| Research Article | ||
Open Vet. J.. 2024; 14(12): 3474-3486 Open Veterinary Journal, (2024), Vol. 14(12): 3474-3486 Research Article Direct and indirect effects of PGF2α administration in male Wistar rats based on increased expression of α-SMA and androgen receptorTeuku Armansyah1, Husnurrizal Husnurrizal2, Sri Wahyuni3, Hafizuddin Hafizuddin2, Tongku Nizwan Siregar2*, Amalia Sutriana1, Arman Sayuti4, Adinda Tri Syahrani5 and Muhammad Bintang Pariansyah51Laboratory of Pharmacology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Laboratory of Reproduction, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Laboratory of Anatomy, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 4Laboratory of Clinic, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 5Study Program of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Tongku Nizwan Siregar. Laboratory of Reproduction, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: siregar [at] usk.ac.id Submitted: 04/10/2024 Accepted: 22/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
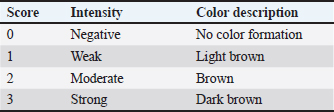
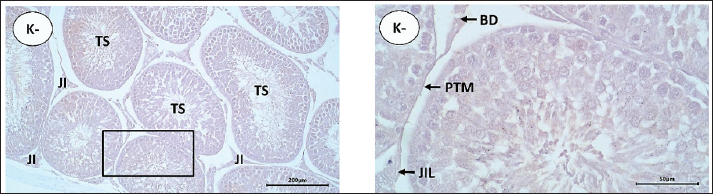
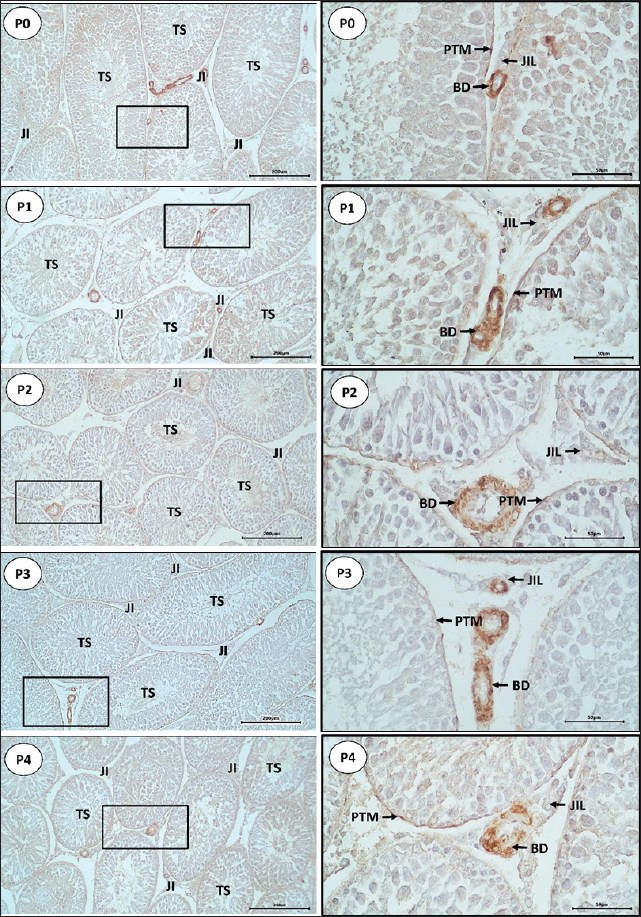
AbstractBackground: Prostaglandin F2α (PGF2α) hormone administration can improve semen quality through increased contractility of smooth muscle cells in testicular tissue and increased testosterone hormone. Immunohistochemically, the increase in contractility of smooth muscle cells and testosterone hormone can be measured by an increase in the expression of alpha-smooth muscle actin (α-SMA) and androgen receptor (AR). Aim: This study aims to determine the distribution and increased α-SMA expression and AR in the testis of Wistar rats after administration of PGF2α which was detected using the immunohistochemistry (IHC) method. Methods: A total of 15 male Wistar rats (Rattus norvegicus) with body weight 200–250 g and 8–10 weeks old were used in this study. All rats were acclimated for 2 weeks. The rats were divided into five treatment groups (n=3). The rat in the control group (P0), testicular collection was carried out 30 minutes after injection of 0.5 ml NaCl. In groups P1, P2, P3, and P4, the rats were intraperitoneal injected with 2.5 mg/kg BW of PGF2α (Lutalyze, Zoetis, USA), and the testis collection was performed 30, 60, 90, and 120 minutes after PGF2α injection, respectively, were processed into histology preparations and stained with IHC staining using avidin-biotin complex peroxidase method. The distribution of α-SMA and AR was analyzed descriptively, while the score difference of α-SMA and AR expression was analyzed using the Kruskal-Wallis test and the Mann-Whitney U Test. Results: The results showed that α-SMA was positively detected in peritubular myoid (PTM) cells on the basal membrane of seminiferous tubules, blood vessel (BV) walls, and connective tissue. Statistical analysis showed that α-SMA expression in PTM cells and testicular connective tissue in P2 (60-minute interval) was significantly different than other treatment groups (P0, P1, P3, and P4) (p < 0.05), while no significant difference was found in the BV walls in all treatment groups (p > 0.05). AR was positively detected in connective tissue, PTM cells, Leydig cells, Sertoli cells, and BVs. Statistical analysis showed significant differences in AR expression in PTM cells and Sertoli cells (p < 0.05) between P0 and P2, P1 and P2, and P2 and P3 or P4. Conclusion: It can be concluded that the administration of PGF2α increases the expression of α-SMA and AR, with the optimal administration interval is 60 minutes for PTM cells and Sertoli cells in Wistar rats testis. Keywords: Androgen receptor, Immunohistochemistry, PGF2α, Wistar rats, α-SMA. IntroductionImproving sperm quality can be achieved in several ways, including the administration of gonadotrophin-releasing hormone (GnRH) (Syafruddin et al., 2020; Armansyah et al., 2021; Sutriana et al., 2022). The addition of prostaglandin F2α (PGF2α) has showed the ability to improve sperm quality both in vivo (Armansyah et al., 2018; Husnurrizal et al., 2021; Sari et al., 2021) and in vitro (Prestiya et al., 2020; Aswadi et al., 2021). In vitro administration of PGF2α has been reported to increase sperm motility by a direct effect on the contractile elements of spermatozoa. Several studies on animal has showed that in vitro administration of PGF2α can improve the quality of boar spermatozoa (Pandur and Pacala, 2012), bull spermatozoa (Karahan et al., 2006; Majeed et al., 2017), horse spermatozoa (Şen and Akcay, 2015), and PE goat spermatozoa (Herawati and Widiarso, 2003; Prestiya et al., 2020). The direct effect of PGF2α on male animals can be attributed to the increase in smooth muscle contraction in the epididymis and contraction in the testicular capsule. The administration of PGF2α causes the relocation of spermatozoa from the cauda epididymis to the ductus deferens due to increased smooth muscle contraction (Hess, 2002). Contraction in the testicular capsule is attributed to be a response to PGF2α, which plays an important role in increasing the number of spermatozoa (Masoumi et al., 2011). Increased contraction of the testicular capsule can be identified by the increase in distribution and expression of α-SMA protein in testicular tissue. Indirectly, PGF2α can improve semen quality by increasing testosterone levels (Aswadi et al., 2021). The increase in testosterone (androgen) is assumed to enhance androgen receptors (AR) in the target cells or tissues of the hormone, especially in testicular tissue. ARs belong to the steroid hormone receptor group and are part of the ligand-activated nuclear receptor superfamily within cells that play a role in regulating the effects of androgens in the processes of proliferation, differentiation, and the function of male reproductive organs (Hasbi and Gustina, 2018). Androgens regulate the function of the testes and male accessory reproductive organs through AR (Arora et al., 2018). These processes can occur when AR binds with natural ligands such as testosterone and dihydrotestosterone (DHT) in target cells (Arini, 2019). The distribution and expression of α-SMA and AR in testicular tissue after PGF2α administration can be detected using the immunohistochemistry (IHC) staining method. IHC is a method used to identify antigens (such as proteins, carbohydrates, and so on) present in tissues or cells based on the principle of the reaction (binding) between antibodies and antigens in the tissue (Bintari and Yuliani, 2020). The distribution of AR has been studied in rat testes, and it has been reported that most AR is found in Leydig cells, with 95% found in peritubular myoid (PTM) cells of the testes. In addition to these two cell types, AR is also found in Sertoli cells, showing specific immunoreactivity expression at certain stages of the seminiferous epithelium. The strongest expression intensity is found in Sertoli cells, while AR is not found in the germinal cells of the seminiferous tubules (Zhu et al., 2000). ARs in goat testicular tissue are found in all testicular cells except for germinal cells (Goyal et al., 1997). In male horse testicular tissue, AR shows immunoreactivity primarily in Leydig cells and Sertoli cells and is also found in PTM cells (Bilińska et al., 2004). Another study reported that AR is positively detected in horse testicular tissue, specifically in the nuclei of Sertoli cells, Leydig cells, and PTM cells, but not in germinal cells (Pearl et al., 2011). Several studies have been performed to evaluate the relation between increased testosterone with sperm quality after PGF2α administration, but the finding showed inconsistency results. These inconsistencies may be due to different times of sperm quality assessment after PGF2α administration. Injection of PGF2α 2 days before semen collection in kacang goats increased testosterone concentration, although it was not followed by a significant improvement in sperm quality (Armansyah et al., 2018). A similar finding was reported by Sari et al. (2019) in Bali cattle. However, when PGF2α was administrated in Aceh cattle, sperm quality was improved without an increase in testosterone concentration (Sari et al., 2021). It is assumed that the interval between PGF2α administration and the increase in testosterone and sperm quality occurs at different times. Differences in α-SMA and AR expression in rat testes based on distribution location can be used to determine the optimal reaction time for PGF2α administration to improve sperm quality, particularly in terms of sperm concentration and motility. Therefore, it is necessary to determine the effect of PGF2α administration at different intervals on sperm quality improvement based on the expression of α-SMA and AR proteins in the testicular tissue of Wistar rats. Materials and MethodsAdministration of PGF2α in Wistar ratsIn this study, 15 male Wistar rats weighed 200–250 g and aged 8–10 weeks were used. All rats were acclimated for 2 weeks. The rats were divided into five treatment groups (n=3 each). Rats testes in control were collected 30 minutes after injection of 0.5 ml NaCl. In groups P1, P2, P3, and P4, the rats were intraperitoneal injected with 2.5 mg/kg BW of PGF2α (Lutalyze, Zoetis, USA), and the testis collection was performed 30, 60, 90, and 120 minutes, respectively. After the treatment, the rats were terminated using Zoletil® 100 (Tiletamine HCl/Zolazepam HCl, Virbac AHIPL, France) at a dose of 40 mg/kg body weight (Ferrari et al., 2005) with modifications. Collection and preparation of testsThe testicular organs of all groups were collected after the rats were terminated, and the testes were separated from the scrotal skin and epididymis. The testicular organs were then fixed using the immersion fixation method with 10% neutral buffered formalin for 3 weeks, referring to the method of Hess and Moore (1993) with modifications. After fixation, the testicular organs were transferred and soaked in a 70% alcohol solution as a stopping point until the dehydration process was carried out. The testicular tissue was processed to histological slides referred to the method of Kiernan (2015) with modifications. Testicular tissue which was previously soaked in 70% alcohol was cut into 0.5 cm pieces and placed into tissue cassettes. The dehydration stage started by immersing the testicular tissue pieces in alcohol with increasing concentrations of 80%, 90%, 95%, and 100% alcohol. After the dehydration process was completed, the tissue was transferred into a xylol solution and then infiltrated with liquid paraffin, followed by embedding the tissue in liquid paraffin to form tissue blocks. The testicular tissue blocks were sectioned using a microtome at a thickness of 5 μm and then adhered to slides coated with poly-L-lysine. All slides were subsequently stained using the Avidin-Biotin Complex (ABC) method of IHC. IHC stainingThe IHC staining procedure using the ABC method refers to the manual procedure of the mouse and rabbit-specific HRP/diaminobenzidine (DAB) (ABC) detection IHC kit (Abcam®) with modifications. The initial stage of staining was started with the deparaffinization of the testicular tissue slides using xylol, followed by rehydration of the tissue in alcohol with decreasing concentrations (starting with 100%, 95%, 90%, 80%, and 70% alcohol), then washed with running water and distilled water. The slides were incubated for 15 minutes with a 3% H2O2 solution and washed with PBS. The slides were then incubated for 15 minutes with a protein block solution and washed again with PBS. The next step, incubated the tissue for 1 hour with the primary antibody (AR antibody) at a dilution of 1:100 and anti-α-SMA antibody at a dilution of 1:100 at room temperature, then wash with PBS. Subsequently, the slides were incubated for 15 minutes with biotinylated goat anti-polyvalent (secondary antibody) at room temperature and re-washed with PBS. After that, the slides were incubated for 15 minutes with streptavidin peroxidase at room temperature, followed by another PBS wash. For visualizing the binding results between the receptor and antibody (indicating immunoreactivity), the chromogen DAB was added to the tissue and incubated for 5 minutes while observing color changes using a light microscope. Positive results or immunoreactivity were indicated by the formation of brown color with varying intensities, showing the expression of the antigen–antibody binding. The slides were then rinsed with PBS twice. The final stage was counterstaining with Mayer’s hematoxylin, dehydration, clearing (using alcohol solutions from low to high concentrations: 70%, 80%, 90%, 95%, absolute alcohol (three times), and xylol (three times)), followed by mounting using a slide adhesive (Entellan®). Observation of IHC stainingThe IHC staining results were observed using a light microscope (Olympus CX31, Japan) equipped with a photography device (SIGMA, Germany) at magnifications of 100× and 400×. The observed parts of the testis included the seminiferous tubules and interstitial tissue of Wistar rat testes. The observation and identification of α-SMA and AR expression in Wistar rat testes were conducted using the intensity score (IS) method, and the immunoreactivity was scored (Table 1). Data analysisThe distribution of α-SMA and AR in the testicular tissue of rats after in vivo administration of PGF2α was analyzed descriptively and presented in the form of histological images. Differences in the expression of α-SMA and AR in the testicular tissue in treatment groups were analyzed using the non-parametric Kruskal-Wallis test, followed by the Mann-Whitney U test (Saruhan et al., 2011). Ethical approvalThis study was conducted with approval from the Ethics Commission responsible for the use of Experimental Animals, Faculty of Veterinary Medicine, Universitas Syiah Kuala with certificate number: 169/KEPH/IX/2022. ResultsThe distribution of α-SMA in the testicular tissue of Wistar rats in this study was detected based on the immunoreactivity results visualized by the formation of brown color due to the reaction between DAB chromogen and the antigen (receptor) complex with antibodies. The distribution and expression data of α-SMA in Wistar rat testes in this study were further compared with the results of negative control staining, which showed no visualization of brown color in the testicular tissue (Fig. 1). There was no immunoreactivity in PTM cells, blood vessels (BVs), and loose connective tissue (LCT). The distribution of α-SMA in the testicular tissue of Wistar rats across all treatment groups is presented in Figure 2. The α-SMA protein was found in specific parts of the testicular tissue, in the PTM cells located on the basal membrane of the seminiferous tubules, connective tissue, and BVs within the testicular interstitial tissue. Results of immunohistochemical staining visualization of α-SMA protein expression in Wistar rat testicular tissue in groups injected with 0.9% NaCl (P0) and PGF2α (P1, P2, P3, P4) showed increased immunoreactivity with varying staining ISs as presented in Table 2. Expression levels in Wistar rat testicular tissue were determined based on the IS values assigned to each observed tissue slide. α-SMA expression in Wistar rat testicular tissue across all treatment groups is presented in Table 2. Table 1. IS for immunoreactivity of α-SMA and AR in Wistar rat testicular tissue (Mudduwa, 2009).
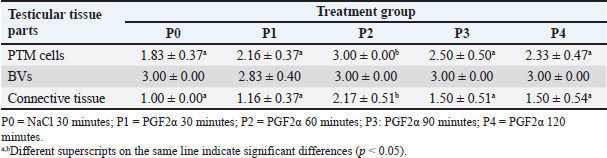
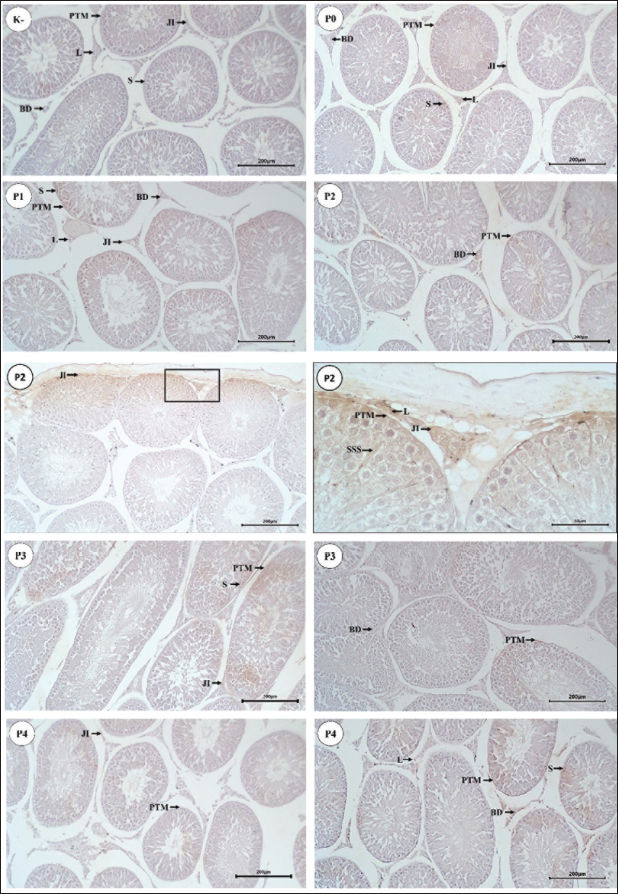
Fig. 1. Staining results of negative control slides (K-) of Wistar rat testicular tissue using IHC staining. BV=blood vessels; LCT=loose connective tissue; PTM=peritubular myoid. Scale bars: 200 µm (K- left) and 50 µm (K- right). The effect of different intervals of PGF2α administration on testis collection time on the increase in AR expression in Wistar rats is presented in Figure 3. The immunoreactivity observations in the testicular tissue of Wistar rats focused on the seminiferous tubules and interstitial tissue. Observations of the seminiferous tubule components indicated that AR was expressed in the nuclei of Sertoli cells and PTM cells. AR expression in the interstitial tissue of the testis was found in Leydig cells, BVs, and connective tissue. Based on the results of this study, AR expression in the testicular tissue of Wistar rats was generally found in the cell nuclei and, to some extent, in the cell cytoplasm. The results of IHC staining visualization of AR expression in Wistar rats, in the group injected with 0.9% NaCl (P0) and the group injected with PGF2α (P1, P2, P3, and P4) at 30, 60, 90, and 120 minutes post-treatment, showed varying levels of immunoreactivity in the tissue with different staining intensities, as presented in Table 3. DiscussionBased on the staining results (Fig. 2), immunoreactivity was observed with brown color visualization in specific parts of the testicular tissue, both in the group of rats injected with 0.9% NaCl (P0) and the group of rats injected with PGF2α (P1, P2, P3, and P4). The α-SMA protein was distributed in the PTM cells located on the basal membrane of the seminiferous tubules, as well as in the walls of BVs and LCT located in the interstitial tissue of the testes (intertubular seminiferous tissue). These results are consistent with the report by Marini et al. (2019) that α-SMA was positively detected (immunoreactive) in the PTM cells surrounding the seminiferous tubules and in the BVs. Olukole et al. (2020) stated that the positive reaction to α-SMA observed in PTM cells and the walls of BVs in the testicular tissue indicates that α-SMA functions in testicular contractility. Strong α-SMA immunoreactivity was found in the PTM cells, the inner capsule of the testes, and BVs, indicating the occurrence of contractile activity in the movement of spermatozoa produced in the seminiferous tubules into the excretory ducts of the male animal’s testes (Olukole et al., 2020). This is consistent with the statements by Losinno et al. (2012) and Madekurozwa (2013) that PTM cells exhibit morphological and functional characteristics of smooth muscle cells and fibroblasts, resulting in the immuno-positive properties of PTM cells towards actin filaments, which are characteristic of smooth muscle cells. The observed α-SMA immunoreactivity in the P1, P2, P3, and P4 rat groups is likely related to the effects of PGF2α induction. Free et al. (1980) reported that PGF2α has a strong effect in increasing venous pressure in rat testes and causes smooth muscle contraction in the testicular capsule and seminiferous tubules to varying degrees. Besides stimulating contraction in the testicular capsule and seminiferous tubules, PGF2α has also been reported to stimulate smooth muscle contraction surrounding the epididymis (Masoumi et al., 2011). Prostaglandin receptors in the epididymis are most abundant in the distal part (Fouchécourt et al., 2002), making this area more sensitive to changes in PGF2α concentration (Masoumi et al., 2011). Prostaglandins (specifically, prostaglandin E2 and F2α), which are also produced by Leydig cells in rats (Frungieri et al., 2015), may play a role in the contraction of peritubular cells to move spermatozoa and testicular fluid to the epididymis (Welter et al., 2013; Rey-Ares et al., 2018). In general, it appears that extrinsic factors (including PGF2α) produced in Leydig cells help stimulate the contractility of peritubular cells to facilitate spermatozoa transfer and enhance male fertility (Heinrich and DeFalco, 2020). Based on Table 2, α-SMA expression was found in PTM cells, the walls of BVs, and connective tissue, with varying staining intensities in each treatment group. Statistically, α-SMA expression in PTM cells and connective tissue in P2 was significantly different (p < 0.05) compared to P0, P1, P2, P3, and P4, whereas expression in the BV walls did not show a significant difference (p > 0.05) among all treatment groups. These different results indicated that α-SMA expression in PTM cells and connective tissue increased in the P2 group with a 60-minute interval between PGF2α administration and testicular tissue collection. The results obtained are consistent with Hess (2002), who reported that the administration of PGF2α reacts more effectively within a range of 40 to 50 minutes and lasts for up to 8 hours.
Fig. 2. Distribution of α-SMA in the testicular tissue of Wistar rats using IHC staining. Arrows indicated α-SMA immunoreactivity in PTM cells on the basal membrane of the seminiferous tubules, BVs, and LCT. Scale bars: 200 µm for P0, P1, P2, P3, and P4 (left column) and 50 µm for P0, P1, P2, P3, and P4 (right column). Table 2. Mean (±SD) IS for α-SMA expression in Wistar rat testicular tissue in five treatment groups.
Expression of α-SMA in PTM cells of Wistar rat testes observed in this study was stronger in the P2 group, while in the P1, P3, and P4 groups, it was expressed with moderate intensity, and weakly expressed in the P0 group which was not injected with PGF2α. According to Sohn et al. (2013), strong α-SMA immunoreactivity in mammalian testes is found in the PTM cells surrounding the seminiferous tubules. Olukole et al. (2020) stated that PTM cells are contractile and play a crucial role in the contraction of seminiferous tubules to transport spermatozoa and testicular fluid. Fernández et al. (2008) also stated that the contraction of seminiferous tubules in transporting spermatozoa involves contractile PTM cells that express α-SMA protein. Expression of α-SMA in the BV walls located in the interstitial tissue of Wistar rat testes was detected with strong expression in all treatment groups (p > 0.05). This is consistent with the statement by Devkota et al. (2006) that α-SMA immunoreactivity in BVs is always detected, making it a choice for determining positive control in detecting α-SMA presence in tissues. α-SMA is an isoform of actin that dominates the smooth muscle cells of BVs and plays a vital role in fibrogenesis (Annegowda et al., 2018). According to Zhu et al. (2019) added that α-SMA becomes the cytoskeleton that regulates the mechanical and elastic properties of BVs with two actin molecular models, G-actin and F-actin, which cause elasticity, relaxation, and structural integrity of BVs. Expression of α-SMA in the connective tissue of Wistar rat testes in the P2 group was expressed with strong intensity compared to P0, P1, P3, and P4 groups, which were expressed with moderate intensity. According to Tung and Fritz (1990), under normal conditions, α-SMA isoactin was easily detected in PTM cells but is difficult to find in other cell types within testicular tissue, such as fibroblasts. However, in this study, α-SMA expression in connective tissue containing fibroblasts was found with strong intensity in the testes of P2 rats. The increased expression in connective tissue containing fibroblasts in P2 was suspected to be due to the effect of PGF2α administration at a 60-minute interval. This is in line with the statement by Hinz et al. (2001) that α-SMA expression correlates with the contractile ability of fibroblasts. Jiroutová et al. (2005) added that fibroblasts may contract using several cytoskeletal proteins typically found in smooth muscle cells, particularly α-SMA protein. Although the strongest α-SMA expression was found in the connective tissue of P2 rat testes, this expression was also found in the connective tissue of other treatment groups. It is suspected that connective tissue containing fibroblasts normally functions as a component of the interstitial tissue of the testes. According to Jiang et al. (2013), fibroblasts proliferate from mesenchymal cells and play a role in forming the interstitial compartment of the testes. Based on the results of this study, there was an increase in α-SMA expression in the testicular tissue of Wistar rats, especially in the P2 group. Therefore, the administration of PGF2α at different intervals may improve sperm quality. This is consistent with the report by El-Badry et al. (2013) that the injection of the PGF2α hormone can result in a significant increase in semen volume, sperm cell concentration, and total spermatozoa per ejaculation. Another study conducted by Husnurrizal et al. (2021) also reported that the increase in sperm motility in Waringin sheep was due to the administration of PGF2α, which inactivates PGF2α on spermatozoa. Herawati and Widiarso (2003) stated that the administration of PGF2α would increase sperm motility because PGF2α can activate the contractile elements of spermatozoa, which are the fibrous layers surrounding the acrosome of the main part of the spermatozoa. Based on this information, the administration of PGF2α can increase α-SMA expression in testicular tissue, which affects the improvement of sperm quality. Based on the data obtained, the administration of PGF2α at intervals of 30 minutes (P1), 60 minutes (P2), 90 minutes (P3), and 120 minutes (P4) influenced the increase in α-SMA expression in the testicular tissue of Wistar rats. In the P1 group (30-minute interval), the performance of PGF2α did not function optimally. This is evidenced by the weak expression of α-SMA in the testicular tissue compared to the P2, P3, and P4 groups. This finding is consistent with the study by Sari et al. (2019) that there was no increase in the semen volume of Bali cattle after the administration of PGF2α at a 30-minute interval. The increased expression of α-SMA in the P2 treatment group compared to the other treatment groups suggests that PGF2α optimally functions at a 60-minute interval. This is supported by Capitan et al. (1990), who found that several semen characteristics of the treatment and post-treatment groups of PGF2α increased significantly at the 1-hour (60-minute) interval. Hashizume and Niwa (1984) also reported an increase in semen volume in pigs after the administration of PGF2α at a 1-hour interval. Additionally, Sari et al. (2019) reported that the administration of PGF2α at a 60–90 minutes interval in Bali cattle significantly increased semen characteristics such as sperm consistency, sperm concentration, the number of sperm per ejaculation, mass motility, individual motility, and the percentage of viable sperm. Thus, it is proven that the optimal interval for the administration of PGF2α is 60 minutes, as evidenced by the increased expression of α-SMA in the testicular tissue of Wistar rats in this study.
Fig. 3. Distribution of AR in Wistar rat testicular tissue. Arrows indicate the location of RA in testicular tissue (P0, P1, P2, P3, and P4), connective tissue (JI), PTM cells, Sertoli cells (S), Sertoli cell cytoplasm (SSS), Leydig cells (L), and blood vessels (BD). Scale bar 200 μm (K-, P0, P1, P2, P3, and P4) and 50 μm (P2). Table 3. IS (mean ± SD) of AR in Wistar rat tissues in five treatment groups.
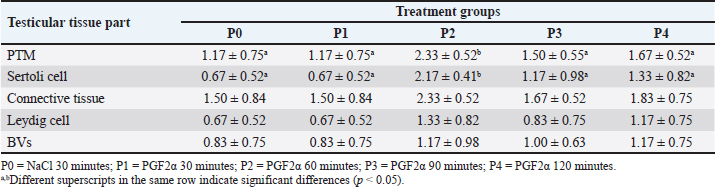
Based on Table 3, AR immunoreactivity was found in connective tissue, PTM cells, Sertoli cells, Leydig cells, and endothelial cells of BVs in all groups. This is consistent with the report by Bilińska et al. (2004) that AR in the testicular tissue of stallions is found in Sertoli cells, Leydig cells, PTM cells, and BVs. Zhu et al. (2000) also reported that most AR was found in Leydig cells, with 95% found in PTM cells of the testis. In addition to these two cell types, AR was also found in Sertoli cells, showing specific immunoreactivity expression in certain stages of the seminiferous epithelium. The strongest intensity of expression was found in Sertoli cells, whereas AR was not found in germ cells of the seminiferous tubules. Shan et al. (1997) reported that the presence of AR in Leydig cells during pubertal development in mice indicates that androgens play a role in Leydig cell differentiation, while AR in Sertoli cells in adult mice highlights the crucial role of androgens in spermatogenesis. Welsh et al. (2012) explained that AR signals to PTM cells, which are important in regulating the development of the structure and function of adult Leydig cells. Additionally, AR is also immunoreactive in BVs of the interstitial tissue of bovine testes, as reported by Welsh et al. (2010). Statistical analysis showed that the IS values of AR expression in PTM cells and Sertoli cells were significantly different (p < 0.05) in each treatment group, whereas the connective tissue, Leydig cells, and BVs did not differ significantly t (p > 0.05). Further analysis of AR expression in PTM cells and Sertoli cells revealed higher IS (p < 0.05) in P2 over other groups. This indicated that RA expression in the testicular tissue of Wistar rats increased in the treatment groups injected with PGF2α for 60–120 minutes. The differences in staining intensity of AR immunoreactivity in the testicular tissue of Wistar rats were related to the different treatment groups. The injection of 0.9% NaCl (P0) was used as a control group to compare the increase in AR expression in the testicular tissue of Wistar rats in each treatment group. AR expression in the testicular tissue of the P1, P2, P3, and P4 groups is suspected to be related to the different times of PGF2α administration, which affects its performance in the testicular tissue of Wistar rats. In P1 (30-minute interval), the performance of PGF2α was not optimal compared to P2, P3, and P4 which is evidenced by the weak expression of AR in the testicular tissue of Wistar rats. This is consistent with the report by Hess (2002) that observed the PGF2α administration will react within 40–50 minutes and will last for 8 hours. Goyal et al. (1997) reported that myoid peritubular cells generally express with moderate intensity, similar to the findings in this study, where myoid peritubular cells expressed with moderate to strong intensity in all groups. However, in treatment P2, the myoid peritubular cells expressed with strong intensity, likely due to the action of PGF2α which regulates various physiological activities, including smooth muscle contraction in the male reproductive tract. This aligns with Capitan et al. (1990) who stated that PGF2α directly affects the mechanism of steroids and local muscle contraction in the lumen of the male reproductive system. Previous studies have observed that the administration of PGF2α is able to regulate smooth muscle contraction in buffalo testes (Henney et al., 1990) and in rabbit testes (Hargrove et al.,1975) AR expression in Sertoli cells of Wistar rats was moderate in P2 and weak in P1, P3, and P4. The increased AR expression in Sertoli cells particularly in P2 was likely related to the influence of PGF2α in increasing testosterone levels, which positively affected AR expression in Sertoli cells. Shan et al. (1997) also reported similar findings, indicating that the presence of AR in tissues shows sensitivity to androgen levels. This study also revealed that AR expression in Leydig cells of Wistar rats was moderate in P2 but weak in P1, P3, and P4. The increased AR expression in Leydig cells from P1 to P2 is likely related to the influence of PGF2α in increasing testosterone levels, which impacts the role of Leydig cells in synthesizing and secreting testosterone. Smith and Walker (2014) also reported that testosterone is produced by Leydig cells in response to luteinizing hormone (LH) stimulation and acts as a paracrine factor diffusing into the seminiferous tubules. The effects of androgens are mediated by AR localized in the nucleus and cytoplasm. AR expression in the BVs of Wistar rats was moderate in P2 but weak in P1, P3, and P4. Generally, AR in BVs is expressed with weak intensity. This condition was also reported by Zhou et al. (2002), who found weak staining intensity in endothelial cells of BVs in adult rats. The increase in AR expression in BVs is likely due to the increase in testosterone, which has a direct effect on BVs. This explanation is supported by Lorigo et al. (2020), who stated that testosterone has direct effects on vascular smooth muscle. Lorigo et al. (2020) also explained that testosterone and DHT can regulate various cellular functions by binding to RA. After crossing the plasma membrane, androgens bind to receptors to form hormone-receptor complexes, which then interact directly with DNA in the nucleus, modulating gene transcription and protein synthesis. Based on the present finding, there was an increase in AR expression in the testicular tissue of Wistar rats, with the strongest expression found in PTM cells and Sertoli cells. This is likely due to the influence of PGF2α administration at different intervals, increasing testosterone levels. One method to increase testosterone concentration is through PGF2α administration. Masoumi et al. (2011) also reported that PGF2α administration could increase testosterone concentration in the serum of male cattle. Another study by Sari et al. (2019) also reported that PGF2α could affect testosterone concentration. Senger (2012) stated that most testosterone is synthesized and secreted by Leydig cells, with small amounts secreted by the adrenal cortex. According to Hasbi and Gustina (2018), testosterone produced by Leydig cells binds to AR in Sertoli cells, secreting androgen-binding protein and inhibin, and aiding in spermatozoa formation. Hill et al. (2004) explained that in vitro studies have shown that testosterone positively regulates its receptors, as indicated by the stimulation of AR expression in Sertoli cells and the reduction of intratesticular testosterone associated with decreased AR protein in Sertoli cells in vivo. Based on these findings, the increase in testosterone after PGF2α administration is also believed to enhance AR activity in testicular tissue. Prostaglandins modulate steroidogenesis in Leydig cells and glucose uptake in Sertoli cells. Therefore, prostaglandins in Leydig and Sertoli cells act as local modulators of testicular activity, regulating efficiency in spermatogenic processes (Frungieri et al., 2015). In vitro experiments on adult hamster Leydig cells incubated with or without LH/human chorionic gonadotropin and testosterone showed increased PGF2α production. This increase was not due to direct LH action but rather to LH stimulating testosterone production. Additionally, the effects of testosterone on hamster Leydig cells occur through ARs (Matzkin et al., 2009). Another study reported that PGF2α modulates androgen production in Leydig cells (Kubota et al., 2011). Androgen activity is mediated by AR (Wang et al., 2009). The decreased AR expression in PTM cells and Sertoli cells after 60 minutes as observed in P3 and P4, is possibly related to the half-life of PGF2α. It has been reported that the half-life of PGF2α is relatively short, possibly less than 1 minute in circulation (Basu, 2010). PGF2α is metabolized quickly and efficiently, mainly in the lungs (Engen, 2004). About 65% of PGF2α injected in heifers is metabolized after a single pass through the lungs, resulting in a half-life of approximately 29 seconds. The metabolic half-life of PGF2α in mares is about 1.5 minutes (94 seconds), longer than the commonly reported half-life (~1 minute) in other species (Shrestha et al., 2012). The half-life of PGF2α is also depends on the administration method. Generally, the half-life of PGF2α is shorter after intramuscular or intravenous administration compared to subcutaneous or intraperitoneal due to differences in absorption, distribution, metabolism, and elimination processes (Gunawan et al., 2007). The increase in testosterone levels due to PGF2α administration is believed to similarly affect testicular tissue by enhancing AR expression in the testicular tissue of Wistar rats. The abundance of testosterone is assumed to stimulate AR binding to androgens, activate and dimerize the receptors, ready to bind again with DNA in the nucleus. Li et al. (2012) explained that the intracellular target of androgens is the AR. After binding with androgens to form AR, it moves to the nucleus and regulates transcription in androgen target genes. According to Nieschlag et al. (2005) and Supakar et al. (1993), the transcription process converts DNA to RNA, initiated by the presence of messenger RNA (mRNA), which carries messages related to the presence of ARs in target tissues. AR is activated by two ligands, testosterone, and DHT, with different binding affinities to AR, causing different activation levels of AR by these ligands. AR induces physiological responses in the same tissue type depending on their location. AR interacts with other nuclear proteins, resulting in the up or down-regulation of specific gene transcription. Up-regulation or activation of transcription increases mRNA synthesis, later translated by ribosomes to produce specific proteins. Therefore, it can be concluded that increased testosterone concentration enhances mRNA AR expression in tissues, leading to cell proliferation. This is supported by previous studies showing that testosterone replacement therapy increases mRNA AR expression in the penis of castrated adult Wistar rats (Indira, 2016). According to Surampudi et al. (2012), the influence of testosterone on AR is highly variable. However, based on the theory that androgens can increase AR in cells, as shown by cell proliferation, the findings from this study demonstrated that the best interval for PGF2α administration to detect an increase in testosterone was 60 minutes, as evidenced by increased AR expression in the testicular tissue of Wistar rats. ConclusionDistribution of α-SMA was found in myoid peritubular cells at the basal membrane of seminiferous tubules as well as in the walls of BVs and connective tissue in the interstitial tissue of the testis. Based on the increased expression of α-SMA in the testicular tissue of Wistar rats, the optimal interval for PGF2α administration is 60 minutes. PGF2α administration can enhance AR expression, and the interval between PGF2α administration and testis collection affects the increase in AR expression, with the strongest expression observed in PTM cells and Sertoli cells of Wistar rat testes at the 60-minute interval. AcknowledgmentsThe authors would like to thank the Rector of Universitas Syiah Kuala for supporting this research through the Professor Research Scheme 2022, grant number: 141/UN11/SPK/PNBP/2021. Conflict of InterestThe authors declare that there is no conflict of interest. FundingThe research work is supported by the fund from Professor Research Scheme 2022 at Syiah Kuala University (No. 141/UN11/SPK/PNBP/2021) Authors’ ContributionsConceptualization: TA, HH, TNS. Methodology: SW, HH, ATS, MBP. Investigation and Data Curation: AS1, TA, AS2. Writing – Review and Editing: TNS, TA, AS1, SW. Project Administration: TNS, HH, AS2. Supervision and Validation: AS1, TNS, SW, TA. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAnnegowda, V.M., Devi, H.U., Rao, K., Smitha, T., Sheethal, H. and Smitha A. 2018. Immunohistochemical study of alpha-smooth muscle actin in odontogenic cysts and tumors. J. Oral Maxillofac. Pathol. 22, 188–192. Arini, L.A. 2019. Studi literatur perbedaan ekspresi messenger ribonucleid acid (mRNA) reseptor androgen setelah pemberian testosteron antara penis dan kelenjar prostat tikus wistar jantan (Rattus norvegicus) pascakastrasi. J. Kes. Reprod. 6, 29–36. Armansyah, T., Barat, E.R.P., Handini, C.V.R., Aliza, D., Sutriana, A., Hamdan, H., Panjaitan, B., Sayuti, A. and Siregar, T.N. 2018. Concentration and motility of spermatozoa and testosterone level of Kacang goat after seminal vesicle extract administration. Open Vet. J. 8, 406–410. Armansyah, T., Putri, S.F., Oktaviany, O., Siregar, T.N., Syafruddin S., Panjaitan, B. and Sayuti, A. 2021. The gonadotropin releasing hormone administration increase testosterone level in Waringin rams. J. Vet. 22, 342–351. Arora, S., Singh, P., Basu, P. and Haldar, C. 2018. Immunohistochemical localization of androgen , meletonin and glucocorticoid receptors in the testis of indian pygmy field mouse Mus terricolor. J. Endocrinol. Reprod. 22, 31–36. Aswadi, A., Husnurrizal, H., Adam, M. and Siregar, T.N. 2021. The effect of PGF2α injection on post-thaw motility in sperm of Nubian goats. Bul. Peternak. 45, 1–5. Basu, S. 2010. Bioactive eicosanoids: role of prostaglandin F2α and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol. Cells 30, 383–391. Bilińska, B., Hejmej, A., Pawlak, M., Sadowska, J. and Tischner, M. 2004. Immunoexpression of androgen receptors in testes of immature and mature stallions. Equine Vet. J. 36, 539–543. Bintari, I.G. and Yuliani, M.G.A. 2020. Deteksi Aeromonas hydrophila pada ginjal mencit Mus musculus dengan teknik imunohistokimia. J. Agriekstensia 19, 114–120. Capitan, S., Antiporda, G. and Momongan, V. 1990. Reaction time, semen output and semen quality of buffalo bulls after pre-collection injection of prostaglandin F2 alpha (PGF2 alpha). Asian-Australas. J. Anim. Sci. 3, 343–346. Devkota, B., Sasaki, M., Takahashi, K.-I., Matsuzaki, S., Matsui, M., Haneda, S., Takahashi, M., Osawa, T. and Miyake, K.-I. 2006. Postnatal developmental changes in immunohistochemical localization of α-smooth muscle actin (SMA) and vimentin in bovine testes. J. Reprod. Dev. 52, 43–49. El-Badry, D.A., Gabr, F.I. and Shaker, M.H. 2013. The effect of oxytocin, prostaglandin F2a or GnRH injection on fresh and frozen-thawed semen characteristics of rams. Assiut Vet. Med. J. 59, 214–229. Engen, R.L. 2004. Dynamics of the cardiovascular system. Dukes’ physiology of domestic animals. Ithaca, NY: Comstock Publishing Associates, pp: 181–191. Fernández, D., Bertoldi, M.V., Gómez, L., Morales, A., Callegari, E. and Lopez, L.A. 2008. Identification and characterization of myosin from rat testicular peritubular myoid cells. Biol. Reprod. 79, 1210–1218. Ferrari, L., Turrini, G., Rostello, C., Guidi, A., Casartelli, A., Piaia, A. and Sartori, M. 2005. Evaluation of two combinations of Domitor, Zoletil 100, and Euthatal to obtain long-term nonrecovery anesthesia in Sprague-Dawley rats. Comp. Med. 55, 256–264. Fouchécourt, S., Charpigny, G., Reinaud, P., Dumont, P. and Dacheux, P.L. 2002. Mammalian lipocalin-type prostaglandin D2 synthase in the fluids of the male genital tract: putative biochemical and physiological functions. Biol. Reprod. 66, 458–467. Free, M.J., Jaffe, R.A. and Morford, D.E. 1980. Sperm transport through the rete testis in anesthetized rats: role of the testicular capsule and effect of gonadotropins and prostaglandins. Biol. Reprod. 22, 1073–1078 Frungieri, M.B., Calandra, R.S., Mayerhofer, A. and Matzkin, M.E. 2015. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction 149, 169–180. Goyal, H.O., Bartol, F.F., Wiley, A.A., Khalil, M.K., Chiu, J. and Vig, M.M. 1997. Immunolocalization of androgen receptor and estrogen receptor in the developing testis and excurrent ducts of goats. Anat. Rec. 249, 54–62. Gunawan, S.G., Setiabudy, R., Nafrialdi, N. and Instiaty, I. 2007. Farmakologi dan Terapi, 5th ed. Jakarta: FKUI Press. Hargrove, J.L., Seeley, R.R. and Ellis, L.C. 1975. Rabbit testicular contractions: bimodal interaction of prostaglandin E1 with other agonists. Am. J. Physiol. 228, 810–814. Hasbi, H. and Gustina, S. 2018. Regulasi androgen dalam spermatogenesis untuk meningkatkan fertilitas ternak jantan. Wartazoa 28(2), 13–22; doi:10.14334/wartazoa.v28i1.1643. Hashizume, T. and Niwa, T. 1984. Effect of administration of prostaglandin F2α (PGF2α) on the properties of sperm rich fraction of boar semen. Japanese J. Anim. Reprod. 30, 182–185. Heinrich, A. and DeFalco, T. 2020. Essential roles of interstitial cells in testicular development and function. Andrology 8, 903–914. Henney, S.R., Killian, G.J. and Deaver, D.V. 1990. Libido, hormone concentrations in blood plasma and semen characteristics in Holstein bulls. Anim. Sci. J. 68, 2784–2792. Herawati, H. and Widiarso, B.P. 2003. Pengaruh penambahan prostaglandin F2α terhadap kualitas sperma pada semen kambing yang diencerkan dengan berbagai larutan. J. Indon. Trop. Anim. Agric. 28, 74–78. Hess, M. 2002. The effects of prostaglandin F2α, oxytocin and gonadotropin releasing hormone on ejaculate characteristics in the dog. Blacksburg, VA: Virginia Polytechnic Institute and State University. Hess, R.A. and Moore, B.J. 1993. Histological methods for evaluation of the testis. In Methods in reproductive toxicology. Eds., Chapin, R.E. and Heindel, J.J. Cambridge, MA: Academic Press Hill, C.M., Anway, M.D., Zirkin, B.R. and Brown, T.R. 2004. Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat sertoli cells. Biol. Reprod. 71, 1348–1358. Hinz, B., Celetta, G., Tomasek, J.J., Gabbiani, G. and Chaponnier, C. 2001. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 12, 2730–2741. Husnurrizal, H., Aritonang, A.S., Siregar, T.N., Armansyah, T. and Hafizuddin, H. 2021. The addition of PGF2α in semen diluent can increase post-thawing motility of spermatozoa of Waringin sheep. Livest. Anim. Res. 19, 210–216. Indira, N.P.R.D.A. 2016. Terapi sulih testosteron meningkatkan ekspresi messenger ribonucleaic acid (mRNA) reseptor androgen pada penis tikus Wistar (Rattus norvegicus) dewasa yang dikastrasi. Intisari Sains Medis 7, 76–80. Jiang, X., Skibba, M., Zhang, C., Tan, Y., Xin, Y. and Qu, Y. 2013. The roles of fibroblast growth factors in the testicular development and tumor. J. Diabetes Res. 2013, 489095. Jiroutová, A., Majdiaková, L., Cermáková, M., Köhlerová, M. and Kanta J. 2005. Expression of cytoskeletal proteins in hepatic stellate cells isolated from normal and cirrhotic rat liver. Acta Med. 48, 137–144. Karahan, İ., Türk, G. and Gür, S. 2006. In vitro effects of prostaglandin F2α and metamizol on motility of diluted bull semen. Turkish J. Vet. Anim. Sci. 30, 271–278. Kiernan, J.A., 2015. Histological and histochemical methods, 5th ed. England: Scion Publishing Ltd. Kubota, H., Sasaki, S., Kubota, Y., Umemoto, Y., Yanai, Y., Tozawa, K,, Hayashi, Y. and Kohri, K. 2011. Cyclooxygenase-2 protects germ cells against spermatogenesis disturbance in experimental cryptorchidism model mice. J. Androl. 32, 77–85. Li, Y., Izumi, K. and Miyamoto, H. 2012. The role of the androgen receptor in the development and progression of bladder cancer. Jpn. J. Clin. Oncol. 42, 569–577. Lorigo, M., Mariana, M., Lemos, M.C. and Cairrao, E. 2020. Vascular mechanisms of testosterone: the non-genomic point of view. J. Steroid Biochem. Mol. Biol. 196, 105496. Losinno, A.D., Morales, A., Fernández, D. and Lopez, L.A. 2012. Peritubular myoid cells from rat seminiferous tubules contain actin and myosin filaments distributed in two independent layers. Biol. Reprod. 86, 150, 151–158. Madekurozwa, M.C. 2013. Post-hatch changes in the immunoexpression of desmin, smooth muscle actin and vimentin in the testicular capsule and interstitial tissue of the Japanese quail (Coturnix coturnix Japonica). Anat. Histol. Embryol. 42, 369–378. Majeed, A., Omar, A., Ahmed, K., Mahmood, R. and Hassan, R. 2017. Effect of addition of prostaglandin PGF2 alpha and oxytocin invitro to diluted and cooled semen of Fresian bull. Euphrates J. Agricult. Sci. 2017, 563–568. Marini, M., Ibba-Manneschi, L., Rosa, I., Sgambati, E. and Manetti, M. 2019. Changes in the telocyte/CD34+ stromal cell and α-SMA+ myoid cell networks in human testicular seminoma. Acta Histochem. 121, 151442. Masoumi, R., Towhidi, A., Javaremi, A.N., Nabizadeh, H. and Zhandi, M. 2011. Influence of PGF2α on semen quality and libido in Holstein bulls. Turkish J. Vet. Anim. Sci. 35, 1–6. Matzkin, M.E., Gonzalez-Calvar, S.I., Mayerhofer, A., Calandra, R.S. and Frungieri, M.B. 2009. Testosterone induction of prostaglandin-endoperoxide synthase 2 expression and prostaglandin F(2alpha) production in hamster Leydig cells. Reproduction 138, 163–175. Mudduwa, L.K.B. 2009. Quick score of hormone receptor status of breast carcinoma: correlation with the other clinicopathological prognostic parameters. Indian J. Pathol. Microbiol. 52, 159–163. Nieschlag, E., Swerdloff, R., Behre, H., Gooren, L., Kaufman, J.M., Legros, J.J., Lunenfeld, B., Morley, J.E., Schulman, C. and Wang, C. 2005. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 8, 56–58. Olukole, S.G., Coker, O.M. and Oke, B.O. 2020. Immunoreactivities to α-SMA and S-100 proteins in the testis of the African four-toed hedgehog (Atelerix albiventris). World Vet. J. 10, 216–222. Pandur, I.D. and Pacala, N. 2012. Sperm motility after the addition of prostaglandin F2α to the landrace boar diluted semen. Anim. Sci. Biotechnol. 45, 222–225. Pearl, C.A., Mason, H. and Roser, J.F. 2011. Immunolocalization of estrogen receptor alpha, estrogen receptor beta and androgen receptor in the pre-, peri- and post-pubertal stallion testis. Anim. Reprod. Sci. 125, 103–111. Prestiya, A., Siregar, T.N., Husnurrizal, H., Wahyuni, S., Sari, E.M., Hafizuddin, H. and Panjaitan, B. 2020. The improvement of sperm motility in Nubian goat after PGF2α administration in andromed semen diluents. J. Agripet. 20, 32–37. Rey-Ares, V., Rossi, S.P., Dietrich, K.G., Köhn, F.M., Schwarzer, J.U., Welter, H., Frungieri, M.B. and Mayerhofer, A. 2018. Prostaglandin E2 (PGE2) is a testicular peritubular cell-derived factor involved in human testicular homeostasis. Mol. Cell. Endocrinol. 473, 217–224. Sari, E.M., Nur, S., Mulkan, M., Gholib, G., Thasmi, C.N. and Siregar, T.N. 2021. Case study the effect of giving PGF2α before the collection of the quality of Aceh cattle semen. J. Agripet. 21, 19–25. Sari, E.M., Tanjung, S., Sari, D.S., Akmal, M., Siregar, T.N. and Thasmi, C.N. 2019. The improvement of semen quality and testosterone level of Bali cattle after prostaglandin F2α administration. J. Kedokt. Hewan. 13, 101–105. Saruhan, B.G., Saǧsoz, H., Akbalik, M.E. and Ketani, M.A. 2011. Distribution of estrogen receptor α and progesterone receptor B in the bovine oviduct during the follicular and luteal phases of the sexual cycle: an immunohistochemical and semi-quantitative study. Biotech. Histochem. 86, 315–325. Şen, Ç.Ç. and Akcay, E. 2015. The effect of oxytocin and prostaglandin hormones added tosemen on stallion sperm quality. Turkish J. Vet. Anim. Sci. 39, 705–709. Senger, P. 2012. Pathways to pregnancy and parturition. Redmond, OR: Current Conceptions, Redmon Inc. Shan, L.X., Bardin, C.W. and Hardy, M.P. 1997. Immunohistochemical analysis of androgen effects on androgen receptor expression in developing Leydig and Sertoli cells. Endocrinology 138, 1259–1266. Shrestha, H.K., Beg, M.A., Burnette, R.R. and Ginther, O. 2012. Plasma clearance and half-life of prostaglandin F2alpha: a comparison between mares and heifers. Biol. Reprod. 87, 11–16. Smith, L.B. and Walker, W.H. 2014. The regulation of spermatogenesis by androgens. Cell Dev. Biol. 30, 2–13. Sohn, J., Sasaki, M., Yasuda, M., Kim, Y., Shin, N.S. and Kimura, J. 2013. Immunolocalization of cytoskeletal proteins in the testes of two Asian cervids: water deer (Hydropotes inermis) and Reeves’ muntjac (Muntiacus reevesi). J. Vet. Med. Sci. 75, 1071–1075. Supakar, P.C., Song, C.S., Jung, M.H., Slomczynska, M.A., Kim, J.M., Vellanoweth, R.L., Chatterjee, B. and Roy, A.K. 1993. A novel regulatory element associated with age-dependent expression of the rat androgen receptor gene. J. Biol. Chem. 268, 26400–26408. Surampudi, P.N., Wang, C. and Swerdloff, R. 2012. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int. J. Endocrinol. 2012, 625434. Sutriana, A., Siregar, T.N., Sirait, F.C., Melia, J. and Hafizuddin, D. 2022. The effect of gonadotropin releasing hormone (GnRH) administration on increasing spermatozoa quality of bali cattle. J. Sain Vet. 40, 307–313. Syafruddin, S., Iryandi, F., Rahmi, R.A.S.A., Husnurrizal, H., Panjaitan, B., Sayuti A. and Siregar, T.N. 2020. The effect of gonadotropin-releasing hormone (GnRH) on semen quality and testosterone level of Nubian goats. Vet. ir Zootech., 77, 16–21. Tung, P.S. and Fritz, I.B. 1990. Characterization of rat testicular peritubular myoid cells in culture: α-Smooth muscle isoactin is a specific differentiation marker. Biol. Reprod. 42, 351–365. Wang, R.S., Yeh, S., Tzeng, C.R. and Chang, C. 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocrinol. Rev. 30, 119–132. Welsh, M., Moffat, L., Belling, K., de França, L.R., Segatelli, T.M., Saunders, P.T.K., Sharpe, R.M. and Smith, L.B. 2012. Androgen receptor signalling in peritubular myoid cells is essential for normal differentiation and function of adult Leydig cells. Int. J. Androl. 35, 25–40. Welsh, M., Sharpe, S.M., Moffat, L., Atanassova, N., Saunders, P.T.K., Kilter, S., Bergh, A. and Smith, L.B. 2010. Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PLoS One 5, e13632. Welter, H., Kampfer, C., Lauf, S., Feil, R., Schwarzer, J.U., Köhn, F.M. and Mayerhofer A. 2013. Partial loss of contractile marker proteins in human testicular peritubular cells in infertility patients. Andrology 1, 318–324. Zhou, Q., Nie, R., Prins, G.S., Saunders, P.T.K., Katzenellenbogen, B.S. and Hess, R.A. 2002. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J. Androl. 23, 870–881. Zhu, L.J., Hardy, M.P., Inigo, I.V., Huhtaniemi, I., Bardin, C.W. and Moo-Young, A.J. 2000. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: a quantitative immunohistochemical study. Biol. Reprod. 63, 368–376. Zhu, Y., Qu, J., He, L., Zhang, F., Zhou, Z., Yang, S. and Zhou, Y. 2019. Calcium in vascular smooth muscle cell elasticity and adhesion: novel insights into the mechanism of action. Front. Physiol. 10, 852. | ||
| How to Cite this Article |
| Pubmed Style Armansyah T, Husnurrizal H, Wahyuni S, Hafizuddin H, Siregar TN, Sutriana A, Sayuti A, Syahrani AT, Pariansyah MB. Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Vet. J.. 2024; 14(12): 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 Web Style Armansyah T, Husnurrizal H, Wahyuni S, Hafizuddin H, Siregar TN, Sutriana A, Sayuti A, Syahrani AT, Pariansyah MB. Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. https://www.openveterinaryjournal.com/?mno=223112 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.31 AMA (American Medical Association) Style Armansyah T, Husnurrizal H, Wahyuni S, Hafizuddin H, Siregar TN, Sutriana A, Sayuti A, Syahrani AT, Pariansyah MB. Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Vet. J.. 2024; 14(12): 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 Vancouver/ICMJE Style Armansyah T, Husnurrizal H, Wahyuni S, Hafizuddin H, Siregar TN, Sutriana A, Sayuti A, Syahrani AT, Pariansyah MB. Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 Harvard Style Armansyah, T., Husnurrizal, . H., Wahyuni, . S., Hafizuddin, . H., Siregar, . T. N., Sutriana, . A., Sayuti, . A., Syahrani, . A. T. & Pariansyah, . M. B. (2024) Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Vet. J., 14 (12), 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 Turabian Style Armansyah, Teuku, Husnurrizal Husnurrizal, Sri Wahyuni, Hafizuddin Hafizuddin, Tongku Nizwan Siregar, Amalia Sutriana, Arman Sayuti, Adinda Tri Syahrani, and Muhammad Bintang Pariansyah. 2024. Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Veterinary Journal, 14 (12), 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 Chicago Style Armansyah, Teuku, Husnurrizal Husnurrizal, Sri Wahyuni, Hafizuddin Hafizuddin, Tongku Nizwan Siregar, Amalia Sutriana, Arman Sayuti, Adinda Tri Syahrani, and Muhammad Bintang Pariansyah. "Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor." Open Veterinary Journal 14 (2024), 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 MLA (The Modern Language Association) Style Armansyah, Teuku, Husnurrizal Husnurrizal, Sri Wahyuni, Hafizuddin Hafizuddin, Tongku Nizwan Siregar, Amalia Sutriana, Arman Sayuti, Adinda Tri Syahrani, and Muhammad Bintang Pariansyah. "Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor." Open Veterinary Journal 14.12 (2024), 3474-3486. Print. doi:10.5455/OVJ.2024.v14.i12.31 APA (American Psychological Association) Style Armansyah, T., Husnurrizal, . H., Wahyuni, . S., Hafizuddin, . H., Siregar, . T. N., Sutriana, . A., Sayuti, . A., Syahrani, . A. T. & Pariansyah, . M. B. (2024) Direct and indirect effects of PGF2α administration in male wistar rats based on increased expression of α-SMA and androgen receptor. Open Veterinary Journal, 14 (12), 3474-3486. doi:10.5455/OVJ.2024.v14.i12.31 |