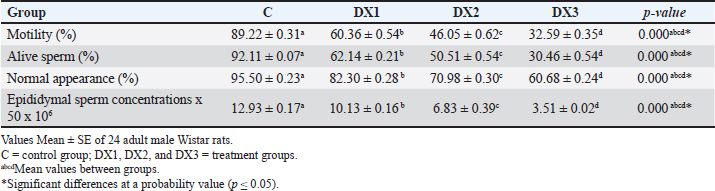
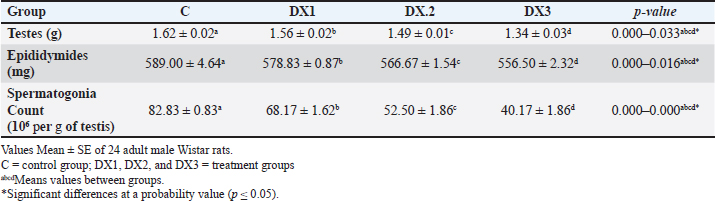
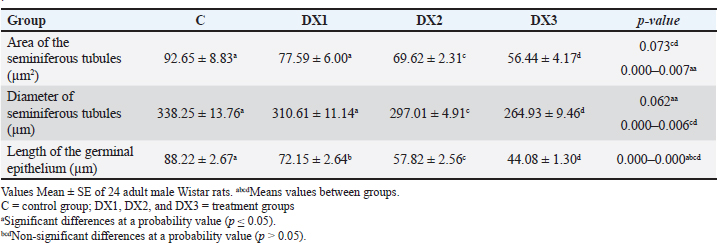
| Research Article | ||
Open Vet. J.. 2025; 15(3): 1140-1149 Open Veterinary Journal, (2025), Vol. 15(3): 1140-1149 Research Article Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar ratsMuntadher Salman Ashour1*, Ashwaq Ahmed Hussein1, Entekhab Hameed Abed Al-Shuwaili1, Amer M. Hussin2, and Harith Humadi Khalaf31Department of Biology, College of Education for Pure Sciences Ibn Al-Haitham, University of Baghdad, Baghdad, Iraq 2Department of Health and Medical Laboratory Techniques, Technical College of Health and Medicine, Imam Ja’afar Al-Sadiq University, Baghdad, Iraq 3Department of Anatomy and Histology, College of Veterinary Medicine, University of Fallujah, Fallujah, Iraq *Corresponding Author: Muntadher Salman Ashour. Department of Biology, College of Education for Pure Sciences Ibn Al-Haitham, University of Baghdad, Baghdad, Iraq. Email: muntadher.s.a [at] ihcoedu.uobaghdad.edu.iq Submitted: 07/11/2024 Accepted: 04/02/2025 Published: 31/03/2025 © 2025 Open Veterinary Journal
AbstractBackground: Chemotherapy drugs, such as Docetaxel, which are crucial for treating cancer, frequently cause unknowingly damage to healthy tissues. Aim: To investigate the histomorphological effects of docetaxel chemotherapy on certain parameters in testes and epididymides. Method: For this purpose, 24 Wistar Albino rats were divided randomly into one control group and three treatment groups. The treatment groups were administered 2.5, 5, and 10 mg/kg of the drug. The experiment lasted for 28 days. The weights of the testes and epididymides were measured. The testicular and epididymal samples were subjected to histological analysis and were examined under a light microscope. Result: The results revealed that the dose of DX1 (2.5 mg/kg) of docetaxel had no adverse effect on the process of spermatogenesis; however, the highest doses of DX2 and DX3 penetrated the blood-testis barrier and disrupted the structural and functional system of spermatogenesis. The results of the present study indicate that the highest dose of docetaxel leads to adverse effects on testes, epididymides, and their fertility parameters. In addition, the testicular tissues of DX2 and DX3 displayed adverse histomorphological changes. The process of spermatogenesis was interrupted, and a progressive decrease in the height of the germinal epithelium was observed. On this basis, the weight of the testes and epididymides decreased. Conclusion: A dose of 2.5 mg/kg of Docetaxel was the drug of choice for chemotherapy. Further studies are needed to investigate the long-term effects of Docetaxel on male fertility. Keywords: Docetaxel, Testis, Epididymis, Histomorphology, Wistar rat, Male fertility. IntroductionThe seminiferous tubules comprise the bulk of the testes (King, 2023; Khalaf et al., 2024). They are essential for spermatogenesis, the production of sperm. Sperms produce spermatozoa through a complicated developmental process. Sperm cells grow with structural support from Sertoli cells. A complex network of blood arteries, lymphatic cells, neurons, and hormone-secreting cells surround seminiferous tubules. Spermatogenesis requires nutrition, oxygen, and hormones, which are transported by interstitial spaces. Leydig cells in the interstitial compartment synthesize testosterone, the primary male sex hormone for male reproductive tissue development and secondary sexual features. These tubules are embedded in sparse interstitial tissue surrounded by a thin layer of contractile myoid cells that play multiple roles (Zhao et al., 2021). These cells support the tubules and maintain their shape. At various stages of spermatogenesis, sperm and fluids are transported inside tubules. Additionally, the contractile properties of these cells facilitate the movement of sperm from the seminiferous tubules to the epididymis, where sperm matures and is stored (Rogers, 2010; Zhao et al., 2021). Male fertility is the ability to produce viable offspring and the high ability to provide semen and sperm traits, in addition to multi-factorial characteristics including (immunological, hormonal, genetics, nutritional, seasonal, and behavioral) (Hussin and Al-Haaik, 2018; Leslie et al., 2025); however, the decreased capacity of viable offspring production may lead to infertility (El-Newary et al., 2022). Infertility is the most critical global problem; furthermore, different reasons lead to decreased fertility rates (Al Chalabi et al., 2020). Infertility is estimated to occur in 8%–12% of couples worldwide, with a male component being the primary contributing reason in approximately 50% of couples. The causes of male subfertility can vary significantly and may be attributed to inherited, acquired, or idiopathic factors that hinder the process of spermatogenesis (Agarwal et al., 2021). Generally, epididymal sperm counts serve as a sensitive and frequently used measure to assess the impact of male reproductive toxicants on the epididymis and testicles (Wang, 2003; Khalaf et al., 2022). Chemotherapy is widely used for the treatment of various cancers. However, side effects can cause severe complications, including infertility. Infertility is a significant issue for patients with cancer, as chemotherapy treatment protocols may lead to long-term health implications (Abdullahi, 2022). Furthermore, chemotherapy drugs may cause metabolic and hormonal alterations due to stress, malnutrition, and fever (Saruhan, 2020). Docetaxel is a chemotherapy drug commonly used to treat various cancers, including breast, prostate, lung, and ovarian (Imran et al., 2020). Moreover, previous studies have shown that docetaxel altered the expression of apoptosis-related genes in mRNA expression in mouse genital organs (Lopes et al., 2014). Docetaxel caused testes damage due to its systemic toxicity (Yardım et al., 2021). Chemotherapeutic drugs are one of the common causes of infertility, such as Filgrastim (Tuimah Alabedi et al., 2021) and Cadmium Chloride (Ali Hameed et al., 2022). Docetaxel commonly damages the male reproductive system, leading to decreased epididymal sperm counts, alive sperm, motility, and abnormal sperm morphology (Zhang et al., 2014). However, the specific effects of Docetaxel on epididymal sperm quality and histological changes in testicular tissue in Wistar rats are not fully understood. This study aimed to focus on the adverse effects of docetaxel on some parameters of the testes and epididymis and to observe changes in the histological characteristics of the testes in Wistar Albino rats. The objective of this study was to better understand the precise changes in reproductive organs caused by docetaxel treatment. This will help us to identify the various pathways contributing to male infertility caused by chemotherapy. This understanding has implications for patients with cancer receiving treatment and for broader initiatives focused on protecting reproductive health. MethodsExperimental designThe present experiment utilized 24 healthy adult male Wistar Albino rats. These rats were purchased from a research center in Najaf, Iraq. The rats were housed in hygienic cages in an animal facility. All rats were exposed to a 12-hours light/dark cycle and were provided by standard food and water ad libitum. Group allocation The animals were divided randomly into four groups (six each), one control (C), and three treatment groups (DX.1, DX.2, and DX.3). The control group received intraperitoneally normal saline. A single dose of (2.5, 5, and 10) mg/kg of Docetaxel was administered to each group. DX dosages were selected according to Sandström et al. (1999); Engels et al. (2004). Experimental duration The experiment lasted for 28 days to evaluate the effect of docetaxel on the reproductive parameters of the testes and epididymis. Ethical approval All experiments were performed according to the guidelines of the Implementing Health Research at the Institutions of the Ministry of Health, Iraq (2018), the WHO Code of Conduct for Responsible Research (2017), and Principles of Human Experimental Techniques (Russell and Burch, 1959), for the Use of Experimental Animals (EC-1, 4-3-2024). MeasurementsEvaluation of sperm parameters At the end of the treatment period, the rats were anesthetized with xylazine and ketamine (5 and 50 mg/kg). The epididymal sperm parameters (Table 1) were studied using standard methods (Al Chalabi et al., 2020). After euthanasia, the cauda epididymides were promptly cut into two halves to discharge the epididymal contents into a 35-mm Petri dish containing 2 ml of RPMI-1640 (pH 7.4). A drop of fluid was placed on a slide to measure sperm motility. The percentage of viable and morphologically aberrant sperm was determined by counting sperm from five random fields. The average was multiplied by 106 according to Tegelenbosch and de Rooij (1993); Wang (2003). To preserve viable sperm activity, all experimental instruments, containers, and surfaces were maintained at 37°C. Sperm motility and numbers were measured by mincing 100 mg of the caudal epididymis in 1 ml of RPMI-1640. A drop of the mixed sample was applied to a slide under a cover slip to measure sperm motility. The index was calculated by counting motile and immotile spermatozoa per unit area. Epididymal counts were also performed and were expressed as millions/ml of suspension. Sperm vitality was assessed by mixing 40 μl of freshly liquefied semen with 10 μl of eosin-nigrosin (Merck-Germany). One drop of the mixture was placed on a clean slide, and 100 sperm were counted at ×100 magnification (Olympus Japan). Sperms dyed pink or crimson were considered dead, whereas unstained ones were considered alive according to Khalaf et al. (2024). Measurement of testicular and epididymal weight The testes and epididymides weights were determined in all four groups to assess the possible effects of docetaxel (Table 2). The data were collected and analyzed using statistical analysis. Histomorphological examination At the end of the experiment, histomorphological examination was performed on the testes and epididymides samples. Tissues were fixed in a 10% formalin solution for 48 hours (Hammodi and Al Aamery, 2022; Khaleel and Alkhazraji, 2022). The routine histological processes were carried out according to Luna (1968), and they involve three steps: dehydration, clearing, and infiltration. Dehydration was performed by immersing the specimens in a series of ethyl alcohol (from 50%, 70%, 90% to 100%) alcohol, 30 minutes for each. The clearing step was Xylene. This solvent displaces the alcohol in the tissue. The clearing process lasted for 30 minutes. In the third step, infiltration was performed using Molten paraffin to displace xylene. Immediately after tissue embedding, the wax was rapidly cooled to reduce the wax crystal size. Multiple changes of the paraffin wax were performed to completely displace the clearing agent xylene. The samples were sectioned using a 7-micron rotary microtome and then stained with hematoxylin and eosin. The stained sections were examined under a light microscope at different magnifications, and photomicrographs were taken using a digital camera (Sony A7RM4A, 26 megapixels) attached to the microscope. The main fertility parameters are sperm motility, alive sperm, normal appearance, and sperm concentration in the epididymis (Bustani and Baiee, 2021; Ali Hameed et al., 2022). For sperm parameter evaluation, the tail of the epididymis was rinsed and incubated in 2 ml of normal saline at 37°C, then segmented into small sizes via the microscissor to release the epididymal sperms from the epididymal tubules for testes evaluation using the protocol (Tuimah Alabedi et al., 2021; Al-Mousaw et al., 2022). Table 1. Morphological findings of docetaxel effect on motility, alive sperm, normal appearance, and concentrations of epididymal sperms
Table 2. Histomorphological findings of docetaxel effect on weight of testes in g and epididymides in mg, and spermatogonia count 106 per g of testis
Table 3. Histomorphological findings of docetaxel effect on total area in μm2, diameter in μm, and germinal epithelium height in μm of the seminiferous tubules.
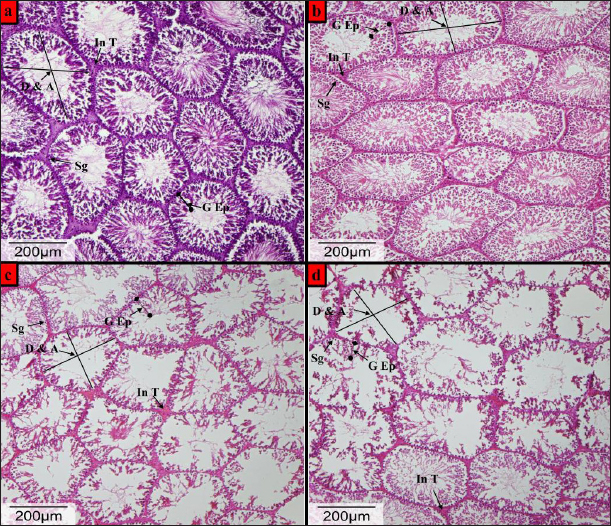
Fig. 1. Photomicrographs of Hematoxylin and Eosin (H&E)-stained testis from the Wistar male rats (a) control group, (b) DX1 group, (c) DX2 group, and (d) DX3 group, showed: Germinal epithelium (G Ep), diameter & total area (D & A), interstitial tissue (In T), spermatogonia (Sg). (b) showing relative histological changes in the height of germinal epithelium and general architecture of the testis. No significant reduction in the area and diameter of seminiferous tubules, and (c and d) showed a progressive adverse effects of chemotherapy on the spermatogenic epithelium, a reduction in total area and diameter of the seminiferous tubules, and damage in the architecture of the testis. H&E-Stain, X-100 x 26-megapixels.
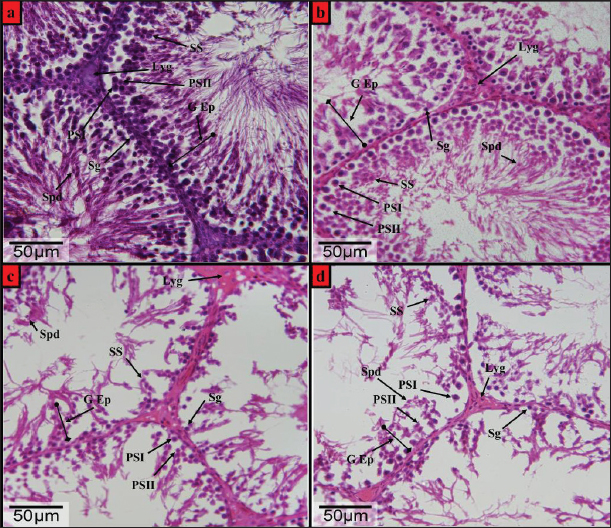
Fig. 2. Photomicrographs of H&E-stained testis from the wistar male rats (a) control group, (b) DX1 group, (c) DX2 group, and (d) DX3 group, showed: Germinal epithelium (G Ep), spermatogonia (Sg), leydig (Lyg), primary spermatocyte-I (PSI), primary spermatocyte-II (PSII), secondary spermatocyte (SS), and spermatid (Spd). (b) showed a relative histological change in the height of germinal epithelium and the general architecture of seminiferous tubules, and there was a significant reduction in the number of spermatogonia. (c and d) showed adverse effect of chemotherapy, a progressive decrease in the height of the germinal epithelium, and damage in the architecture of the seminiferous tubules. H&E-Stain, X-400 x 26-megapixels.
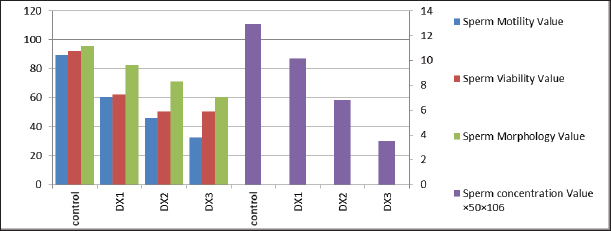
Fig. 3. Showed a statistical comparison among means of the groups: Control, DX1, DX2, and DX3. There was a progressive decrease in the values of motility, alive sperm, sperm morphology, and concentration of epididymal sperms. DX2 and DX3 showed a significant effect of Docetaxel.
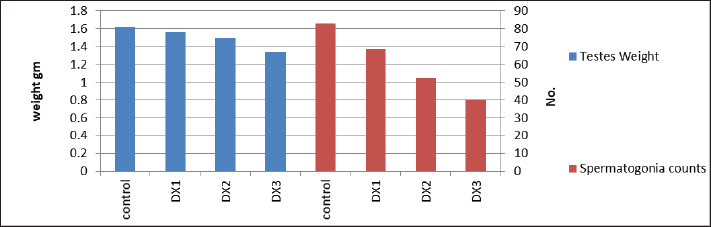
Fig. 4. Showed a statistical comparison among means of the groups: Control, DX1, DX2, and DX3. There was a progressive decrease in the values of the weight of testes in g and spermatogonia count 106 per g of testis. DX2 and DX3 showed a significant effect of Docetaxel.
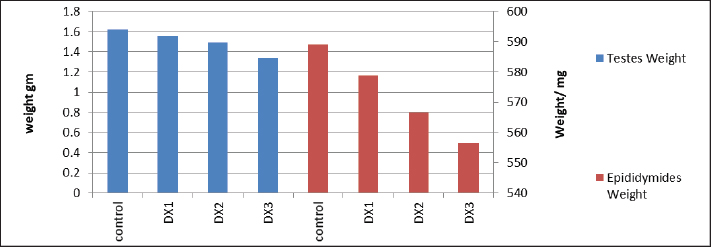
Fig. 5. Showed a statistical comparison among means of the groups: Control, DX1, DX2, and DX3. There was a progressive decrease in the values of the weights of testes in g, and epididymides in mg. DX2 and DX3 showed a significant effect of Docetaxel.
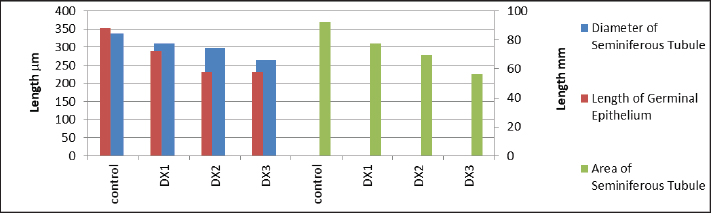
Fig. 6. Showed a statistical comparison among means of the groups: Control, DX1, DX2, and DX3. There was a progressive decrease in the values of total area in μm2, and diameter in μm of the seminiferous tubules, and germinal epithelium length in μm. DX2 and DX3 showed a significant effect of Docetaxel. Statistical analysis The data were tabulated in a datasheet of the Statistical Package for the Social Sciences (IBM, SPSS) version 25.0, which was used for statistical analysis. The mean and standard errors of continuous variables were reported, and significant differences were tested using the analysis of variance test, followed by the least significant difference test. These tests were used to determine the differences between the various concentrations of treatment and control groups in terms of spermatogonia counts, weights of testes, and epididymides (SAS, 2012). ResultsThe current results showed that the low dose (2.5 mg/kg) docetaxel (DX1) had no adverse effects on the fertility of male rats, as shown in Figures 1a and b, 2a and b. Furthermore, histological examination of the testes showed little effect in the seminiferous tubules of the control and DX1 groups compared with DX2 and DX3 groups. In DX2 and DX3 groups, the periphery of the seminiferous tubules appeared thinner and polygonal compared with control group, as shown in Figures 1 and 6 and Table 3. In addition, the germinal epithelium was degenerated compared with control group, as shown in Figures 2b–d and 6 and Table 3. Histological examination of the testes revealed significant changes in DX1, possibly due to apoptosis. However, no significant histological damage was noticed in DX1 group, as shown in Figures 1b and 2b. Table 2 and Figures 4 and 5 showed a progressive decrease in the weight of the testes and epididymides in DX 2 and DX3 groups. Spermatogonia counts decreased in all treatment groups, especially in DX1 group, as shown in Figures 2 and 4 and Table 2. The administration of docetaxel at the highest doses caused a significant decrease in epididymal sperm parameters. The Testes of control group showed normal testicular architecture with well organized seminiferous tubules and Leydig cells, as shown in Figures 1a and 2a. In contrast, the testicular tissues of rats treated with the highest doses of Docetaxel showed degeneration of seminiferous tubules, atrophy, and reduced Leydig cell counts (Figures 1c and d, 2c and d). DiscussionThe study found that Docetaxel at a low dose (2.5 mg/kg) did not affect rats’ fertility negatively (Figures 1a and b, 2a and b). The treatment groups (DX2 and DX3) had lower epididymal parameters and less effect on the seminiferous tubules (Figures 3–6 and Tables 1 and 2). However, the DX1 group did not present any significant damage or changes. However, considerable damage occurred in DX2 and DX3 groups (Figures 1c and d, 2c and d). This is consistent with the findings of (Zhang et al., 2014; Altintas et al., 2015; Sarıözkan et al., 2017). Docetaxel had chemotherapeutic properties represented by the induction of apoptotic cell death and the inhibition of mitosis by promoting the phosphorylation of Bcl-2, a protein known for its antiapoptotic properties, in malignant cells (Sarıözkan et al., 2017). Hence, these findings in DX2 and DX3 suggest that docetaxel may have detrimental effects on male reproductive health and should be used with caution in clinical practice. Docetaxel administration resulted in a significant decline in epididymal sperm parameters, particularly in DX2 and DX3 groups, indicating a progressive decrease in testes and epididymides (Table 2 and Figures 4 and 5). These findings were consistent with those of previous studies showing chemotherapy-induced infertility in male rats (Abdelaziz et al., 2020). The mechanism underlying the reduction in epididymal sperm parameters could be the direct toxic effects of the drug on the testicular and epididymal tissues. Chemotherapeutic drugs, i.e., Docetaxel, have been shown to induce apoptosis and oxidative stress in different cell types (Al Chalabi et al., 2020; Al-Mousaw et al., 2022). The findings of the present study agreed with the findings of (Gelmon, 1994), who demonstrated a reduction in sperm parameters. In addition, Altintas et al. (2015) reported that the administration of Docetaxel leads to a significant decrease in sperm count, motility, and abnormal sperm morphology in rats (Altintas et al., 2015). Furthermore, Sariözkan et al. (2016) reported that docetaxel administration resulted in significant histopathological changes in the testes of adult rats (Sariözkan et al., 2016). The present results were also typically correlated with Moradi et al. (2021), who reported a progressive decline in the spermatogonia count in rodents affected by chemotherapy. A previous study reported a proportional relationship between the spermatogonia count and the weights of the testes and epididymides; thus, a reduction will follow any upgrading decrease in spermatogonia in the diameter of the seminiferous tubules (Robb et al., 1978). This result was similar to the findings in (Tables 2 and 3, Figures 1b–d, 4 and 5). On the other hand, Takzare et al. (2016) reported that the highest doses of morphine and naloxone led to a progressive statistical decrease in the spermatogonia count and the weights of testes and epididymides, consistent with the herein results. The control group’s testes exhibited normal structure (Figures 1a and 2a), whereas rats treated with high doses of Docetaxel showed degeneration of seminiferous tubules, atrophy, and reduced Leydig cell counts (Figures 1c and d, 2c, and d). The current results agreed with prior research that confirmed the occurrence of testicular degeneration after chemotherapy treatment (Altintas et al., 2015; El-Amir et al., 2019; Özyilmaz Yay et al., 2019). The findings of this study revealed that the administration of Docetaxel at doses of 5 and 10 mg/kg could be lead to testicular injury and damage to male reproductive parameters within 28 days. The alterations in the structure of testicular tissue could be ascribed to the direct harmful impact of Docetaxel on Leydig cells and the epithelium responsible for spermatogenesis (Boekelheide, 2005; Sarıözkan et al., 2017). Additionally, it is possible that the damaged testis was influenced by docetaxel-induced oxidative stress and apoptosis. The results of the current study were consistent with several other publications that have assessed the effects of chemotherapy on fertility parameters and testicular tissue (Huyghe et al., 2004; Chatzidarellis et al., 2010; Jacobs and Vaughn, 2012; Meistrich, 2013; Altintas et al., 2015; Polland and Berookhim, 2016; Sarıözkan et al., 2017; Tue Nguyen et al., 2018; Ghafouri-Fard et al., 2021). The effect of doxorubicin, a chemotherapy medication, on male fertility parameters in Wistar rats was explored by (Badkoobeh et al., 2013). Their findings demonstrated a decrease in the number, movement, and survival of sperm. Moreover, histological analysis demonstrated testicular degeneration in the testis of Wistar rats administered doxorubicin (Lee et al., 2012). Similarly, the effects of cisplatin, another chemotherapy drug, on male fertility parameters in Wistar rodents were assessed (Aksu et al., 2016). Sperm parameters decreased, and histological alterations, such as seminiferous tubule atrophy and germ cell apoptosis, were observed in testicular tissue. In their study, Khoei et al. (2018) examined the impact of methotrexate on various parameters associated with male Swiss albino mice’s fertility. The findings revealed a decline in the number, movement, and survival of sperm. Furthermore, a histological study found that testicular degeneration was characterized by an increase in apoptosis and a reduction in the proliferation of germ cells. Prior research has examined the impact of crocin, a saffron extract, on various aspects of male fertility in adult male rodents. These studies had shown a decline in sperm numbers, mobility, and cell survival (Al-Fartwsy et al., 2022; Mohammadpour et al., 2023). Both of these results aligned with the outcomes of this current research, suggesting that the administration of chemotherapy medications at large doses could elicit an adverse effect on testicular health, epididymides, and male fertility parameters. ConclusionThe current study showed that administering docetaxel at large doses harms the reproductive indices and histology of testicular tissue in male Wistar rats. The findings indicate that the testicular toxicity of docetaxel is associated with oxidative stress and may vary depending on the dosage. The implications of the study findings have significant importance for the therapeutic application of docetaxel in patients with cancer within the reproductive age group who may face potential long-term fertility concerns. Additional research is required to examine the mechanisms underlying the harmful impact of Docetaxel on testicular tissue and to devise methods to reduce or prevent its adverse effects. Furthermore, novel treatment approaches should be developed to alleviate the reproductive toxicity of chemotherapy. The formation of collaborative partnerships between oncologists, reproductive specialists, and fundamental scientists holds significance in advancing the understanding of the impact of chemotherapy on male fertility and devising accurate interventions to safeguard reproductive well-being during cancer treatment. It is crucial to adopt a proactive and comprehensive approach to obtain the most favorable treatment outcomes and mitigate adverse reproductive consequences. The objective of this approach is to offer comprehensive healthcare services to individuals diagnosed with cancer, including their oncological and reproductive health. Conflict of interestThe authors declare that they have no competing interests. FundingNo funding was received. Authors’ contributionsConceptualization, M.S.A.; Methodology, M. S.A.; Validation, M. S.A.; Formal Analysis, H.H.K.; Investigation, A.A.H.; Data Curation, E.H.A.A.; Writing—Original Draft Preparation, M.S.A.; Writing—Review and Editing, M.S.A.; Supervision, A.M.H.; Project Administration, H.H.K.; Funding Acquisition, M.S.A. All authors have read and agreed to the publication of the manuscript. Ethical approvalAll experiments were performed according to the guidelines of the Implementing Health Research at the Institutions of Ministry of Health, Iraq (2018), the WHO Code of Conduct for Responsible Research (2017), and Principles of Human Experimental Techniques (Russell and Burch, 1959), for the Use of Experimental Animals (EC-1, 4-3-2024). Data availabilityThe authors confirm that data supporting the findings of this study are available in the manuscript. ReferencesAbdelaziz, A.S., Kamel, M.A., Ahmed, A.I., Shalaby, S.I., El-Darier, S.M., Magdy Beshbishy, A., Batiha, G.E., Alomar, S.Y. and Khodeer, D.M. 2020. Chemotherapeutic potential of Epimedium brevicornum extract: the cGMP-specific PDE5 inhibitor as anti-infertility agent following long-term administration of tramadol in male rats. Antibiotics (Basel) 9(6), 318; doi:10.3390/antibiotics9060318. Abdullahi, S.U. 2022. Cancer chemotherapy: a review update of the mechanisms of actions, prospects and associated problems. J. Biomed. 1(01), 001–016. Agarwal, A., Baskaran, S., Parekh, N., Cho, C.L., Henkel, R., Vij, S., Arafa, M., Panner Selvam, M.K. and Shah, R. 2021. Male infertility. Lancet 397(10271), 319–333; doi:10.1016/S0140-6736(20)32667-2. Aksu, E.H., Kandemir, F.M., Altun, S., Küçükler, S., Çomaklı, S. and Ömür, A.D. 2016. Ameliorative effect of carvacrol on cisplatin-induced reproductive damage in male rats. J. Biochem. Mol. Toxicol. 30(10), 513–520; doi:10.1002/jbt.21816. Al Chalabi, S.M.M., Maahmood, R.I. and Abdul-latiff, R.F. 2020. The effect of some plants extract on hormonal and testicular function in rats. IJPR 12(02), 1223–1228. Al-Fartwsy, A.R.H., Mohammadpour, A.A. and Parham, A. 2022. Evaluation of mice testosterone and sperm parameters affected with Crocin. Comp. Clin. Pathol. 31(1), 109–114; doi:10.1007/s00580-021-03313-1. Al-Mousaw, M., Bustani, G.S., Barqaawee, M.J.A. and AL-Shamma, Y.Mh. 2022. Evaluation of histology and sperm parameters of testes treated by lycopene against cyclophosphamide that induced testicular toxicity in Male rats. Proc. 2021 ISCKU. Conf. 2386(1), 020040; doi:10.1063/5.0067059. Ali Hameed, M., Sabah Al-Khalidi, Z., Abdulhussein Hasan, M. and Sabah Bustani, G. 2022. Effect of kisspeptin-54 on testicular degeneration induced by cadmium chloride. Arch. Razi. Inst. 77(1), 461–466; doi:10.22092/ARI.2021.356811.1918. Altintas, R., Ciftci, O., Aydin, M., Akpolat, N., Oguz, F. and Beytur, A. 2015. Quercetin prevents docetaxel-induced testicular damage in rats. Andrologia 47(3), 248–256; doi:10.1111/and.12253. Badkoobeh, P., Parivar, K., Kalantar, S.M., Hosseini, S.D. and Salabat, A. 2013. Effect of nano-zinc oxide on doxorubicin- induced oxidative stress and sperm disorders in adult male Wistar rats. Iran. J. Reprod. Med. 11(5), 355–364. Boekelheide, K. 2005. Mechanisms of toxic damage to spermatogenesis. J. Natl. Cancer Inst. Monogr. (34), 6–8; doi:10.1093/jncimonographs/lgi006. Bustani, G.S. and Baiee, F.H. 2021. Semen extenders: an evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World 14(5), 1220–1233. Chatzidarellis, E., Makrilia, N., Giza, L., Georgiadis, E., Alamara, C. and Syrigos, K.N. 2010. Effects of taxane-based chemotherapy on inhibin B and gonadotropins as biomarkers of spermatogenesis. Fertil. Steril. 94(2), 558–563; doi:10.1016/j.fertnstert.2009.03.068. El-Amir, Y.O., Yahia, D. and Yousef, M.S. 2019. Protective effect of avenanthramides against cisplatin induced testicular degeneration in rats. JAVR 9(1), 14–22. Available via https://advetresearch.com/index.php/AVR/article/view/347 El-Newary, S.A., Aly, M.S., Hameed, A.R.A.E., Kotp, M.S., Youssef, A.A. and Ali, N.A. 2022. Sperm quality and testicular histopathology of Wistar albino male rats treated with hydroethanolic extract of Cordia dichotoma fruits. Pharm. Biol. 60(1), 282–293; doi:10.1080/13880209.2021.2008455. Engels, F.K., Ten Tije, A.J., Baker, S.D., Lee, C.K., Loos, W.J., Vulto, A.G., Verweij, J. and Sparreboom, A. 2004. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin. Pharmacol. Ther. 75(5), 448–454; doi:10.1016/j.clpt.2004.01.001. Gelmon, K. 1994. The taxoids: paclitaxel and docetaxel. Lancet 344(8932), 1267–1272; doi:10.1016/s0140-6736(94)90754-4. Ghafouri-Fard, S., Shoorei, H., Abak, A., Seify, M., Mohaqiq, M., Keshmir, F., Taheri, M. and Ayatollahi, S.A. 2021. Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants. Biomed. Pharmacother. 142, 112040; doi:10.1016/j.biopha.2021.112040. Hammodi, N.M.J. and Al Aamery, R.A. 2022. Histological and immunohistochemical study of thyroid gland in Caucasian squirrel (Sciurus anamalus) (Gmelin, 1778) by using marker (Anti-Thyroglobulin, Code IR5090). PENS 10(6), 104–112. Hussin, A.M. and Al-Haaik, A. 2018. Seasonal histological and Morphometrical changes in the Testis of Adult Awassi Ram: angiogenesis. JEZS 5(5), 1108–1112. Huyghe, E., Matsuda, T., Daudin, M., Chevreau, C., Bachaud, J.M., Plante, P., Bujan, L. and Thonneau, P. 2004. Fertility after testicular cancer treatments: results of a large multicenter study. Cancer 100(4), 732–737; doi:10.1002/cncr.11950. Imran, M., Saleem, S., Chaudhuri, A., Ali, J. and Baboota, S. 2020. Docetaxel: an update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. JDDST 60, 101959; doi:10.1016/j.jddst.2020.101959. Jacobs, L.A. and Vaughn, D.J. 2012. Hypogonadism and infertility in testicular cancer survivors. J. Natl. Compr. Canc. Netw. 10(4), 558–563; doi:10.6004/jnccn.2012.0053. Khalaf, A.A., Ogaly, H.A., Ibrahim, M.A., Abdallah, A.A., Zaki, A.R. and Tohamy, A.F. 2022. The reproductive injury and oxidative testicular toxicity induced by chlorpyrifos can be restored by zinc in male rats. Biol. Trace. Elem. Res. 200(2), 551–559; doi:10.1007/s12011-021-02704-3. Khalaf, H.H., Al-Juhaishi, O.A. and Ashour, M.S. 2024. Investigation of the morphological and histological features of the testes of pigeon (Columba livia domestica) in pre-puberty and post-puberty. OVJ 14(9), 2163–2169; doi:10.5455/OVJ. 2024.v14.i9.5. Khaleel, I.M. and Alkhazraji, K.I.A. 2022. A comparative histomorphological and histochemical study of the ventriculus between Iraqi adult geese (Anser anser) and guinea fowls (Numidia meleagris). Rev. Bionatura. 7(3), 1–7; doi:10.21931/RB/2022.07.03.37. Khoei, H.H., Fakhri, S., Parvardeh, S., Shams Mofarahe, Z.S., Baninameh, Z. and Vardiani, M. 2018. Astaxanthin prevents the methotrexate-induced reproductive toxicity by targeting oxidative stress in male mice. Toxin Rev. 38(3), 248–254. King, D. 2023. Seminiferous tubule. Southern Illinois University Carbondale, School of Medicine/Anatomy. U.S.A., Illinois. Available via https://histology.siu.edu/erg/testis.htm Lee, K.M., Lee, I.C., Kim, S.H., Moon, C., Park, S.H., Shin, D.H., Kim, S.H., Park, S.C., Kim, H.C. and Kim, J.C. 2012. Melatonin attenuates doxorubicin-induced testicular toxicity in rats. Andrologia 44(Suppl 1), 796–803; doi:10.1111/j.1439-0272.2011.01269.x. Leslie, S.W., Soon-Sutton, T.L. and Khan, M.A.B. 2025. Male infertility. In StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY- NC-ND 4.0) (http://creativecommons.org/licenses/by-nc-nd/4.0/) Available via https://www.ncbi.nlm.nih.gov/books/NBK562258/ Lopes, F., Smith, R., Anderson, R.A. and Spears, N. 2014. Docetaxel induces moderate ovarian toxicity in mice, primarily affecting granulosa cells of early growing follicles. Mol. Hum. Reprod. 20(10), 948–959; doi:10.1093/molehr/gau057. Luna, L.G. 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd ed. New York City, NY: Blakiston Division, McGraw-Hill. pp: 12–21. http://books.google.com/books?id=19NOAQAAIAAJ Meistrich, M.L. 2013. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 100(5), 1180–1186; doi:10.1016/j.fertnstert.2013.08.010. MOH. 2018. The ethical guide for implementing health research at the Institution of the Ministry of Health. Baghdad, Iraq: Ministry of Health, page: 18. https://moh.gov.iq/upload/886.pdf Mohammadpour, A.A., Al-Fartwsy, A.R.H. and Parham, A. 2023. Effect of crocin on the spermatogenesis indices of mice testis: a histopathological and histomorphological study. EJVS 54(2), 297–308; doi:10.21608/ejvs.2023.165515.1400. Moradi, M., Goodarzi, N., Faramarzi, A., Cheraghi, H., Hashemian, A.H. and Jalili, C. 2021. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. Biomed. Pharmacother. 138, 111481; doi:10.1016/j.biopha.2021.111481. Özyilmaz Yay, N.O., Şener, G. and Ercan, F. 2019. Resveratrol treatment reduces apoptosis and morphological alterations in cisplatin induced testis damage. J. Res. Pharm. 23(4), 621–631. Polland, A. and Berookhim, B.M. 2016. Fertility concerns in men with genitourinary malignancies: treatment dilemmas, fertility options, and medicolegal considerations. Urol. Onco. 34(9), 399–406; doi:10.1016/j.urolonc.2016.05.007. Robb, G.W., Amann, R.P. and Killian, G.J. 1978. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 54(1), 103–107; doi:10.1530/jrf.0.0540103. Rogers, K. 2010. The reproductive system. In The human body. Chicago, IL: Britannica Educational Publishing. Russell, W.M.S. and Burch, R.L. 1959. The principles of human experimental technique. Wheathampstead, UK: Universities Federation for Animal Welfare, 238 pp. Available via https://www.cabidigitallibrary.org/doi/full/10.5555/19592204037 Sandström, M., Simonsen, L.E., Freijs, A. and Karlsson, M.O. 1999. The pharmacokinetics of epirubicin and docetaxel in combination in rats. Cancer Chemother. Pharmacol. 44(6), 469–474; doi:10.1007/s002800051120. Sarıözkan, S., Türk, G., Eken, A., Bayram, L.Ç., Baldemir, A. and Doğan, G. 2017. Gilaburu (Viburnum opulus L.) fruit extract alleviates testis and sperm damages induced by taxane-based chemotherapeutics. Biomed. Pharmacother. 95, 1284–1294; doi:10.1016/j.biopha.2017.09.057. Sariözkan, S., Türk, G., Güvenç, M., Yüce, A., Özdamar, S., Cantürk, F. and Yay, A.H. 2016. Effects of cinnamon (C. zeylanicum) bark oil against taxanes-induced damages in sperm quality, testicular and epididymal oxidant/antioxidant balance, testicular apoptosis, and sperm DNA integrity. Nutr. Cancer 68(3), 481–494; doi:10.1080/01635581.2016.1152384. Saruhan, Ç. 2020. Cancer, side effects of chemotherapy and nursing care. IJHSRP 5(1), 51–63; doi:10.33457/ijhsrp.670942. SAS. 2012. Statistical analysis system, user’s guide. In Statistical. Version 9.1th ed. Cary, NC: SAS. Inst. Inc. Takzare, N., Samizadeh, E., Shoar, S., Majidi Zolbin, M., Naderan, M., Lashkari, A. and Bakhtiarian, A. 2016. Impacts of morphine addiction on spermatogenesis in rats. Int. J. Reprod. Biomed. 14(5), 303–308. Tegelenbosch, R.A. and de Rooij, D.G. 1993. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 290(2), 193–200; doi:10.1016/0027-5107(93)90159-d. Tue Nguyen, H.M.T., Heidenberg, D.J. and Sikka, S.C. 2018. Chapter 17 - Androgen receptor modulators: the impact of environment and lifestyle choices on reproduction. In Bioenvironmental issues affecting men’s reproductive and sexual health. Eds., Sikka, S.C. and Hellstrom, W.J.G. Cambridge, MA: Academic Press, pp: 277–292; doi:10.1016/B978-0-12-801299-4.00017-7. Tuimah Alabedi, G.S., Al-Baghdady, H.F., Alahmer, M.A., Bustani, G.S. and Al-Dhalimy, A.M.B. 2021. Effects of Ocimum tenuiflorum on induced testicular degeneration by filgrastim in wistar rats. Arch. Razi. Inst. 76(5), 1555–1559; doi: 10.22092/ari.2021.356079.1772. Wang Y. 2003. Epididymal sperm count. Curr. Protoc. Toxicol. Chapter 16, Unit16.6; doi:10.1002/0471140856.tx1606s14. WHO. 2017. Code of conduct for responsible research. Geneva, Switzerland: World Health Organization, pp: 26. Yardım, A., Kucukler, S., Özdemir, S., Çomaklı, S., Caglayan, C., Kandemir, F.M. and Çelik, H. 2021. Silymarin alleviates docetaxel-induced central and peripheral neurotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Gene 769, 145239; doi:10.1016/j.gene.2020.145239. Zhang, L., Yang, Y., Song, Y., Yang, H., Zhou, G., Xin, Y., You, Z. and Xuan, Y. 2014. Nanoparticle delivery systems reduce the reproductive toxicity of docetaxel in rodents. Nano Life 4(04), 1441012; doi:10.1142/S1793984414410128. Zhao, X., Wen, X., Ji, M., Guan, X., Chen, P., Hao, X., Chen, F., Hu, Y., Duan, P., Ge, R.S. and Chen, H. 2021. Differentiation of seminiferous tubule-associated stem cells into leydig cell and myoid cell lineages. Mol. Cell. Endocrinol. 525, 111179; doi:10.1016/j.mce.2021.111179. | ||
| How to Cite this Article |
| Pubmed Style Ashour MS, Hussein AA, Al-shuwaili EHA, Hussin AM, Khalaf HH. Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Vet. J.. 2025; 15(3): 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 Web Style Ashour MS, Hussein AA, Al-shuwaili EHA, Hussin AM, Khalaf HH. Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. https://www.openveterinaryjournal.com/?mno=227690 [Access: January 16, 2026]. doi:10.5455/OVJ.2025.v15.i3.6 AMA (American Medical Association) Style Ashour MS, Hussein AA, Al-shuwaili EHA, Hussin AM, Khalaf HH. Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Vet. J.. 2025; 15(3): 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 Vancouver/ICMJE Style Ashour MS, Hussein AA, Al-shuwaili EHA, Hussin AM, Khalaf HH. Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Vet. J.. (2025), [cited January 16, 2026]; 15(3): 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 Harvard Style Ashour, M. S., Hussein, . A. A., Al-shuwaili, . E. H. A., Hussin, . A. M. & Khalaf, . H. H. (2025) Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Vet. J., 15 (3), 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 Turabian Style Ashour, Muntadher Salman, Ashwaq Ahmed Hussein, Entekhab Hameed Abed Al-shuwaili, Amer M. Hussin, and Harith Humadi Khalaf. 2025. Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Veterinary Journal, 15 (3), 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 Chicago Style Ashour, Muntadher Salman, Ashwaq Ahmed Hussein, Entekhab Hameed Abed Al-shuwaili, Amer M. Hussin, and Harith Humadi Khalaf. "Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats." Open Veterinary Journal 15 (2025), 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 MLA (The Modern Language Association) Style Ashour, Muntadher Salman, Ashwaq Ahmed Hussein, Entekhab Hameed Abed Al-shuwaili, Amer M. Hussin, and Harith Humadi Khalaf. "Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats." Open Veterinary Journal 15.3 (2025), 1140-1149. Print. doi:10.5455/OVJ.2025.v15.i3.6 APA (American Psychological Association) Style Ashour, M. S., Hussein, . A. A., Al-shuwaili, . E. H. A., Hussin, . A. M. & Khalaf, . H. H. (2025) Histomorphological evaluation of docetaxel effects on testes and epididymides in Wistar rats. Open Veterinary Journal, 15 (3), 1140-1149. doi:10.5455/OVJ.2025.v15.i3.6 |