| Research Article | ||
Open Vet. J.. 2024; 14(12): 3525-3538 Open Veterinary Journal, (2024), Vol. 14(12): 3525-3538 Research Article Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickensPhan Vu Hai1*, Hoang Thi Anh Phuong2, Pham Hoang Son Hung1, Tran Thi Na1, Ngo Huu Lai3, Nguyen Dinh Thuy Khuong1, Tran Ngoc Liem1 and Nguyen Xuan Hoa11Faculty of Animal Husbandry and Veterinary Medicine, University of Agriculture and Forestry, Hue University, Hue city, Vietnam 2Faculty of Animal Husbandry and Veterinary Medicine, Tay Nguyen University, Buon Ma Thuat city, Vietnam 3Vietnam Department of Animal Health, Region IV Animal Health Department, Da Nang city, Vietnam *Corresponding Author: Phan Vu Hai. Faculty of Animal Husbandry and Veterinary Medicine, University of Agriculture and Forestry, Hue University, Hue city, Vietnam. Email: phanvuhai [at] hueuni.edu.vn Submitted: 20/10/2024 Accepted: 29/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
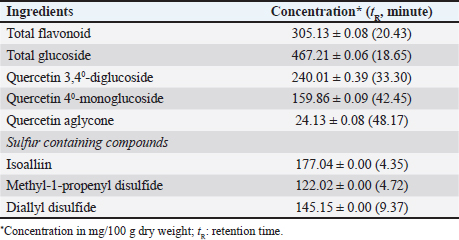
AbstractBackground: The increasing prevalence of antibiotic resistance in poultry pathogens necessitates the development of sustainable alternatives to antibiotics. Probiotics, particularly Lactobacillus spp., have shown promise in combating bacterial infections in poultry. Purple onion extract (OE) possesses antibacterial properties and can potentially enhance the probiotic efficacy of Lactobacillus strains. Aim: This study aimed to develop a biological product based on Lactobacillus-fermented OE (LFOE) as a sustainable alternative to antibiotics for the control of Salmonella-induced diarrhea in poultry. Methods: Lactobacillus strains were isolated from native free-range chicken feces and screened for their antibacterial activity against Salmonella pullorum NCTC10705 and Salmonella typhimurium FC13827, as well as their survival rate in OE. Six promising strains were selected and further characterized for their ability to ferment OE and their co-aggregation ability against the pathogenic bacteria using scanning electron microscopy (SEM). 16S rRNA gene sequencing was performed for bacterial identification. The selected strain was used for fermentation in OE, and the resulting product was freeze-dried into a biological preparation. In vivo studies in chicks were conducted to assess the safety and intestinal persistence of LFOE. Results: From an initial pool of 68 Lactobacillus strains, six promising candidates (L. plantarum 1582, L. plantarum WCFS1, L. plantarum JDM1, L. acidophilus NCFM, L. agilis DSM 20509, and L. agilis La3) were selected based on their antibacterial activity and high survival rate in OE. SEM confirmed the ability of these strains to ferment OE and co-aggregate with pathogenic bacteria. 16S rRNA gene sequencing confirmed their taxonomic identity as Lactobacillus. L. plantarum 1582, selected for its superior probiotic properties, was used to ferment LFOE, which proved safe for chicks and demonstrated the strain’s ability to survive temporarily in the intestine. Conclusion: This study successfully developed a biopreparation based on LFOE as a potential alternative to antibiotics for the control of Salmonella-induced diarrhea in poultry. However, regular re-supplementation is required to maintain probiotic efficacy due to the transient nature of intestinal colonization. Keywords: Antibiotic resistance, Chicken, Lactobacillus, Onion, Salmonella. IntroductionSalmonella, a Gram-negative bacterium belonging to the Enterobacteriaceae family, causes intestinal diseases like dysentery and typhoid in poultry, which can be transmitted to humans (Nair et al., 2018). Controlling Salmonella in poultry production is crucial, especially since the WHO has listed it as a pathogen requiring antibiotic susceptibility testing before treatment (WHO, 2023). Antibiotic resistance is on the rise due to the overuse of antibiotics in livestock, rendering many common antibiotics ineffective against Salmonella spp. (Farhat et al., 2023). This resistance compromises treatment efficacy, alters the intestinal microbiota, and negatively impacts animal health (Kim et al., 2017). Consequently, there is a need to develop alternatives to antibiotics, such as herbal medicines, as bacteria are less likely to develop resistance to these natural compounds (Brijesh et al., 2009; Singh, 2013). Purple onion, an agricultural product prevalent in the Central region of Vietnam, is a rich source of health-promoting bioactive compounds, including phenolic compounds, particularly flavonoids, and sulfur-containing organic compounds with antibacterial and antioxidant properties (Wilson and Demmig-Adams, 2007). Recent studies have demonstrated the bacteriostatic and bactericidal effects of purple onion against E. coli causing diarrhea in broilers, even in strains resistant to gentamicin and amoxicillin (Hai et al., 2020a). Additionally, supplementing broiler diets with 0.5% and 0.7% purple onion extract increased antibody titers against the Newcastle disease virus (Hai and Hoa, 2020). Furthermore, dietary supplementation with 300 mg/kg of the extract improved chicken performance and health (Hai et al. 2020b). However, some plant extracts, including purple onion, can disrupt the balance of beneficial gut bacteria (Myers et al., 2009), necessitating the exploration of beneficial biological compounds to restore the intestinal microbiota. Probiotics play a vital role in regulating the microbiota and inhibiting pathogen growth (Wang et al., 2018), while prebiotics are selectively metabolized by beneficial intestinal bacteria (Sunu et al., 2019). Fermenting herbs with probiotics can enhance the bioactivity and efficacy of functional ingredients. Pickled purple onions, a traditional dish in Vietnamese cuisine, rely on spontaneous fermentation with indigenous lactic acid bacteria. However, spontaneous fermentation can lead to uncontrolled transformations and the generation of harmful microorganisms or toxins (Xiang et al., 2019). Isolating probiotic strains from natural hosts is preferable as these strains are well adapted to the host’s gastrointestinal environment (Reuben et al., 2019). Creating probiotic strains tailored to specific hosts is crucial for maximizing health advantages and improving animal production efficiency (Dowarah et al., 2018). Currently, there is a lack of research on the effects of probiotic fermentation of purple onion substrates at concentrations lethal to enteric pathogens. This study aimed to evaluate the synergistic effects of potential Lactobacillus (LAB) strains isolated from chicken feces in fermented purple OE against Salmonella spp. in chickens, contributing to a sustainable approach for disease control in poultry production. Materials and MethodsMaterialsBacterial strains Probiotic strains: Sixty-eight Lactobacillus strains were isolated from the feces of native free-range chickens raised without probiotic supplementation. The strains were screened based on morphological and biochemical characteristics. Pathogenic bacteria: Pathogenic bacteria were isolated from the feces of native chickens exhibiting diarrhea suspected to be caused by Salmonella spp. Two isolates, carrying the virulence genes InvA and Stn, were selected for further analysis. Identification of the isolates was performed using the 16S PCR method, which involves amplifying the 16S rRNA gene region, analyzing the PCR product via agarose gel electrophoresis, purifying it, and sequencing. The obtained sequences were compared with the GenBank database using BLAST to determine the species. The isolates were identified as Salmonella pullorum NCTC10705 (GenBank ID: UGWX01000002.1) and S. Typhimurium FC13827 (GenBank ID: MK886517.1). Strain maintenance: All bacterial strains were maintained in sterile glycerol (20%) and stored at −80°C at the Microbiology Laboratory, Faculty of Animal Husbandry and Veterinary Medicine, University of Agriculture and Forestry, Hue University, Vietnam. Purple OE preparationPurple onions (Allium cepa L. var. aggregatum - NCBI Genbank ID: NC_057575.1), aged 4–5 months, were cultivated under biosafety conditions according to VietGAP standard TCVN 11892-1:2017 in Dien Mon, Phong Dien district, Thua Thien Hue province, Vietnam. The onion extraction process was adapted from Yadav et al. (2015) with modifications. Briefly, after washing and removing substandard bulbs, the onions were surface-sterilized by soaking in a 5% NaCl solution for 120 minutes. One hundred grams of onions were then crushed to obtain the extract by Philips HR3770/00 Juicer (capacity: 1500W, 2 l), which was filtered through two layers of 25 micron PET gauze and centrifuged at 5,000 rpm for 15 minutes to remove insoluble debris. The resulting extract was sterilized using UV irradiation (30 mW/cm² for 15 minutes) to obtain purple OE, which was stored at 4°C. Quantification of the main bioactive compounds in OE was performed using HPLC at the Center for Drug and Food Testing (HueQC, TT-Hue) (Table 1). Production of a fermented OE biopreparationThe production process for the fermented OE biopreparation was adapted from Mangisah et al. (2021). Pure selected LAB cultures were propagated on MRS agar plates and incubated anaerobically at 38°C for 48 hours. Skim milk was diluted with distilled water at a ratio of 1:14 (v/v) and mixed thoroughly to create a growth medium for the bacteria, providing a source of lactose. LAB from two agar plates was inoculated into 200 ml of the diluted skim milk and incubated anaerobically at 38°C for 2 days. To produce the fermented product, OE was combined with the LAB-inoculated skim milk at a ratio of 1:2 (v/v) and mixed thoroughly. Fermentation was carried out at 30°C for 18 hours with shaking at 100 rpm, achieving an initial LAB inoculum density exceeding 108 CFU/ml. Post-fermentation, the product was freeze-dried at a pressure of 63 Pa for 96 hours utilizing an HT-FD freeze dryer (Hai Tan Joint Stock Company, Vietnam). Table 1. Quantification of major bioactive compounds in OE.
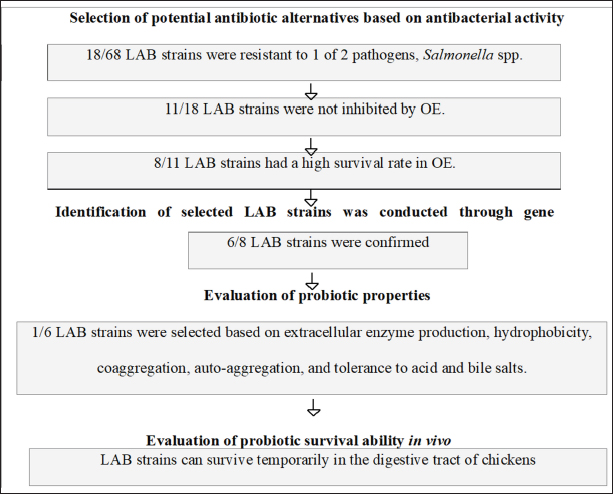
MethodsAntibacterial sensitivity assessment Antibacterial activity of OE and LAB against gastrointestinal pathogens The antibacterial activity of OE against common poultry gastrointestinal pathogens was evaluated using the agar well diffusion method as described by Aujoulat et al. (2011). Briefly, Muller Hinton agar (MHA) plates were overlaid with a 0.5 OD630 suspension of Salmonella spp. to achieve a bacterial density of 1 × 108 CFU/ml. After 15–20 minutes, six wells (30 mm apart) were punched into the MHA agar. Each well was filled with 100 μl of an overnight MRS broth culture of the selected LAB strains (adjusted to 0.5 OD630) to achieve a bacterial density of 1 × 108 CFU/ml. The lid ajar was left for from 3 to 5 minutes with no more than 15 minutes in the sterile cabinet and the plates were incubated at 37°C for 20 hours. The antibacterial activity was evaluated by measuring the diameter of the inhibition zone (DIZ, mm) using the formula: DIZ=Diameter of the inhibition zone—Diameter of the well. Antibacterial activity was considered significant when DIZ ≥ 10 mm (Georgieva et al., 2015). LAB survival in OEThe survival of LAB strains in OEs was assessed as described by Fadare et al. (2022). MRS medium supplemented with 12.5% (v/v) OE was prepared. The test samples were inoculated with 100 µl of LAB suspension (1 × 108 CFU/ml) and incubated in the OE-supplemented MRS broth at 37°C for 24 hours. Control samples were inoculated into MRS broth without OE. Growth efficiency was determined using the plate count method. The survival rate (%) of LAB was calculated using the formula: Survival rate (%)=100 × (log CFU of test sample / log CFU of control sample). Bactericidal and synergistic effects of OEThe bactericidal and synergistic effects of OE were investigated using scanning electron microscopy (SEM) following a modified protocol from Kim et al. (2020). Bacterial suspensions of S. typhimurium FC13827 and L. plantarum 1582 (100 μl, 0.5 McFarland standard) were treated with 12.5% (v/v) OE and incubated for 8 hours at 37°C. The cultures were then centrifuged at 10,000 rpm for 10 minutes and washed twice with 0.1 M phosphate buffer. A 25 μl aliquot of the cell suspension was smeared onto a coverslip and incubated overnight at 37°C. The cells were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer at room temperature for 2 hours and washed three times. Subsequently, the cells were fixed with 2% osmium tetroxide in phosphate buffer, washed three times, and dehydrated through a graded ethanol series (30%–100%). The dried samples were then examined using a ZEISS Leo 1560 SEM (Germany). Molecular identification of LAB strainsThe test was conducted at DNA Sequencing Company (Vietnam). The selected LAB strains were identified genetically by sequencing the 16S rRNA gene. A 1500 bp fragment of the 16S rRNA gene was amplified using the forward primer 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and the reverse primer 1492R (5’-TACGGCTACCTTGTTACGACTT-3’) as described by Shokryazdan et al. (2014). PCR reactions were performed in a total volume of 18.5 μl, containing 10 μl NZYTaq 2x Green Master Mix, 0.5 μl of each primer, 6 μl of DNase-free water, and 2 μl of DNA template. PCR products (10 μl) were analyzed by electrophoresis and visualized under UV illumination using the ImageMaster system (Pharmacia Biotech, UK). The DNA dye used was Ethidium Bromide ((UltraPure™, Thermo Fisher Scientific), the DNA standard was a 1 kb DNA ladder (New England Biolabs), and the agarose gel concentration was 1%. The 1.5 kb PCR product was purified and sequenced using the Sanger method. The resulting sequences were compared with the NCBI GenBank database using the BLAST tool to identify the LAB strains. Evaluation of probiotic properties of LAB strainsExtracellular enzyme production The ability of LAB strains to produce extracellular enzymes was assessed using the agar well diffusion method (Taheri et al., 2012). LAB strains were cultured in MRS broth, and the cultures were centrifuged to obtain cell-free supernatants containing extracellular enzymes. The supernatants were then added to wells punched in MRS agar plates supplemented with either starch (for amylase activity) or gelatin (for protease activity). Enzyme production was evaluated after 24 hours of incubation by observing the formation of a clear zone of substrate degradation around the wells. Cell surface hydrophobicity Cell surface hydrophobicity was determined according to the method described by Thapa et al. (2004), using microbial adhesion to xylene. LAB strains were grown in 10 ml of MRS broth, centrifuged at 6,000 rpm for 5 minutes, and washed twice with Ringer’s solution. The cells were resuspended in Ringer’s solution to an optical density (OD600) of approximately 0.08. One milliliter of xylene was added to 5 ml of the bacterial suspension, vortexed vigorously for 2 minutes, and allowed to stand for 5 minutes. The aqueous phase was gently extracted, and the OD600 was measured. The hydrophobicity percentage was determined using the following equation: Hydrophobicity (%)=[(OD0 – OD) / OD0] × 100, where OD0 and OD represent the optical densities before and following the addition of xylene, respectively. Self-aggregation and co-aggregation Self-aggregation and co-aggregation abilities were assessed using the methods described by Mallappa et al. (2019). For self-aggregation, LAB strains were cultured overnight, centrifuged at 8,500 rpm for 10 minutes, washed, and resuspended in phosphate-buffered saline. The suspensions were incubated at 37°C for 4 hours. A 0.2 ml aliquot of the suspension was taken before and after incubation, and the OD600 was measured. The auto-aggregation percentage was calculated as follows: Auto-aggregation (%)=1 – [At / Ao] x 100 where Ao and At are the optical densities before and after incubation, respectively. Co-aggregation assays were performed similarly to the self-aggregation assay. Suspensions of S. pullorum and S. typhimurium in brain heart infusion (BHI) broth were prepared and standardized to approximately 1 × 108 CFU/ml. One milliliter of LAB suspension was mixed with 1 ml of Salmonella suspension and vortexed for 10 seconds. The mixture was allowed to settle, and the OD600 was measured after 5 hours of incubation at 37°C. A control containing only 2 ml of bacterial suspension (without mixing) was also included. The co-aggregation percentage was calculated using the formula: Co-aggregation (%)=(OD600 (x) + OD600 (y) – OD600 (x + y)) / (OD600 (x) + OD600 (y)) × 100 where x and y represent the LAB and Salmonella suspensions, respectively. Acid and bile tolerance Acid and bile tolerance were evaluated according to the method of Mallappa et al. (2019). LAB strains were cultured overnight and then inoculated into 10 ml of MRS broth adjusted to pH 2 and 50 ml of MRS broth containing 0.3% (w/v) bile salts (Himedia, India). The initial inoculum concentration was adjusted to 0.5 McFarland standard turbidity (~1.5 × 108 CFU/ml). The cultures were incubated at 37°C for 3 hours. Samples (300 μl) were collected at 0, 1, 2, and 3 hours to monitor growth kinetics by measuring the OD600. Simultaneously, 100 μl samples were taken for viable cell counts using the plate count method. Viability of probiotics in the chicken intestine Thirty-six one-day-old 3F-Viet chicks were randomly divided into two groups of 18 chicks each, with each group further divided into three cages (6 chicks per cage). The experimental group received 1 ml of the probiotic product containing 109 CFU/ml of LAB, while the control group received 1 ml of distilled water. The chicks were housed in iron cages (0.9 × 0.5 × 0.5 m) under continuous lighting and a constant temperature of 35°C throughout the experimental period (1-3 days old). Before the experiment, the cages and flooring system were sterilized using a gas torch and Povidine 10% disinfectant. The chickens were fed a diet formulated from local ingredients, including rice bran, corn flour, peanut meal, and soybean meal, meeting the standards of the Vietnam Ministry of Agriculture and Rural Development (10 TCVN 661-2005). Feed and water were sterilized by UV irradiation (300 µW-s/cm² for 30 minutes) before use and provided ad libitum. At 24, 48, and 72 hours, three chicks from each group (one chick per cage) were randomly selected and euthanized. Samples were collected from the ileum, cecum, and colon to determine Lactobacillus counts using the plate count method. The average LAB count was calculated per gram of each intestinal section. Statistical analysisAll assays were performed in triplicate, and data are presented as mean ± standard deviation (SD) and percentage. Viable cell counts were transformed to log10 CFU/ml. Statistical analysis was conducted using IBM SPSS Statistics (Version 22). One-way ANOVA followed by Tukey’s post hoc test was used to determine significant differences between groups. Results were considered statistically significant at α ≤ 0.05. Ethical approvalAll procedures involving chickens in this study were conducted in accordance with the ethical standards and guidelines approved by the Animal Ethics Committee of Hue University, Vietnam (Approval No.: HUVNO39.1). ResultsThe results of the criteria for identifying and selecting potential Lactobacillus strains as antibiotic alternatives in broiler farming are presented in the diagram of Figure 1.
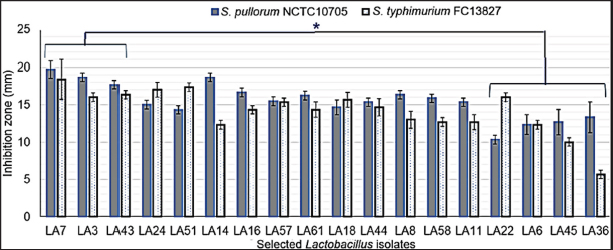
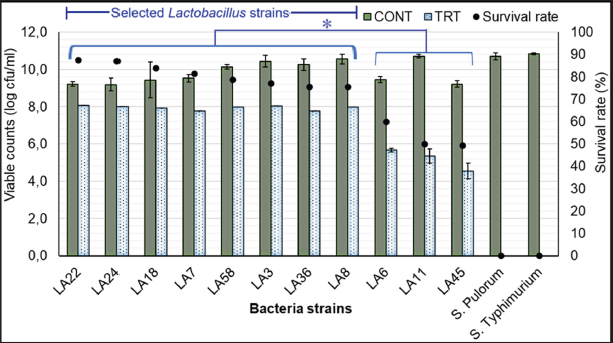
Fig. 1. Results of selection criteria for potential Lactobacillus strains. Selection of LAB strains based on antibiotic alternative propertiesTesting the ability of LAB strains to resist pathogenic bacteria in chickens Antagonistic activity against S. pullorum and S. typhimurium causing digestive diseases was tested in 68 isolated LAB strains. Using the agar well diffusion assay, 18 LAB strains (26.86%) demonstrated inhibitory activity against at least one of the pathogens (DIZ ≥ 10 mm) at varying levels (Fig. 2). These strains were selected for further experiments. In contrast, 49 strains (73.14%) showed no inhibitory effect. Among the selected strains, three strains - LA3, LA7, and LA43 - exhibited strong antibacterial activity against both pathogens, with DIZ averaging 17–19 mm, significantly higher (p < 0.05) than the other four strains (LA6, LA22, LA36, and LA45), which had DIZ of only 7–12 mm. Consequently, the latter four strains were excluded from the list of potential candidates. Evaluation of the antibacterial activity of OE Figure 3 presents the antibacterial activity of 100% OE against digestive pathogens (S. typhimurium and S. pullorum) and the 18 LAB strains. The agar diffusion test indicated that OE initially inhibited the growth of these intestinal pathogens, producing inhibition zones of approximately 15–17 mm, which were larger than those of the selected LAB strains. Notably, 11 LAB strains (LA3, LA6, LA7, LA8, LA11, LA18, LA22, LA24, LA36, LA45, and LA58) showed low inhibition by OE (DIZ < 10 mm), suggesting their potential for submerged fermentation in OE. These strains were selected for subsequent trials. Evaluation of LAB strain compatibility with OE The effect of OE at a concentration of 12.5% on both co-cultures and individual cultures of the two enteric pathogens—Salmonella spp. was assessed via submerged fermentation, based on bacterial counts and survival rates (Fig. 4). Results revealed that eight strains (LA3, LA7, LA8, LA18, LA22, LA24, LA36, and LA58) had survival rates nearly equivalent to the control, with viable bacterial counts around 10 log CFU/ml. In contrast, three strains (LA6, LA11, and LA45) displayed lower survival rates, approximately 50%–60%, which were significantly lower (p < 0.05) than the other strains, leading to their exclusion from further experiments. Meanwhile, the activity of 12.5% OE against intestinal pathogens was substantial, as the pathogen population density decreased over time, with no detectable pathogens after 20 hours of incubation.
Fig. 2. Antibacterial activity of selected LAB strains. Note: values with different superscript letters (a, b or A, B, C) indicate statistically significant differences (p < 0.05).
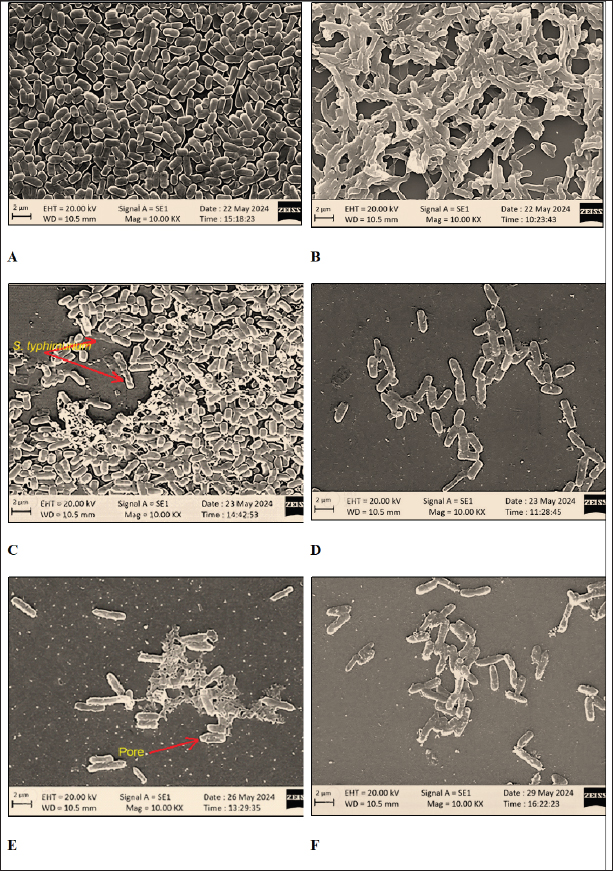
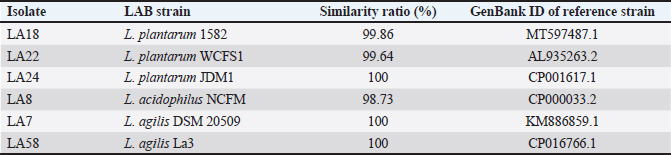
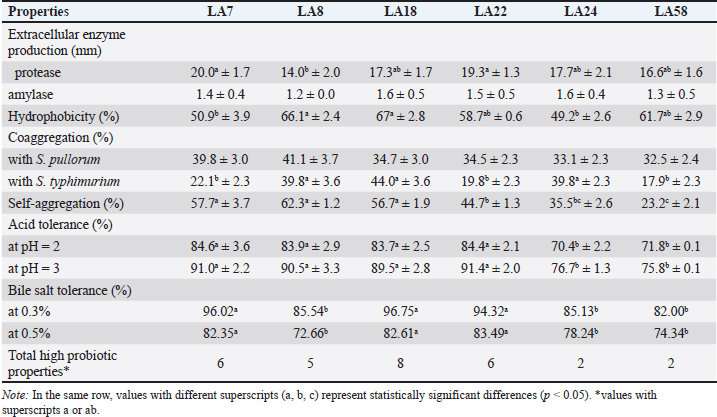
Fig. 3. Inhibition of enteric pathogens and LAB strains by OE (100% concentration). Visualization of the interaction between OE, LAB, and pathogenic bacteria SEM was employed to visualize the interaction among OE, LAB, and pathogenic bacteria. Distinct differences in morphology were observed between L. plantarum (short rods, approximately 1.5 μm in length) and S. typhimurium (long rods, approximately 2.2 μm in length) when cultured individually (Fig. 5A, B) and in co-culture (Fig. 5C). In the co-culture, the number of S. typhimurium cells was significantly reduced compared to L. plantarum (Fig. 5C). Moreover, S. typhimurium cells exhibited clear signs of damage, including cell membrane swelling (Fig. 5D), membrane pore formation (Fig. 5E), and membrane sloughing (Fig. 5F). In contrast, S. typhimurium cells in the control sample (Fig. 5B) maintained nearly intact cell membranes. Molecular identification of LAB strainsSix LAB strains, chosen due to their significant antibacterial effectiveness against S. pullorum and Salmonella typhimurium, lack of inhibition by OE, and safety for use in animal feed, were identified at the genus level using PCR assays. All six strains were confirmed to belong to the genus Lactobacillus (Table 2). Species-level identification was achieved through 16S rRNA gene sequencing. The six LAB strains, initially designated as LA8, LA22, LA24, LA18, LA7, and LA58, were identified as L. plantarum 1582, L. plantarum WCFS1, L. plantarum JDM1, L. acidophilus NCFM, L. agilis DSM 20509, and L. agilis La3, respectively (Fig. 6). The 16S rRNA gene sequences were deposited in the NCBI GenBank database under the accession IDs MT597487.1, AL935263.2, CP001617.1, CP000033.2, KM886859.1, and CP016766.1. Selection of LAB strains based on probiotic propertiesAs presented in Table 3, The protease activity of strains LA7 and LA22 (inhibition zone ~20 mm) was significantly higher (p < 0.05) than that of strain LA8 (~14 mm). Meanwhile, amylase activity was comparable (p=0.214) across the LAB strains, with inhibition zones ranging from 1.2 to 1.6 mm. Regarding hydrophobicity, strains LA8 and LA18 exhibited high levels of water repellency (66.07% and 66.98%, respectively), which were significantly higher (p < 0.05) than those of strains LA7 and LA24 (~50%). Co-aggregation ability with S. pullorum was similar across the selected strains, ranging from 32.5% to 41.1%. However, for S. typhimurium, strains LA8, LA18, and LA24 showed significantly higher co-aggregation ability (39.8%–44.0%) (p < 0.05) compared to the remaining strains (17.9%–22.1%).
Fig. 4. Survival rates of LAB strains in OE medium (12.5% v/v) compared to the control group after 24 hours of incubation at 37°C. Note: *indicates a statistically significant difference (p < 0.05). In this study, all LAB strains isolated from native chicken manure demonstrated good acid tolerance at pH 2 and pH 3, and resistance to bile salts at concentrations of 0.3% and 0.5%, with survival rates exceeding 70%. Among these, strains LA7, LA8, LA18, and LA22 exhibited significantly higher survival rates (p < 0.05) under acidic conditions compared to LA24 and LA58. However, for bile salt tolerance, strains LA8, LA24, and LA58 had significantly lower survival rates (p < 0.05) than the other strains. Overall, the probiotic evaluation showed that strain LA18 ( L. plantarum 1582) exhibited superior probiotic properties (eight characteristics) compared to the other strains (2–6 characteristics). As a result, LA18 was selected for use in the production of biological test products. Probiotic survival in the chicken intestineThroughout the 72-hour observation period following the administration of L. plantarum 1582 fermented OE biopreparation to chickens at a dose of L. plantarum 1582 109 CFU/ml, no adverse clinical signs were observed, and the chickens maintained a normal appetite, indicating that the LAB strain used is safe and does not produce any negative reactions. Figure 7 illustrates the survival of L. plantarum 1582 with the administration of the fermented OE biopreparation in the ileum, cecum, and colon of chickens at 24, 48, and 72 hours post-administration. Overall, probiotic survival in the chicken intestine decreased over time, although both strains maintained relatively high counts after 24 hours (5.73–5.80 log CFU/g) and 48 hours (5.46–5.81 log CFU/g). However, a significant decrease (p < 0.05) in bacterial numbers was observed after 72 hours (4.78–5.47 log CFU/g). The density of LAB was higher in the ileum and cecum compared to the colon (5.27–5.80 vs. 4.78–5.73 log CFU/g). DiscussionThe gut microbiota of indigenous free-range chicken breeds differs significantly from that of commercial breeds due to a variety of factors (Paul et al., 2021). The diverse natural diet consumed by free-range chickens enhances their microbial diversity, which in turn strengthens their immune system. As a result, free-range chicken breeds exhibit greater resistance to various infectious diseases compared to commercial breeds (Kannaki et al., 2021). One of the key components of the chicken gut microbiota is Lactobacillus, with concentrations reaching up to 109 g–1 in the cecum (Barnes, 1979). The evaluation of antibacterial activity is a crucial step in the search for potential bacterial strains to replace antibiotics. In this study, 26.86% of the 68 LAB strains isolated from free-range chicken manure demonstrated inhibitory activity (DIZ ≥ 10 mm) against S. pullorum NCTC10705 and S. typhimurium FC13827. This finding aligns with study of Mulaw et al. (2020), where LAB isolated from pig manure exhibited antibacterial activity against intestinal pathogens, including Salmonella spp. LAB inhibits pathogenic bacteria through multiple mechanisms, including lactic acid production from carbohydrate fermentation, which lowers the environmental pH, thereby inhibiting bacterial growth (O’Shea et al., 2012). Additionally, LAB produces bacteriocins (Alvarez-Sieiro et al., 2016), H2O2 (Hertzberger et al., 2014), and other organic acids such as acetic acid and propionic acid (Makras and De Vuyst, 2006), further contributing to its antibacterial effects. Besides, the competitive exclusion mechanism, where beneficial LAB strains compete with pathogens for nutrients and attachment sites, also plays a vital role in preventing pathogen colonization in the gut.
Fig. 5. Scanning electron micrographs of L. plantarum and S. typhimurium. (A) L. plantarum cultured alone; (B) S. typhimurium cultured alone; (C) L. plantarum and S. typhimurium co-cultured in OE medium; (D) S. typhimurium in OE medium; (E) S. typhimurium in cell-free culture supernatant of L. plantarum; (F) S. typhimurium in cell-free culture supernatant of L. plantarum and OE.
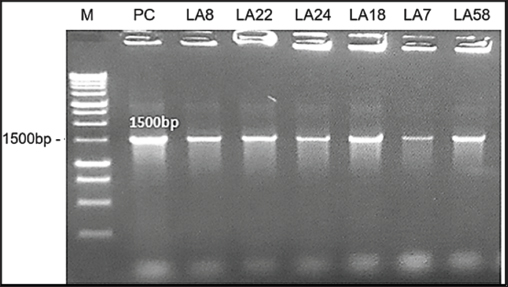
Fig. 6. Agarose gel electrophoresis of PCR products after 16S rRNA gene amplification. Lane M: 1 kb DNA ladder; Lane PC: positive control; Lanes 1–6: PCR products from LAB strains LA8, LA22, LA24, LA18, LA7, and LA58 (1500 bp).
Fig. 7. Survival of L. plantarum 1582 in the ileum, cecum, and colon of chickens. Note: values with different superscript letters (a–c or A, B) indicate statistically significant differences (p < 0.05) at each time point or within each intestinal section. Onion has long been recognized for its antimicrobial properties due to the presence of compounds such as flavonoids, saponins, and sulfur-containing compounds (Yang et al., 2012). In this study, OE demonstrated strong inhibitory effects against S. pullorum and S. typhimurium, with DIZ about 15–17 mm, suggesting its potential as a natural antibiotic alternative. These findings are consistent with previous research on the antimicrobial properties of purple onion. Rattanachaikunsopon and Phumkhachorn (2008) reported that chive’s bulb effectively inhibits the growth of various gastrointestinal pathogens. Specifically, onion exhibits strong antibacterial activity against Salmonella spp. isolated from broiler chickens with diarrhea (Hai et al., 2020a). Plants in the Aliaceae family, including onions, are known to synthesize antibacterial compounds such as saponins, flavonoids, and sulfur-containing compounds during secondary metabolism (Yang et al., 2012). These compounds are also responsible for the antimicrobial activity of garlic, which disrupts bacterial cell membranes and inhibits the synthesis of essential proteins and enzymes (Melguizo-Rodríguez et al., 2022). Previous studies have shown that flavonoids, especially quercetin, can inhibit Gram-negative bacteria such as Salmonella (Cushnie and Lamb, 2005). The antibacterial effect of quercetin is thought to be due to its ability to inhibit enzymes, disrupt cell membranes, and inhibit bacterial DNA synthesis (Rauha et al., 2000). The results of gas chromatography analysis (Table 1) revealed that the OE contained various compounds with significant antibacterial potential. Notably, the total flavonoid content was relatively high (305.13 mg/100 g), with flavonols such as quercetin 3,4’-diglucoside, quercetin 4’-monoglucoside, and quercetin aglycone, alongside sulfur-containing compounds, particularly isoalliin (177.04 mg/100 g). Isoalliin can hydrolyze into allicin, a potent antibacterial agent. The combination of these compounds is likely to produce a synergistic effect, enhancing the antibacterial activity of the extract. Meanwhile, 11 LAB strains (LA3, LA6, LA7, LA8, LA11, LA18, LA22, LA24, LA36, and LA45) exhibited inhibition zones with diameters of less than 10 mm, suggesting that the OE had little to no antibacterial effect on these strains, making them potential candidates for submerged fermentation with OE. This finding is consistent with previous studies, which have shown that plant extracts may affect the survival of LAB strains without completely inhibiting their growth (Borges et al., 2016). This persistence may be explained by several factors. First, LAB strains may possess specific resistance mechanisms, such as degrading enzymes or membrane proteins that prevent the penetration of antimicrobial compounds (Arqués et al., 2015). Second, the biofilm formed by LAB acts as a physical barrier against environmental stressors, including antimicrobial compounds (Williams and Ciorba, 2010). Finally, the presence of other compounds in OEs, such as antioxidants, may reduce the inhibitory effect of antimicrobial compounds on LAB (Jacobsen et al., 1999). The evaluation of bacterial survival in combination with OE at a 12.5% concentration showed that 8 out of 11 LAB strains had the highest survival rate after 20 hours of incubation. This contrasts with the study by Belguith et al. (2010), which reported that Salmonella strains exposed to garlic bulb extract concentrations (11–13 mg/ml) initially overcame inhibition and resumed growth. This discrepancy may be attributed to the lower extract concentration in the previous study compared to the present study (65–125 mg/ml). Conversely, when LAB were cultured with 12.5% OE (v/v), the number of viable cells initially decreased by 1–2 logs, but then increased again. This suggests that the LAB strains were able to overcome the inhibitory phase and continue growing, possibly by metabolizing some of the compounds in the extract. A similar growth pattern was observed in the study by Belguith et al. (2010), where LAB strains initially experienced inhibition but resumed growth after 4–8 hours of exposure to garlic bulb extract. SEM images further demonstrated the synergistic effect of OE and LAB in inhibiting the growth and causing cellular damage to S. typhimurium. Genus-level PCR analysis identified six of the eight selected strains as belonging to the Lactobacillus genus, specifically L. plantarum, L. acidophilus, and L. agilis. The ability of LAB strains to produce extracellular enzymes is a crucial probiotic characteristic, as it supports digestion and reduces fecal excretion (Lee et al., 2001). The findings of this study align with previous research by Kim et al. (2008), which demonstrated that all LAB strains isolated from chicken cecum were capable of producing protease and amylase enzymes. Variations in enzymatic activity among LAB strains may be attributed to their origin; for instance, strains isolated from fermented starchy foods typically exhibit strong starch-degrading capabilities (Sanni et al., 2002). Additionally, the degree of enzyme association with the bacterial cell wall can influence their biochemical properties. Lee et al. (2001) found that the amylolytic activity of L. acidophilus L23 was closely associated with intestinal mucosal cells, suggesting that the enzyme is integrated into the cell wall. Table 2. Identification of LAB strains by 16S rRNA gene sequencing.
Table 3. Probiotic properties of the isolated LAB strains.
Hydrophobicity on the cell surface affects overall adhesion and may facilitate contact between the host and probiotic epithelial cells, allowing probiotics to compete with pathogenic bacteria and produce digestive enzymes (Sánchez-Ortiz et al., 2015). High hydrophobicity indicates that bacteria can better bind to the intestinal mucosa and is classified into three categories: low (<33%), medium (33%–66%) or high (>66%) (Bouchard et al., 2015). In this study, 2 strains LA8 and LA18 exhibited high hydrophobicity (66.07% and 66.98%), and 2 strains LA7 and LA24 were at the intermediate level (about 50%). The ability of probiotics to form cell aggregates through auto-aggregation (aggregation of bacteria of the same strain) or coaggregation (aggregation of bacteria of genetically different strains) may also contribute to the persistence of probiotic strains in the gut. Furthermore, coaggregation may exert antagonistic activity against pathogenic microorganisms (Sidira et al., 2015). In this study, against S. typimurium, strains LA8, LA18, and LA24 showed high coaggregation ability (39.8%–44.0%). Acid and bile salt tolerance are two important factors for probiotic bacteria to survive and grow in the gastrointestinal environment of chickens. The pH of the glandular stomach and muscular stomach is 1.9–4.5 and the feed has to move within 2 hours, while the concentration of bile salts in the upper part of the small intestine (duodenum) in chickens can range from 0.1% to 0.5% (Scanes, 2015). According to Prabhurajeshwar and Chandrakanth (2017), bacteria originating from the host are often better adapted to their digestive conditions, helping them colonize more effectively than bacteria from other sources. This study demonstrated that LAB strains isolated from the feces of native chickens exhibited strong acid tolerance at pH levels of 2 and 3, as well as good survival rates exceeding 70% in the presence of bile salts at concentrations of 0.3% and 0.5%. LA18 ( L. plantarum 1582) was selected for its resistance to acid and bile salts and its production of high levels of organic acids, which are more compatible with the poultry digestive tract, leading to increased growth performance and simultaneously improved immune system. In addition, these isolates showed a high ability to adhere to intestinal epithelial cells and competitively exclude S. pullorum và S. typhimurium from invading the intestinal mucosa. During the 72-hour observation period after LAB supplementation at a dose of 109 CFU/ml, chickens did not show any abnormal clinical symptoms and maintained normal feed consumption. Although Fuller (1989) mentioned the possibility of side effects such as diarrhea or anorexia when using probiotics, these phenomena were not observed in this study, suggesting that the dosage and method of LAB administration were safe. However, the survival of L. plantarum 1582 in the chicken intestinal tract varied between locations. Bacterial density decreased rapidly in the small intestine, likely due to digestive enzymes and bile, but persisted longer in the cecum and large intestine, where a more stable environment and support from indigenous microflora were observed. This reinforces the view of Valerio et al. (2006) that probiotics only reside temporarily in the intestinal tract and need to be regularly replenished to maintain efficacy. ConclusionFrom an initial pool of 68 LAB strains isolated from native chicken feces, 18 strains were selected based on their antibacterial activity against poultry diarrheal pathogens. Among these, 11 strains were not inhibited by OE, and 8 strains demonstrated high survival rates in OE, indicating their potential for fermenting OE. LA18 strain ( L. plantarum 1582) exhibited superior probiotic properties, including the production of digestive enzymes, adhere to intestinal epithelial cells, and withstand acidic conditions and bile salts. In vivo studies in chicks confirmed the safety of the L. plantarum 1582 fermented OE biopreparation and demonstrated its ability to temporarily survive in the chicken intestine. These findings highlight the potential of combining probiotics with herbal extracts as an alternative to antibiotics for controlling gastrointestinal diseases in poultry. AcknowledgmentsThe authors appreciate and acknowledge the laboratory and technical assistance provided by the Faculty of Animal Sciences, Universtiy of Agriculture and Forestry, Hue University. The authors are grateful to the Vietnam Ministry of Education and Training for the research grants and funding (project code: B2023-DHH-24). FundingThe research was funded by the Vietnam Ministry of Education and Training (project code: B2023-DHH-24). Authors’ contributionsAll authors contributed to the study’s conception and design. PVH developed the original hypotheses, designed the experiments, and collaborated in interpreting the results; NDTK, HTAP, and PHSH collected the data for this study, conducted the statistical analyses, and collaborated in the interpretation of the results; NXH, TNL, and NHL collaborated in interpreting the results, and finalized the manuscript. All authors have read and approved the finalized manuscript. Conflict of interestThe authors declare that there is no conflict of interest related to this article. Data availabilityData supporting the findings of this study are available from the corresponding author under the Project funding, upon reasonable request. ReferencesAlvarez-Sieiro, P., Montalbán-López, M., Mu, D. and Kuipers, O.P. 2016. Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. Arqués, J.L., Rodríguez, E., Langa, S., Landete, J.M. and Medina, M. 2015. Antimicrobial activity of lactic acid bacteria in dairy products and gut: effect on pathogens. Biomed. Res. Int. 2015, 584183. Aujoulat, F., Lebreton, F., Romano, S., Delage, M., Marchandin, H., Brabet, M., Bricard, F., Godreuil, S., Parer, S. and Jumas-Bilak, E. 2011. Comparative diffusion assay to assess efficacy of topical antimicrobial agents against Pseudomonas aeruginosa in burns care. Ann. Clin. Microbiol. Antimicrob. 10(3), 27. Barnes, E.M. 1979. The intestinal microflora of poultry and game birds during life and after storage. J. Appl. Bacteriol. 46, 407–419. Belguith, H., Kthiri, F., Chati, A., Sofah, A.A., Hamida, J.B. and Landoulsi, A. 2010. Study of the effect of aqueous garlic extract (Allium sativum) on some Salmonella serovars isolates. Environ. Sci. Agric. Food Sci. 22(3), 189–206. Borges, A., Abreu, A.C., Dias, C., Saavedra, M.J., Borges, F. and Simões, M. 2016. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules (Basel, Switzerland). 21(7), 877. Bouchard, D.S., Seridan, B., Saraoui, T., Rault, L., Germon, P., Gonzalez-Moreno, C., Nader-Macias, F.M., Baud, D., François, P. and Chuat, V. 2015. Lactic acid bacteria isolated from bovine mammary microbiota: potential allies against bovine mastitis. Plus One. 10(12), e0144831. Brijesh, S., Daswani, P., Tetali, P., Antia, N. and Birdi, T. 2009. Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: validating its traditional usage. BMC Compl. Altern. Med. 9(47), 1–12. Cushnie, T.P. and Lamb, A.J. 2005. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 26(5), 343–356. Dowarah, R., Verma, A.K., Agarwal, N., Singh, P. and Singh, B.R. 2018. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. Plos One. 13(3), e0192978. Fadare, O.S., Singh, V., Enabulele, O.I., Shittu, O.H. and Pradhan, D. 2022. In vitro evaluation of the synbiotic effect of probiotic Lactobacillus strains and garlic extract against Salmonella species. LWT. 153, 112439. Farhat, M., Khayi, S., Berrada, J., Mouahid, M., Ameur, N., El-Adawy, H. and Fellahi, S. 2023. Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum in poultry: review of pathogenesis, antibiotic resistance, diagnosis and control in the genomic era. Antibiotics (Basel, Switzerland). 13(1), 23. Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteria 66, 365–378. Georgieva, R., Yocheva, L., Tserovska, L., Zhelezova, G., Stefanova, N., Atanasova, A., Danguleva, A., Ivanova, G., Karapetksov, N., Rumyan, N. and Karaivanova, E. 2015. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Equip. 29(2), 84–91. Hai, P.V., Chao, N.V., Khuong, N.D.T., Liem, T.N, Dung, H.T, Le, T.T.T., Anh, L.X. and Hung, P. H.S. 2020a. Antimicrobial activity of chive and ginger extract on Escherichia coli and Salmonella spp. isolated from diarrhoeic broiler chickens. Hue Univ. J. Sci.: Agri. Rural Develop. 128, 105–111. Hai, P.V. and Hoa, N.X. 2020. Effect of Allium Schoenoprasum extract on immune status against Newcastle virus and growth performance of broiler chicken. J. Agri. Sci. Tech., Hue Univ. Agri. Fores. 4, 2058–2064. Hai, P.V., Pham, H.S.H., Ho, T.D., Tran, N.L., Nguyen, D.T.K. and Nguyen, X.H. 2020b. The dietary supplement efficiency of essential oil of chive (Allium macrostemon) on the productivity and health performance of broilers. Can Tho Univ. J. Sci. 12, 1–6. Hertzberger, R., Arents, J., Dekker, H.L., Pridmore, R.D., Gysler, C., Kleerebezem, M. and Mattos, M. 2014. H2O2 production in species of the Lactobacillus acidophilus group: a central role for a novel NADH-dependent flavin reductase. Appl Environ Microbiol. 80(7), 2229–2239. Jacobsen, C.N., Rosenfeldt Nielsen, V., Hayford, A.E., Møller, P.L., Michaelsen, K.F., Paerregaard, A., Sandström, B., Tvede, M. and Jakobsen, M. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65(11), 4949–4956. Kannaki, T.R., Priyanka, E. and Haunshi, S. 2021. Research note: disease tolerance/resistance and host immune response to experimental infection with Pasteurella multocida A:1 isolate in Indian native Nicobari chicken breed. Poult. Sci. 100(8), 101268. Kim, H. B., Borewicz, K., White, B. A., Singer, R. S., Sreevatsan, S., Tu, Z. J. and Isaacson, R. E. 2017. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet. Microbiol. 153(1-2), 124–134. Kim, S.H., Kim, D.W., Park, S.Y., Kim, J.H., Kang, G.H., Kang, H.K., Yu, D.J., Na, J.C. and Lee, S.J. 2008. Characterization of Lactobacilli isolated from chicken ceca as probiotics. J. Anim. Sci. Tech. 50(4), 509–518. Kim, S.W., Ha, Y.J., Bang, K.H., Lee, S., Yeo, J.H., Yang, H.S., Kim, T.W., Lee, K.P. and Bang, W.Y.J.T. 2020. Potential of bacteriocins from Lactobacillus taiwanensis for producing bacterial ghosts as a next generation vaccine. Toxins (Basel) 12(7), 432. Lee, Y. K. and Salminen, S. 2001. The coming of age of probiotics. Trends Food Sci. Technol. 12(11), 411–415. Makras, L. and De Vuyst, L. 2006. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int. Dairy J. 16, 1049–1057. Mallappa, R.H., Singh, D.K., Rokana, N., Pradhan, D., Batish, V.K. and Grover, S. 2019. Screening and selection of probiotic Lactobacillus strains of Indian gut origin based on assessment of desired probiotic attributes combined with principal component and heatmap analysis. LWT 105, 272–281. Mangisah, I., Yunianto, V.D., Sumarsih, S. and Sugiharto, S. 2021. Supplementation of garlic powder and Lactobacillus casei to improve nutrient digestibility, physiological conditions, and performance of broiler during starter phase. J. Indonesian Trop. Anim. Agri. 46(4), 336–346. Melguizo-Rodríguez, L., García-Recio, E., Ruiz, C., De Luna-Bertos, E., Illescas-Montes, R. and Costela-Ruiz, V.J. 2022. Biological properties and therapeutic applications of garlic and its components. Food Funct. 13, 2415–2426. Mulaw, G., Muleta, D., Tesfaye, A. and Sisay, T. 2020. Protective effect of potential probiotic strains from fermented Ethiopian food against Salmonella Typhimurium DT104 in mice. Int. J. Microbiol. 2020, 7523629. Myers, S.R., Hawrelak, J. and Cattley, T. 2009. Essential oils in the treatment of intestinal dysbiosis: a preliminary in vitro study. Alter. Med. Rev. 14(4), 380–384. Nair, D., Venkitanarayanan, K. and Kollanoor Johny, A. 2018. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 7(10), 167. O’Shea, E.F., Cotter, P.D., Stanton, C., Ross, R.P. and Hill, C. 2012. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 152, 189–205. Paul, S.S., Chatterjee, R.N., Raju, M., Prakash, B., Rama Rao, S.V., Yadav, S.P. and Kannan, A. 2021. Gut microbial composition differs extensively among Indian native chicken breeds originated in different geographical locations and a commercial broiler line, but breed-specific, as well as across-breed core microbiomes, are found. Microorganisms 9(2), 391–412. Prabhurajeshwar, C. and Chandrakanth, R. 2017. Probiotic potential of Lactobacilli with antagonistic activity against pathogenic strains: an in vitro validation for the production of inhibitory substances. Biomed. J. 40(5), 270–283. Rattanachaikunsopon, P. and Phumkhachorn, P. 2008. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum). Biosci. Biotechnol. Biochem. 72(11), 2987–2991. Rauha, J.P., Remes, S., Heinonen, M., Hopia, A., Kähkönen, M., Kujala, T., Pihlaja, K., Vuorela, H. and Vuorela, P. 2000. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 56(1), 3–12. Reuben, R.C., Roy, P.C., Sarkar, S.L., Alam, R.U. and Jahid, I.K.J.B. 2019. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microb. 19(253), 1–20. Sánchez-Ortiz, A., Luna, A., Campa-Córdova, Á., Escamilla-Montes, R., Flores Miranda, M.D.C. and Mazón-Suástegui, J.M. 2015. Isolation and characterization of potential probiotic bacteria from pustulose ark (Anadara tuberculosa) suitable for shrimp farming. Latin Ametica J. Aquat. Res. 43(1), 123–136. Sanni, A. I., Morlon-Guyot, J. and Guyot, J. P. 2002. New efficient amylase-producing strains of Lactobacillus plantarum and L. fermentum isolated from starchy fermented foods. Int. J. Food Microbiol. 72(1-2), 53–62. Scanes, C.G. 2015. Preface, In: Sturkie’s avian physiology (Sixth edition). San Diego, CA: Academic Press. Shokryazdan, P., Kalavathy, R., Sieo, C., Alitheen, N., Liang, J., Jahromi, M. and Ho, Y. 2014. Isolation and characterization of Lactobacillus strains as potential probiotics for chickens. Pertanika J. Trop. Agric. Sci. 37 (1), 141–157. Sidira, M., Galanis, A., Ypsilantis, P., Karapetsas, A., Galani, E. and Kourkoutas, Y. 2015. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yogurt production. LWT - Food Sci. Technol. 62(1), 458–464. Singh, B.R. 2013. Antimicrobial drug resistance against Eucalyptus citriodora gum in strains of common microbes of public health concern isolated from food, animals and environment. Natural Prod. 9(4), 153–160. Sunu, P., Sunarti, D., Mahfudz, L.D. and Yunianto, V.D. 2019. Prebiotic activity of garlic (Allium sativum) extract on Lactobacillus acidophilus. Vet. World. 12(7), 2046. Taheri, A., Robinson, S., Parkin, I. and Gruber, M. 2012. Revised selection criteria for candidate restriction enzymes in genome walking. PloS One. 7, e35117. Thapa, N., Pal, J., Tamang, J.P. and Biotech. 2004. Microbial diversity in Ngari, Hentak and Tungtap, fermented fish products of North-East India. World J. Microbiol. Biotech. 20, 599–607. Valerio, F., De Bellis, P., Lonigro, S.L., Morelli, L., Visconti, A. and Lavermicocca, P. 2006. In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 72, 3042–3045. Wang, L., Zhang, H., Rehman, M.U., Mehmood, K., Jiang, X., Iqbal, M., Tong, X., Gao, X. and Li, J. 2018. Antibacterial activity of Lactobacillus plantarum isolated from Tibetan yaks. Microb. Pathog. 115(3), 293–298. WHO, 2023. List of antibiotic-resistant priority pathogens. Geneva, Swizterland: WHO. Available via http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/. Williams, M.D., Ha, C.Y. and Ciorba, M.A. 2010. Probiotics as therapy in gastroenterology: a study of physician opinions and recommendations. J. Clin. Gastroenterol. 44(9), 631–636. Wilson, E.A. and Demmig-Adams, B. 2007. Antioxidant, anti-inflammatory, and antimicrobial properties of garlic and onions. Nutri. Food Sci. 37(3), 178–183. Xiang, H., Sun-Waterhouse, D., Waterhouse, G.I.N., Cui, C. and Ruan, Z. 2019. Fermentation-enabled wellness foods: a fresh perspective. Food Sci. Human Wellness. 8(2), 203–243. Yadav, S., Trivedi, N.A. and Bhatt, J.D.J.A. 2015. Antimicrobial activity of fresh garlic juice: an: in vitro study. Ayu. 36(5), 203–207. Yang, E.J., Kim, S.I., Park, S.Y., Bang, H.Y., Jeong, J.H., So, J.H., Rhee, I.K. and Song, K.S.J.F. 2012. Fermentation enhances the in vitro antioxidative effect of onion (Allium cepa) via an increase in quercetin content. Food. Chem. Toxicol. 50(6), 2042–2048. | ||
| How to Cite this Article |
| Pubmed Style Hai PV, Phuong HTA, Hung PHS, Na TT, Lai NH, Khuong NDT, Liem TN, Hoa NX. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J.. 2024; 14(12): 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 Web Style Hai PV, Phuong HTA, Hung PHS, Na TT, Lai NH, Khuong NDT, Liem TN, Hoa NX. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. https://www.openveterinaryjournal.com/?mno=225342 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.35 AMA (American Medical Association) Style Hai PV, Phuong HTA, Hung PHS, Na TT, Lai NH, Khuong NDT, Liem TN, Hoa NX. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J.. 2024; 14(12): 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 Vancouver/ICMJE Style Hai PV, Phuong HTA, Hung PHS, Na TT, Lai NH, Khuong NDT, Liem TN, Hoa NX. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 Harvard Style Hai, P. V., Phuong, . H. T. A., Hung, . P. H. S., Na, . T. T., Lai, . N. H., Khuong, . N. D. T., Liem, . T. N. & Hoa, . N. X. (2024) Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J., 14 (12), 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 Turabian Style Hai, Phan Vu, Hoang Thi Anh Phuong, Pham Hoang Son Hung, Tran Thi Na, Ngo Huu Lai, Nguyen Dinh Thuy Khuong, Tran Ngoc Liem, and Nguyen Xuan Hoa. 2024. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Veterinary Journal, 14 (12), 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 Chicago Style Hai, Phan Vu, Hoang Thi Anh Phuong, Pham Hoang Son Hung, Tran Thi Na, Ngo Huu Lai, Nguyen Dinh Thuy Khuong, Tran Ngoc Liem, and Nguyen Xuan Hoa. "Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens." Open Veterinary Journal 14 (2024), 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 MLA (The Modern Language Association) Style Hai, Phan Vu, Hoang Thi Anh Phuong, Pham Hoang Son Hung, Tran Thi Na, Ngo Huu Lai, Nguyen Dinh Thuy Khuong, Tran Ngoc Liem, and Nguyen Xuan Hoa. "Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens." Open Veterinary Journal 14.12 (2024), 3525-3538. Print. doi:10.5455/OVJ.2024.v14.i12.35 APA (American Psychological Association) Style Hai, P. V., Phuong, . H. T. A., Hung, . P. H. S., Na, . T. T., Lai, . N. H., Khuong, . N. D. T., Liem, . T. N. & Hoa, . N. X. (2024) Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Veterinary Journal, 14 (12), 3525-3538. doi:10.5455/OVJ.2024.v14.i12.35 |