| Research Article | ||
Open Vet. J.. 2024; 14(12): 3498-3504 Open Veterinary Journal, (2024), Vol. 14(12): 3498-3504 Research Article Progression of pulmonary arterial hypertension: A study in modelAgus Cahyono1,2, Irwanto3*, Mahrus A. Rahman3 and Widjiati Widjiati41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Clinical Medicine, Faculty of Medicine, Universitas Surabaya, Surabaya, Indonesia 3Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Irwanto. Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: irwanto [at] fk.unair.ac.id Submitted: 22/10/2024 Accepted: 21/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
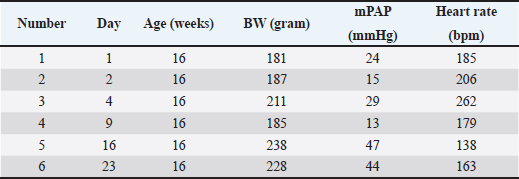
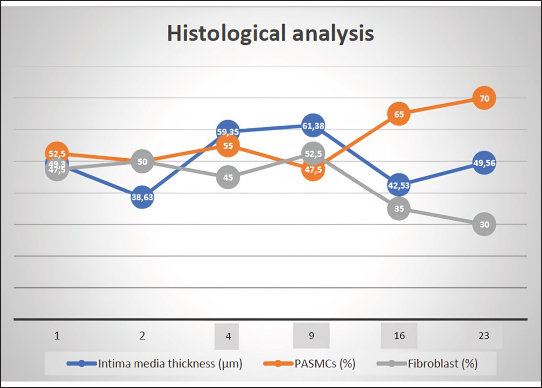
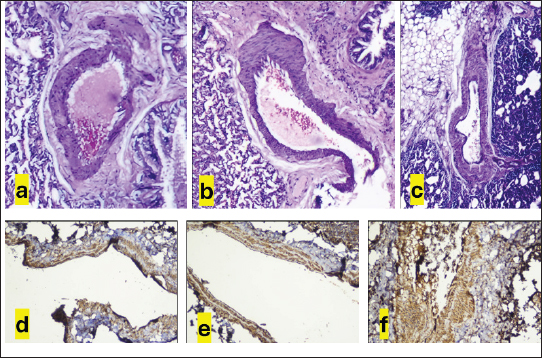
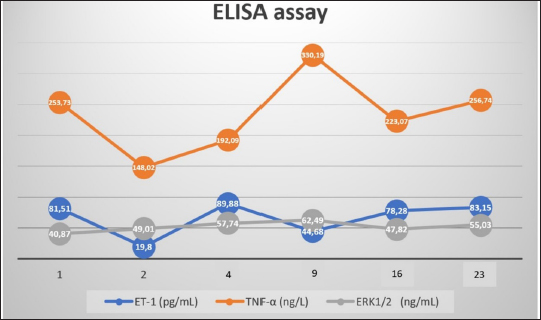
AbstractBackground: The pathophysiology of pulmonary arterial hypertension (PAH) is complex. Pathology and molecular biology signatures during its progression are interesting to study. Aim: This study will describe PAH progression from the first until the fourth week in a model focussing on endothelin-1 (ET-1), tumor necrosis factor α (TNF-α), extracellular signal-regulated kinase 1/2 (ERK1/2), intima media thickness (IMT), and proliferation of pulmonary arterial smooth muscle cells (PASMCs) and fibroblast. Methods: Six male Wistar rats aged 4 months old with a range bodyweight (BW) of 180–230 g were used in this experiment. Rats were injected with Monocrotaline (MCT) 60 mg/kg of BW subcutaneously to induce PAH. Rats were anesthetized with Ketamin 50 mg/kg BW and Xylazin 5 mg/kg BW intramuscularly before cathetherization. Right heart cathetherization was performed at days 1st, 2nd, 4th, 9th, 16th, and 23rd after MCT injection. After completion of cathetherization, intracardiac exsanguination was performed and blood serum was analyzed by ELISA for ET-1, TNF-α, and ERK1/2. Lungs were harvested and parafinated blocked before being analyzed for IMT and proliferation of PASMCs and fibroblast. Results: At first until the second week after MCT injection, mean pulmonary arterial pressure fluctuated. However, after 3rd until 4th week after MCT injection, its value becomes established over 40 mmHg. This is also followed by the level of ET-1 over 78 pg/ml, level of TNF-α over 223 ng/l, level of ERK1/2 over 47 ng/ml, IMT over 42 µm, ratio of PASMCs over 65%, and ratio of fibroblast 30%–35%. Conclusion: Pulmonary hypertension was established at week 3rd-4th after MCT injection in a model. Keywords: Pulmonary hypertension, Progression, Model. IntroductionPulmonary hypertension (PH) is defined by a mean pulmonary arterial pressure (mPAP) of more than 20 mmHg at rest (Humbert et al., 2023). Its prevalence in the adult population is 5 per million adults and in the pediatric population is 2–16 per million children (Hansmann, 2017). As in adults, pediatrics has a similar pathobiology of PH. However, idiopathic pulmonary arterial hypertension, pulmonary arterial hypertension (PAH) associated with congenital heart disease, and developmental lung diseases are predominant in children (Rosenzweig et al., 2019). The pathobiology of PH is complex. It is a progression from the subclinic to the clinic phase that is subtle. One may notice PH when it comes to an advanced degree of severity (Humbert et al., 2006; Ivy et al., 2013; Babu, 2019). Understanding the pathobiology of PAH is important to find potential novel treatments (Babu, 2019). The basic pathobiology of PAH is endothelial dysfunction. This will later cause pulmonary vascular remodeling. The remodeled vascular is more vasoconstrictive and has a thicker wall. The proliferation of pulmonary artery smooth muscle cells (PASMCs) and fibroblasts plus increasing of vasoreactive modulators contribute to this event. When the causes are persistent, the remodeling process will be progressive and at some point, irreversible. Mediators are many to cause remodeling and their role in the pathobiology of PAH is more understandable recently (Ivy et al., 2013; Humbert et al., 2019; Zahid et al., 2020, Sweatt et al., 2021). Many researches try to uncover the pathobiology of PAH. From the research, some key mediators have been discovered. Interleukin-1 (IL-1) is responsible in remodeling process by induction of fibroblast proliferation (Mitchell et al., 2007; Bui et al., 2019). Meanwhile, Serotonin and Interleukin-6 (IL-6) are important mediators for PASMCs proliferation (Savale et al., 2009; West et al., 2016; Sakarin et al., 2020). Furthermore, tumor necrosis factor α (TNF-α), Vascular Endothelial Growth Factor (VEGF), tumor growth factor β (TGF-β), and Platelet Derived Growth Factor (PDGF) are responsible in this process by induction of fibroblast and PASMCs proliferation (Farkas et al., 2009; Biernacka et al., 2011; Zhao et al., 2013; Pilling et al., 2015; Li et al., 2016; Rieg et al., 2018; Bell et al., 2020; Winter et al., 2020; Takamura et al., 2021). Lack of Nitrit Oxyde (NO) and increased level of Endothelin-1 (ET-1) will cause vasocontriction (Klinger et al., 2013; Chester and Yacoub, 2014; Klinger and Kadowitz, 2017). ET-1 itself acts as vasoconstrictor and inducer of fibroblast and PASMCs proliferation (Meoli and White, 2010; Duangrat et al., 2023). From the aforementioned mediators, ET-1 and TNF-α are interesting to study. These two mediators act in more than one way to cause vascular remodeling. Together, these two mediators inducing extracellular signal regulated kinase 1/2 (ERK1/2) to cause the proliferation of fibroblast and PASMCs with the end results of pulmonary arterial wall thickening (Meoli and White, 2010; Wu et al., 2011; Wang et al., 2017; Duangrat et al., 2023). ET-1 is also known as the strongest endogenous vasoconstrictor (Chester and Yacoub, 2014). Using a model will clearly depict PAH progression that is similar in pediatric patients since in humans the beginning of PAH is difficult to determine. This study will describe PAH progression from the first until the fourth week in a model focusing on ET-1, TNF-α, ERK1/2, and pulmonary artery remodeling. Materials and MethodsAnimal studiesSix male Wistar rats aged 4 months old with a range bodyweight (BW) of 180–230 g were used in this experiment. Rats were housed at 24°C, 50%–70% humidity in room oxygen with a 12-hour light/dark cycle, and given free access to standard laboratory food and water. Rats were injected with Monocrotaline (MCT, MedChemExpress, catalog number HY-N0750) 60mg/kg of BW subcutaneously. MCT was diluted in 1 M Hydrocloride acid and 1 M Sodium hydroxide was added to create a pH of 7.4. Right heart cathetherization was performed at days 1st, 2nd, 4th, 9th, 16th, and 23rd after MCT injection. Rats were anesthetized with Ketamin 50 mg/kg BW and Xylazin 5 mg/kg BW intramuscularly. After that, the rats were put on a supine position. Fur was shaved and skin was incised vertically from 1st to 5th costae until the pectoralis muscle was seen. Pectoralis muscle was bluntly put aside and retracted laterally until the intercostalis muscle is seen. Intravenous catheter no 22G (after washed with heparin) was then introduced through 4th parasternal intercostalis space to the right ventricle. The dark red blood will come out from the right ventricle and immediately, the catheter is connected to tranducer and Mac 51 cable. The cathether then directed to the pulmonary artery and the pulmonary arterial wave will be seen on DASH 4000 monitor. Pulmonary arterial pressure was then recorded. After completion of cathetherization, a blood sample was taken from the intracardiac (±3.5 ml). The rats were euthanized by this exsangunation. The lungs were harvested and put into formalin before processed to paraffin block. Blood was then centrifuged by refrigerated centrifuse (Kubota K3520) with 3,500 rpm and 4°C for 10 minutes. The serum was kept in −20°C refrigerator before ELISA analysis. The study was conducted from March to April 2024 at the Animal Laboratory Faculty of Veterinary Medicine Airlangga University, Surabaya, East Java, Indonesia. Histological analysisThe Parrafin block of each lung lobe was sliced to 5 µm thickness. These slides were washed with xylene for 5 minutes three times to clear parrafin then dripped three times into methanol and washed 1 minute in the water. After the procedure, the slides were stained with hematoxylin and eosin at room temperature for 10 minutes and 6 minutes consecutively. A pulmonary artery with a diameter of 50–200 µm were evaluated using a light microscope. Eight pulmonary artery, for each rat, were randomly selected. Four areas of intima media thickness (IMT) were measured at x400 magnification for their thickness and count for their average. Slides were also stained with alpha smooth muscle actin (α-SMA, GeneTex, catalog number GTX636885) antibody to evaluate the ratio of the smooth muscle cell and fibroblasts. ELISA assaySerum ET-1 concentrations were measured using a Rat ET-1 competitive ELISA kit (catalog number ER0019, Fine Test), and serum TNF-α concentrations were measured using Rat TNF-α ELISA kit (Catalog number E0764Ra, Bioassay Technology Laboratory). Meanwhile, serum ERK1/2 concentrations were measured using Mouse ERK1/2 ELISA kit (Catalog number E2815Mo, Bioassay Technology Laboratory). For ET-1 measurement, frozen samples were thawed at room temperature before ELISA assay. The plate was washed twice before adding the standard and sample. Fifty microliters of biotin-labeled antibody working solution was added to each well that contained 50 µl of standard or sample. The plate was then shaked for 1 minute and statically incubated at 37°C for 45 minutes. After that, the plate was washed 3 times and immersed for 1 minute each time. One hundred microliters of HRP-Streptavidin Conjugate working solution was added into each well and then the plate was sealed and statically incubated at 37°C for 45 minutes. The plate was washed five times after that and immersed for 1 minute each time. Ninety TMB substrate solution was then added and the plate was sealed and incubated at 37°C for 10–20 minutes. Eventually, 50 µl stop solution was added and read at 450 nm immediately. Regression equations were formulated from a standard curve of optical density and standard solution concentrations. The equation was used to calculate sample concentration. For TNF-α and ERK1/2 measurement, the step was similar to that of ET-1. The serum volume required for measurement was 40 µl. Ethical approvalThis study was approved by the Animal Care and Use Committee Faculty of Veterinary Medicine Airlangga University no 2.KEH.161.10.2023. ResultsClinical dataData of rat’s clinical condition at cathetherization is described in Table 1. On 1st day after MCT injection, mPAP reaches 24 mmHg, which is consistent with PAH definition. However, between 1st and 2nd week, mPAP is still fluctuative. When entering 3rd and 4th week, mPAP is established at 44 and 47 mmHg. IMT and PASMCs/Fibroblast ratio IMT is described on µm and PASMC/fibroblast ratio is described on percentage. The progression of PAH development is depicted on Figure 1. Figure 2a–c are representative of histological views of IMT (day 1st, day 16th, and day 23rd consecutively), and d, e, f are representative of PASMCs/Fibroblast ratio (day 1st, day 16th, and day 23rd consecutively) ELISA assay. Inflammatory mediators characterize PAH. ET-1, TNF-α, and ERK1/2 fluctuate during the progression of PAH. ERK1/2 is the least fluctuative mediator. However, at day 23rd, these 3 mediators are higher when compared to day 16th (Fig. 3). DiscussionPAH is an interesting subject to study. Clinical manifestations, pulmonary arterial pathology, and molecular abnormalities characterize PAH progression (Humbert et al., 2006; Ivy et al., 2013; Hansmann, 2017; Babu, 2019). However, it is not always possible to study the progression PAH in human subjects, since the onset of the disease is less predictable (Humbert et al., 2006). An animal model is chosen for the study to understand the pathobiology of PAH. Wistar rats are commonly used for PAH model since their pulmonary vascular shares similarities with human. MCT was chosen to induce PAH in a model due to its effectiveness (Boucherat et al., 2022). To illustrate PAH progression, which is the dominant type of PH in pediatrics population (Rosenzweig et al., 2019), 16-week-old rats were used in this research. A 4-week-old rats is equal to a 2.5-month-old human, it means 16 weeks rats is equal to 10 years 10-year-old human (Andreollo et al., 2012). mPAP measurement using right heart Catheterization confirmed evidence of PAH at day 1 after MCT injection, since PH definition is when mPAP of more than 20 mmHg at rest (Humbert et al., 2023). Even though the value fluctuates at the following day, it is established at day 16th and 23rd. This fact reflects that vasoconstriction is might be dominant at the first day and proliferation of PASMCs and fibroblast is more apparent at 3rd and 4th week (Yoshida et al., 2020). Pathobiology of PAH consists of vasoconstriction, proliferation of PASMCs and fibroblast. These entity due to stimulation of mediators involved in PAH (Humbert et al., 2006; Ivy et al., 2013; Babu, 2019). From hystological views, IMT and proliferation of PASMCs and fibroblast depict the pathology of PAH. Intima media reflects the most area of the pulmonary artery that are affected by either PASMCs or fibroblast proliferation (Humbert et al., 2019; Zahid et al., 2020). However, since PASMCs and fibroblast is proliferating in PAH (Humbert et al., 2019; Zahid et al., 2020; Sweatt et al., 2021), we conducted a study to measure PASMCs and fibroblast ratio in the pulmonary artery. The study showed us that IMT fluctuates from the 1st day until 23rd day after MCT injection. PASMC became prominent at day 16th and 23rd after the MCT injection. This fact is also underscored by the decreasing of fibroblast ratio at the same period. During PAH progression, none of the rats showed obvious clinical signs. This can be explained by the fact that the pulmonary artery has not been fully remodeled (Humbert et al., 2006). Fluctuation of mediators also accompanied IMT and mPAP. Tumor necrosis factor alpha (TNF-α) and ERK 1/2 fluctuated in accordance with IMT. Meanwhile, ET-1 fluctuated in accordance with mPAP. These facts showed that TNF-α and ERK 1/2 were mediators more related with IMT and ET-1 was a mediator related with mPAP. Previous research showed TNF-α and ET-1 act together to induce ERK 1/2 with the end result of pulmonary vascular thickening. ET-1 also known as the strongest vasoconstrictor. These 3 mediators are important in PAH (Meoli and White, 2010; Wu et al., 2011; Chester and Yacoub, 2014; Wang et al., 2017; Duangrat et al., 2023). Table 1. Clinical data of experimental rats.
Fig. 1. Histological analysis. This graphic depicts progression of pulmonary arterial from first day to 23rd day of MCT injection. PASMCs: pulmonary arterial smooth muscle cells.
Fig. 2. Intima media and PASMCs/Fibroblast picture. Hematoxylin and eosin staining (a, b, c) depicts IMT at day 1st, 16th, and 23rd consecutively and α-SMA staining (d, e, f) depicts PASMCs/Fibroblast ratio at day 1st, 16th, and 23rd consecutively. At 23rd day, IMT become prominent and arterial wall is dominated by PASMCs. IMT, purple colored; PASMCs brown colored.
Fig. 3. ELISA assay. This graphic depicts inflammatory mediators fluctuation during PAH progression. At first until 3rd week ET-1, TNF-α, and ERK1/2 are fluctuating. However, at 4th week, these 3 mediators are simultaneously increased. We focused on the study of 3 mediators and their effects on the progression of PAH. The role of other mediators such as IL-1, IL-6, Serotonin, VEGF, TGF-β, PDGF, and lack of NO are also significant in the pathobiology of PAH. Their orchestration cause remodelling of pulmonary arterial wall (Mitchell et al., 2007; Farkas et al., 2009; Savale et al., 2009; Biernacka et al., 2011; Klinger et al., 2013; Klinger and Kadowitz, 2017; Zhao et al., 2013; Pilling et al., 2015; Li et al., 2016; West et al., 2016; Rieg et al., 2018; Bui et al., 2019; Bell et al., 2020; Sakarin et al., 2020; Winter et al., 2020; Takamura et al., 2021). IL-1will induce fibroblast proliferation (Mitchell et al., 2007; Bui et al., 2019); meanwhile, Serotonin and IL-6 will induce PASMCs to proliferate (Savale et al., 2009; West et al., 2016; Sakarin et al., 2020). The proliferation of PASMCs and fibroblast simultaneously will be induced by the work of VEGF, TGF-β, and PDGF (Farkas et al., 2009; Biernacka et al., 2011; Zhao et al., 2013; Pilling et al., 2015; Li et al., 2016; Rieg et al., 2018; Bell et al., 2020; Winter et al., 2020; Takamura et al., 2021). On the other hand, a lack of NO will cause the pulmonary artery to easily constrict (Klinger et al., 2013; Klinger and Kadowitz, 2017). Further research to recognize these mediators role in PAH progression is widely open. AcknowledgmentThe authors thank Anang Murdjito, Vet. Med and Azizah, Vet. Med for providing assistance during the research. Conflict of interestThere is no conflict of interest. FundingThere is no specific financial support funded the research. Authors’ contributionsAC, I, and MAR developing concept and methodology. AC, MAR, and W performing the investigation. MAR and I performing data curation. AC and MAR writing the original draft. I and W reviewing and editing draft. All authors have reviewed and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAndreollo, N., Santos, E.D., Araujo, M. and Lopes, L. 2012. Rat’s age versus human’s age: what is the relationship? Arq. Bras. Cir. Dig. 25, 49–51. Babu, A.F. 2019. Molecular basis of pulmonary hypertension. Int. J. Clin. Cardiol. 6, 145. Bell, R.D., White, R.J., Garcia-Hernandez, M.L., Wu, E., Rahimi, H., Marangoni, R.G., Slattery, P., Duemmel, S., Nuzzo, M., Huertas, N., Yee, M., O’Reilly, M.A., Morrel, C., Ritchlin, C.T., Scwarz, E.M. and Korman, B.D. 2020. TNF induces obliterative pulmonary vascular disease in a novel model of connective tissue disease associated pulmonary arterial hypertension (CTD-PAH). Arthritis Rheumatol. 72(10), 1759–1770. Biernacka, A., Dobaczewski, M. and Frangogiannis, N.G. 2011. TGF-β signaling in fibrosis. Growth Factors. 29(5), 196–202. Boucherat, O., Agrawal, V., Lawrie, A. and Bonnet, S. 2022. The latest in animal models of pulmonary hypertension and right ventricular failure. Circ. Res. 130, 1466–1486. Bui, C.B., Kolodziej, M., Lamanna, E., Elgass, K., Sehgal, A., Rudloff, I., Schwenke, D.O., Tsuchimochi, H., Kroon, M.A.G.M., Cho, S.X., Maksimenko, A., Cholewa, M., Berger, P.J., Young, M.J., Bourke, J.E., Pearson, J.T, Nold, M.F. and Nold-Petry, C.A. 2019. Interleukin-1 receptor antagonist protects newborn mice against pulmonary hypertension. Front. Immunol. 10, 1480–1494. Chester, A.H. and Yacoub, M.H. 2014. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 29, 62–78. Duangrat, R., Parichatikanond, W., Likitnukul, S. and Mangmool, S. 2023. Endothelin-1 induces cell proliferation and myofibroblast differentiation through the ETAR/Gαq/ERK signaling pathway in human cardiac fibroblasts. Int. J. Mol.Sci. 24(5), 4475–4492. Farkas, L., Farkas, D., Ask, K., Moller, A., Gauldie, J., Margetts, P., Inman, M. and Kolb, M. 2009. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Invest. 119, 1298–1311. Hansmann, G. 2017. Pulmonary hypertension in infants, children, and young adults. J. Am. Coll. Cardiol. 69(20), 2551–2569. Humbert, M., Guignabert, C., Bonnet, S., Dorfmuller, P., Klinger, J.R., Nicolls, M.R. and Olschewski, A.J. 2019. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur. Respir. J. 53, 1801887. Humbert, M., Kovacs, G., Hoeper, M.M., Badagliacca, R., Berger, R.M.F., Brida, M., Carlsen, J., Coats, A.J.S., Escribano-Subias, P., Ferrari, P., Ferreira, D.S., Ghofrani, H.A., Giannakoulas, G., Kiely, D.G., Mayer, E., Meszaros, G., Nagavci, B., Olsson, K.M., Pepke-Zaba, J., Quint, J.K., Radegran, G., Simonneau, G., Sitbon, O., Tonia, T., Toshner, M., Vachiery, J.L., Noordegraaf, A.V., Delcroix, M., Rosenkranz, S. and ESC/ERS Scientific Document Group. 2023. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 61, 2200879. Humbert, M., Sitbon, O., Chaouat, A., Bertocchi, M., Habib, G., Gressin, V., Yaici, A., Weitzenblum, E., Cordier, J.F., Chabot, F., Dromer, C., Pison, C., Reynaud-Gaubert, M., Haloun, A., Laurent, M., Hachulla, E. and Simonneau, G. 2006. Pulmonary hypertension in France: result from national registry. Am. J. Respir. Crit. Care Med. 173, 1023–1030. Ivy, D.D., Abman, S.H., Barst, R.J., Berger, R.M.F., Bonnet, D., Fleming, T.R., Haworth, S.G., Raj, J.U., Rosenzweig, E.B., Neick, I.S., Steinhorn, S.H. and Beghetti, M. 2013. Pediatric pulmonary hypertension. J. Am. Coll. Cardiol. 62, 117–126. Klinger, J.R., Abman, S.H. and Gladwin, M.T. 2013. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. 2013. Am. J. Respir. Crit. Care Med. 188(6), 639–646. Klinger, J.R. and Kadowitz, P.J. The nitric oxide pathway in pulmonary vascular disease. Am. J. Cardiol. 120, S71–S79. Li, L., Zhang, X., Li, X., Lv, C., Yu, H., Xu, M., Zhang, M., Fu, Y., Meng, H. and Zhou, J. 2016. TGF-β1 inhibits the apoptosis of pulmonary arterial smooth muscle cells and contributes to pulmonary vascular medial thickening via the PI3K/Akt pathway. Mol. Med. Rep. 13, 2751–2756. Meoli, D.F. and White, R.J. 2010. Endothelin-1 induces pulmonary but not aortic smooth muscle cell migration by activating ERK1/2 MAP kinase. Can. J. Physiol. Pharmacol. 88, 830–839. Mitchell, M.D., Laird, R.E., Brown, R.D. and Long, C.S. 2007. IL-1β stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am. J. Physiol. Heart Circ. Physiol. 292, H1139–H1147. Pilling, D., Vakil, V., Cox, N. and Gomer, R.H. 2015. TNF-α–stimulated fibroblasts secrete lumican to promote fibrocyte differentiation. Proc. Natl. Acad. Sci. 112(38), 11929–11934. Rieg, A.D., Suleiman, S., Anker, C., Verjans, E., Rossaint, R., Uhlig, S. and Martin, C. 2018. PDGF-BB regulates the pulmonary vascular tone: impact of prostaglandins, calcium, MAPK and PI3K/AKT/mTOR signalling and actin polymerisation in pulmonary veins of guinea pigs. Respir. Res. 19(1), 120–138. Rosenzweig, E.B., Abman, S.H., Adatia, I., Beghetti, M., Bonnet, D., Haworth, S., Ivy, D.D. and Berger, R.M.F. 2019. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur. Respir. J. 53, 1801916. Sakarin, S., Surachetpong, S.D. and Rungsipipat, A. 2020. The expression of proteins related to serotonin pathway in pulmonary arteries of dogs affected with pulmonary hypertension secondary to degenerative mitral valve disease. Front. Vet. Sci. 7, 612130. Savale, L., Tu, L., Rideau, D., Izziki, M., Maitre, B., Adnot, S. and Eddahibi, S. 2009. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res. 10, 6–18. Sweatt, A.J., Reddy, R., Rahaghi, F.N., Al-Naamani, N. and American Thoracic Society Pulmonary Circulation Assembly Early Career Working Group. 2021. What’s new in pulmonary hypertension clinical research: lessons from the best abstracts at the 2020 American Thoracic Society International Conference. Pulm. Circ. 11(3), 1–27. Takamura, N., Renaud, L., da Silveira, W.A. and Feghali-Bostwick, C. 2021. PDGF promotes dermal fibroblast activation via a novel mechanism mediated by signaling through MCHR1. Front. Immunol. 12, 745308. Wang, L., Yang, D., Tian, J., Gao, A., Shen, Y., Ren, X., Li, X., Jiang, G. and Dong, T. 2017. Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer. Oncotarget 8(16), 26323–26333. West, J.D., Carrier, E.J., Bloodworth, N.C., Schroer, A.K., Chen, P., Ryzhova, L.M., Gladson, S., Shay, S., Hutcheson, J.D. and Merryman, W.D. 2016. Serotonin 2B receptor antagonism prevents heritable pulmonary arterial hypertension. PLoS One 11(2), e0148657. Winter, M.P., Sharma, S., Altmann, J., Seidl, V., Panzenbock, A., Alimohammadi, A., Zelniker, T., Redwan, B., Nagel, F., Santer, D., Stieglbauer, A., Podesser, B., Sibilia, M., Helbich, T., Prager, G., Ilhan-Mutlu, A., Preusser, M. and Lang, I.M. 2020. Interruption of vascular endothelial growth factor receptor 2 signaling induces a proliferative pulmonary vasculopathy and pulmonary hypertension. Basic Res. Cardiol. 115(6), 58. Wu, S.T., Sun, G.H., Hsu, C.Y., Huang, C.S., Wu, Y.H., Wang, H.H. and Sun, K.H. 2011. Tumor necrosis factor-a induces epithelial –mesenchymal transition of renal cell carcinoma cells via a nuclear factor kappa B-independent mechanism. Exp. Biol. Med. 236, 1022–1029. Yoshida, T., Matsuura, K., Goya, S., Ma, D., Shimada, K., Kitpipatkun, P., Namiki, R., Uemura, A., Suzuki, K. and Tanaka, R. 2020. Metformin prevents the development of monocrotaline-induced pulmonary hypertension by decreasing serum levels of big endothelin-1. Exp. Ther. Med. 20(6), 149–157. Zahid, K.R., Raza, U., Chen, J., Raj, U.J. and Gou, D. 2020. Pathobiology of pulmonary artery hypertension: role of long non-coding RNAs. Cardiovas. Res. 116, 1937–1947. Zhao, T., Zhao, W., Chen, Y., Li, V.S., Meng, W. and Sun, Y. 2013. Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 304, H1719–H1726. | ||
| How to Cite this Article |
| Pubmed Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Progression of pulmonary arterial hypertension: A study in model. Open Vet. J.. 2024; 14(12): 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 Web Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Progression of pulmonary arterial hypertension: A study in model. https://www.openveterinaryjournal.com/?mno=225411 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.33 AMA (American Medical Association) Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Progression of pulmonary arterial hypertension: A study in model. Open Vet. J.. 2024; 14(12): 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 Vancouver/ICMJE Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Progression of pulmonary arterial hypertension: A study in model. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 Harvard Style Cahyono, A., Irwanto, . I., Rahman, . M. A. & Widjiati, . W. (2024) Progression of pulmonary arterial hypertension: A study in model. Open Vet. J., 14 (12), 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 Turabian Style Cahyono, Agus, Irwanto Irwanto, Mahrus A. Rahman, and Widjiati Widjiati. 2024. Progression of pulmonary arterial hypertension: A study in model. Open Veterinary Journal, 14 (12), 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 Chicago Style Cahyono, Agus, Irwanto Irwanto, Mahrus A. Rahman, and Widjiati Widjiati. "Progression of pulmonary arterial hypertension: A study in model." Open Veterinary Journal 14 (2024), 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 MLA (The Modern Language Association) Style Cahyono, Agus, Irwanto Irwanto, Mahrus A. Rahman, and Widjiati Widjiati. "Progression of pulmonary arterial hypertension: A study in model." Open Veterinary Journal 14.12 (2024), 3498-3504. Print. doi:10.5455/OVJ.2024.v14.i12.33 APA (American Psychological Association) Style Cahyono, A., Irwanto, . I., Rahman, . M. A. & Widjiati, . W. (2024) Progression of pulmonary arterial hypertension: A study in model. Open Veterinary Journal, 14 (12), 3498-3504. doi:10.5455/OVJ.2024.v14.i12.33 |