| Short Communication | ||
Open Vet. J.. 2025; 15(3): 1495-1504 Open Veterinary Journal, (2025), Vol. 15(3): 1495-1504 Short Communication Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primerHalida Rahmania1, Rien Larasati Arini1, Abdul Rohman1,2* and Rumiyati Rumiyati11Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Center of Excellence, Institute of Halal Industry and Systems (IHIS), Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Abdul Rohman. Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: abdulkimfar [at] gmail.com Submitted: 23/10/2024 Accepted: 18/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
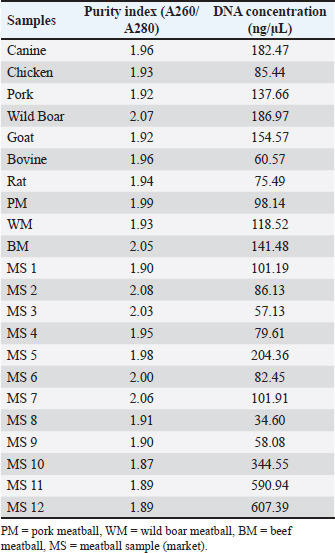
AbstractBackground: Indonesia, home to the world’s largest Muslim population, enforces Law No. 33 of 2014, mandating halal certification for all products in the country. However, meatballs, a popular Indonesian dish, frequently fall victim to adulteration with non-halal meats, such as pork and wild boar due to economic incentives. Aim: This study aims to develop an analytical technique employing real-time polymerase chain reaction (PCR) with specific primers to detect pork and wild boar meat within beef meatballs. Method: The research commenced with the design of specific primers for pork and wild boar DNA via IDT software. Subsequent phases encompassed DNA isolation, specificity, linearity, limit of detection, efficiency, and repeatability assessments to evaluate the method. Results: The D-Loop 539 and D-Loop 409 primers successfully detected the pork and wild boar DNA in both raw meat and meatballs, with optimal annealing temperatures of 61.2°C and 60.6°C, respectively. The D-loop 539 primer amplified pork DNA and pork-infused meatballs, boasting a detection threshold of 0.1 ng, with a coefficient of variation (CV) of 0.25%. Similarly, the D-loop 409 primer successfully amplified wild boar DNA and meatball sample, showcasing detection limits of 1 and 0.1 ng, accompanied by CV values of 0.37% and 0.44%. All primers passed the critical PCR validation tests, making them suitable for analysis of meatball samples across 12 sub-districts in Sleman-Yogyakarta. The observed results showed negative amplification signals for both pork and wild boar components. Conclusion: Using the D-Loop 539 and D-Loop 409 primers, this method is capable to detect the presence of pork and wild boar meat within beef meatballs This research contributes to the authentication of halal products in alignment with the provisions of Law No. 33 of 2014, thereby endorsing consumer confidence in product integrity. Keywords: D-loop mitochondria, Real-time PCR, Halal, Adulteration, Meatballs. IntroductionIndonesia has a big Muslim community, reaching around 229 million individuals, or 87% of the Indonesian population, considered the world’s largest Muslim population and nearly 13% of the total global Muslim population (Statista Research Department, 2023). Due to the high number of Muslim population, the demand for Halal food and products is significant. As the mixture of non-halal ingredients in food can become a serious problem in society, thus halal authentication is truly essential for consumers and researchers to fulfill the need of obeying the religious laws and beliefs (Subara and Jaswir, 2018). Based on Indonesian Law No. 33 (2014), all products circulating in Indonesia must have a halal certificate. This has an important meaning because halal products are products that meet the requirements of Islamic law. In the case of counterfeiting beef with non-halal meat, article 18 also prohibits using materials from forbidden animals, such as pigs, blood, or animals that are slaughtered not in accordance with the Shari’a. Meat-based foods such as meatballs are highly popular in Indonesia (Guntarti et al., 2020). However, meatballs are often mixed with non-halal meat such as wild boar and pork, for economic purposes, as the price of beef in the market is much higher than other meat. Amidst this condition, halal authentication is crucial to maintain product integrity. Finding a proper method to quickly and accurately detect the adulteration of halal food is undeniably important. Several methods have been reported to evaluate the authentication of halal foods. Fourier transform-infrared spectroscopy and differential scanning calorimetry are some of the examples that are commonly used to analyze the suspected samples (Guntarti et al., 2017). However, these methods have some limitations as further confirmation is needed to highly certain the sample composition. Molecular biology methods have also been developed to detect non-halal components in food, such as Enzyme-Linked Immunosorbent Assay (ELISA) and polymerase chain reaction (PCR). Unfortunately, the ELISA method might not be suitable for processed products that experience high heating due to protein denaturation (Perestam et al., 2017). The PCR method is the main choice for the analysis of types of meat in food products since DNA can maintain its stability at high temperatures (Kumar et al., 2015). In this case, mitochondrial DNA (mtDNA)-based PCR via the D-Loop region was chosen due to the high level of polymorphism in mtDNA, allowing to differentiate animal species effectively (Fajardo et al., 2010; Pereira et al., 2011). Moreover, mitochondrial D-Loops are more resistant to degradation due to heating than nuclear DNA (Foran, 2006). Real-time PCR, as an extension of PCR, provides rapid quantitative analysis (Tanabe et al., 2007; Che Man et al., 2012). By using this method, it is expected that high accuracy and specificity can be achieved so that the process of food products authentication can be obtained to ensure halalness and protect consumers from counterfeiting. Materials and MethodsDNA isolationDNA extraction was conducted on seven meat types, specifically canine (Canis domesticus), chicken (Gallus gallus), pork (Sus scrofa domesticus), boar (Sus scrofa), goat (Capra hircus), bovine (Bos taurus), and rat (Rattus norvegicus). The isolated DNA will later be used for the initial stage of the primer specificity test using real-time PCR. In addition, DNA isolation was also carried out on 12 samples of meatballs on the market. DNA was isolated using a silica gel membrane-based isolation method by FavorPrepTM Tissue Genomic DNA Extraction Mini Kit (Favorgen, Taiwan), which included the cell lysis process, protein degradation, DNA binding with a silica matrix, and contaminant elimination. The isolated DNA then later quantified by NanoQuant PlateTM in conjunction with TE buffer as the blank. The UV spectrophotometer automatically recorded readings at λ 230, 260, and 280 nm to evaluate the A260/A280 ratios, coupled with concentrations expressed in ng/µl. The purity index was determined by the A260/A280 ratio, which is considered good within the range of 1.8 to 2.0; as the ratio value of less than 1.8 indicates the presence of protein contaminants in DNA isolates, while more than 2.0 suggested the presence of RNA contaminants (Olson and Morrow, 2012). Primer designingThe DNA primers were designed using the Integrated DNA Technologies software (IDT, California, USA). Design criteria encompassed essential parameters such as %GC content, product length, primary length, melting temperature (Tm), self-dimer potential, and hairpin formation. D-Loop fragment DNA sequences from Sus scrofa and Sus scrofa domesticus mtDNA were sourced from the National Center for Biotechnology Information (NCBI) official website (http://www.ncbi.nlm.nih.gov). The optimal length for an ideal primer was expected to be in range between 18 and 30 oligonucleotides, as this range considered sufficient for facilitating primer binding to the template at the annealing temperature (Ta), ultimately resulting in the production of a specific sequence. The Tm of forward and reverse primers typically falls within 2°C to 4°C above 60°C. This Tm value will later guide the determination of the Ta during PCR. The selected primer pairs were then subjected to a Basic Local Alignment Search Tool-nucleotide (BLASTn) or BLAST-suite analysis against mtDNA sequences from species S. scrofa, S. domesticus, and other comparative animals, including Canis lupus familiaris (taxid: 9615), Gallus gallus (taxid: 9031), Capra hircus (taxid: 9925), Rattus norvegicus (taxid: 10116), and Bos taurus (taxid: 9913). The outcome of the BLAST analysis led to the identification of the most specific primer pair. Analysis using real-time PCRPCR amplification of the target DNA was conducted using the CFX96 PCR system (Biorad, USA) in a single run with gradient temperature settings, allocating each primer to a distinct row. The analysis employed a 10 µl reaction mixture containing SensiFastTM SYBR, comprising 5 µl of 2x SensiFast SYBR No-Rox Kit (Meridian Biosciences, USA), 0.4 µl of forward primer and 0.4 µl of reverse primer (both at a final concentration of 0.4 µM), 1 µl of DNA at a concentration of 10 ng/µl, and 3.2 µl of nuclease-free water. The process was initiated with pre-denaturation at 95°C for 3 minutes. The Ta was optimized within the range of 51.2°C to 61.2°C, held for 10 seconds at each temperature. The elongation phase ensued at 72°C for 20 seconds. The reaction was carried out in 25 cycles of denaturation at the same temperature (95°C) for 5 seconds according to the temperature program. Validation of quantitative PCR (qPCR) with species-specific primerThe efficacy of the designed primer was confirmed through the evaluation of validation parameters encompassing specificity, repeatability, linearity, efficiency value, and sensitivity (limit of detection/LoD). The acceptance criteria for each parameter were established in accordance with the standards set by Codex Alimentarius (FAO, 2010). Subsequently, the validated methodology was employed for the analysis of commercially available meatball products in Yogyakarta, Indonesia. Results and DiscussionDNA isolationTo determine the potential adulteration of meatballs, the DNA of standard for raw meats and market samples were first isolated. We focused on mtDNA due to its much higher copy number than nuclear DNA (Wiesner et al., 1992; Zhang et al., 2020). As a result, the DNA was successfully isolated from the samples (including the standard of seven meat types and 12 market samples), with a concentration ranging from 34.60 to 607.39 ng/µl. All successfully isolated DNA were considered to have a good purity, with a range from 1.87 to 2.08, respectively. This high purity of the DNA market samples confirmed that the quality of DNA was not compromised by the heat processing in meatball making process. The detailed of purity index and DNA concentration of collected samples is shown in Table 1. Table 1. Evaluation of the concentration and purity of extracted DNA from raw beef and commercial meatball samples.
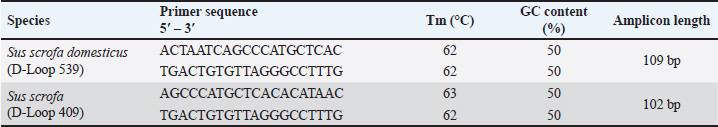
Table 2. Primers of D-loop 539 and 409 targeting D-loop fragment on mtDNA of S. scrofa and S. domesticus.
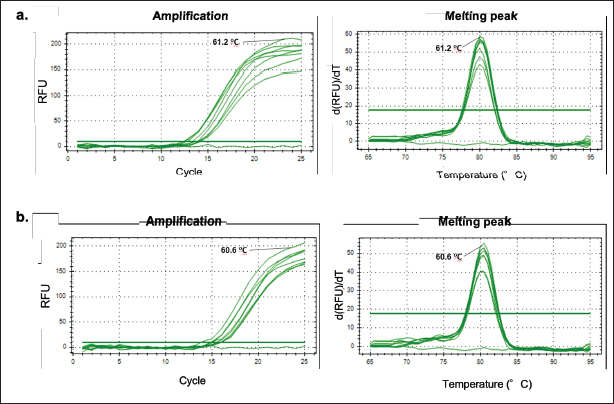
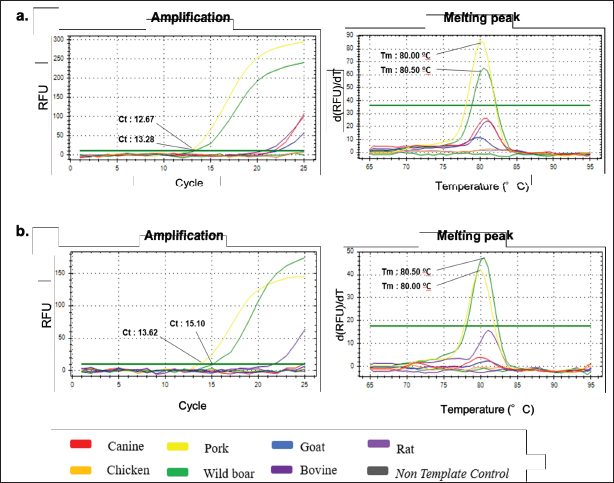
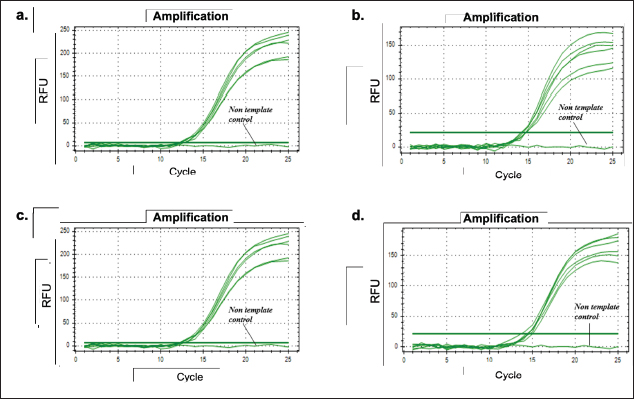
Primer designThe process of designing primers was conducted through computational methods to obtain specific primer pairs for the D-Loop sequence of two distinct subspecies: pork (Sus scrofa domesticus) taxid: 9,825 and wild boar (Sus scrofa) taxid: 9,823. This BLASTn analysis was carried out using the official NCBI website and its provided nucleotide software. The sequences of primer pairs for each species are shown in Table 2, with the Tm around 62°C–63°C. Annealing temperature (Ta) optimizationThe primer pair, namely D-Loop 409 (for boar) and D-Loop 539 (for pork), which underwent initial in-silico testing, was subsequently fine-tuned for optimal primer Ta within a specified range of 51.2°C–61.2°C. Typically, the appropriate Ta usually obtains by reducing the Tm value by 3°C–5°C (Herman and Rosim, 2018). The results of the primary optimization obtained the optimum sticking temperature at 61.2oC for pork and 60.6oC for wild boars, as these temperatures produced the maximum intensity while producing the lowest quantification cycle (Cq) value (Fig. 1). This temperature will later be used in the following validation procedure. Method validationSpecificity Specification tests are carried out to determine whether the designed primers are specific for the DNA of the species being tested at the optimal temperature for each primer. This test was performed by amplified on all DNA isolates from fresh tissue, namely canine (Canis familiaris), chicken (Gallus gallus), pork (Sus scrofa domesticus), wild boar (Sus scrofa), goat (Capra hircus), bovine (Bos taurus), and rat (Rattus novergicus). The result of each primer amplification to each species can be seen in Figure 2. Primer D-Loop 539 (designed for pork DNA) exhibited the highest peak intensity in pork DNA samples, registering a relative fluorescence units (RFU) value of 86.23 at an Ta of 61.2°C. This resulted in a cycle threshold (Ct) value of 12.67 and a Tm of 80.00°C. However, D-Loop 539 primer also displayed amplification in wild boar DNA, albeit with a lower RFU value of 65.30, a Ct value of 13.28, and a Tm of 80.50°C (Fig. 3a). A similar scenario was observed with the D-Loop 409 primer (designed for boar DNA) (Fig. 3b). This primer demonstrated the highest peak height in boar DNA but still exhibited amplification in pork DNA.
Fig. 1. Amplification curve of annealing temperature optimization at different temperatures. (a) D-Loop 539 primer (for pork) is optimum at 61.2°C; (b) D-Loop 409 primer (for boar) is optimum at a temperature of 60.6°C.
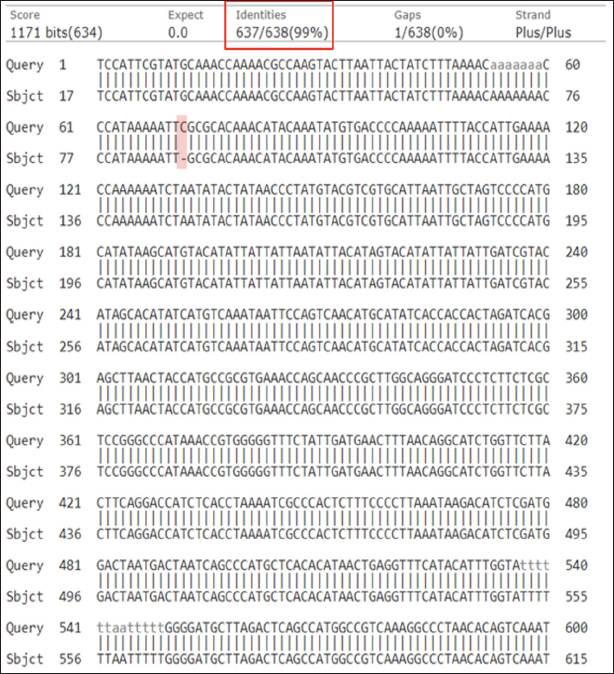
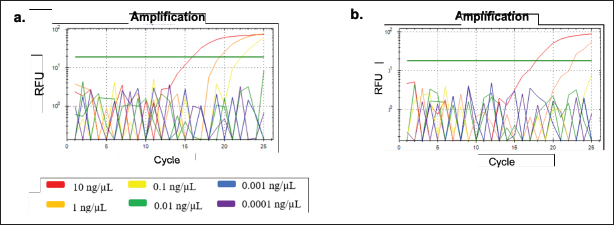
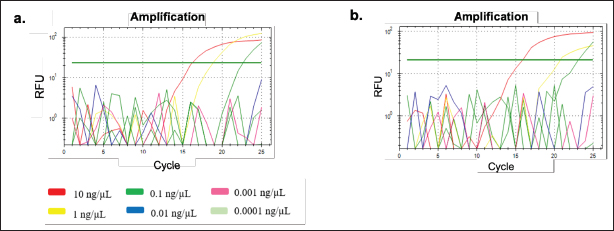
Fig. 2. Amplification curve and melting peak specificity test of primers against six other species. (a) D-Loop 539 (pork); (B) D-Loop 409 (wild boar). In the case of D-Loop 409, the primary RFU value for wild boar was 47.44 at an annealing temperature of 60.6°C, with a Ct value of 12.67 and Tm of 80.00°C. For pork DNA, the primary RFU value was slightly lower at 42.13, maintaining the same Ct values and Tm of 12.67 and 80.00°C, respectively. This phenomenon can be attributed to the fact that wild boars and pork belong to the same species, “Sus scrofa.” Wild boars, also known as S. scrofa, serve as the ancestors of domestic pigs (Sus scrofa domesticus) (Payne et al., 2000). The genetic sequences of wild boars and pork are highly similar, sharing about 99% resemblance, with only minor variations in nucleotide bases (Fig. 3). Nevertheless, the two primers, D-Loop 539 and 409, still managed to differentiate between pork and wild boar DNA. This distinction is particularly valuable concerning dietary laws, as it can detect the presence of wild boar within the same biological family. This capability ensures the identification of any type of wild boar or pork present in processed food items using the D-Loop 539 and 409 primers. Sensitivity A sensitivity assessment was conducted involving six sets of DNA dilutions derived from fresh meat and 100% meatballs product of pork and wild boar. These dilutions were prepared at concentrations of 10, 1, 0.1, 0.01, 0.001, and 0.0001 ng/µl. The objective of this study was to establish the lowest detectable DNA concentration within a sample, which is often referred to as the LoD (Hübner et al., 2001; FAO, 2010). The sensitivity test for fresh meat can be seen in Figure 4, while for meatballs, it is shown in Figure 5, suggested that the LoD of primer D-Loop 539 on DNA of fresh meat and pork meatballs is 0.1 ng/µl while the primer D-Loop 409 on fresh meat and wild boar meatballs is 1 ng/µl.
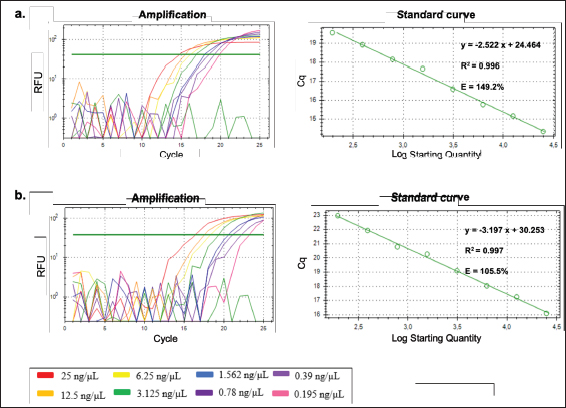
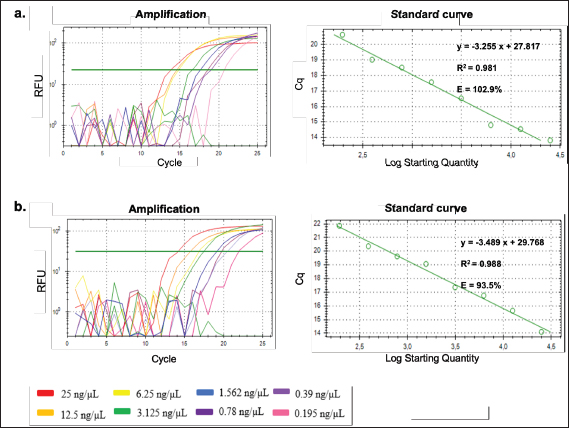
Fig. 3. Alignment results of pork and wild boar DNA sequences with NCBI. Efficiency assessments of each D-Loop 539 and D-Loop 409 DNA primers were conducted using 8 serial dilutions of DNA from pork, wild boar, and 100% pork meatball, and 100% wild boar meatball samples at concentrations of 100, 25, 12.5, 6.25, 3.125, 1.562, 0.78, 0.39, and 0.195 ng/µl. As shown in Figure 6a, the regression curve of Cq values against a logarithm of DNA concentration extracted from fresh meat pork (D-Loop 539 primer) exhibited an equation with an R-squared value of 0.996, a slope of −2.522, a y-intercept of 25.464, and an efficiency value of 149.2%. Similarly, D-Loop 409 primer demonstrates an equation with an R-squared value of 0.997, a slope of −3.197, a y-intercept of 30.253, and an efficiency value of 105.5% (Fig. 6b).
Fig. 4. Amplification at different concentrations of fresh meat DNA for sensitivity testing using primers (a) D-Loop 539 (pork) and (b) D-Loop 409 (wild boar).
Fig. 5. Amplification at different concentrations of meatball DNA for sensitivity testing using primers (a) D-Loop 539 (pork) and (b) D-Loop 409 (wild boar).
Fig. 6. Amplification curve and melting peak from efficiency test by serial dilution of fresh meat of pork DNA template using primer D-Loop 539 (a) and wild boar DNA template using primer D-Loop 409 (b).
Fig. 7. Amplification curve and melting peak from efficiency test by serial dilution of pork meatball DNA template using primer D-Loop 539 (a), and wild boar meatball DNA template using primer D-Loop 409 (b).
Fig. 8. Amplification using D-Loop 539 primer for repeatability analysis for pork meat (a) and pork meatball (b) and D-loop 443 primer for repeatability analysis for boar meat (c) and boar meatball (d). Table 3. Repeatability test results for the repeatability of amplification responses to 100% fresh meat and meatball DNA.
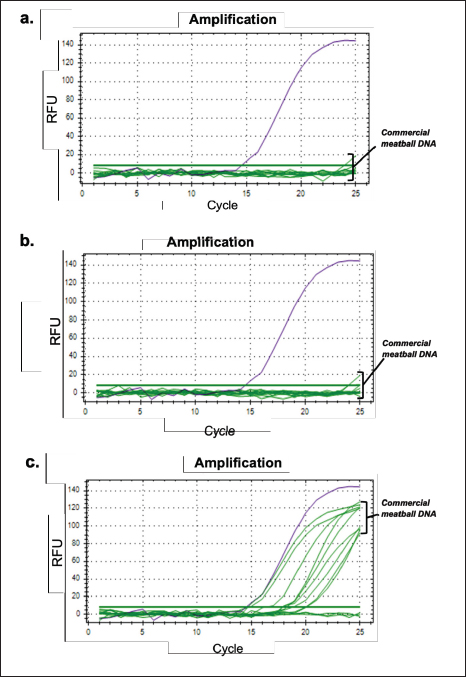
On the other hand, the regression curve for the D-Loop 539 primer against 100% pork meatball samples yields an equation with an R-squared value of 0.981, a slope of −3.255, a y-intercept of 27.817, and an efficiency value of 102.8% (Fig. 7a). Conversely, D-Loop 409 primer against 100% wild boar meatball samples generates an equation with an R-squared value of 0.988, a slope of −3.489, a y-intercept of 29.768, and an efficiency value of 93.5% (Fig. 7b). Most cases of each primer’s R-squared value meet the criteria for both qualitative and quantitative assessments in the real-time PCR method. Moreover, the efficiency values derived from D-Loop 539 and 409 primers for meatball samples generally satisfy recommended standards. However, there is an exception with the efficiency value of D-Loop 539 primer against fresh meat samples (pork), which is unusually high. Such a high-efficiency value suggests the presence of an inhibitor affecting the replication of the target DNA during the reaction. The primary focus of this study leans toward qualitative analysis. This approach is taken because even if the quantitative test outcomes do not fully comply, particularly the efficiency test, it may not significantly impact the overall findings. The primary objective is to accurately determine the presence of non-halal species in food products, like meatballs, to adhere to halal regulations, as halal regulation has zero tolerance on the presence of any traceable non-halal species. Repeatability In this study, a repeatability test was conducted using a DNA template concentration of 10 ng. The evaluation of repeatability was based on the coefficient of variation (CV), which was calculated from 6 tests performed on each DNA sample sourced from both fresh meat and meatballs, all at the specified concentration. For the D-Loop 539 primer’s amplification response to DNA isolates from pork meat and pork meatballs (Fig. 8a and b), the resulting CV values were both 0.25%. Similarly, tests performed with the D-Loop 409 primer against DNA isolates from boar meat and boar meatballs (Fig. 8c and d) yielded CV values of 0.37% and 0.44%, respectively. Both CV values from both fresh meat DNA and 100% meatball DNA met the recommended criteria for using the PCR method, namely CV≤25% (FAO, 2010; Hübner et al., 2001). The resulting Ct, SD, and CV data are shown in Table 3. Analysis of meatball sampleThe validated qPCR method using primers D-Loop 539 and 409 was used to detect the presence of pork and wild boar in 12 samples of commercial meatballs from Sleman-Yogyakarta. The experiment used a negative control (without template control, NTC) and a positive control (beef meatball DNA). Analysis was carried out at the optimal condition that was previously optimized. In addition, analysis was also carried out using a beef primer (D-Loop 922) with a similar condition developed in the previous study. The results showed that 12 meatball samples from 12 sub-districts in Sleman-Yogyakarta tested using primers D-Loop 539 and D-Loop 409 gave a negative amplification response against pork and wild boar (Fig. 9a and b). The amplification response was only positive for cattle using primer D-Loop 922, which is shown in Figure 9c.
Fig. 9. Amplification response on two samples of commercial meatballs with primer (a) D-Loop 539 (pork); (b) D-Loop 409 (wild boar); and (c) D-Loop 922 (cattle). ConclusionThis study succeeded in designing and validating two specific primers targeting the D-loop region of mtDNA for different species for qualitative purpose. The D-Loop 539 primer, which was optimized at 61.2°C, effectively amplified 0.1 ng of pork DNA and 100% of pork meatball DNA with a CV value of 0.25%. The D-Loop 409 primer, with an attachment temperature of 60.6°C, efficiently amplified 1 ng of wild boar meat DNA and 0.1 ng of 100% pork meatball DNA, producing CV values of 0.37% and 0.44% for DNA wild boar meat and 0.44% 100% wild boar meatball DNA, respectively. Both primers met the validation criteria for the real-time PCR method. In testing on 12 meatball samples from various sub-districts in Sleman-Yogyakarta using primers D-Loop 539 and D-Loop 409, no amplification response was seen in pork and wild boar. Positive amplification was only detected in cattle using the D-Loop 922 primer. This method has been tested and can be used as a standard method to analyze the presence of pork and wild boar in food products to support halal authentication. AcknowledgmentThe authors would thank to Universitas Gadjah Mada, especially Faculty of Pharmacy for giving great support for conducting this research. Conflict of interestThe authors declare that they have no conflict of interest. FundingThis research received no specific grant. Authors’ contributionConceptualization—A.R., R. Methodology—H.R., A.R. Validation—H.R., R.L.A., A.R. Resources—A.R., R. Data curation—H.R. R.L.A., Writing manuscript—H.R., R.L.A. All authors reviewed the manuscript. Data availabilityAll data underlying the results are available as part of the article and no additional source data are required. ReferencesChe Man, Y.B., Mustafa, S., Khairil Mokhtar, N.F., Nordin, R. and Sazili, A.Q. 2012. Porcine-specific polymerase chain reaction assay based on mitochondrial D-loop gene for identification of pork in raw meat. Int. J. Food Prop. 15(1), 134–144; doi: 10.1080/10942911003754692 Fajardo, V., González, I., Rojas, M., García, T. and Martín, R. 2010. A review of current PCR-based methodologies for authentication of meats from game animal species. Trends Food Sci. Technol. 21, 408–421; doi: 10.1016/j.tifs.2010.06.002 FAO. 2010. Guidelines on performance criteria and validatin of methods for detection, identification, and quantification of specific DNA sequences and specific proteins in foods: CAC/GL 74-2010. Rome, Italy: FAO. Available via https://www.fao.org/fileadmin/user_upload/gmfp/resources/CXG_074e.pdf Foran, D.R. 2006. Relative degradation of nuclear and mitochondrial DNA: an experimental approach. J. Forensic Sci. 51, 766–770; doi: 10.1111/j.1556-4029.2006.00176.x Guntarti, A., Ahda, M., Kusbandari, A. and Atmadja, D. 2020. Aplikasi metode FTIR kombinasi kemometrika untuk analisis lemak daging tikus pada nugget ayam. JHSR 1, 1–8; doi: 10.12928/jhsr.v1i1.1837 Guntarti, A., Rohman, A., Martono, S. and Yuswanto, A. 2017. Authentication of wild boar meat in meatball formulation using differential scanning calorimetry and chemometrics. J. Food. Pharm. Sci. 5(1), 8–12; doi: 10.14499/jfps Herman, M.N. and Roslim, D.I. 2018. Optimizing temperature annealing for four primary RAPD in Mungbean (Vigna radiata L.). Jurnal Dinamika Pertanian. 34(1), 41–46. Hübner, P., Waiblinger, H.U., Pietsch, K. and Brodmann, P. 2001. Validation of PCR methods for quantitation of genetically modified plants in food. J. AOAC Int. 84(6), 1855–1864; doi:10.1093/jaoac/84.6.1855 Indonesian Law No. 33 year 2014 on Halal Product Assurance. 2014. Jakarta: Government of Indonesia. https://peraturan.bpk.go.id/Details/38709/uu-no-33-tahun-2014 Kumar, A., Kumar, R.R., Sharma, B.D., Gokulakrishnan, P., Mendiratta, S.K. and Sharma, D. 2015. Identification of species origin of meat and meat products on the DNA basis: a review. Crit. Rev. Food Sci. Nutr. 55, 1340–1351; doi: 10.1080/10408398.2012.693978 Olson, N.D. and Morrow, J.B. 2012. DNA extract characterization process for microbial detection methods development and validation. BMC Res. Notes. 5, 668; doi: 10.1186/1756-0500-5-668 Payne, J., Francis, C.M., Phillips, K. and Kartikasari, S.N. 2000. Panduan Lapangan Mamalia di Kalimantan, Sabah, Sarawak and Brunei Darussalam. Malaysia: The Sabah Society. Pereira, J.C, Chaves, R., Bastos, E., Leitão, A. and Guedes-Pinto, H. 2011. An efficient method for genomic DNA extraction from different molluscs species. Int. J. Mol. Sci. 12, 8086–8095; doi: 10.3390/ijms12118086 Perestam, A.T., Fujisaki, K.K., Nava, O. and Hellberg, R.S. 2017. Comparison of real-time PCR and ELISA-based methods for the detection of beef and pork in processed meat products. Food Control. 71, 346–352; doi: 10.1016/j.foodcont.2016.07.017 Statista Research Department. 2023. World population review: share of the Muslim population in Southeast Asia in 2023, by Country. Statista. Available via https://www.statista.com/statistics/1113906/southeast-asia-muslim-population-forecasted-share-by-country/ (Accessed 4 January 2024) Subara, D. and Jaswir, I. 2018. Gold nanoparticles: synthesis and application for halal authentication in meat and meat products. Int. J. Adv. Sci. Eng. Inf. Techno. 8, 1633; doi: 10.18517/ijaseit.8.4-2.7055 Tanabe, S., Hase, M., Yano, T., Sato, M., Fujimura, T. and Akiyama, H. 2007. A real-time quantitative PCR detection method for pork, chicken, beef, mutton, and horseflesh in foods. Biosci. Biotechnol. Biochem. 71, 3131–3135; doi: 10.1271/bbb.70683 Wiesner, R.J., Ruegg, J.C. and Morano, I. 1992. Counting target molecules by exponential polymerase chain reaction, copy number of mitochondrial DNA in rat tissues. Biochem. Biophys. Res. Commun. 183(2), 553–559; doi: 10.1016/0006-291X(92)90517-O Zhang, Y., Qu, Q., Rao, M., Zhang, N., Zhao, Y. and Tao, F. 2020. Simultaneous identification of animal-derived components in meats using highThroughput sequencing in combination with a custom-built mitochondrial genome database. Sci. Rep. 10, 8965; doi: 10.1038/s41598-020-65724-4 | ||
| How to Cite this Article |
| Pubmed Style Rahmania H, Arini RL, Rohman A, Rumiyati R. Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Vet. J.. 2025; 15(3): 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 Web Style Rahmania H, Arini RL, Rohman A, Rumiyati R. Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. https://www.openveterinaryjournal.com/?mno=225798 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i3.39 AMA (American Medical Association) Style Rahmania H, Arini RL, Rohman A, Rumiyati R. Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Vet. J.. 2025; 15(3): 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 Vancouver/ICMJE Style Rahmania H, Arini RL, Rohman A, Rumiyati R. Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Vet. J.. (2025), [cited January 25, 2026]; 15(3): 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 Harvard Style Rahmania, H., Arini, . R. L., Rohman, . A. & Rumiyati, . R. (2025) Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Vet. J., 15 (3), 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 Turabian Style Rahmania, Halida, Rien Larasati Arini, Abdul Rohman, and Rumiyati Rumiyati. 2025. Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Veterinary Journal, 15 (3), 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 Chicago Style Rahmania, Halida, Rien Larasati Arini, Abdul Rohman, and Rumiyati Rumiyati. "Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer." Open Veterinary Journal 15 (2025), 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 MLA (The Modern Language Association) Style Rahmania, Halida, Rien Larasati Arini, Abdul Rohman, and Rumiyati Rumiyati. "Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer." Open Veterinary Journal 15.3 (2025), 1495-1504. Print. doi:10.5455/OVJ.2025.v15.i3.39 APA (American Psychological Association) Style Rahmania, H., Arini, . R. L., Rohman, . A. & Rumiyati, . R. (2025) Analysis of non-halal meat adulteration in beef meatball using real-time PCR and mitochondrial D-loop gen-specific primer. Open Veterinary Journal, 15 (3), 1495-1504. doi:10.5455/OVJ.2025.v15.i3.39 |