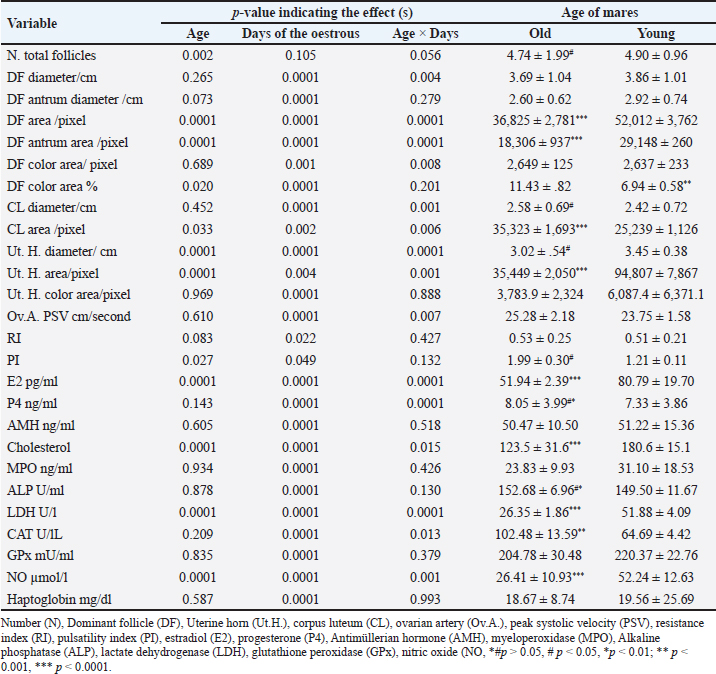
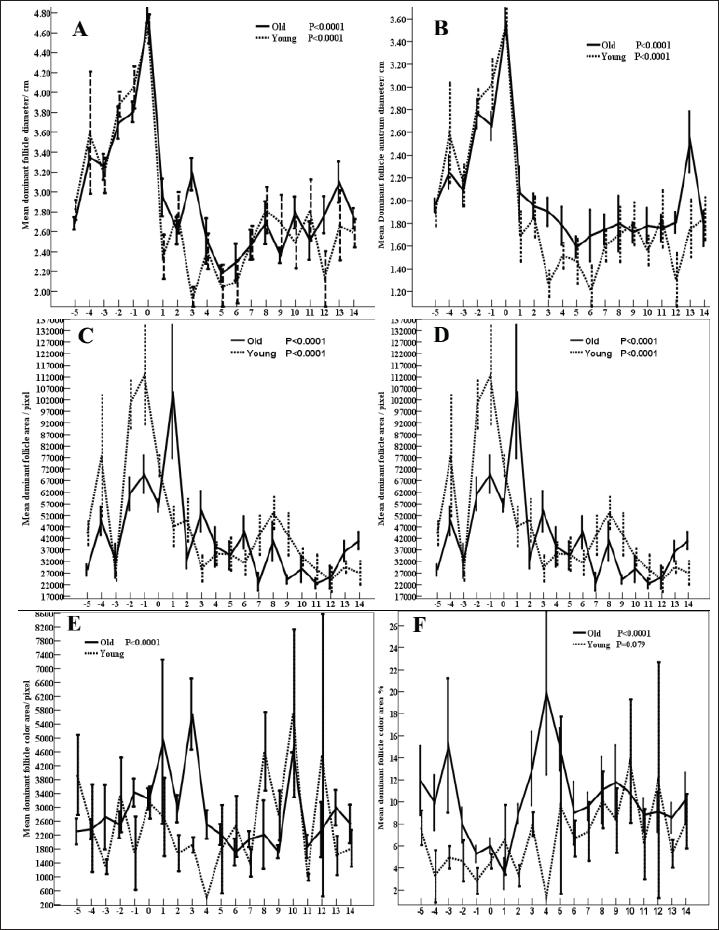
AbstractBackground: Senility influences fertility in women and companion animals, especially horses. Aim: This study aimed to investigate the effect of aging in horses on the daily changes in the dominant follicle (DF) dynamics and hemodynamics, antimüllerian hormone (AMH), enzymes, antioxidants, and ovarian hormones during the estrous cycle. Methods: Ovaries of old mares (n=5, age >20 years) and young native mares (n=6, age <10 years) were scanned during 6 different estrous cycles from March 2022 to August 2023 with Doppler ultrasound. The DF diameter and color area, the corpus luteum (CL) diameter, and the uterine horn area and color area were determined. In collected blood samples with each ultrasound and Doppler scanning, AMH, estradiol (E2), progesterone (P4), cholesterol, myeloperoxidase, catalase, glutathione peroxidase, lactate dehydrogenase (LDH), alkaline phosphatase, and nitric oxide (NO) concentrations were determined. Results: Age significantly affected the DF area ( p < 0.0001), color area ( p < 0.0001), color area % (p=0.020), CL area (p=0.033), uterine horn area ( p < 0.0001), ovarian artery pulsatility index (PI, p < 0.05), E2 (p < 0.001), cholesterol ( p < 0.0001), LDH ( p < 0.0001), and NO ( p < 0.0001). Aging tended (p > 0.05) to decrease the DF antrum diameter but significantly decreased (p < 0.05) its area, antrum area, and color area % in addition to the decrease (p < 0.01) in the uterine horn diameter and area, estradiol, total cholesterol, LDH, NO, aging increased (p < 0.05) CL area and the ovarian artery PI, and tended (p > 0.05) to increase the ovarian artery resistance index. Conclusion: Aging in mares did not disturb AMH, the ovarian macro-environmental dynamics, follicle growth, and recruiting but the disrupted blood flow mediators, enzymes, and antioxidant status may affect the intrafollicular mediators and influence the oocyte quality. Keywords: Mares, Aging, Antimüllerian hormone, Ovarian hormones, Luteal dynamics. IntroductionWithin the equine oocytes and follicular granulosa cells, aging impaired mitochondrial metabolic functions and increased oxidative stress (Catandi et al., 2023). The increased abundance of glutamic acid and triglycerides is associated declined abundance of ceramides in aged mares’ oocytes and somatic follicular cells in addition to the decrease in the abundance of alanine in all follicular cell types (Catandi et al., 2023). These alterations in amino acids carbohydrates and lipids impaired the transfer of essential carbohydrates and free fatty acid substrates from cumulus cells to the oocytes leading to the disruption of trans zonal projections between all these cell types (Catandi et al., 2023). Though aging or senility were not related to the decrease in the ovarian reserve and fertility in mares (Claes et al., 2016) previous research has reported that the increase in the maternal age was accompanied by a reduction in the ovarian reserve (Ball, 2011). In cyclic mares, higher concentrations of cholesterol were determined in the follicular fluid in comparison to the determination of low concentration in blood serum meanwhile the diameter of the dominant follicle (DF) increased from 2.0 to >4.0 cm (Satué et al., 2019). This difference was not reported in buffaloes, where cholesterol concentrations did not vary with the increase in the diameter of medium-sized follicles to a larger and dominant one (Abd Ellah et al., 2010). Antimüllerian hormone (AMH) is one of the hormones that was determined to determine the ovarian reserve in women. In mares, it was reported that AMH is secreted from the granulosa cells of growing and small antral follicles (Ball et al., 2008). The determination of AMH enabled the prediction of follicular growth in mares (Vernunft et al., 2011). In seasonal breeding mares and winter anestrus, to predict the onset of ovarian cyclicity in the transitional period, AMH was evaluated in the blood plasma as an early indicator of the development and ovulation of the ovarian follicles from the anovulatory phase to the cyclic phase at the start of their breeding season (Claes et al., 2016). Later report stated that the absence of any difference in the concentrations of AMH before and after the first ovulation in the breeding season did not support the use of AMH as a biomarker to predict the onset of cyclicity in non-pregnant mares or to detect early pregnancy in either mare with age <10 years or those of age >20 years (Fouché et al., 2022). MH regulated the number of primordial follicles involved in every growing follicular wave pool and its concentrations correlated with the antral follicle reserve on both ovaries (Vernunft et al., 2011). Pregnancy rate and AMH concentrations showed no association with age of the animal. AMH was very low and similar concentrations were reported in either normal cyclic or pregnant mares in addition to the absence of any effects of estrous cycle phase or the gestation age on its serum concentrations but its increased concentrations were greatly related to the development of granulosa cell tumors (Almeida et al., 2011). Myeloperoxidase (MPO) is an indicator of neutrophil activation during inflammation and infections such as endometritis. As an inflammatory marker, MPO was constantly detected in estrous mares in their uterine lumen under physiological conditions. When MPO concentrations were assayed in low-volume uterine wash from mares in the absence of endometritis during different phases of the estrous cycle or during their anestrus, it was reported that the higher concentrations that were recorded during the estrous phase and anestrus declined greatly during their diestrus (Parrilla Hernández et al., 2023). In the endometrial epithelial cells and neutrophils, MPO immune expression increased association with the increased glandular secretions during the estrous phase in mid and basal glands but the predominant intracytoplasmic apical reinforcement and the sporadic immune-positive glands in the diestruos phase (Parrilla Hernández et al., 2023). During normal physiological conditions of the estrous cycle, the endometrial cells produce the uterine MPO (Parrilla Hernández et al., 2023). Increased MPO concentrations during the estrous phase were not associated with uterine hyper-edema, intraluminal fluid, or positive cytology so it was not recommended to be used as a diagnostic tool for diagnosing endometritis (Parrilla Hernández et al., 2014). Therefore, this study’s objectives were to find out the effect of aging or senility on the daily fluctuations of AMH, ovarian hormones, follicular and luteal dynamics, oxidants-antioxidants, enzymatic status, and MPO during the estrous cycle of native young and senile mares. Material and MethodsAnimals, housing, and feedingBefore starting this study, the protocol was approved by the Animal Care and Ethical Use Committee of the Faculty of Veterinary Medicine at Cairo University (Approval ID: Vet- CU- 03162023708 and Vet-CU- 01122022586). Mares were examined before conducting this study to confirm that they are clinically healthy and normally cycling mares. Native mares included in this study were divided into old mares (N=5) of 20–26 years old. Old mares were granted for conducting research from the Police Horsey (El Basateen, Cairo). Young mares (N=6) of 6–10 years old were purchased from private horse studs (Al Haram, Giza). Mares were kept on the research farm of the Theriogenology Department, Faculty of Veterinary Medicine, Cairo University. Animals were kept in an open yard during the Day and housed indoors at night with artificial light. All animals were dewormed regularly and received medications for blood parasites. The study was conducted from October 2022 to August 2023. Ultrasonographic (US) and reproductive doppler examinationsFor performing reproductive DopplerUS examinations to determine ovarian and uterine dynamics and hemodynamics, the ultrasound-Doppler scanner (Sonovet R3, Madison, Samsung, South Korea) equipped with 12 MHz B-mode transrectal linear-array trans-rectal transducer was used. Traditional grey scale ultrasound was used to count the numbers and diameters of ovarian follicles throughout the estrous cycle. Once the DF reached 30 mm in diameter and was associated with uterine oedema and estrous behavior, the DF diameter and color signals were determined until reaching ovulation (Day 0). Ovulation day (Day 0) was determined by the day before the disappearance of the largest dominant preovulatory follicle reaching its maximum diameter with the presence of another smaller follicle (subordinate) or a corpus luteum (CL) developing at its site (Abo El-Maaty and Abdelnaby, 2017). After detecting ovulation, the CL developed at the site of the preovulatory follicle starts its growth phase and the increase in diameter (cm) was sequentially determined until Day 14 of ovulation was determined from its first appearance till the approach of the next ovulation (Abdelnaby and Abo El-Maaty, 2017b). Also, the CL area and color signal (pixel) were recorded using the color Doppler mode. Ovarian and uterine hemodynamicsThe DF dynamics and hemodynamics were determined for all animals from Day - 5 before ovulation till Day 14. The diameter and the antral diameter (cm) of the DF were determined in each ultrasound scan (Abdelnaby and Abo El-Maaty, 2017a). The DF area, antrum area, and color vascularization area (pixel) were estimated (Abo El-Maaty and Abdelnaby, 2017). The granulosa area was estimated by subtracting the antrum area from the follicle area. The estimation of follicular vascularization color percentage was calculated by dividing the DF color area (pixel) by the follicle area (pixel). With each ultrasound and Doppler examination, the uterine body and uterine horns diameters, area, and color signal (pixel) were determined (Abdelnaby et al., 2016). The ovarian arteries’ diameters, blood flow velocities, and blood flow indices were determined using the pulsed-wave Spectral Doppler mode (Bollwein et al., 2004). The ovarian artery peak systolic velocity (PSV cm/second), the end-diastolic velocity (END cm/second), and the time average mean velocity (TAMV cm/second), the pulsatility index (PI; PSV-EDV/TAMV) and resistance index (RI; PSV-EDV/PSV) indices and the blood flow volumes (BFV ml/minute) were determined for every ovarian according to Bollwein et al. (2004). The images and video clips were stored in the memory of the ultrasound and then were exported using a portable removable disk for later analysis using image analysis software. Image analysis software (Photoshop CC) was used to estimate the area, color area, and antrum area in pixels of the DF, in addition to the area and color area of the CL, uterine body, and uterine horns. Blood samplingBlood samples were collected from the jugular vein of each mare and placed into a tube containing anticoagulant for assessment of hormonal profiles (Progesterone, Estradiol) in plasma and another blank tube for harvesting serum tube to measure nitric oxide (NO), biochemical oxidants, and antioxidants analysis. Blood samples were kept on ice until centrifugation (10 minutes at 3,000 r.p.m) within 2 hours from collection. Plasma and sera were stored at −20°C until they were assayed. Hormone assayingThe ELISA assay commercial diagnostic kits were used to measure progesterone (P4) and estradiol (E2) hormones, Horse AMH, and Horse MPO. The range of P4 and E2 assays were between 0.0 to 40 ng/ml and 9.7 to 200 pg/ml. The sensitivity, intra- and inter-assay precisions of P4 and E2 assays were 0.045 ng/ml, 6.81%, and 7.25%, and 9.714 pg/ml, 6.86%, and 5.59%, respectively. Haptoglobin, lactate dehydrogenase (LDH), total cholesterol, total proteins, albumin, alkaline phosphatase (ALP), glutathione peroxidase (GPx), catalase (CAT), and NO were assayed using the colorimetric diagnostic kits. Statistical analysisData are presented as Mean ± SEM. Analysis of variance (ANOVA) was used to study the effect of days of estrous cycle on the measured parameters using SPSS 20.0. Duncan’s Multiple Range test was used to differentiate between significant means at p < 0.05. The effect of age (young vs. old) was performed using an independent sample t-test. Univariate General linear model was used to study the effect of age and days during the estrous cycle (Days -5 to 14) on the measured variables. Two-way ANOVA using repeated measure Univariate General Linear Mode Age (2 groups), Days of estrous cycle (20 days; Days -5 to 14), and the interaction of Age × Days of estrous cycle (2 groups ×20 days) was performed. ResultsAge (p < 0.002) of the mare and Age × Days (p=0.056) of the estrous cycle affected the ovarian follicle numbers (Table 1). Estrous days of old mares tended (p > 0.05; Fig. 4A) to influence the number of total follicles. Age of the mare showed no effect on DF diameter (DF, Fig. 1A) and DF color area (Fig. 1E) but influenced its area (p < 0.0001; Fig. 1C), antrum area (p < 0.0001; Fig. 1D), color area % (p=0.02; Fig. 1F) and tended to affect DF antrum diameter (p > 0.05). Generally, days of estrous (Table 1) and estrous days of old mares impacted (p < 0.0001; Fig. 1) DF diameter, DF antrum diameter, DF area, antrum area, DF color area, and DF color area % (Fig 1 A–F). The estrous days of young mares influenced ( p < 0.0001) DF diameter, antrum diameter, area, and antrum area (Fig. 1 A–D) but the estrous days of young mares tended to influence (p > 0.05) DF color area % (Fig. 1F) and did not affect the DF color area/pixels (Fig. 1E). Young mares have got DF of small diameter than old mares on days 3, 5,6, 12 and day 13 compared to old mares (Fig. 1A). DF antrum diameter of young mares is lower than those of old mares (Fig. 1B) on days 3, 6, and 12. DF area (Fig. 1C) and the DF antrum area (Fig. 1D) of young mares are higher than old mares on days −4, −2, −1, and 8. DF of old mares showed higher color area % (Fig. 1F) throughout the estrous cycle from Day −5 to Day 14 except Day 10. The CL diameter (CL, Table 1) is influenced by the Days of the estrous cycles ( p < 0.0001) and the interaction of Age × Days of estrous cycle (p < 0.001). The CL area is influenced by age (p=0.033), days of estrous (p=0.002), and Age × Days of estrous cycle (p=0.006). Days of the estrous of old mares impacted CL diameter (p < 0.001; Fig. 2A) and CL area (p < 0.0001; Fig. 2B). CL area tended to be influenced by days of the estrous of young mares (p > 0.05; Fig. 2B). A decrease in the CL diameter of young mares can be observed on Day 13 and an increase on Day 10 (Fig. 2A) compared to old ones. CL areas of young mares are lower or nearly similar to those of old ones except on Days 7 and 9 (Fig. 2B). The ovarian artery PSV, Table 1 is influenced by the days of the estrous cycle ( p < 0.0001) and Age × Days of estrous cycle (p=0.007). The estrous days of old mares influenced ( p < 0.0001) PSV (Fig. 2C). PSVs of old mares are lower than that of young ones on Days −4, −2 and Days 2–4 but peaked on Days 1, 8, and 12–14 (Fig. 2C). The ovarian artery PI of the ovarian artery (PI) is influenced by the age of the mare (p < 0.05), days of the estrous cycle (p < 0.05, Table 1). Days of the estrous of young mares ( p < 0.0001) and those of old mares (p=0.018) impacted the ovarian artery PSV (Fig. 2D). PI of old mares is higher than young mares on all days of the estrous cycle except Days −5, −1, 3, 6, and 8 (Fig. 2D). Table 1. Effect of age, days of oestrous (Days), Age × Days of oestrous, days of oestrous within young or old mares on different ovarian follicular count, DF, CL, uterine, and hormonal parameters.
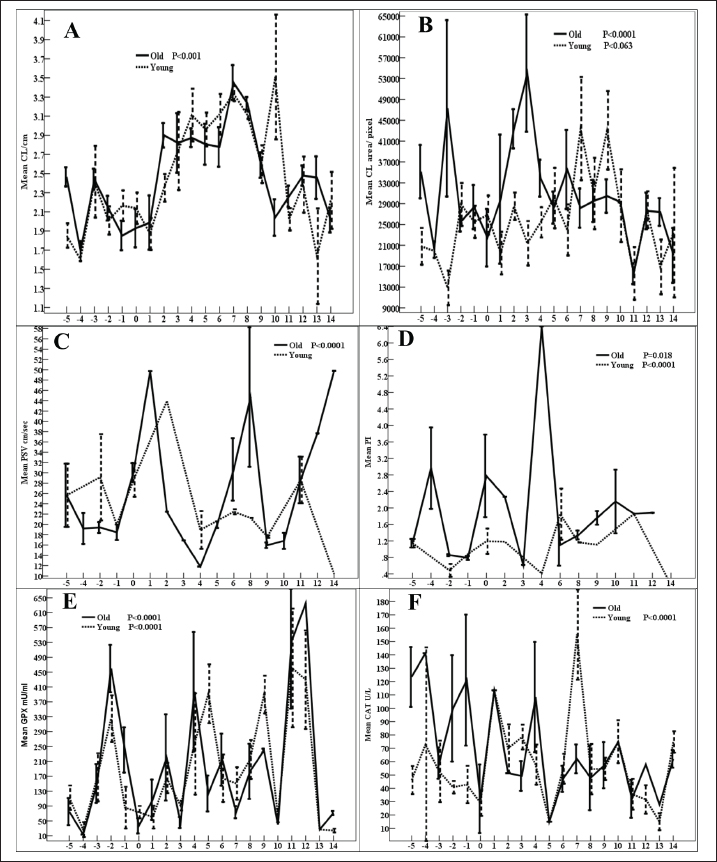
The uterine horn diameter (Table 1) and area are influenced by the age of the mare ( p < 0.0001), days of the estrous cycle (p < 0.001), and Age × Days of estrous cycle (p < 0.01). Days of the estrous of young (p < 0.05, Fig. 3E) and old ( p < 0.0001) mares impacted the uterine horn area. Old mares got smaller uterine horn areas than young mares throughout the estrous cycle (Fig. 3E). The uterine horn color area is influenced by the days of the estrus. Days of young and old (Fig. 3F) mares’ estrous cycles. On Day 9, the uterine horn color area of old mares (19,923 ± 7,283) is higher than those (8,847.86 ± 3,285) of young ones (Fig. 3F). GPx, Figure 2E are impacted by days of the estrous cycle ( p < 0.0001). GPX of young mares is higher than those of old mares on Days 5 and 9 but lower than mares on Days −2 and −1. CAT, Table 1 is influenced by days of the estrous cycle ( p < 0.0001) and Age × Days of estrous cycle (p=0.013). Days of the estrous cycle of young mares showed low (p < 0.0001, Fig. 2F) activities during Days −5 to 0 preovulation and on Day 12. Young mares got lower CAT during the preovulatory interval compared to old mares with exceptionally higher concentrations on Day 7.
Fig. 1. DF diameter/cm (A), Antrum diameter/cm (B), DF area/pixel (C), antrum area/ pixel (D), DF color area/pixel, and color area % (F) in old and young mares.
Fig. 2. Mean CL diameter/cm (CL, A), CL area/pixel (B), ovarian artery (PSV, C), ovarian artery (PI, D), (GPx mU/ml, E), and (CAT U/l, F).
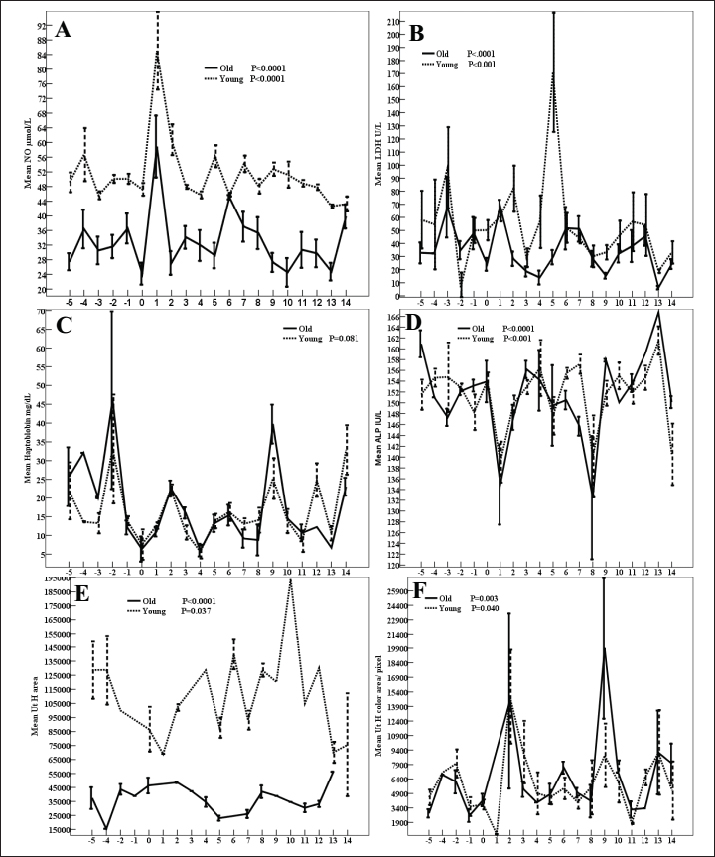
Fig. 3. Mean (NO µmol/l, A), (LDH U/l, B), haptoglobin mg/dl (C), (ALP, D), Uterine horn area /pixels (E), and uterine horn color area/pixel (Ut H, F). NO, Figure 3A is affected by the age of the mares, days of the estrous cycle (p < 0.001), and Age × Days of estrous cycle (Table 1). Days of the estrous cycle of either young or old mares influenced the levels of NO (Fig. 3A). Young mares obtained higher NO than young mares throughout the estrous cycle except Day 6 and 14. LDH is impacted ( p < 0.0001) by age, days of the estrous cycle, and Age × Days of estrous cycle. Days of young (p < 0.001) and old ( p < 0.0001) mares’ estrous cycles influenced LDH (Fig. 3B) with a significant increase in LDH of young mares on Days 2–5 (Fig. 3B). Haptoglobin is impacted by the days of the estrous cycle ( p < 0.0001). Haptoglobin tended (p > 0.05) to be impacted by days of the young mare’s estrous cycle (Fig. 3C). Haptoglobin of young mares is nearly similar or low compared to those of old mares throughout the days of the estrous cycle except Days 7, 8, 12, and 14. ALP of young mares is higher than old ones on Day −3, Day 7, and Day 10. ALP, is impacted by days of the estrous cycle ( p < 0.0001). Days of (Fig. 3D) old ( p < 0.0001) and young (p < 0.001) mares estrous cycles influenced ALP. ALP of young mares is nearly similar or low compared to those of old mares except Days −3, −1, and 4–6. The AMH is influenced by Days of the estrous cycle (p < 0.0001, Table 1). Days of estrous cycle of young (p=0.008, Fig. 4B), and those of old-mares (p=0.014) impacted AMH. AMH of young mares is higher than those of old mares on Day −3, 4, and 10 but lower values are observed on Day 5. MPO is influenced by Days of the estrous cycle ( p < 0.0001). A similar impact of days of young- (p=0.006), and days of the old-mares’ estrous cycles ( p < 0.0001) on MPO (Fig. 4C) are noted. Compared to old mares, young mares obtained high MPO on Day - 4 but low MPO is recorded on Days 13 and 14. Age of the mare, Days pf estrous, and Age × Days of estrous cycle influenced estradiol (E2 pg/ml, p < 0.0001, Table 1). Days of young (p=0.009) and old ( p < 0.0001) mare’s estrous cycle influenced E2 (Fig. 4D). In old mares, (Fig. 4D) reached the lowest concentrations ( p < 0.0001) on Day −5 (34.88 ± 2.38) and its maximum concentrations on Day 6 (72.59 ± 2.23) and Day 8 (116.44 ± 10.38). In young mares, low (p=0.009) E2 were recorded on Day −3 (45.01 ± 5.46) and Day 11 (63.84 ± 17.81) and high values on Days −5 (94.92 ± 7.42), -4 (98.59 ± 27.67), Day 5 (100.36 ± 5.33), Day 3 (101.67 ± 9.46), and Day 13 (103.47 ± 13.03). Young mares indicated higher E2 than old mares except Day 8 (Fig. 4D). Both old and young mares attained the same E2 on Day -3). Days of the estrous cycle ( p < 0.0001) and Age × Days of estrous cycle ( p < 0.0001) affected the concentrations of progesterone (Table 1). Days of the estrous cycle of both young mares and old mares ( p < 0.0001) affected the concentrations of progesterone (P4, Fig. 4E). Young mares got lower progesterone during the preovulation and obtained higher concentrations from Days 7–10 and 13–14. Total cholesterol is affected by the age of the mares, days of the estrous cycle ( p < 0.0001), Age × Days of estrous cycle (p=0.015). Days of the estrous cycle of either young (p-0.028) or old ( p < 0.0001) mares (Fig. 4F) influenced total cholesterol concentrations. Young mares have got higher total cholesterol concentrations than old mares throughout the estrous cycle except Day 1 and 6. DiscussionIn aged mares, the overall effect of aging on the DF dimensions in association with the circulating ovarian hormones, NO, total cholesterol, and LDH were reported in our previous research (Alkhadrawy et al., 2024). The daily DF dynamics are studied in detail throughout the estrous cycle of this study. Results of this study indicated that aged mares had a higher number of follicles but their DF were lower in diameter and antrum diameters compared to that of young mares. The DF of young mares is selected of smaller diameter and antrum than those of old mares. Aging or senility are from the important factors affecting oocyte quality. In previous studies, aging was reported to impact the ovarian follicular environment, the communication between the oocyte and follicular granulosa cells, and the quality of the oocyte (Campos-Chillon et al., 2015; El-Hayek et al., 2018; Krisher, 2019; Alberico and Woods, 2022). The increase in the maternal age was associated with reduced women fertility (Krisher, 2019). In aged mares, reduced fertility is associated reduction in the quality of the oocyte (Carnevale et al., 1993; Carnevale et al., 2020). The maturation of equine oocyte associated with a prolonged rise in LH before ovulation during the preovulatory follicular phase and the presence of uterine oedema (Fanelli et al., 2022). In the mare, reproductive aging is associated with a decline in the fertility, change in the reproductive cycles, and altered the quality of the oocyte (Carnevale et al. 1993; Carnevale and Ginther 1995; Carnevale 2008). The altered oocyte quality in aged mares could be referred to the decrease in the gene expressions essential of essential genes related to follicular and oocyte maturation before ovulation (Campos-Chillon et al., 2015), and impaired both oocyte metabolic activity and capacity of mares (Catandi et al., 2021). The increase in the percentage of unsuccessful equine embryo recovery from older mares (Boakari et al., 2021) could be referred to the altered gene expression of genes essential for early embryo development 8 days after conception (Derisoud et al., 2022). Moreover, a decrease in the number of aspirated follicles from mares by ovum pick up (OPU) started from reaching age of 16 years till the age of 25 years and reached the minimum number and the lowest number associated the lowest recovered oocytes upon reaching the age 26–30 years compared to those <16 years (Fonte et al., 2024) and the oocyte mitochondrial DNA (mtDNA) of old mares during in vitro oocyte maturation contribute in the decreased fertility in senile mares (Rambags et al., 2014). A reduction in the number of aspirated follicles, recovered oocytes, blastocysts per OPU session, and blastocyst rates in mares older than >20 years was recorded (Fonte et al., 2024). The increase in the age of the mare is related to the increased in the damage in the mitochondrial degeneration of the oocytes (Rambags et al., 2006). Contrary, the absence of any effect of the mare age on the mtDNA MII copy number was reported (Catandi et al., 2021). In mares of the current study, both old or young mares have nearly the same mean ovarian follicular development throughout the estrous cycle. In contrast, ovarian aging was reported in humans was characterized by a decrease in the number of the primordial follicles, the ovarian reserve, across their life usually associate with alteration in the endocrine hormones (Telfer et al., 2023).
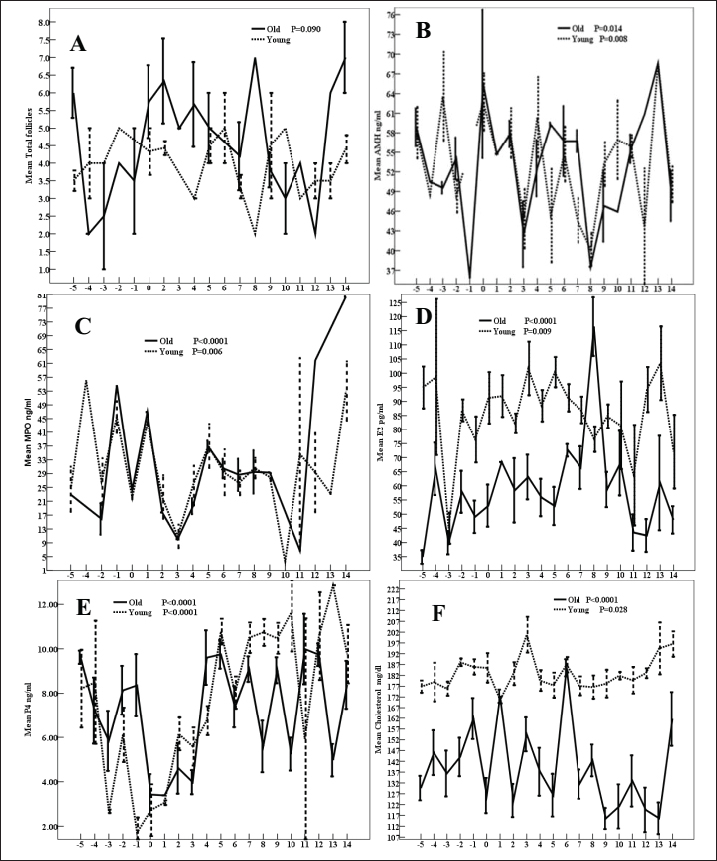
Fig. 4. Mean total ovarian follicles (A), (AMH, B), (MPO, C), estradiol (E2, D), progesterone (P4, E), and total cholesterol (F) in old and young mares. In women, a decline in AMH increased with the increase in age and in the context of aging where AMH measurement was used to identify women with a very low ovarian reserve (de Vet et al., 2002). NO declined in our aged mares throughout the estrous cycle except Day 6 and 14 both aged and young mares had similar values. The increase of NO during the preovulatory phase of the estrous cycle was also reported in its levels increased in both aged and young mares of this study was also observed in young mares during the preovulatory follicular phase (Abdelnaby and Abo El-Maaty, 2017a) and future DF selection and the early CL development after ovulation (Abo El-Maaty and Abdelnaby, 2017). The old age of the mare of this study showed a significant effect in decreasing the DF area, antrum area and increasing its color area %. In jennies, the increase in the blood flow in the wall of the DF from Day −3 to −1 agrees with the increase in the blood flow color area and blood flow color area % in the wall of the DF of our young mares (Magalhaes et al., 2023). Similarly, Asahi et al. (2024) recorded no age effects on GPx in riding horses of age >17 years and of those <10 years. LDH proved its importance in maintaining the continuity of glycolysis and controlling the system of cellular energy release (Augoff et al., 2015). In contrast to the significant effect of age (p < 0.001) in reducing the concentrations of LDH in aged mares during the estrous cycle, no age effects were noted in aged or young riding horses (Asahi et al., 2024). The significant increase in total cholesterol in mares of age >20 years of this study is in agreement with the slight increase in the riding horses of age >17 years compared to those <10 years (Asahi et al., 2024). The significant increase in MDA of aged riding horses >17 years is similar to the increased NO in our aged mares (Asahi et al., 2024). Haptoglobin is one of the equine major acute-phase proteins. It increased after stress, infection, and inflammation. Serum amyloid A is also an important member of acute phase proteins and its concentrations did not vary in riding horses with different ages from < 10 to >17 years (Asahi et al., 2024). AMH was considered as one of the markers for that can be used to assess the ovarian reserve and functions as its decrease inhibits the primordial follicle recruitment and decreases the sensitivity of follicles for the FSH-dependent selection for dominance and future ovulations (Visser et al., 2005; Visser and Themmen, 2005; Visser, 2006). AMH inhibited the growth of antral follicles and the selection of the DFs in monkeys (Xu et al., 2016). ConclusionAging in mares did not influence the ovulating follicle diameter, antrum diameter on Day 5 after ovulation but the future DFs of higher diameter and color area % were selected compared to young mares. Aging increased the area, the antrum area, color area, and color area % of the deviated DF on days 13 and 14. Aging delayed the development of the CL (diameter) during the early luteal phase and delayed its luteolysis during the late luteal phase but did not change the mature CL diameter associated with reduced blood flow, estradiol, cholesterol, and NO and increased PI. Aging increased the activity of CAT, haptoglobin during preovulatory follicle development. AcknowledgmentsMany thanks to the researchers in the National Research Center, Veterinary Research Institute, Animal Reproduction, and Artificial Insemination Department for allowing the Doppler Ultrasound and training until the completion of this work. Conflict of interestThe authors declare that they do not have any conflict of interest. FundingThis research did not receive any funding. Authors’ contributionsAmal M. Aboelmaaty put the research conceptualization, performed the formal analysis, data curation, and methodology, wrote the draft, and reviewed the final version before submission. Abdalla E. A. Elgharieb performed ultrasound and Doppler examinations in old mares. Hazem A. El-Debaky performed the analysis of the hormones, biochemistry, and statistical analysis. Jamal M. H. Alkhadrawy performed ultrasound and Doppler examinations in young mares. Mostafa M. Abou-Ahmed and Abdelraouf M. Ghallab revised the final version of the manuscript. Data availabilityData will be available upon request. ReferencesAbdelnaby, E.A. and Abo El-Maaty, A.M. 2017a. Dynamics of follicular blood flow, antrum growth and angiogenic mediators in mares from deviation to ovulation. J. Equine Vet. Sci. 55, 51–59. Abdelnaby, E.A. and Abo El-Maaty A.M. 2017b. Luteal blood flow and growth in correlation to circulating angiogenic hormones after spontaneous ovulation in mares. Bulg. J. Vet. Med. 20, 97–101. Abdelnaby, E.A., Abo El-Maaty, A.M., Ragab, R.S.A. and Seida, A.A. 2016. Assessment of uterine vascular perfusion during the estrous cycle of mares in connection to circulating leptin, and nitric oxide concentrations. J. Equine Vet. Sci. 39, 25–32. Abd Ellah, M.R., Hussein, H.A. and Derar D.R. 2010. Ovarian follicular fluid constituents in relation to stage of estrus cycle and size of the follicle in buffalo. Vet. World. 3, 263. Abo El-Maaty, A.M. and Abdelnaby, E.A. 2017. Follicular blood flow, antrum growth and angiogenic mediators in mares from ovulation to deviation. Anim. Reprod. 14, 1043–1056. Alberico, H.C. and Woods, D.C. 2022. Role of granulosa cells in the aging ovarian landscape, a focus on mitochondrial and metabolic function. Front. Physiol. 12, 800739. Alkhadrawy, J.M.H., Aboelmaaty, A.M., Abou-Ahmed, M.M. and Ghallab, A.M. 2024. Effect of breeding season and age on follicular dynamics and hemodynamics in embryo donor mares subjected to luteolysis after embryo flushing. Open Vet. J. 14, 852–865. Almeida, J., Ball, B.A., Conley, A.J., Place, N.J., Liu, I.K., Scholtz, E.L., Mathewson, L., Stanley, S.D. and Moeller, B.C. 2011. Biological and clinical significance of anti-mullerian hormone determination in blood serum of the mare. Theriogenology 76, 1393–1403. Asahi, Y., Arai, T. and Tanaka, Y. 2024. Changes in plasma metabolite concentrations and enzyme activities in aging riding horses. Front. Vet. Sci. 11, 1345548. Augoff, K., Hryniewicz-Jankowska, A. and Tabola, R. 2015. Lactate dehydrogenase 5, an old friend and a new hope in the war on cancer. Cancer Lett. 358, 1–7. Ball, B. 2011. Embryonic loss. Equine Reprod. 2, 2327–3238. Ball, B.A., Conley, A.J., Grundy, S.A., Sabeur, K. and Liu, I.K.M. 2008. Expression of anti-Müllerian hormone (AMH) in equine granulosa-cell tumors and in normal equine ovaries. Theriogenology 70, 968–977. Boakari, Y.L., El-Sheikh Ali, H., Schnobrich, M., Lofrumento, K., Scoggin, C., Bradecamp, E., Scoggin, K., Esteller-Vico, A., Claes, A., Lawrence, L. and Ball, B. 2021. Relationships between blood and follicular fluid urea nitrogen concentrations and between blood urea nitrogen and embryo survival in mares. Theriogenology 160, 142–150. Bollwein, H., Kolberg, B. and Stolla, R. 2004. The effect of exogenous estradiol benzoate and altrenogest on uterine and ovarian blood flow during the estrous cycle in mares. Theriogenology 61, 1137–1137. Campos-Chillon, F., Farmerie, T.A., Bouma, G.J., Clay, C.M. and Carnevale, E.M. 2015. Effects of aging on gene expression and mitochondrial DNA in the equine oocyte and follicle cells. Reprod. Fertil. Dev. 27, 925–933. Carnevale, E.M. 2008. The mare model for follicular maturation and reproductive aging in the woman. Theriogenology 69, 23–30. Carnevale, E., Bergfelt, D. and Ginther, O. 1993. Aging effects on follicular activity and concentrations of FSH, LH, and progesterone in mares. Anim. Reprod. Sci. 31, 287–299. Carnevale, E.M., Catandi, G.D., and Fresa, K. 2020. Equine aging and the oocyte, a potential model for reproductive aging in women. J. Equine Vet. Sci. 89, 103022. Carnevale, E.M. and Ginther, O.J. 1995. Defective oocytes as a cause of subfertility in old mares. Biol. Reprod. 52, 209–214. Catandi, G.D., Bresnahan, D.R., Peters, S.O., Fresa, K.J., Maclellan L.J., Broeckling C.D. and Carnevale, E.M. 2023. Equine maternal aging affects the metabolomic profile of oocytes and follicular cells during different maturation time points. Front. Cell Dev. Biol. 11, 1239154. Catandi, G.D., Obeidat, Y.M., Broeckling, C.D., Chen, T.W., Chicco, A.J. and Carnevale, E.M. 2021. Equine maternal aging affects oocyte lipid content, metabolic function and developmental potential. Reproduction 161, 399–409. Claes, A., Galli, C., Colleoni, S., Necchi, D., Lazzari, G., Deelen, C., Beitsma, M. and Stout, T., 2016. Factors influencing oocyte recovery and in-vitro production of equine embryos in a commercial OPU/ICSI program. J. Equine Vet. Sci. 41, 68–69. Derisoud, E., Jouneau, L., Dubois, C., Archilla, C., Jaszczyszyn, Y., Legendre, R., Daniel, N., Peynot, N., Dahirel, M., Auclair-Ronzaud, J., Wimel, L., Duranthon, V. and Chavatte-Palmer, P. 2022. Maternal age affects equine day 8 embryo gene expression both in trophoblast and inner cell mass. BMC Genomics. 23, 443. de Vet, A., Laven, J.S., de Jong, F.H., Themmen, A.P. and Fauser, B.C. 2002. Antimüllerian hormone serum levels, a putative marker for ovarian aging. Fertil Steril. 77, 357–362. El-Hayek, S., Yang, Q., Abbassi, L., FitzHarris, G. and Clarke, H.J. 2018. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr. Biol. 28, 1124–1131. Fanelli, D., Tesi, M., Rota, A., Beltramo, M., Conte, G., Giorgi, M., Barsotti, G., Camillo, F. and Panzani, D. 2022. hCG is more effective than the GnRH agonist buserelin for inducing the first ovulation of the breeding season in mares. Equine Vet. J. 54, 306–311. Fonte, J.S., Alonso, M.A., Junior, M.P.M., Gonçalves, M.A., Pontes, J.H., Bordignon, V., Fleury, P.D.C. and Fernandes, C.B. 2024. Successful equine in vitro embryo production by ICSI—effect of season, mares’ age, breed, and phase of the estrous cycle on embryo production. Theriogenology 223, 47–52. Fouché, N., Gerber, V., Bruckmaier, R.M., Erni-Wespi, B., Zander, Y., Vidondo, B., Sieme, H., Claes, A., Kaeser, R. and Burger, D. 2022. Assessment of anti-Müllerian hormone in mares’ transitional period and in relation to fertility in elderly mares. Theriogenology 179, 97–102. Krisher, R.L. 2019. Maternal age affects oocyte developmental potential at both ends of the age spectrum. Reprod. Fertil. Dev. 31, 1–9. Magalhaes, H.B., Canisso, I.F.and. Dell-Aqua JA Jr. 2023. The temporal associations of b-mode and power-doppler ultrasonography, and ovarian steroid changes of the periovulatory follicle and corpus luteum during luteogenesis and luteolysis in jennies. J. Equine Vet. Sci. 122, 104224. Parrilla Hernández, S., Franck, T., Munaut, C., Feyereisen, É., Piret, J., Farnir, F., Reigner, F., Barrière, P. and Deleuze, S. 2023. Characterization of myeloperoxidase in the healthy equine endometrium. Animals (Basel) 13, 375. Rambags, B.P.B., van Boxtel, D.C.J., Tharasanit, T., Lenstra, J.A., Colenbrander, B. and Stout T.A.E. 2006. Maturation in vitro leads to mitochondrial degeneration in oocytes recovered from aged but not young mares. Anim. Reprod. Sci. 94, 359–361. Rambags, B.P.B., van Boxtel, D.C.J., Tharasanit, T., Lenstra, J.A., Colenbrander, B. and Stout, T.A.E. 2014. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology 81, 959–965. Satué, K., Fazio, E., Ferlazzo, A. and Medica P. 2019. Hematochemical patterns in follicular fluid and blood stream in cycling mares, a comparative note J. Equine Vet. Sci. 80, 20–26. Telfer, E.E., Grosbois, J., Odey, Y.L., Rosario, R. and Anderson, R.A. 2023. Making a good egg, human oocyte health, aging, and in vitro development. Physiol. Rev. 103(4), 2623–2677. Vernunft, A., Schneider, F. and Kanitz, W. 2011. Anti-muellerian hormone (AMH) can help to predict follicular growth in mares. Reprod. Domest. Anim. 46, 44. Visser, J. 2006. Role of anti-Müllerian hormone in follicle recruitment and maturation. J. Gynecol. Obstet. Biol. Reprod. (Paris). 5 (Pt 2), 2S30–2S34. Visser, J.A., de Jong, F.H., Laven, J.S. and Themmen, A.P. 2006.bAnti-Müllerian hormone, a new marker for ovarian function. Reproduction 131(1), 1–9. Visser, J.A. and Themmen, A.P. 2005. Anti-Müllerian hormone and folliculogenesis. Mol. Cell Endocrinol. 234(1-2), 81–86. Xu, J., Bishop, C.V., Lawson, M.S., Park, B.S. and Xu, F. 2016. Anti-müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum. Reprod. 31, 1522–1530. | ||
| How to Cite this Article |
| Pubmed Style Aboelmaaty AM, Elgharieb AEA, El-debaky HA, Alkhadrawy JMH, Abou-ahmed MM, Ghallab AM. Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Vet. J.. 2024; 14(11): 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 Web Style Aboelmaaty AM, Elgharieb AEA, El-debaky HA, Alkhadrawy JMH, Abou-ahmed MM, Ghallab AM. Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. https://www.openveterinaryjournal.com/?mno=222067 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.44 AMA (American Medical Association) Style Aboelmaaty AM, Elgharieb AEA, El-debaky HA, Alkhadrawy JMH, Abou-ahmed MM, Ghallab AM. Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Vet. J.. 2024; 14(11): 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 Vancouver/ICMJE Style Aboelmaaty AM, Elgharieb AEA, El-debaky HA, Alkhadrawy JMH, Abou-ahmed MM, Ghallab AM. Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 Harvard Style Aboelmaaty, A. M., Elgharieb, . A. E. A., El-debaky, . H. A., Alkhadrawy, . J. M. H., Abou-ahmed, . M. M. & Ghallab, . A. M. (2024) Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Vet. J., 14 (11), 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 Turabian Style Aboelmaaty, Amal M., Abdalla E. A. Elgharieb, Hazem A. El-debaky, Jamal M. H. Alkhadrawy, Mostafa M. Abou-ahmed, and Abdelraouf M. Ghallab. 2024. Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Veterinary Journal, 14 (11), 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 Chicago Style Aboelmaaty, Amal M., Abdalla E. A. Elgharieb, Hazem A. El-debaky, Jamal M. H. Alkhadrawy, Mostafa M. Abou-ahmed, and Abdelraouf M. Ghallab. "Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares." Open Veterinary Journal 14 (2024), 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 MLA (The Modern Language Association) Style Aboelmaaty, Amal M., Abdalla E. A. Elgharieb, Hazem A. El-debaky, Jamal M. H. Alkhadrawy, Mostafa M. Abou-ahmed, and Abdelraouf M. Ghallab. "Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares." Open Veterinary Journal 14.11 (2024), 3132-3143. Print. doi:10.5455/OVJ.2024.v14.i11.44 APA (American Psychological Association) Style Aboelmaaty, A. M., Elgharieb, . A. E. A., El-debaky, . H. A., Alkhadrawy, . J. M. H., Abou-ahmed, . M. M. & Ghallab, . A. M. (2024) Fluctuations of antimüllerian hormone, ovarian follicular reserve, and antioxidant status throughout the estrous cycle in aged mares. Open Veterinary Journal, 14 (11), 3132-3143. doi:10.5455/OVJ.2024.v14.i11.44 |