| Research Article | ||
Open Vet. J.. 2024; 14(12): 3440-3448 Open Veterinary Journal, (2024), Vol. 14(12): 3440-3448 Research Article Wild birds of Al-Jouf region may harbor zoonotic parasitesFayez Muhammad Shaldoum*, Meshari Mohamad Alsharari and Barakat Meli AlrashidiDepartment of Biology, College of Science, Jouf University, Sakakah, Saudi Arabia *Corresponding Author: Fayez Muhammad Shaldoum. Department of Biology, College of Science, Jouf University, Sakakah, Saudi Arabia. Email: fshaldoum [at] ju.edu.sa Submitted: 03/10/2024 Accepted: 21/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Wild Birds have hazards of carrying parasites that are possibly transmitted to man. Aim: The aim of this work is to recover parasites from the possibly infected wild birds. Methods: Three wild birds of each type of quail, dove, and pigeon were caught in the Al-Jouf region. They were sacrificed and the contents of their gut were studied using microscopy. Results: Two different species of Cestodes (tapeworms) of order Cyclophyllidea were recovered from wild quail and dove. Only one Mesocestoides sp. worm has been recovered from one wild quail. Mesocestoididae is a family of Cestoda in the order Cyclophyllidea. Members of this family are gut parasites of small mammals and occasionally birds. Cestodes in the genus Mesocestoides are common in carnivores but only very rarely infect humans. Ten Raillietina sp. worms have been recovered from a wild dove. The majority of Raillietina spp. infect avian definitive hosts. It may cause rare accidental innocuous infections in humans. Conclusion: There is much to be learned about the possible transmission of these cestodes from birds to humans, and further research in this area could have important implications for both human and animal health. Projects must be adopted for making a survey of what Parasites may wild animals carry to raise the level of health, environmental, and economic awareness of the community. Keywords: Cestodes, dove, Zoonotic parasites, Pigeon, Quail, Aljouf. IntroductionWild birds move freely in the surrounding environment and travel across the borders between Countries without restrictions. These wild birds have the hazard of carrying parasites that are possibly transmitted to man (Mathison and Pritt, 2018; Vogt, 2023). The effect of infection with these parasites on humans and other animals of economic importance to humans such as camels, sheep, cattle, horses, and poultry is of great importance (Torgerson, 2013). These parasites are known to cause serious health problems in birds, such as decreased body weight, organ damage, reduced reproductive success, and even death (McLaughlin, 2008). Studies have revealed that cestodes have been detected in a wide range of bird species globally, from waterbirds to raptors, and songbirds. For instance, a review study conducted in Argentine on the cestode parasites of Argentinean wild birds found that the highest number of cestode was recorded in the family Hymenolepididae, with 12 species belonging to two genera, followed by Dilepididae, with eight species belonging to three genera. Of the 1,042 species of birds reported in Argentina, only 29 (2.8%) were reported as hosts of adult cestodes. There were two families of birds with the highest number of reported species, Laridae and Anatidae (Drago et al., 2021). Another review about a total of 27 studies, eight were carried out in Europe, six in Asia, five in North America, four in Africa, and four in South America, have found that 52 parasite species were reported in 72 avian species in zoological settings, globally. Parasites were found in zoo birds globally and in seven common free-ranging avian species [mallard (Anas platyrhynchos), Eurasian blackbird (Turdus merula), common starling (Sturnus vulgaris), Eurasian jackdaw (Corvus monedula), house sparrow (Passer domesticus), European robin (Erithacus rubecula), and rock dove (Columba livia)]. These parasites included cestodes (Dicranotaenia coronula, Diorchis stefanskii, Fimbriaria fasciolaris, and Raillietina cesticillus, Sobolevicanthus gracilis) (Jativa et al., 2020). Cestodes are a significant concern in the health of wild and domesticated birds and can cause significant economic losses in the poultry industry. There are several cestodes that infect birds including: Hymenolepis spp., These cestodes infect a wide range of avian hosts and can cause severe damage to the gastrointestinal tract (Vergara et al., 2021). This parasite has been detected in a wide range of bird species globally, including pigeons, doves, crows, and gulls (al-Sallami, 1991; Bondarenko et al., 2018; Ali et al., 2020). The parasite is known to have a significant impact on avian health, with studies reporting reduced body weight and organ damage in birds infected with the tapeworm (Zahrani et al., 2013). Another cestode that has been detected in birds is the taeniid tapeworm, Taenia spp., which is known to infect a wide range of bird species, including owls, hawks, and falcons (Smith, 1996; Kinsella et al., 1998, and 2001; Kravchenko et al., 2023). This parasite is known to cause serious health problems in birds, including neurological disorders and reduced reproductive success (Kinsella et al., 1995). Raillietina spp., These cestodes are found in the small intestines of birds and can cause weight loss, malnutrition, and reduced egg production (Katoch et al., 2012). Choanotaenia spp., these cestodes are commonly found in birds and can cause severe pathology in their hosts (Jajere et al., 2018). The Choanotaenia spp. has also been detected in several bird species, including passerines and woodpeckers (Baer, 1959; Badawy, 2000; Sykes, 2015). Davaineidae, these cestodes are found in the intestines of wild and domesticated birds and can cause severe enteritis and necrosis (Ramnath et al., 2014; Al-Quraishy et al., 2021). Moreover, other cestodes detected in birds include the Diphyllobothrium spp., which is commonly found in waterbirds and has been shown to cause serious health problems in birds, including liver damage and reduced reproductive success (Dick et al., 2001; Scholz et al., 2019). Bird’s cestodes can be transmitted or infect humans through ingestion of contaminated food or water. The most common species involved in human infection from birds are Diphyllobothrium latum and Diphyllobothrium nihonkaiense, which are tapeworms that are commonly found in fish-eating birds such as gulls and cormorants. In humans, these tapeworms can cause diphyllobothriasis, which is an intestinal infection that can lead to abdominal discomfort, diarrhea, and malnutrition (Scholz et al., 2009). There are also other bird cestodes such as Raillietina tetragona, Raillietina echinobothrida, and Hymenolepis diminuta that have been reported to infect humans in some cases. These cestodes usually do not cause serious harm to humans but can still cause mild gastrointestinal symptoms (Kuchta et al., 2013; Dupouy and Peduzzi, 2014; Inci et al., 2018). Mesocestoididae is a family of Cestoda (tapeworms) in the order Cyclophyllidea. Members of this family are gut parasites of small mammals and occasionally birds (Millán and Casanova, 2007; Jones, 2011; Jones et al., 2017; Heneberg et al., 2019). Cestodes in the genus Mesocestoides are common in carnivores but only very rarely infect humans. The aim of this research was to find out what parasites may live in the intestines of some birds that fly over the sakaka area - Al-Jouf province - northern Saudi Arabia. Materials and MethodsThere are different methods to recover cestodes from the intestinal lumen of birds (Salazar et al., 2023). These include the following. Dissection methodThe dissection method involves cutting open the bird’s intestine and carefully examining it for cestodes. This method is suitable for low-density infections and is often used in field studies. Gastrointestinal tract inversion methodIn this method, the gastrointestinal tract is inverted onto a Petri dish containing saline solution. The cestodes are then separated from the intestinal wall and collected in the saline solution. This method is suitable for high-density infections and is commonly used in laboratory studies. Flotation methodThe flotation method involves mixing the intestinal contents with a flotation solution, such as sugar or sodium chloride, and allowing the cestodes to float to the surface. The cestodes are then collected and examined under a microscope. This method is suitable for low-density infections and is commonly used in clinical settings. Sedimentation methodThe sedimentation method involves allowing the intestinal contents to settle in a tube or container, and then collecting the cestodes from the sediment. This method is suitable for low-density infections and is commonly used in laboratory studies. Enzymatic digestion methodThe enzymatic digestion method involves using enzymes, such as pepsin or trypsin, to break down the intestinal contents and release the cestodes. The cestodes are then collected and examined under a microscope. This method is suitable for low-density infections and is commonly used in laboratory studies. In the current research, we have mixed the dissection method with the gastrointestinal tract inversion method. Three wild birds of each type of quail, doves, and pigeons were caught from Al-Hammad area, west of Sakaka city, Al-Jouf governorate in the north of the Kingdom, during the month of October 2019, (Fig. 1). We then sacrificed and slaughtered these animals for the dissecting, removal of their guts and examination of the gastrointestinal tract, and presence of parasites in an isotonic saline solution. Make a cut in the intestine carefully to allow worms to swim out. Then transfer the gut to another dish with saline increase this cut to fully invert the intestine and be sure that any worm will move out to the saline solution. Recovered worms were studied under a binocular dissecting microscope equipped with an imaging digital device (Motic China Group Co., Ltd.).
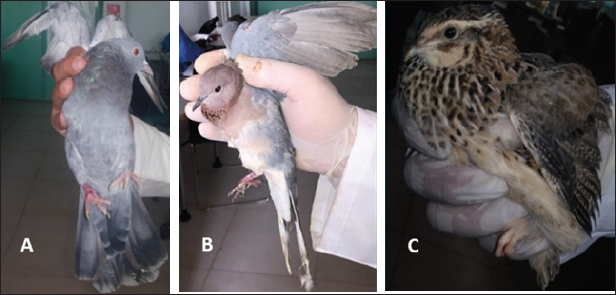
Fig. 1. (A) Wild pigeon, (B) wild dove, (C) wild quail.
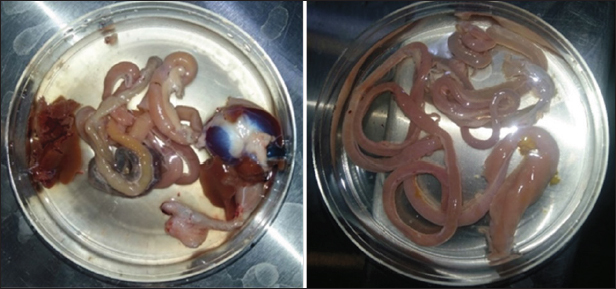
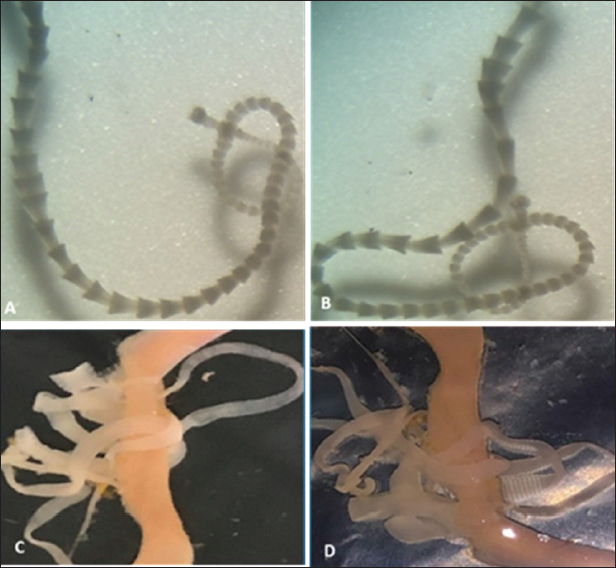
Fig. 2. The bird’s intestines after slaughter and dissection are isolated in isotonic saline in a petri dish. Ethical approvalNot needed for this study. ResultsDiscriminating among cestode species that are transmitted from birds to humans can be achieved through various methods, including morphological, molecular, and ecological analysis. These results showed that the pigeons were free of parasites, and one quail was infected with only one tapeworm (Fig. 3a and b), as well as one dove extensively infected with ten tapeworms (Fig. 3c and d). In the current research, we have relied on morphological examination and ecological analysis to discriminate among cestodes that have been recovered from the studied wild birds. By isolating these worms and examining them alive in saline under the microscope, we found that the type of tapeworm from quail is different from that of the dove (Figs. 4 and 5). Morphological study for the single worm recovered from a quail in the current work indicates that it is Mesocestoides sp. since the scolex is unarmed and has no distinct rostellum and no hooks. On the scolex, there are four elongated muscular suckers. Each mature segment contains a single set of reproductive organs. In gravid segments, the eggs are contained in a distinctive parauterine organ located centrally towards the posterior end of the segment, with uterine remnants anteriorly and posteriorly (Fig. 4). Mesocestoididae is a family of Cestoda in the order Cyclophyllidea.
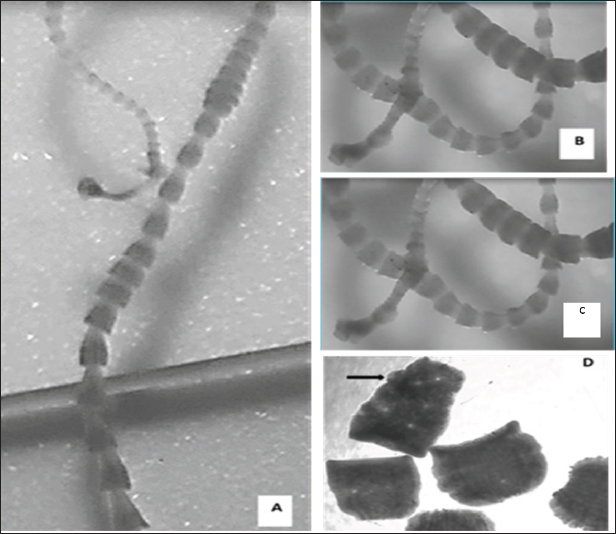
Fig. 3. (A, B) Tapeworm isolated from wild quail intestines swims alive in saline, C-D tapeworms from wild dove intestine after incision along gastrointestinal tract. On the other hand, morphological study for the ten worms recovered from a dove in the current work indicates that it is Raillietina spp. since the scolex is a bulbous knob-like structure bearing suckers and a rostellum. The scolex is followed by an unsegmented neck or growth region. The neck is then followed by numerous trapezoidal proglottids which become rounder towards the posterior region of the strobila, creating a “beaded” appearance. Gravid proglottids are barrel-shaped and are filled with egg capsules (Fig. 5). DiscussionCestodes, also known as tapeworms, are a type of parasitic worm that infect a wide range of animals, including birds and humans. They are characterized by their segmented body, with each segment containing both male and female reproductive organs. The morphology of the scolex in cestodes of birds may play a role in their transmission to humans, but other factors such as host range and geographic distribution also need to be considered. Cestodes of birds can be classified into two groups based on their scolex morphology - those with both hooks and suckers and those with only suckers. One study conducted on cestodes of birds found that those with both hooks and suckers on their scolex were more commonly found in raptors than in other avian hosts. This is believed to be due to the predatory nature of raptors, which puts them in contact with other infected prey species more frequently (Ferrer et al., 2004). In terms of transmission to humans, some species of cestodes that infect birds can also infect and cause disease in humans. For example, Hymenolepis nana and Hymenolepis diminuta are two species of cestodes that are commonly found in birds and rodents but can also infect humans. These tapeworms have only suckers on their scolex and are transmitted to humans through the ingestion of contaminated food or water (Talazadeh et al., 2023).
Fig. 4. A single Mesocestoides sp. worm recovered from quail with (A), low magnification showing whole worm, (B, C), higher magnification showing head armed with four suckers (no hooks) and different Proglottids. (D), mature proglottids with Genital openings on one side. Another species of cestode that infects birds and can also infect humans is Diphyllobothrium latum. This tapeworm has bothria on its scolex and is transmitted to humans through the ingestion of raw or undercooked fish. D. latum infections have been reported in many parts of the world, particularly in areas where fish is a dietary staple (Scholz et al., 2019). Discriminating among cestode species that are transmitted from birds to humans requires mostly a combination of different methods, including morphological, molecular, and ecological analyses. These methods can help accurately identify different cestode species and provide insights into their transmission dynamics and epidemiology. Morphological examination involves the examination of the external and internal anatomy of the cestode species, which can help identify specific features unique to different species. For instance, the morphology of the scolex and segments of the cestode can help distinguish between different species. This method has been used in the identification of different species of cestodes, including Dipylidium caninum, Hymenolepis diminuta, and Hymenolepis nana (Sepulveda and Kinsella, 2013). Molecular analysis can also be used to discriminate between different cestode species. Molecular techniques such as polymerase chain reaction, DNA sequencing, and restriction fragment length polymorphism have been used to analyze and differentiate between different cestode species (Wu et al., 2013; Takeuchi et al., 2015; Younis et al., 2021). This method can help identify species that have similar morphological features but differ genetically. Ecological analysis involves the examination of the host range, geographical distribution, and life cycle of different cestode species. This method is particularly useful in the identification of cestodes transmitted from birds to humans since birds are known to harbor different cestode species. For instance, cestode species such as Diphyllobothrium latum and Spirometra spp. are known to be transmitted from fish-eating birds to humans (Chai, 2010).
Fig. 5. Ten Raillietina spp. worms recovered from quail Bird with (A), low magnification showing a whole worm; (B), medium magnification showing head, immature and mature Proglottids; (C), higher magnification showing head armed with four suckers and rostellum with hooks; (D), higher magnification showing mature proglottids and (E), higher magnification showing gravid Proglottids with embryos. However, in the current research, we have followed both morphological examination and ecological analysis to discriminate among cestodes that have been recovered from the wild birds. The morphological differences were clear enough to identify the parasite. In this research, we also used as few birds as possible, in order not to harm the wildlife, and the presence of infection with a parasite in a small random sample indicates the spread of infection with this type of parasite in the corresponding bird host at Al-Jouf geographical territory, Saudi Arabia. Current results indicated that worms recovered from dove and quail show the characteristics of order Cyclophyllidea (Taenoidea) because of the presence of a head equipped with four suckers (in case of quail) as well as the presence of rostellum with hooks (in case of dove), these results agree with findings of (Zhang et al., 2021). Definitive hosts are mostly birds and mammals and include a Two- or Three-host life cycle, with invertebrates or vertebrates as intermediate hosts. Some species are capable of paratenic parasitism in vertebrates (UCONN, 2024). Members of the family Mesocestoididae, as the worm recovered from quail in the current study, are gut parasites of small mammals and occasionally birds. Cestodes in the genus Mesocestoides are common in carnivores but only very rarely infect humans (Millán et al., 2003). The majority of Rallying spp., as these recovered from dove in the current study, infect avian definitive hosts. It may cause rare accidental innocuous infections in humans (Sapp and Bradbury, 2020). It should be noted that the danger of this type of worm varies for humans (Haukisalmi, 2015; Inci et al., 2018), some of them are compatible only with the animal host, the primary host, and harm is limited to the animal only, and even the risk to the animal varies from limited to severe damage and the loss for humans is only economic loss. The real problem is that some species of these tapeworms of the order “Cyclophyllidea” may be transmitted even in rare cases to humans and cause human health problems. Also, it varies in seriousness, from gastrointestinal weakness and anemia that can be remedied by treatment and elimination of worms that settle down only in the human intestines (Lloyd et al., 2014). The serious risk of straying the larvae of these worms, which are cystic larvae and affect various tissues of the human body such as the lung, liver, viscera, muscles, and even the central nerves and brain (Heyneman, 1996). This risk leads to serious complications that do not cure; even Surgical intervention sometimes leads to the death of the patient (Hamamoto et al., 2022). Cases that may be successful with chemotherapy need many years and despite the success of treatment with the death of larvae, but their cysts remain as long as the life of the patient as well as damage caused by the incidence of these tissues (Butala et al., 2021). The World Health Organization has always driven the world to pay attention to tapeworm infection and related diseases. It is a type of infection that is neglected by research despite its seriousness. ConclusionThere is much to be learned about the possible transmission of these cestodes from birds to humans, and further research in this area could have important implications for both human and animal health. Projects must be adopted for making a survey of what Parasites may wild animals carry to raise the level of health, environmental, and economic awareness of the community. AcknowledgmentsThe authors extend their appreciation to the Deanship of Scientific Research at Jouf University for funding this work through research grant No (DSR2020-04-2578). Conflict of interestIt is declared by the authors that no conflicts of interest exist. FundingResearch grant No (DSR2020-04-2578) funded by the Deanship of Scientific Research at Jouf University, Kingdom of Saudi Arabia. Authors’ contributionsFM: Designed the study, supervision, parasite detection and identification, and writing-review and editing the manuscript., MM: Practical work, parasites-recovery and drafted the manuscript., BM: Conceptualization and review the manuscript. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAli, M., Ibrahim, R., Alahmadi, S. and Elshazly, H. 2020. Ectoparasites and Intestinal Helminths of Pigeons in Medina, Saudi Arabia. J. Parasitol. 106(6), 20-64. Al-Quraishy, S., Abdel-Gaber, R., Dkhil, M. A., Abdel-Baki, A. S., Alotaibi, M., Alhafidh, W. and Al-Houshany, N. 2021. Detection of Raillietina saudiae from the domestic pigeon in Saudi Arabia through 18S and 28S rDNA genes. Lett. Appl. Microbiol. 72(1), 90–97. al-Sallami S. 1991. A possible role of crows in the spread of diarrhoeal diseases in Aden. J. Egypt Public Health Assoc. 66(3-4), 441–449. Badawy, B. A. 2000. House Sparrows (Passer domesticus niloticus) As a Source of Some Parasitic Diseases to the Domesticated Birds at Giza Governorate, Egypt. Assiut Vet. Med. J. 42(84), 180506. Baer, J. G. 1959. Helminthes parasites., Exploration du Parc national Congo Belge Miss Baer and Gerber 1, pp: 1-80. Bondarenko, S., Kondrat’eva, L.F. and Khober, E.P. 1987. The gull parasite Hymenolepis (s. 1.) haldemani Schiller, 1951 and its life cycle. Acta Parasitologica Lituanica 22, 82-91 Butala, C., Brook, T. M., Majekodunmi, A. O. and Welburn, S. C. 2021. Neurocysticercosis: Current Perspectives on Diagnosis and Management. Front. Vet. Sci. 8, 615703. Chai, J.-Y. 2010. Fish-borne Parasitic Diseases. Hanyang Med. Rev. 30(3), 223. Deplazes, P., Eichenberger, R. M. and Grimm, F. 2019. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int. J. . Parasites Wildl. 9, 342–358. Dick, T. A., Nelson, P. A. and Choudhury, A. 2001. Diphyllobothriasis: Update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian J. Trop. Med. Public Health. 32(2), 59-76. Drago, F., Dueñas Díaz, M., Draghi, R. and Núñez, V. 2021. Checklist of the cestode parasites of wild birds of Argentina. J. Helminthol. 95, E43. Dupouy-Camet, J. and Peduzzi, R. 2014. Helminth-Trematode: Diphyllobothrium. In Encyclopedia of Food Safety, Elsevier, pp: 130–133. Ferrer, D., Molina, R., Adelantado, C. and Kinsella, J. M. 2004. Helminths isolated from the digestive tract of diurnal raptors in Catalonia, Spain. Vet. Rec. 154(1), 17–20. Hamamoto Filho, P. T., Rodríguez-Rivas, R. and Fleury, A. 2022. Neurocysticercosis: A Review into Treatment Options, Indications, and Their Efficacy. Res. Rep. Trop. Med. 13, 67–79. Haukisalmi, V. 2015. Checklist of tapeworms (Platyhelminthes, Cestoda) of vertebrates in Finland. ZooKeys, 533, 1–61. Heneberg, P., Georgiev, B. B., Sitko, J. and Literák, I. 2019. Massive infection of a song thrush by Mesocestoides sp. (Cestoda) tetrathyridia that genetically match acephalic metacestodes causing lethal peritoneal larval cestodiasis in domesticated mammals. Parasit. Vectors. 12(1), 230. Heyneman D. 1996. Cestodes. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; Chapter 89. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8399/ Inci, A., Doganay, M., Ozdarendeli, A., Duzlu, O. and Yildirim, A. 2018. Overview of Zoonotic Diseases in Turkey: The One Health Concept and Future Threats. Turkiye. Parazitol. Derg. 42(1), 39–80. Jajere, S. M., Lawal, J. R., Atsanda, N. N., Hamisu, T. M. and Goni, M. D. 2018. Prevalence and burden of gastrointestinal helminthes among grey-breasted helmet guinea fowls (Numida meleagris galeata) encountered in Gombe state, Nigeria. Int. J. Vet. Sci. Med. 6(1), 73–79. Jativa, P., Morgan, E., Barrows, M., tegui, G. and a, J. 2020. Free-ranging avifauna as a source of generalist parasites for captive birds in zoological settings: An overview of parasite records and potential for cross-transmission. J. Adv. Vet. Anim. Res. 7(3), 482. Jones, O. 2011. Prevalence of dog gastrointestinal parasites and risk perception of zoonotic infection by dog owners in Wondo Genet, Southern Ethiopia. J. Public Health Epidemiol. 3, 550-555. Katoch, R., Yadav, A., Godara, R., Khajuria, J. K., Borkataki, S. and Sodhi, S. S. 2012. Prevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J. Parasit. Dis. 36(1), 49–52. Kinsella, J. M., Foster, G. W. and Forrester, D. J. 1995. Parasitic helminths of six species of hawks and falcons in Florida. J. Raptor. Res. 29, 117-122. Kinsella, J. M., Foster, G. W. and Forrester, D. J. 2001. Parasitic helminths of five species of owls from florida, U.S.A. Comp. Parasitol. 68, 130-134. Kinsella, J. M., Foster, G. W., Cole, R. A. and Forrester, D. J. 1998. Helminth parasites of the bald eagle, Haliaeetus leucocephalus, in Florida. J. Helminthol. Soc. Washington 65, 65-68. Komorová, P., Sitko, J., Špakulová, M., Hurníková, Z., Sałamatin, R. and Chovancová, G. 2017. New data on helminth fauna of birds of prey (Falconiformes, Accipitriformes, Strigiformes) in the Slovak Republic. Helminthologia 54(4), 314–321. Kravchenko, I. A., Musaev, M. B. and Ankudinova, E. S. 2023. Helminth fauna in diurnal birds of prey of the order Falconiformes. Russ. J. Parasitol. 17(2), 198–205. Kuchta, R., Brabec, J., Kubáčková, P. and Scholz, T. 2013. Tapeworm Diphyllobothrium dendriticum (Cestoda)—Neglected or Emerging Human Parasite? PLoS Negl. Trop. Dis. 7(12), e2535. Lloyd, A. E., Honey, B. L., John, B. M. and Condren, M. 2014. Treatment Options and Considerations for Intestinal Helminthic Infections. J. Pharm. Technol. 30(4), 130–139. Mathison, B. A. and Pritt, B. S. 2018. A Systematic Overview of Zoonotic Helminth Infections in North America. Lab. Med. 49(4), e61–e93. McLaughlin, J. D. 2008. Cestodes. In Parasitic Diseases of Wild Birds, Wiley, pp: 261–276. Millán, J. and Casanova, J. C. 2007. Helminth parasites of the endangered Iberian lynx ( Lynx pardinus ) and sympatric carnivores. J. Helminthol. 81(4), 377–380. Millán, J., Gortazar, C. and Casanova, J. C. 2003. First occurrence of Mesocestoides sp. in a bird, the red-legged partridge, Alectoris rufa, in Spain. Parasitol. Res. 90(1), 80–81. Ramnath, Jyrwa, D. B., Dutta, A. K., Das, B. and Tandon, V. 2014. Molecular characterization of the Indian poultry nodular tapeworm, Raillietina echinobothrida (Cestoda: Cyclophyllidea: Davaineidae) based on rDNA internal transcribed spacer 2 region. J. Parasit. Dis. 38(1), 22–26. Salazar-Silva, C. H., Oyarzún-Ruiz, P., Rodríguez, R., Torres-Fuentes, L. G., Cicchino, A., Mironov, S., Muñoz-Leal, S. and Moreno, L. 2023. External and gastrointestinal parasites of the black-faced ibis Theristicus melanopis (Pelecaniformes: Threskiornithidae) in the Los Ríos region, southern Chile. Vet. Parasitol. Reg. Stud. Reports. 42, 100893. Sapp, S. G. H. and Bradbury, R. S. 2020. The forgotten exotic tapeworms: a review of uncommon zoonotic Cyclophyllidea. Parasitology 147(5), 533–558. Scholz, T., Garcia, H. H., Kuchta, R. and Wicht, B. 2009. Update on the Human Broad Tapeworm (Genus Diphyllobothrium), Including Clinical Relevance. Clin. Microbiol. Rev. 22(1), 146–160. Scholz, T., Kuchta, R. and Brabec, J. 2019. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: Recent progress and future challenges. Int. J. Parasitol. Parasites Wildl. 9, 359–369. Sepulveda, M. S. and Kinsella, J. M. 2013. Helminth Collection and Identification from Wildlife. J. Vis. Exp. 82, 51000. Smith, S. A. 1996. Parasites of birds of prey: Their diagnosis and treatment. Semin. Avian Exotic. Pet. Med. 5(2), 80022-3 Sykes, J. M. 2015. Piciformes (Honeyguides, Barbets, Woodpeckers, Toucans). In Fowler’s Zoo and Wild Animal Medicine, Volume 8, Elsevier, pp: 230–236. Takeuchi-Storm, N., Mejer, H., Al-Sabi, M. N. S., Olsen, C. S., Thamsborg, S. M. and Enemark, H. L. 2015. Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration McMaster technique. Vet. Parasitol. 214(3–4), 327–332. Talazadeh, F., Razijalali, M., Roshanzadeh, N. and Davoodi, P. 2023. Survey on the gastrointestinal parasites in Passeriformes and Psittaciformes with a focus on zoonotic parasites. J. Hellenic Vet. Med. Soc. 74(3), 6237–6245. Torgerson, P. R. 2013. One world health: Socioeconomic burden and parasitic disease control priorities. Vet. Parasitol. 195(3–4), 223–232. UCONN. 2024. A Survey of the Tapeworms (Cestoda: Platyhelminthes) from Vertebrate Bowels of the Earth. https://tapeworms.uconn.edu/cyclophyllidea.html [Accessed 18 April 2024]. Vergara, D., Alvarez, J. and Cordero, A. 2021. Prevalence of gastrointestinal parasites in three groups of domestic poultry managed under backyard system in the Savanna subregion, Department of Sucre, Colombia. J. Adv. Vet. Anim. Res. 8(4), 551. Vogt, N. A. 2023. Wild Birds and Zoonotic Pathogens. In Zoonoses: Infections Affecting Humans and Animals. Springer International Publishing, pp: 1–31. Wu, X., Fu, Y., Yang, D., Xie, Y., Zhang, R., Zheng, W., Nie, H., Yan, N., Wang, N., Wang, J., Gu, X., Wang, S., Peng, X. and Yang, G. 2013. Identification of neglected cestode Taenia multicepsmicroRNAs by illumina sequencing and bioinformatic analysis. BMC Vet. Res. 9(1), 162. Younis, A. E., Saad, A. I., El-Akhal, I. R. M. and Saleh, N. M. K. 2021. A parasitological survey of zoonotic cestodes carried by house rats in Aswan, Egypt, reveals cryptic diversity at the molecular level. Vet. World 14(8), 2169. Zahrani, M. R., Ashour, A. A. and Shobrak, M. Y. 2013. Tapeworms of Rock Dove and Domestic Chicken in Taif Area, Saudi Arabia. J. Egypt. Soc. Parasitol. 42(3), 507–513. Zhang, D., Wu, G., Yang, X., Tian, W. and Huo, N. 2021. Molecular phylogenetic identification and morphological characteristics of Raillietina echinobothrida (Cestoda: Cyclophyllidea: Davaineidae) in commercial chickens in North China. Parasitol. Res. 120(4), 1303–1310. | ||
| How to Cite this Article |
| Pubmed Style Shaldoum FM, Alsharari MM, Alrashidi BM. Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Vet. J.. 2024; 14(12): 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 Web Style Shaldoum FM, Alsharari MM, Alrashidi BM. Wild birds of Al-Jouf region may harbor zoonotic parasites. https://www.openveterinaryjournal.com/?mno=222855 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.28 AMA (American Medical Association) Style Shaldoum FM, Alsharari MM, Alrashidi BM. Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Vet. J.. 2024; 14(12): 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 Vancouver/ICMJE Style Shaldoum FM, Alsharari MM, Alrashidi BM. Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 Harvard Style Shaldoum, F. M., Alsharari, . M. M. & Alrashidi, . B. M. (2024) Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Vet. J., 14 (12), 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 Turabian Style Shaldoum, Fayez Muhammad, Meshari Mohamad Alsharari, and Barakat Meli Alrashidi. 2024. Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Veterinary Journal, 14 (12), 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 Chicago Style Shaldoum, Fayez Muhammad, Meshari Mohamad Alsharari, and Barakat Meli Alrashidi. "Wild birds of Al-Jouf region may harbor zoonotic parasites." Open Veterinary Journal 14 (2024), 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 MLA (The Modern Language Association) Style Shaldoum, Fayez Muhammad, Meshari Mohamad Alsharari, and Barakat Meli Alrashidi. "Wild birds of Al-Jouf region may harbor zoonotic parasites." Open Veterinary Journal 14.12 (2024), 3440-3448. Print. doi:10.5455/OVJ.2024.v14.i12.28 APA (American Psychological Association) Style Shaldoum, F. M., Alsharari, . M. M. & Alrashidi, . B. M. (2024) Wild birds of Al-Jouf region may harbor zoonotic parasites. Open Veterinary Journal, 14 (12), 3440-3448. doi:10.5455/OVJ.2024.v14.i12.28 |