| Research Article | ||
Open Vet. J.. 2024; 14(12): 3614-3624 Open Veterinary Journal, (2024), Vol. 14(12): 3614-3624 Research Article Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy catsMio Ishizaka1*, Huai-Hsun Hsu2, Yuichi Miyagawa1 and Naoyuki Takemura11The Laboratory of Veterinary Internal Medicine II, School of Veterinary Medicine, Faculty of Veterinary Science, Nippon Veterinary and Life Science University, Musashino-shi, Japan 2Taiwan National Chung Hsing University Veterinary Medical Teaching Hospital, National Chung Hsing University, Taichung, Taiwan *Corresponding Author: Mio Ishizaka, School of Veterinary Medicine, Faculty of Veterinary Science, Nippon Veterinary and Life Science University, Musashino-shi, Japan. Email: ishizakamio95 [at] gmail.com Submitted: 07/10/2024 Accepted: 27/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
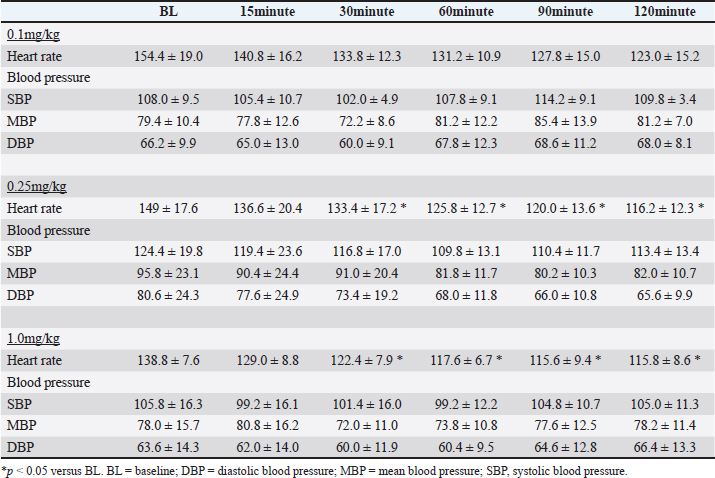
AbstractBackground: Omecamtiv mecarbil (OM), a selective cardiac myosin activator, is gaining attention as a potential heart failure (HF) treatment because it can enhance cardiac contractility without adverse effects. Concerns were raised about arrhythmias with conventional HF treatments in cats. Despite OM’s promise in veterinary medicine, no studies have confirmed its efficacy in cats. Aim: This study aimed to investigate the pharmacodynamics of OM in cats. Methods: Five clinically healthy cats were used. OM’s efficacy was examined in three doses: 0.1, 0.25, and 1.0 mg/kg. To minimize the effect on heart rates, the cats were under general anesthesia, and baseline measurements were taken after the heart rate and blood pressure had stabilized. OM was administered as a single intravenous injection. Echocardiography was performed 15, 30, 60, 90, and 120 minutes after administration. Heart rate and blood pressure were measured before each echocardiographic examination. Results: The heart rates decreased at all doses; significant reductions were seen at 0.25 and 1.0 mg/kg. All doses enhanced cardiac contractility, showing a dose-dependent effect. Blood pressure remained unchanged. Conclusion: OM enhances cardiac contractility in cats, with 0.25 mg/kg being the optimal dose. Keywords: Omecamtiv mecarbil, Feline, Heart failure, Cardiomyopathy. IntroductionHeart failure (HF) is a pathological condition caused by structural and functional damage to the heart, resulting in a reduction in the ejection fraction and congestion (Sinescu and Axente, 2010). The decrease in cardiac output leads to multiple organ dysfunction because of hypoperfusion in various organs (Sinescu and Axente, 2010). Congestion can also lead to ascites and/or pleural effusion, occasionally resulting in dyspnea. In veterinary medicine, the standard approach to HF treatment typically includes the administration of inotropic drugs and diuretics (Fuentes et al., 2020). However, in cats, the utilization of inotropic drugs may be restricted because of concerns about potential proarrhythmic effects linked to an elevation in intracellular calcium ion (Ca2+) levels (Fujii et al., 2021). Inotropic agents induce an increase in Ca2+ levels and myocardial oxygen consumption, leading to side effects such as lethal arrhythmias (Tisdale et al., 1995; Landstrom et al., 2017; Chong et al., 2018). In addition, continuous use of catecholamines can downregulate beta receptors, necessitating dosage escalation and increasing the incidence of adverse effects (Bristow et al., 1982). Omecamtiv mecarbil (OM) is a selective cardiac myosin activator that is currently gaining attention as a potential therapeutic agent for the treatment of HF in humans (Teerlink, 2009). OM binds directly to cardiac myosin and traps it in a long-lived actin-bound state, which inhibits its ATPase cycle time (Swenson et al., 2017; Woody et al., 2018). Essentially, the OM-bound myosin molecules bind to the actin thin filaments and facilitate cooperative thin filament activation, which allows the binding of OM-free, fully active myosin molecules to the activated thin filaments (Woody et al., 2018; Day et al., 2022). The overall result is an increase in contractile force at a given calcium concentration in cardiac muscles (Malik et al., 2011; Teerlink et al., 2016b; Swenson et al., 2017; Teerlink et al., 2021). OM stabilizes the state where it does not interact with the actin filament before contraction, leading to reduced adenosine triphosphate consumption (Teerlink, 2009; Malik et al., 2011; Teerlink et al., 2020). Furthermore, OM specifically binds to the allosteric site of myosin, accumulating precontracted cardiac myosin heads and increasing the number of myosin heads, thereby causing increased contractile force (Teerlink, 2009; Malik et al., 2011; Teerlink et al., 2020). In humans and dogs, OM increases contractile strength and cardiac output without causing an increase in Ca2+ levels or myocardial oxygen consumption, distinguishing it from conventional HF treatments (Shen et al., 2010; Teerlink et al., 2016a). OM may have the potential to treat HF in veterinary medicine, similar to its application in human medicine. In addition, cats with cardiomyopathy often exhibit intracellular calcium handling abnormalities and are more susceptible to arrhythmias than dogs (Schober et al., 2021; Snoberger et al., 2021). Therefore, OM may hold promise as a therapeutic agent for the treatment of HF in cats with cardiomyopathy; however, its efficacy needs to be confirmed in this species. Accordingly, this study aimed to investigate the pharmacodynamics of OM in cats. Materials and MethodsAnimalsThis study was performed using five cats managed in our laboratory (all mixed-breed animals; age, 12.5 ± 0.5 years; body weight, 3.9 ± 0.5 kg) that were regarded as clinically healthy based on a physical examination, complete blood count, biochemistry, and echocardiography. Throughout the study, they were housed individually in stainless-steel cages and fed dry food at 8 a.m. and 6 p.m. Water was freely available. Study protocolIn previous studies, OM (CK-1827452, MedChemExpress, NJ) was examined for its pharmacokinetics and pharmacodynamics in HF dog models. Based on the findings of those reports, three doses, namely, 0.1, 0.25, and 1.0 mg/kg, were employed in this study. To minimize the effect on heart rate, all cats underwent transthoracic echocardiography under general anesthesia. The normal heart rate in cats ranges from 140 to 220 beats per minute, and they can easily become tachycardic with external stimulation. As tachycardia can affect echocardiographic measurements, pharmacodynamic studies were conducted under general anesthesia to mitigate this effect. If echocardiography were performed on conscious cats, their heart rate would be highly susceptible to environmental stimuli, including human presence, behavioral changes, and auditory cues, often resulting in a 1.5–2.0-fold increase. The marked variability in heart rate under such conditions would make it exceedingly difficult to accurately assess the effects of drugs over time. Therefore, short-term anesthesia was used to evaluate drug efficacy. Anesthesia was induced by pre-administration of midazolam (0.3 mg/kg, IV) (Nichi-Iko Pharmaceutical Co., Ltd, Toyama, Japan) and induction with alfaxalone (2–5 mg/kg, to effect) (Meiji Animal Health Co., Ltd, Kumamoto, Japan). The depth of anesthesia for all subjects was maintained with continuous infusion of alfaxalone at a rate of 0.07–0.09 mg/kg/min to achieve a state where jaw tone decreased, but spontaneous respiration was maintained, and the palpebral reflex was present. Body temperature was maintained within a narrow range of 37.0°C–38.0°C for all animals during the 2-hour evaluation period. After confirming that the heart rate and blood pressure were stable for 60 minutes following the maintenance of anesthesia, baseline (BL) echocardiography was performed. Subsequently, OM was administered, and measurements of blood pressure, heart rate, and echocardiography were performed at 15, 30, 60, 90, and 120 minutes after administration. A 1-week washout period was instituted between evaluations of each drug. Hemodynamic stability, as assessed by heart rate, blood pressure, and body temperature, was monitored in all cats at the experimental anesthetic depth before the study’s commencement. Heart rate and blood pressureEach measurement was conducted using an animal vital signs monitor (AM140 Type2: Fukuda M.E. Corporation, Tokyo, Japan). EchocardiographyEach measurement is presented as the average of five consecutive measurements. All echocardiographic examinations were performed using an ultrasonic diagnostic device (Aplion a Verifia V: Cannon Medical Systems, Tochigi, Japan). The following parameters were measured by echocardiographic examination: interventricular septum in diastole, left ventricular (LV) internal dimension in diastole, LV posterior wall thickness in diastole, interventricular septum in systole, LV internal dimension in systole (LVIDs), LV posterior wall thickness in systole (LVPWs), diameter of the left atrium, diameter of the aortic root, LA/Ao ratio, aortic flow, pulmonary artery flow, end-systolic wall stress (ESWS), mitral valve lateral annulus Tei index (MV late Tei index), mitral valve septal annulus Tei index (MV sep Tei index), tricuspid valve annulus Tei index (TV Tei index), maximum velocity of early transmitral flow (MV E wave), maximum velocity of late transmitral flow, maximum velocity of early tricuspid flow, maximum velocity of late tricuspid flow, E/A ratio, velocity of the MV and TV annulus (s’, systolic; e’, early diastolic), E/e’, fractional shortening (FS), LV ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), septal and lateral mitral annular plane systolic excursion (MAPSE), mitral annulus movement (MAM), pre-ejection period (PEP), ejection time (ET), PEP/ET, global longitudinal strain (GLS), and global circumferential strain (GCS). In addition, ESWS was calculated as an index of vascular resistance using the following formula: ESWS (dyn/cm2)=0.334 × systolic blood pressure × (LVIDs)/{LVPWs (1 + LVPWs/LVIDs)} (Reichek et al., 1982). Statistical analysisStatistical analyses were performed using IBM SPSS Statistics for Windows version 24 (IBM Japan, Tokyo; EZR, Saitama Medical Centre, Jichi Medical University, Saitama, Japan). The Shapiro–Wilk test was used to determine the normality of each variable. One-way repeated-measure analysis of variance (ANOVA) was conducted with the Bonferroni post hoc test to compare each variable. Data are represented as the mean ± standard deviation. Significance was considered at p-value < 0.05. Ethical approvalThis study adhered to the Guidelines of the Declaration of Helsinki and was approved by the Nippon Veterinary and Life Science University Experimental Animal Committee (No.2023S-47). ResultHeart rate and blood pressureThe results for heart rate and blood pressure at all doses are shown in Table 1. At a dose of 0.1 mg/kg, although a significant difference was observed with one-way repeated-measure ANOVA, Bonferroni post hoc correction did not reveal significant differences at each measurement point compared to BL. At 0.25 mg/kg, significant differences were observed between each measurement point, with a significant decrease noted at 30, 60, 90, and 120 minutes compared with BL. Similarly, at 1.0 mg/kg, significant differences were observed between each measurement point, with a significant decrease noted at 30, 60, 90, and 120 minutes compared with BL. No significant differences in blood pressure at any of the doses were found. EchocardiographyThe echocardiographic results for each dose are presented in Tables 2–4. At a dose of 0.1 mg/kg, significant differences were observed in several systolic function parameters using one-way repeated-measure ANOVA, and post hoc tests revealed a significant increase only in GCS compared with BL at 15 minutes. At 0.25 mg/kg, significant differences were found in many systolic function parameters using one-way repeated-measure ANOVA, and post hoc tests showed significant increases in FS, LVEF, TV s’, MV sep s’, MAPSE sep, ET, GLS, and GCS at each measurement point compared with BL. At 1.0 mg/kg, significant differences were observed in many systolic function parameters using one-way repeated-measures ANOVA, and post hoc tests revealed significant increases in FS, TAPSE, MAPSE late, MAPSE sep, ET, GLS, and GCS at each measurement point compared with BL. At all doses, no hypotension or abnormalities in echocardiographic parameters were observed. Furthermore, there were no gastrointestinal symptoms or adverse effects on kidney and liver functions in blood tests following the experiment. Table 1. Heart rate and blood pressure at each dose of OM.
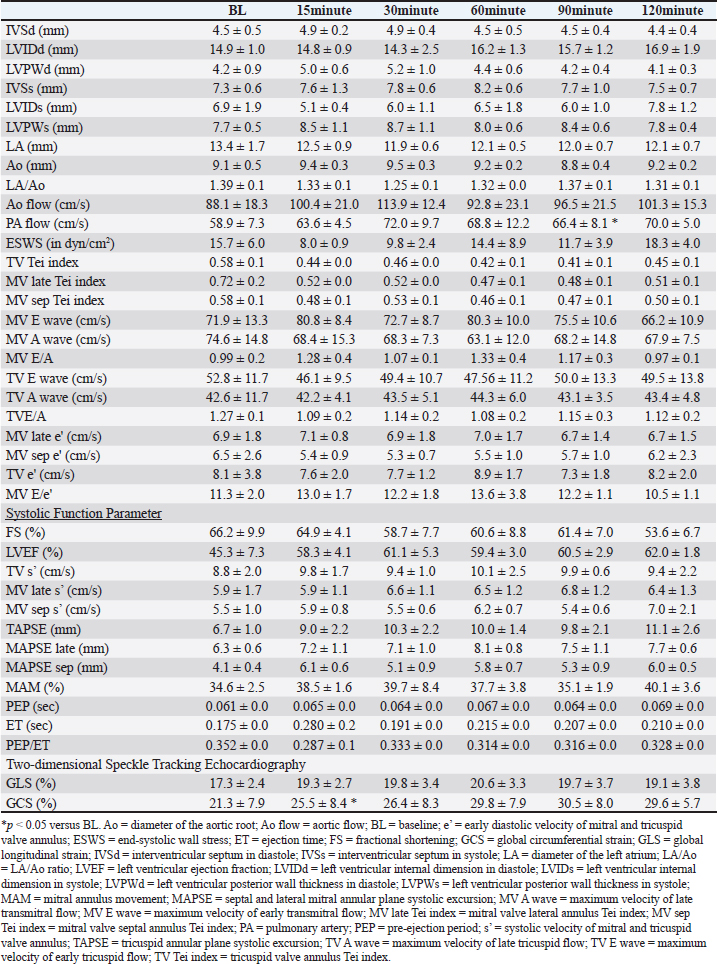
Table 2. Echocardiographic parameters with OM at a dose of 0.1 mg/kg.
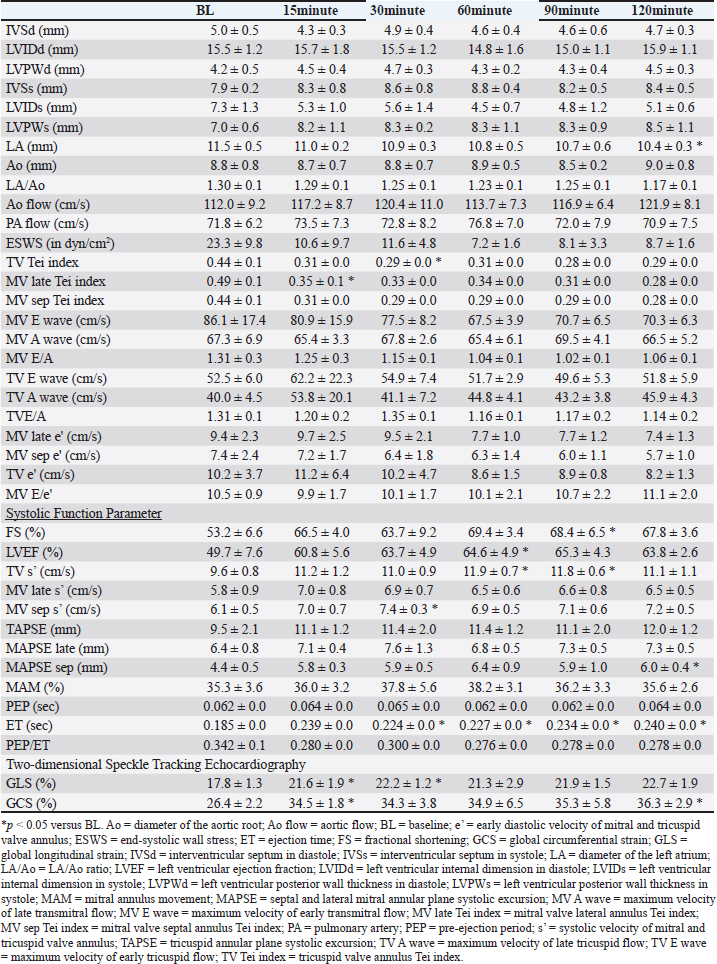
Table 3. Echocardiographic parameters with OM at a dose of 0.25 mg/kg.
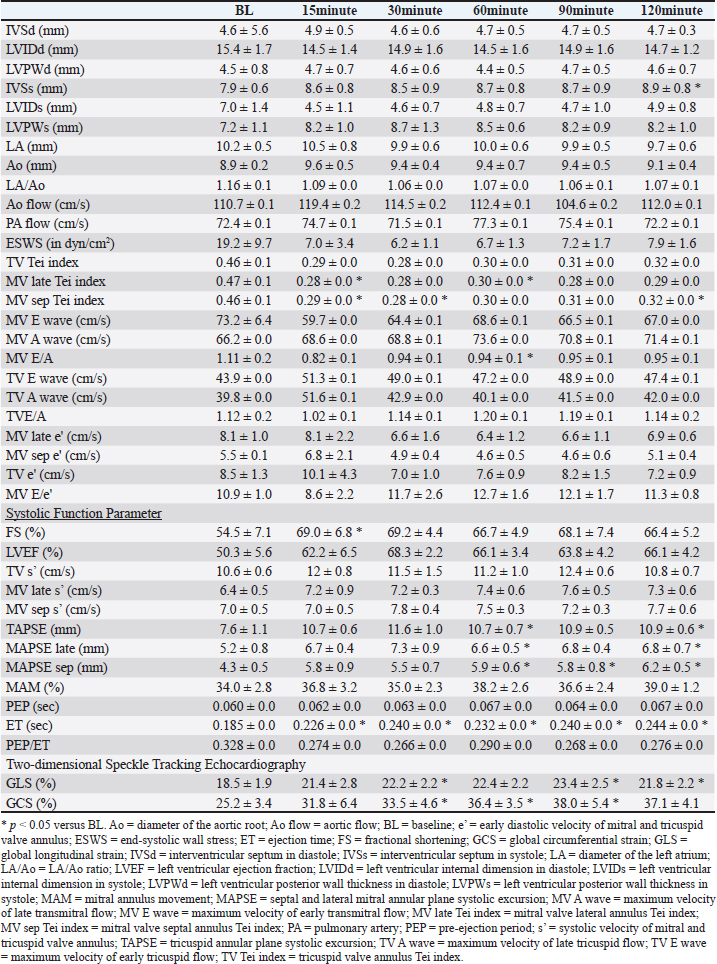
Table 4. Echocardiographic parameters with OM at a dose of 1.0 mg/kg.
DiscussionThis study evaluated the pharmacodynamics of OM in healthy cats, referencing the dosage range used in past studies on HF dogs. In our pharmacodynamic study of OM in clinically healthy cats, we observed a decrease in heart rate and positive inotropic effects. Although no significant differences were observed at a dosage of 0.1 mg/kg, significant reductions of up to 20% were noted at doses of 0.25 and 1.0 mg/kg compared with BL. In humans and canine HF models, a decrease of 12%–15% in heart rate has been reported after intravenous administration of OM, which is consistent with the results of this study (Teerlink, 2009; Shen et al., 2010). This reduction in heart rate is associated with an increase in LV ET and stroke volume (Shen et al., 2010). OM, as a myosin activator, augments the number of independent myosin heads binding to actin filaments. By binding with high affinity to the allosteric site of myosin before contraction, OM stabilizes the myosin–actin complex sixfold, accelerating the rate at which actin is pulled into a bound state. This prolongation of the overall contraction time reduces the heart rate while maintaining the increased cardiac output. Furthermore, despite the decrease in heart rate observed in this study, the systemic blood pressure remained normal. Blood pressure is determined by the heart rate, stroke volume, and peripheral vascular resistance. When the stroke volume and peripheral vascular resistance remain unchanged, a decrease in heart rate results in decreased blood pressure. In this study, although a significant decrease in heart rate was observed, blood pressure remained unchanged. This may be attributed to an increase in stroke volume and/or an increase in peripheral vascular resistance. Despite the decrease in peripheral vascular resistance observed in this study, blood pressure was maintained, which was likely due to the increase in stroke volume. Similar to the results of this study, OM did not cause fluctuations in blood pressure in patients with HF (Teerlink et al., 2021; Felker et al., 2022). A study reported that OM does not affect renal function or potassium homeostasis (Felker et al., 2022). In this study, blood tests performed after the experiments also showed no changes in renal function parameters. In HF, a decrease in cardiac output leads to a reduction in blood pressure, which is reflexively detected by chemoreceptors, resulting in increased sympathetic nervous system activity. In humans and dogs, this heightened sympathetic activity contributes to the development of HF (Umana et al., 2003; Gronda et al., 2022). Similarly, in cats, stress-induced increases in sympathetic nervous system activity can trigger systemic vasoconstriction and increased cardiac output, which in turn increases ventricular and atrial pressures and pulmonary capillary wedge pressure, leading to signs of HF (Ferasin and DeFrancesco, 2015). In addition, tachyarrhythmias can significantly reduce cardiac output due to decreased diastolic filling time (Kagami et al., 2022; Côté et al., 2023). In cats with hypertrophic cardiomyopathy (the most common cardiac disease in cats), those with tachycardia have shorter survival times than those with a heart rate <200 beats per minute (Rush et al., 2002). Therefore, in animals with HF, controlling the heart rate and suppressing sympathetic nervous system activity are necessary. OM can reduce the heart rate in cats with HF and tachycardia without causing hypotension, thereby decreasing myocardial workload and increasing stroke volume. This highlights the need for further research and clinical trials of OM in cats with HF. The effects of OM on cardiac function were assessed using echocardiography. At a dosage of 0.1 mg/kg, GCS significantly increased at 15 minutes compared with BL, and a trend toward increased GCS was observed at other evaluation time points. The increase in ET suggests a prolongation of the total systolic time due to OM (Malik et al., 2011; Gao et al., 2020). Furthermore, the trends of increase in TV s’, MV late s’, MV sep s’, TAPSE, MAPSE late, MAPSE sep, and GLS, as well as the significant increase in GCS, indicate an enhancement of myocardial contractility. Studies administering OM to patients with HF have reported improvements in GLS and GCS (Biering-Sørensen et al., 2020). Although no studies have measured TV s’, MV late s’, MV sep s’, TAPSE, MAPSE late, and MAPSE sep after OM administration in humans and animal models, these parameters reflect cardiac contractile function in human and veterinary medicine (Schober and Fuentes, 2001; Nicolle et al., 2005; Koestenberger et al., 2011; Daskalov et al., 2012; Hu et al., 2013; Kadappu and Thomas, 2015; Spalla et al., 2017). In this study, the observed increases in these parameters, comparable to GLS and GCS, suggest enhanced contractility with the 0.1-mg/kg dose of OM. Therefore, a 0.1-mg/kg dose may enhance myocardial contractility and possibly lead to an increase in cardiac output. At 0.25 mg/kg, FS, LVEF, TV s’, MV sep s’, MAPSE sep, ET, GLS, and GCS significantly increased at each evaluation point compared with the BL. The significant increase in ET suggests an extension of the total systolic time by OM, similar to 0.1 mg/kg. The increasing trends in MV late s’ and TAPSE and the significant increase in TV s’, MV sep s’, MAPSE sep, GLS, and GCS indicate an increase in myocardial contractility. The increase in FS has been observed in the OM-treated groups of patients with HF and severe chronic aortic regurgitation in rats (Cleland et al., 2011; Teerlink et al., 2011; El-Oumeiri et al., 2018). In healthy individuals, FS increased at a dosage of 0.125 mg/kg (Teerlink et al., 2011), which is similar to the results of this study. In patients with HF, GLS and GCS improved after 20 weeks of treatment with an oral OM formulation (25–50 mg, twice a day) (Biering-Sørensen et al., 2020). The average plasma concentration of the oral OM formulation at a dosage of 50 mg was 165 ng/mL (Teerlink et al., 2016b), which is equivalent to approximately 0.0625–0.125 mg/kg/h when administered intravenously (Teerlink et al., 2011). Therefore, in this study, the observed increases in GLS and GCS occurred at doses similar to past reports. To our knowledge, no studies have measured TV s’, MV late s’, MV sep s’, and TAPSE after OM administration, and these parameters reflect cardiac contractile function in human and veterinary medicine (Schober and Fuentes, 2001; Nicolle et al., 2005; Koestenberger et al., 2011; Daskalov et al., 2012; Hu et al., 2013; Kadappu and Thomas, 2015; Spalla et al., 2017). The significant increase in LVEF was similar to the increase in cardiac output observed in model animals. The increase in LVEF at a dosage of 0.1 mg/kg was approximately 40%, whereas at 0.25 mg/kg, it showed a maximum increase of 46%. This suggests that 0.25 mg/kg may have more potent effects of OM than 0.1 mg/kg. PEP/ET correlates with other LV function parameters and is an independent indicator of overall LV function regardless of the heart rate (Lewis et al., 1977; Spodick et al., 1984). The decreasing trend in PEP/ET is thought to be due to the significant increase in ET, suggesting enhanced LV function. Therefore, the dosage of 0.25 mg/kg in this study may enhance myocardial contractility and demonstrate equivalent effects at equivalent dosages in previous reports, potentially making it the minimal optimal dosage in cats. At the dosage of 1.0 mg/kg, FS, TAPSE, MAPSE late, MAPSE sep, ET, GLS, and GCS significantly increased at each evaluation point compared with BL. The significant increase in ET suggests an extension of the total systolic time by OM, similar to those at dosages of 0.1 and 0.25 mg/kg. The increasing trends in TV s’, MV sep s’, and MAM and the significant increase in FS, TAPSE, MAPSE late, MAPSE sep, ET, GLS, and GCS indicate an increase in myocardial contractility. OM dose-dependently enhances myocardial contractility, and at the maximum dosage of 1.0 mg/kg, it may have affected more evaluation points of contractile function parameters than 0.1 and 0.25 mg/kg. In rats and canine HF model, diastolic dysfunction has been reported to occur with high-dose OM use (Fülöp et al., 2021; Ráduly et al. 2023), which is due to prolonged myosin and actin binding times, leading to a slower release of actin from myosin, resulting in decreased diastolic velocity. A significant decrease in MV E/A was observed at the dosage of 1.0 mg/kg in this study. E/A is an indicator of diastolic function, and its decrease suggests early diastolic dysfunction (Schober and Chetboul, 2015; Nagueh et al., 2016; Mitter et al., 2017). However, other indicators of diastolic function, such as e’ and E/e’, did not change. In human medicine, the evaluation of diastolic dysfunction involves the assessment of the septal or lateral e’, E/e’ using the average values of septal and lateral sides, mitral regurgitation velocity, and left atrial volume index. Diastolic dysfunction is diagnosed if abnormalities are observed in three or more indicators (Ommen et al., 2000; Tsang et al., 2002; Nagueh et al., 2016; Mitter et al., 2017). In cats, the main parameters for evaluating diastolic dysfunction include left atrial size, E/A, pulmonary vein flow velocity, isovolumetric relaxation time, e’, aortic regurgitation, and E/e’ (Chetboul et al., 2006; Disatian et al., 2008; Schober and Chetboul, 2015). However, owing to the waveform fusion caused by tachycardia and insufficient research in cats, a complete evaluation is challenging. Therefore, affirming diastolic dysfunction based solely on the observed decrease in MV E/A in this study is difficult. In the pharmacokinetic evaluation conducted in this study across three dosage regimens, a decrease in heart rate and an increase in cardiac contractility were observed at all doses. Based on these results, 0.25 mg/kg is the recommended dosage for cats, as it exhibits a significantly low potential for side effects and a reliable enhancement in cardiac contractility. This dosage is nearly equivalent to those used in previous studies involving patients with HF and animal models. Although no apparent diastolic dysfunction was observed at the maximum dosage of 1.0 mg/kg in this study, doses exceeding 1.0 mg/kg may lead to adverse effects and are therefore not recommended. More studies are needed to determine the threshold at which diastolic dysfunction occurs in cats with increased dosage. This study has several limitations. First, the results from our analysis of a small sample size of five cats may have insufficient statistical power. However, significant enhancement of myocardial contractility was observed at all doses in all five cats, and considering animal welfare, the incorporated number was deemed appropriate. Second, owing to the lack of pharmacokinetic testing of blood drug levels, the relationship between the administered doses and blood concentrations remains unknown. ConclusionIn the pharmacokinetic study of OM in clinically healthy cats, enhanced myocardial contractility was observed at doses of 0.1, 0.25, and 1.0 mg/kg. In addition, 0.25 and 1.0 mg/kg exhibited a significant decrease in heart rate. Among these doses, 0.25 mg/kg demonstrated the potential for the beneficial effects of OM without the occurrence of adverse effects, suggesting it to be the optimal dose for cats. AcknowledgmentsThe authors thank the students of the Laboratory of Veterinary Internal Medicine II at Nippon Veterinary and Life Science University for their support in our study. We would like to thank Cannon Medical Systems for their support. In addition, we would like to thank Enago (www.enago.jp) for the English language review. Conflict of interestThe authors declare no conflict of interest. FundingThis study received no external funding. Authors’ contributionsConceptualization, M.I., H.H., Y.M., and N.T.; methodology, M.I., H.H., Y.M., and N.T.; software, M.I.; validation, M.I., H.H., Y.M., and N.T.; formal analysis, M.I., H.H., Y.M., and N.T.; investigation, M.I., H.H., Y.M., and N.T.; data curation, M.I., H.H., Y.M., and N.T.; writing–original draft preparation, M.I.; writing—review and editing, M.I., H.H., Y.M., and N.T.; visualization, M.I.; supervision, M.I., Y.M., and N.T.; and project administration, M.I., Y.M., and N.T. All authors have read and agreed to the published version of the manuscript. Institutional review board statementThis study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Laboratory Animal Care and Use at Nippon Veterinary and Life Science University (Approval No. 2023S-47, approval date). Data availabilityThe data presented in this study are available upon request from the corresponding author. ReferencesBiering-Sørensen, T., Minamisawa, M., Claggett, B., Liu, J., Felker, G.M., McMurray, J.J.V., Malik, F.I., Abbasi, S., Kurtz, C.E., Teerlink, J.R. and Solomon, S.D. 2020. Cardiac myosin activator omecamtiv mecarbil improves left ventricular myocardial deformation in chronic heart failure: the COSMIC-HF trial. Circ. Heart Fail. 13, e008007. Bristow, M.R., Ginsburg, R., Minobe, W., Cubicciotti, R.S., Sageman, W.S., Lurie, K., Billingham, M.E., Harrison, D.C. and Stinsonet, E.B. 1982. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. Med. 307, 205–211. Chetboul, V., Sampedrano, C.C., Tissier, R., Gouni, V., Saponaro, V., Nicolle, A.P. and Pouchelonet, J.L. 2006. Quantitative assessment of velocities of the annulus of the left atrioventricular valve and left ventricular free wall in healthy cats by use of two-dimensional color tissue Doppler imaging. Am. J. Vet. Res. 67, 250–258. Chong, L.Y.Z., Satya, K., Kim, B. and Berkowitz, R. 2018. Milrinone dosing and a culture of caution in clinical practice. Cardiol. Rev. 26, 35–42. Cleland, J.D.F., Teerlink, J.R., Senior, R., Nifontov, E.M., Mc Murray, J.J.V., Lang, C.C., Tsyrlin, V.A., Greenberg, B.H., Mayet, J., Francis, D.P., Shaburishvili, T., Monaghan, M., Saltzberg, M., Neyses, L., Wasserman, S.M., Lee, J.H., Saikali, K.G., Clarke, C.P., Goldman, J.H., Wolff, A.A. and Maliket, F.I. 2011. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 378, 676–683. Côté, E., Ettinger, S.J. and Feldman, E.C. 2023. Syncope. In Ettinger’s textbook of veterinary internal medicine, 9th ed. Eds., Ettinger, S.J., Feldman, E.C. and Cote, E. Amsterdam: Elsevier, pp. 186–188. Daskalov, I.R., Petrovsky, P.D. and Demirevska, L.D. 2012. Mitral annular systolic velocity as a marker of preclinical systolic dysfunction among patients with arterial hypertension. Cardiovasc. Ultrasound 10, 46. Day, S.M., Tardiff, J.C. and Ostap, E.M. 2022. Myosin modulators: emerging approaches for the treatment of cardiomyopathies and heart failure. J. Clin. Invest. 132, e148557. Disatian, S., Bright, J.M. and Boon, J. 2008. Association of age and heart rate with pulsed-wave Doppler measurements in healthy, nonsedated cats. J. Vet. Intern. Med. 22, 351–356. El-Oumeiri, E., Entee, K.M., Annoni, F., Herpain, A., Eynden, F.V., Jespers, P., Nooten, G.V. and van de Borne, P. 2018. Effects of the cardiac myosin activator Omecamtiv-mecarbil on severe chronic aortic regurgitation in Wistar rats. BMC Cardiovasc. Disord. 18, 99. Felker, G.M., Solomon, S.D., Claggett, B., Diaz, R., McMurray, J.J.V., Metra, M., Anand, I., Crespo-Leiro, M.G., Dahlström, U., Goncalvesova, E., Howlett, J.G., MacDonald, P., Parkhomenko, A., Tomcsányi, J., Abbasi, S.A., Heitner, S.B., Hucko, T., Kupfer, S., Malik, F.I. and Teerlink, J.R. 2022. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALACTIC-HF randomized clinical trial. JAMA Cardiol. 7, 26–34. Ferasin, L. and DeFrancesco, T. 2015. Management of acute heart failure in cats. J. Vet. Cardiol. 17, S173–S189. Fuentes, V.L., Abbott, J., Chetboul, V., Côté, E., Fox, P.R., Häggström, J., Kittleson, M.D., Schober, K. and Stern, J.A. 2020. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 34, 1062–1077. Fujii, Y., Sugimoto, K., Omichi, M., Kanai, K. and Orito, K. 2021. A pilot study investigating the effect of pimobendan on the cardiac rhythm and selected echocardiographic parameters of healthy cats. J. Vet. Cardiol. 35, 74–83. Fülöp, G.Á., Oláh, A., Csipo, T., Kovács, Á., Pórszász, R., Veress, R., Horváth, B., Nagy, L., Bódi, B., Fagyas, M., Helgadottir, S.L., Bánhegyi, V., Juhász, B., Bombicz, M., Priksz, D., Nanasi Jr, P., Merkely, B., Édes, I., Csanádi, Z., Papp, Z., Radovits, T. and Tóth, A. 2021. Omecamtiv mecarbil evokes diastolic dysfunction and leads to periodic electromechanical alternans. Basic Res. Cardiol. 116, 24. Gao, B.X., Sutherland, W., Vargas, H.M. and Qu, Y. 2020. Effects of omecamtiv mecarbil on calcium-transients and contractility in a translational canine myocyte model. Pharmacol. Res. Perspect. 8, e00656. Gronda, E., Dusi, V., D’Elia, E., Iacoviello, M., Benvenuto, E. and Vanoli, E. 2022. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 24, E4–E11. Hu, K., Liu, D., Herrmann, S., Niemann, M., Gaudron, P.D., Voelker, W., Ertl, G., Bijnens, B. and Weidemann, F. 2013. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 14, 205–212 Kadappu, K.K. and Thomas, L. 2015. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ. 24, 224–233. Kagami, K., Obokata, M., Harada, T., Kato, T., Wada, N., Adachi, T. and Ishii, H. 2022. Diastolic filling time, chronotropic response, and exercise capacity in heart failure and preserved ejection fraction with sinus rhythm. J. Am. Heart Assoc. 11, e026009. Koestenberger, M., Nagel, B., Ravekes, W., Urlesberger, B., Raith, W., Avian, A., Halb, V., Cvirn, G., Fritsch, P. and Gamillscheg, A. 2011. Systolic right ventricular function in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology 100, 85–92. Landstrom, A.P., Dobrev, D. and Wehrens, X.H.T. 2017. Calcium signaling and cardiac arrhythmias. Circ. Res. 120, 1969–1993. Lewis, R.P., Rittogers, S.E., Froester, W.F. and Boudoulas, H. 1977. A critical review of the systolic time intervals. Circulation 56, 146–158. Malik, F.I., Hartman, J.J., Elias, K.A., Morgan, B.P., Rodriguez, H., Brejc, K., Anderson, R.L., Sueoka, S.H., Lee, K.H., Finer, J.T., Sakowicz, R., Baliga, R., Cox, D.R., Garard, M., Godinez, G., Kawas, R., Kraynack, E., Lenzi, D., Lu, P.P., Muci, A., Niu, C., Qian, X., Pierce, D.W., Pokrovskii, M., Suehiro, I., Sylvester, S., Tochimoto, T., Valdez, C., Wang, W., Katori, T., Kass, D.A., Shen, Y.T., Vatner, S.F. and Morgans, D.J. 2011. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 331, 1439–1443. Mitter, S.S., Shah, S.J. and Thomas, J.D. 2017. A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 69, 1451–1464. Nagueh, S.F., Smiseth, O.A., Appleton, C.P., Byrd 3rd, B.F., Dokainish, H., Edvardsen, T., Flachskampf, F.A., Gillebert, T.C., Klein, A.L., Lancellotti, P., Marino, P., Oh, J.K., Popescu, B.A. and Waggoner, A.D. 2016. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 29, 277–314. Nicolle, A.P., Sampedrano, C.C., Fontaine, J.J., Tessier-Vetzel, D., Goumi, V., Pelligand, L., Pouchelon, J.L. and Chetboul, V. 2005. Longitudinal left ventricular myocardial dysfunction assessed by 2D colour tissue Doppler imaging in a dog with systemic hypertension and severe arteriosclerosis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52, 83–87. Ommen, S.R., Nishimura, R.A., Appleton, C.P., Miller, F.A., Oh, J.K., Redfield, M.M. and Tajik, A.J. 2000. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102, 1788–1794. Ráduly, A.P., Tóth, A., Sárkány, F., Horváth, B., Szentandrássy, N., Nánási, P.P., Csanádi, Z., Édes, I., Papp, Z. and Borbély, A. 2023. Omecamtiv mecarbil augments cardiomyocyte contractile activity both at resting and systolic Ca2+ levels. ESC Heart Fail. 10, 1326–1335. Reichek, N., Wilson, J., Sutton, M.S.J., Plappert, T.A., Goldberg, S. and Hirshfeld, J.W. 1982. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation 65, 99–108. Rush, J.E., Freeman, L.M., Fenollosa, N.K. and Brown, D.J. 2002. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J. Am. Vet. Med. Assoc. 220, 202–207. Schober, K.E. and Chetboul, V. 2015. Echocardiographic evaluation of left ventricular diastolic function in cats: hemodynamic determinants and pattern recognition. J. Vet. Cardiol. 17, S102–S133. Schober, K.E. and Fuentes, V.L. 2001. Mitral annulus motion as determined by M-mode echocardiography in normal dogs and dogs with cardiac disease. Vet. Radiol. Ultrasound 42, 52–61. Schober, K.E., Rush, J.E., Fuentes, V.L., Glaus, T., Summerfield, N.J., Wright, K., Lehmkuhl, L., Wess, G., Sayer, M.P., Loureiro, J., MacGregor, J. and Mohren, N. 2021. Effects of pimobendan in cats with hypertrophic cardiomyopathy and recent congestive heart failure: results of a prospective, double-blind, randomized, nonpivotal, exploratory field study. J. Vet. Intern. Med. 35, 789–800. Shen, Y.T., Malik, F.I., Zhao, X., Depre, C., Dhar, S.K., Abarzúa, P., Morgans, D.J. and Vatner, S.F. 2010. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ. Heart Fail. 3, 522–527. Sinescu, C. and Axente, L. 2010. Heart failure–concepts and significance. Birth of a prognostic model. J. Med. Life 3, 421–429. Snoberger, A., Barua, B., Atherton, J.L., Shuman, H., Forgacs, E., Goldman, Y.E., Winkelmann, D.A. and Ostap, E.M. 2021. Myosin with trophictrophic cardiac mutation R712L has a decreased working stroke which is rescued by omecamtiv mecarbil. Elife 10, e63691. Spalla, I., Payne, J.R., Borgeat, K., Pope, A., Fuentes, V.L. and Connolly, D.J. 2017. Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 31, 691–699. Spodick, D.H., Doi, Y.L., Bishop, R.L. and Hashimoto, T. 1984. Systolic time intervals reconsidered. Reevaluation of the preejection period: absence of relation to heart rate. Am. J. Cardiol. 53, 1667–1670. Swenson, A.M., Tang, W., Blair, C.A., Fetrow, C.M., Unrath, W.C., Previs, M.J., Campbell, K.S. and Yengo, C.M. 2017. Omecamtiv mecarbil enhances the duty ratio of human β-cardiac myosin resulting in increased calcium sensitivity and slowed force development in cardiac muscle. J. Biol. Chem. 292, 3768–3778. Teerlink, J.R. 2009. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail. Rev. 14:289–98. Teerlink, J.R., Clarke, C.P., Saikali, K.G., Lee, J.H., Chen, M.M., Escandon, R.D., Elliott, L., Bee, R., Habibzadeh, M.R., Goldman, J.H., Schiller, N.B., Malik, F.I. and Wolff, A.A. 2011. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet 378, 667–675. Teerlink, J.R., Diaz, R., Felker, G.M., McMurray, J.J.V., Metra, M., Solomon, S.D., Legg, J.C., Büchele, G., Varin, C., Kurtz, C.E., Malik, F.I. and Honarpour, N. 2020. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC-HF. JACC Heart Fail. 8, 329–340. Teerlink, J.R., Diaz, R., Felker, G.M., McMurray, J.J.V., Metra, M., Solomon, S.D., Adams, K.F., Anand, I., Arias-Mendoza, A., Biering-Sørensen, T., Böhm, M., Bonderman, D., Cleland, J.G.F., Corbalan, R., Crespo-Leiro, M.G., Dahlström, U., Echeverria, L.E., Fang, J.C., Filippatos, G., Fonseca, C., Goncalvesova, E., Goudev, A.R., Howlett, J.G., Lanfear, D.E., Li, J., Lund, M., Macdonald, P., Mareev, V., Momomura, S., O’Meara, E., Parkhomenko, A., Ponikowski, P., Ramires, F.J.A., Serpytis, P., Sliwa, K., Spinar, J., Suter, T.M., Tomcsanyi, J., Vandekerckhove, H., Vinereanu, D., Voors, A.A., Yilmaz, M.B., Zannad, F., Sharpsten, L., Legg, J.C., Varin, C., Honarpour, N., Abbasi, S.A., Malik, F.I. and Kurtz, C.E. 2021. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N. Eng. J. Med. 384, 105–116. Teerlink, J.R., Felker, G.M., McMurray, J.J.V., Ponikowski, P., Metra, M., Filippatos, G.S., Ezekowitz, J.A., Dickstein, K., Cleland, J.G.F., Kim, J.B., Lei, L., Knusel, B., Wolff, A.A., Malik, F.I. and Wasserman, S.M. 2016a. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC-AHF study. J. Am. Coll. Cardiol. 67, 1444–1455. Teerlink, J.R., Felker, G.M., McMurray, J.J.V., Solomon, S.D., Adams Jr, K.F., Cleland, J.G.F., Ezekowitz, J.A., Goudev, A., Macdonald, P., Metra, M., Mitrovic, V., Ponikowski, P., Serpytis, P., Spinar, J., Tomcsányi, J., Vandekerckhove, H.J., Voors, A.A., Monsalvo, M.L., Johnston, J., Malik, F.I. and Honarpour, N. 2016b. Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 388, 2895–2903. Tisdale, J.E., Patel, R., Webb, C.R., Borzak, S. and Zarowitz, B.J. 1995. Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog. Cardiovasc. Dis. 38, 167–180. Tsang, T.S.M., Barnes, M.E., Gersh, B.J., Bailey, K.R. and Seward, J.B. 2002. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 90, 1284–1289. Umana, E., Solares, C.A. and Alpert, M.A. 2003. Tachycardia-induced cardiomyopathy. Am. J. Med. 114, 51–55. Woody, M.S., Greenberg, M.J., Barua, B., Winkelmann, D.A., Goldman, Y.E. and Ostap, E.M. 2018. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat. Commun. 9, 3838. | ||
| How to Cite this Article |
| Pubmed Style Ishizaka M, Hsu H, Miyagawa Y, Takemura N. Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Vet. J.. 2024; 14(12): 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 Web Style Ishizaka M, Hsu H, Miyagawa Y, Takemura N. Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. https://www.openveterinaryjournal.com/?mno=223493 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.42 AMA (American Medical Association) Style Ishizaka M, Hsu H, Miyagawa Y, Takemura N. Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Vet. J.. 2024; 14(12): 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 Vancouver/ICMJE Style Ishizaka M, Hsu H, Miyagawa Y, Takemura N. Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 Harvard Style Ishizaka, M., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2024) Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Vet. J., 14 (12), 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 Turabian Style Ishizaka, Mio, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. 2024. Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Veterinary Journal, 14 (12), 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 Chicago Style Ishizaka, Mio, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats." Open Veterinary Journal 14 (2024), 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 MLA (The Modern Language Association) Style Ishizaka, Mio, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats." Open Veterinary Journal 14.12 (2024), 3614-3624. Print. doi:10.5455/OVJ.2024.v14.i12.42 APA (American Psychological Association) Style Ishizaka, M., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2024) Pharmacodynamics of single-dose omecamtiv mecarbil administered intravenously in clinically healthy cats. Open Veterinary Journal, 14 (12), 3614-3624. doi:10.5455/OVJ.2024.v14.i12.42 |